Abstract
The scope of this investigation was to understand the role of aquaporin 5 (AQP5) for maintaining lens transparency and homeostasis. Studies were conducted using lenses of wild-type (WT) and AQP5 knockout (AQP5-KO) mice. Immunofluorescent staining verified AQP5 expression in WT lens sections and lack of expression in the knockout. In vivo and ex vivo, AQP5-KO lenses resembled WT lenses in morphology and transparency. Therefore, we subjected the lenses ex vivo under normal (5.6 mM glucose) and hyperglycemic (55.6 mM glucose) conditions to test for cataract formation. Twenty four hours after incubation in hyperglycemic culture medium, AQP5-KO lenses showed mild opacification which was accelerated several fold at 48 hours; in contrast, WT lenses remained clear even after 48 hours of hyperglycemic treatment. AQP5-KO lenses displayed osmotic swelling due to increase in water content. Cellular contents began to leak into the culture medium after 48 hours. We reason that water influx through glucose transporters and glucose cotransporters into the cells could mainly be responsible for creating hyperglycemic osmotic swelling; absence of AQP5 in fiber cells appears to cause lack of required water efflux, challenging cell volume regulation and adding to osmotic swelling. This study reveals that AQP5 could play a critical role in lens microcirculation for maintaining transparency and homeostasis, especially by providing protection under stressful conditions. To the best of our knowledge, this is the first report providing evidence that AQP5 facilitates maintenance of lens transparency and homeostasis by regulating osmotic swelling caused by glucose transporters and cotransporters under hyperglycemic stressful conditions.
1. Introduction
Cataract or lens opacity is the leading cause of visual impairment throughout the world. Over 24.4 million Americans age 40 and above are affected by cataract and more than 50% at age 80 suffer from cataract. Diabetes mellitus affects more than 285 million people worldwide [1] and is a major risk factor for cataract [2]. Onset of cataract is ~20 years earlier in diabetic patients than in non-diabetics [3]. In diabetes, high blood sugar or hyperglycemia results in ocular lens swelling leading to visual impairment. Mechanism of lens swelling has not been addressed satisfactorily thus far.
The lens of adult mammalian eye is devoid of vasculature to remain transparent for focusing objects. It consists of an epithelial cell monolayer (Fig.1A) that extends from the anterior pole to the equatorial surface. Multilayered fiber cells form bulk of the lens, the oldest cells being deep in the interior. Secondary fiber cells differentiate from the equatorial epithelial cells and cover the older fiber cells, the youngest being at the outermost layer. To meet metabolic demands, the avascular lens tissue is postulated to have unique microcirculatory mechanisms, based on the asymmetrically distributed ion pumps, transporters, water channels and gap junction channels [4,5].
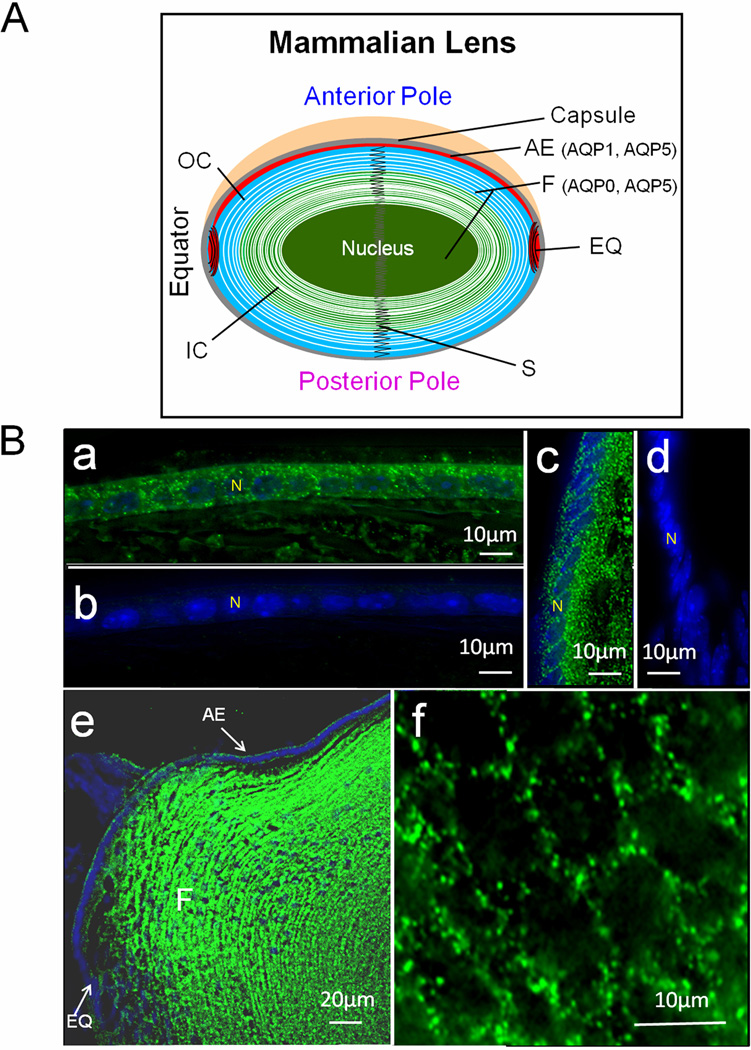
Fig. 1.
A. Schematic representation of the expression patterns of AQP0, AQP1 and AQP5 in adult mouse lens. B. Immunostaining using anti-AQP5 antibody in lens cryosections. a: WT epithelial cells; b: AQP5-KO epithelial cells; c: WT lens equatorial region showing AQP5 in epithelial and differentiating fiber cells; d: AQP5-KO equatorial epithelial cells; e: Sagittal section, WT lens epithelial and fiber cells showing immunoreactivity to anti-AQP5 antibody; f: Cross-sectional view of WT cortex fiber cells showing AQP5 expression. a–f: FITC-conjugated secondary antibody; green, antibody binding; blue, nuclear staining by DAPI. White arrows – antibody binding. AE, anterior epithelial cells; EQ, equatorial epithelial cells; F, fiber cells; IC- Inner cortex; N-nucleus; OC- outer cortex; S- suture.
Aquaporin (AQP) water channels allow passage of water and/or small neutral solutes across cell membranes based on osmotic gradient, thus taking part in lens microcirculation. Mutations or lack of expression of aquaporins in mammals cause pathophysiological conditions indicating their important role/s in cellular water homeostasis. Until recently, it was thought each type of lens cell expresses exclusively one specific member of the aquaporin family i.e., AQP0 in the fiber cells and AQP1 in the epithelial cells. The spatial expression of a third member of the aquaporin family, namely AQP5, in both epithelial and fiber cells was demonstrated lately [6.7] even though its presence was identified earlier by RT-PCR [8] and mass spectrometry [9]. In lens, anterior epithelial cells express AQP1 and AQP5 [10]. AQP1 functions as a water channel in the epithelial cells [11,12,13]. Knockout of AQP1 caused lens cataract only under stressful conditions [13]. Lens fiber cells express AQP0 and AQP5. AQP0 provides water permeability [11,14], and cell-to-cell adhesion [15,16]. Mutations as well as knockout of AQP0 caused lens cataract.
AQP5 is expressed in several secretory tissues, retina, cornea, and lens. AQP5-KO mouse model studies have corroborated the role of AQP5 in salivary secretion [17,18] and corneal thickness [19]. Phosphorylation of AQP5 results in internalization of the protein from plasma membrane [6]. Even though spatial distribution of AQP5 in lens has been studied, there is no research yet to find out whether it has any role in maintaining lens transparency and homeostasis. The aim of the current investigation was to explore the involvement of AQP5 in lens transparency and homeostasis.
In this study, we tested WT and AQP5-KO lenses under hyperglycemic condition ex vivo and demonstrated for the first time that presence of AQP5 is critical for maintaining lenticular osmotic balance as was evident from cataractogenesis of the experimental lenses in contrast to control. Moreover, we provide possible answers for: Why and how does swelling occur in lens under hyperglycemic condition? This study opens up a realm of future explorations with the potential for developing therapeutic strategies for senile and diabetic cataracts.
2. Materials and methods
2.1. Animals, lens isolation and immunofluorescent staining
Wild type (WT; C57BL/6J: Jackson Laboratory) and AQP5 knockout (AQP5-KO) mice (provided by Dr. A.G. Menon [18]) were used. All procedures were performed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by Stony Brook University Animal Care and Use Committee. Lenses were dissected out from eyes under a light microscope without damaging lens capsules. Immunofluorescent staining was performed as described previously [6].
2.2. Ex vivo model of cataract formation
Hyperglycemia-induced ex vivo cataractogenesis was studied as previously described [13,20]. Lenses from WT and AQP5-KO mice were dissected out aseptically, rinsed in saline and transferred to 12-well culture plates containing culture medium supplemented with 5.6 mM (normal, control) or 55.6 mM glucose (hyperglycemic). Incubations were done at 37°C in a humidified atmosphere with 5% CO2. Lens opacity was quantified using a digital camera-fitted stereo-zoom microscope [12].
2.3. Lens water content, transparency and focusing ability
Water content of four month-old WT and AQP5-KO mouse lenses was quantified by measuring wet and dry weights after 60 hours of hyperglycemic stress. Lenses (8 each from WT and AQP5-KO mice) were weighed for wet weight, dried in a vacuum oven (98°C, 24 hrs) and weighed again for dry weight. Percentage of water content was calculated [21]: (wet weight − dry weight)*100/wet weight), expressed as mean ± SD and analyzed using Paired t-test.
Lens transparency was quantified as described by Varadaraj et al.[12]. In brief, lenses treated with normal or hyperglycemic medium for 48 hours were photographed digitally and analyzed (SigmaScan Pro, Version 5.0). The transparency index of each lens was calculated by dividing total intensity by number of pixels and was high in less-transparent lenses.
Focusing ability was assessed qualitatively using grid images of normal glucose (5.6mM)-treated and hyperglycemic (55.6mM glucose) WT and AQP5-KO lenses after 24 hours. Imaging was performed under dark field [21].
Results and Discussion
Immunofluorescent staining of WT and AQP5-KO mouse lens cryosections (Fig.1B) verified our previous observations [6]. AQP5 expression in the WT lens epithelial cells (Fig.1B a) was corroborated by negative immunostaining of AQP5-KO lens (Fig.1B b). WT lens equatorial region showed positive staining (Fig.1B c) in contrast to AQP5-KO (Fig.1B d). Overall, AQP5 was expressed in the epithelial and fiber cells (Fig.1B e). Enlarged image of cortical fiber cells demonstrated AQP5 in the plasma membrane (Fig.1B f). In epithelial cells, expression of AQP5 was several fold less than AQP1 [6].
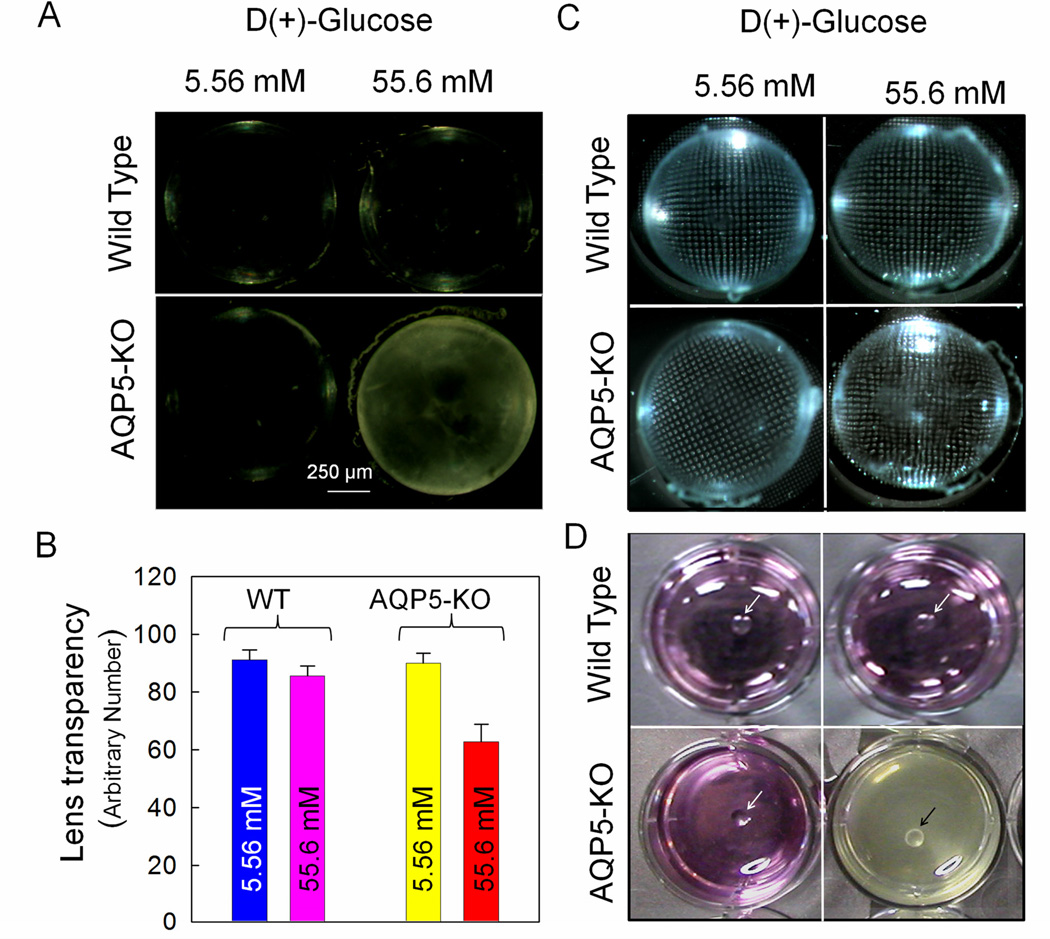
Expression of a second aquaporin in fiber cells (apart from AQP0) and epithelial cells (apart from AQP1) prompted us to explore its functional significance. Since AQP5-KO mouse lenses appeared as normal as those of WT, we followed hyperglycemia-induced ex vivo cataractogenesis model to investigate whether AQP5 plays a role in lens microcirculation. The glucose concentration selected for hyperglycemia induction was 55.6 mM, as observed in diabetic cataracts in vivo [22] and had been used customarily to induce cataract [13] as well as to test the cataractogenic effects of drugs [23]. We tested two concentrations for WT and AQP5-KO lenses; 5.56 mM which is equivalent to the normal glucose level, and 55.6 mM hyperglycemic level. After 24 hours of incubation, only high glucose-treated lenses of AQP5-KO mouse showed mild opacification. AT 48 hours, hyperglycemic AQP5-KO lenses developed severe opacity while other lenses remained clear (Fig.2A). Quantification of lens transparency revealed significantly less transparency in AQP5-KO lenses treated with high glucose (Fig.2B) than other lenses. Lens transparency was 31% less compared to WT normal glucose (5.56 mM)-treated, 27% less compared to WT high glucose (55.6mM)-treated and 30% less compared to normal glucose-treated AQP5-KO lenses (P < 0.001). An earlier report [13] found accelerated cataractogenesis in AQP1 knockout mouse lenses exposed to hyperglycemic medium. As expected, development of cataract affected focusing ability. Hyperglycemic AQP5-KO lenses exhibited distorted focusing of grid compared to the rest of the lenses (Fig.2C).
Fig. 2.
Effect of acute hyperglycemia on mouse lens transparency. (A) WT and AQP5-KO lenses after 48 hours of incubation in normal (5.56 mM glucose) or hyperglycemic (55.6 mM glucose) culture medium. (B) Quantification of lens transparency of WT and AQP5-KO lenses incubated for 48 hours in normal or hyperglycemic culture medium. Mean ± SD. *P< 0.001. (C) Focusing of metal grid transmitted through lenses of WT and AQP5-KO mice after 24 hours of incubation in normal or hyperglycemic condition. Note: AQP5-KO lens exposed to 55.6 mM glucose shows distorted grid lines due to fiber cell swelling and cataract formation. (D). Photographs of WT and AQP5-KO lenses after 60 hours of incubation in normal and hyperglycemic conditions. AQP5-KO mouse lens leaked cellular contents into the culture dish. Arrows point to the lenses in culture dishes.
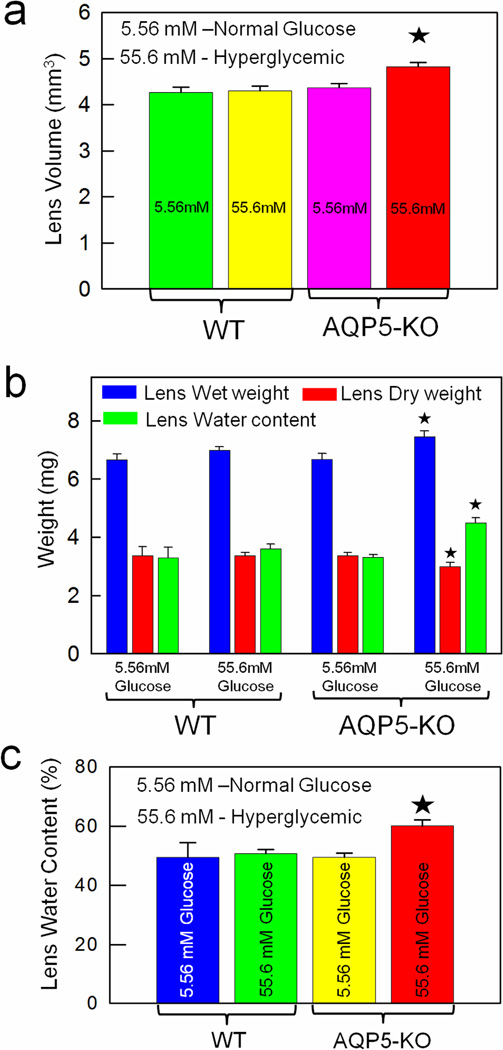
A noticeable effect of hyperglycemic exposure was swelling of AQP5-KO lenses which developed lesions, leaked out cytosolic contents that formed a white precipitate changing the pH and color of the culture medium (Fig.2D). Therefore, we measured lens volume before leakage began. Figure 3a shows increase in volume of hyperglycemic (55.6mM glucose) AQP5-KO lenses (~12%) due to increased water influx in comparison to normal glucose-treated AQP5-KO lenses. Wet weight, dry weight and water content of WT and AQP5-KO lenses under normal and hyperglycemic conditions were quantified after 60 hours of incubation (Fig.3b). Compared to AQP5-KO lenses treated with normal glucose medium, wet weight (15%) and water content (12%) of high-glucose-treated AQP5-KO lenses increased significantly (P<0.01) whereas dry weight decreased (~12%) significantly (P< 0.01). WT lenses treated with normal or high glucose yielded comparable values as the normal glucose-treated AQP5-KO lenses. WT and AQP5-KO lenses treated with high glucose had 49 ± 5% and 60 ± 2%, water content, respectively (Fig. 3c). Water content of AQP5-KO lens was significantly higher (P< 0.001) than that of other groups suggesting absence of AQP5 could be responsible for lens swelling and cataractogenesis. In all probability, AQP5 seems a necessary component in lens microcirculation to maintain transparency and homeostasis. AQP1 knockout mouse lenses treated with high glucose also exhibited increase in water content [13]. In addition, diabetic human [24] and rat [25] lenses showed increased water content in hyperglycemic environment.
Fig. 3.
Qualitative biometric measurements of WT and AQP5-KO mouse lenses incubated under normal and hyperglycemic condition. Comparison of: (a) Lens volume; (b) Wet weight, dry weight and water content; (c) Water content percentage. *Statistically significant.
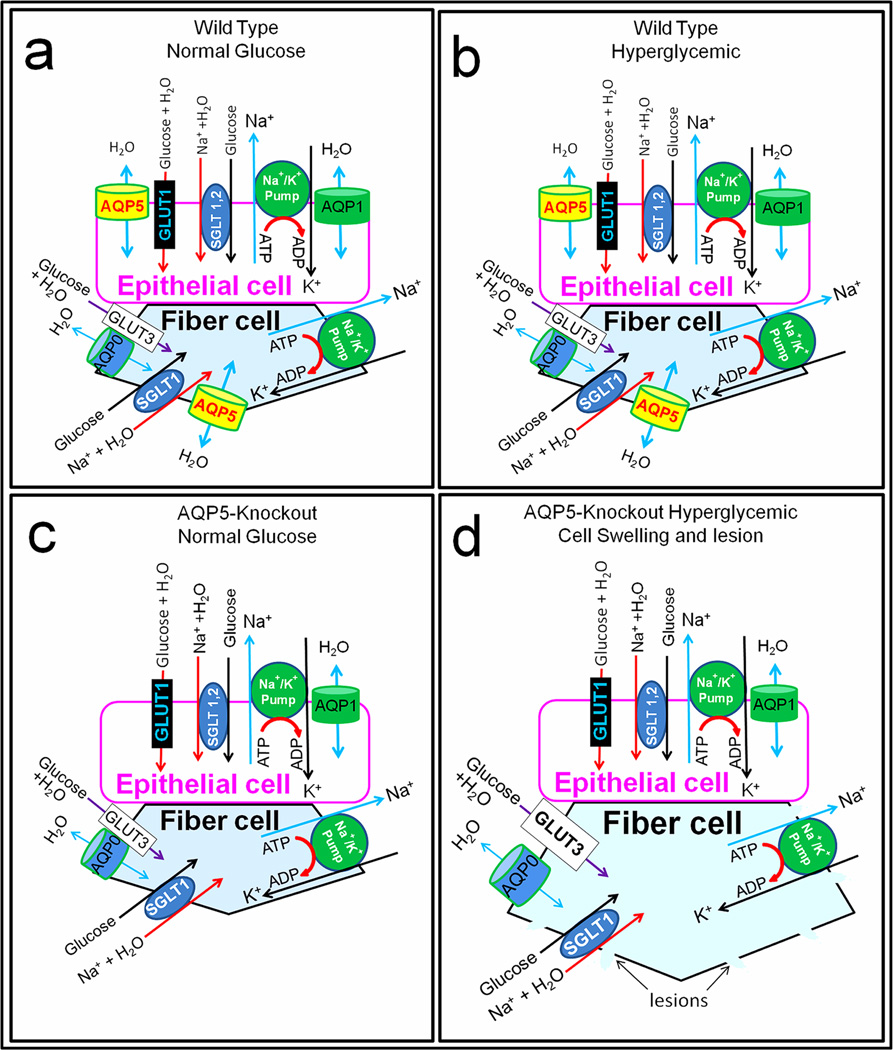
What is the mechanism behind hyperglycemia-induced cataractogenesis? Based on current and previous studies we propose schematic models (Fig.4) to illustrate the possible molecular mechanism by which the tested lenses remained transparent or developed opacity/cataract. The possible events in a representative epithelial cell and fiber cell are depicted in each model. Figure 4a represents the WT lens treated with normal glucose concentration and did not develop lens opacity. Epithelial cells express large quantities of AQP1 and trace amounts of AQP5 [6]; glucose transporter GLUT1 [26–28] and sodium-dependent glucose cotransporters, SGLT1 and SGLT2 [28,29] are also expressed and transport water along with glucose [30–32]. For example, SGLT1 can transport one glucose, two sodium and 378 molecules of water in rabbit and 234 in human [32]. In WT mouse lens under normal glucose condition (Fig. 4a), normal levels of glucose and sodium along with water enter lens epithelial cells. Glucose is used for normal metabolism. Na+/K+-ATPase, which is abundantly expressed in the epithelial cells [33], pumps excess sodium out and causes excess water to leave through AQP1 and AQP5 water channels creating cellular homeostasis. In lens fiber cells under normal glucose condition (Fig.4a), as in the epithelial cells, normal levels of Na+ and water enter along with glucose through appropriate channels GLUT3 [26,27,28] and SGLT1 [28,29]. Glucose is metabolized, and normal functioning of Na+/K+-ATPase [33], AQP0 and AQP5, ensures that excess sodium is pumped out and excess water escapes through the water channels without causing osmotic cell swelling.
Fig. 4.
Schematic models illustrating possible regulation of cellular osmotic stress through AQP5 water efflux to maintain lens homeostasis and transparency. (a,b,c,d) In epithelial cells, water enters along with glucose through glucose transporter GLUT1, and with glucose and sodium through sodium glucose cotransporters SGLT1 and 2. Na+/K+-ATPase pumps the excess sodium out. In (a) and (b) AQP1 and AQP5 are present for water efflux to maintain homeostasis while in (c) and (d) only AQP1 is present; being a highly efficient water channel, lack of AQP5 might not have affected the homeostasis of the cell in (c) and (d). In fiber cells, water enters along with glucose through GLUT3, and with glucose and sodium through SGLT1. Na+/K+-ATPase pumps excess sodium out in (a), (b) and (c). In (a) and (b) AQP0 and AQP5 are present for water efflux to maintain homeostasis. In (c) and (d) AQP5 is knocked out and only AQP0 is present. In (c) with normal glucose, AQP0 was able to create enough water outflow and there was no cataract development. However, in (d) AQP0 being a poor water transporter, absence of AQP5 affected water efflux causing water retention and swelling under hyperglycemic conditions. Na+/K+-ATPase probably could not pump excess sodium out due to lack of enough water efflux. Accumulation of water, sodium and glucose caused osmotic stress resulting in fiber cell lesions, leakage and subsequently, cataract.
Under hyperglycemic condition (Fig.4b) WT lenses did not develop cataract; presumably, similar events described above take place in the WT lens epithelial cells and fiber cells. Glucose and water enter through GLUT1 in epithelial cells and GLUT3 in fiber cells; glucose, sodium and water enter through SGLT1 and SGLT2 in epithelial cells and through SGLT1 in fiber cells. It has been reported that under hyperglycemic conditions, GLUT3 in fiber cells but not GLUT1 in the epithelial cells was upregulated [27]. Continual entry of glucose, sodium and water activates Na+/K+-ATPase to pump excess sodium out and force water to exit through AQP1 and AQP5 in the epithelial cells and through AQP0 and AQP5 in the fiber cells, thus maintaining homeostasis. Under hyperglycemic condition (55.6mM glucose) excess glucose is converted to sorbitol in rat and other animal models that express large quantities of the enzyme aldose reductase (AR) and accumulation of sorbitol causes osmotic stress [34,35]. It has been reported that mouse lens expresses only very low quantities of AR which is the case in humans also [35,36]. Ruiz-Ederra and Verkman [13] also showed that wild type mouse lens did not develop cataract when subjected to hyperglycemic conditions.
AQP5-KO mouse lens subjected to normal glucose treatment did not develop opacity/cataract (Fig.4c). The events described above for epithelial and fiber cells of the WT (Fig 4a) appear to take place even in the absence of AQP5 (Fig.4c). Presumably, in the epithelial cells, AQP1 which is expressed much more than AQP5 and as efficient as AQP5 in water permeability, is able to compensate for the absence of AQP5. Similarly in the fiber cells, AQP0 a much less efficient water channel compared to AQP5 but expressed abundantly in the membranes is able to compensate and create homeostasis in the absence of AQP5 under the normal glucose condition.
Figure 4d demonstrates the events leading to AQP5-KO mouse lens developing opacity/cataract under hyperglycemic conditions. In AQP5-KO lens epithelial cells, as mentioned above, a large amount of sodium and water enter along with glucose. A small portion of the excess glucose may be converted into sorbitol which may not create a considerable osmotic gradient but continual entry and accumulation of glucose, sodium and water activates Na+/K+-ATPase to pump the extra sodium out; water leaves through AQP1, which is expressed in large quantities in epithelial cells and 40-fold more efficient than AQP0 expressed in fiber cells, thus establishing homeostasis in epithelial cells. Lack of AQP5, which is expressed in very low quantities might not have caused deleterious effects in epithelial cells. A study by Ruiz-Ederra and Verkman [13] showed that AQP1 knockout mouse also developed hyperglycemia-induced lens opacity/cataract. Deficiency of AQP1 which is expressed in large quantities in lens epithelial cells compared to AQP5 might have led to osmotic swelling and stress causing opacity/cataract. This suggests that AQP1 could be the primary water channel in the lens epithelial cells and AQP5 could be a secondary water channel.
The main reason for cataract formation appears to be lack of AQP5, a highly efficient water channel in the fiber cells. In AQP5-KO lens (Fig.4d), along with glucose large quantities of Na+ and water enter the fiber cells; glucose to sorbitol conversion is very low in mouse due to low quantities of AR enzyme. However, osmotic stress develops due to continuous intake of glucose, sodium and water. This activates Na+/K+-ATPase in the fiber cells to pump excess sodium out and make water egress through AQP0. Even though AQP0 is present in large quantities, being a much less efficient water channel than AQP5 and performing an additional function of cell-to-cell adhesion [16,21,37], AQP0 probably is unable to accomplish enough water efflux to compensate for the absence of AQP5, leading to accumulation of water and sodium along with glucose and instigating osmotic stress-induced fiber cell swelling. Relentless swelling results in loss of cellular homeostasis, development of fiber cell membrane lesions and lens opacity/cataract. This further emphasizes that the primary role of AQP0 could be cell-to-cell adhesion and secondary function could be water permeability in the lens fiber cell membrane. Hyperglycemia-induced cataractogenesis of AQP5-KO lens suggests AQP5 could be the primary water channel of the fiber cells and its presence is crucial for cell volume regulation. As a secondary effect of fiber cell lesion, leakage and necrosis, the epithelial cells might have suffered osmotic stress and breakage. Currently we are investigating the in vivo functions of AQP0 and AQP5 using double knockouts and mutant AQP0 and AQP5 models.
Reports indicate reduction in the expression of AQP1 in kidney [13,38] and AQP5 in lung [39] due to aging. This implies the possibility that reduction/modification in functional lens AQP5 due to aging could induce age-onset (senile) lens opacity/cataract in human. Our data showed increased lens volume and water content as well as loss of transparency in AQP5-KO lens due to hyperglycemic stress. Increased water content in lens nuclear region due to aging has been documented [40]; we speculate aging-related alteration/reduction in functional AQP5 could be a reason for the increase in water content.
Diabetic patients are prone to lens cataract, corneal epitheliopathy, retinopathy and glaucoma. A diabetic rat model study reported upregulation of AQP5 expression in retinal pigment epithelial (RPE) cells [41]. This upregulation may allow a higher rate of fluid transport across RPE cells to reduce/prevent subretinal edema which if left unchecked could cause retinal detachment. This observation further supports our interpretation that hyperglycemia-induced osmotic stress was thwarted in WT lens most probably by the involvement of AQP5-mediated water efflux; AQP5 deficiency in knockout lens caused cataract due to continual water influx, lens swelling, leakage and uninhibited osmotic stress.
To our knowledge this investigation is the first to show the possible involvement of AQP5 in maintaining lens transparency and homeostasis through its protective role during stressful conditions like diabetes-induced hyperglycemia. This study also identifies the water influx glucose transporters and cotransporters cause under hyperglycemic conditions in the absence of AQP5, resulting in osmotic swelling and disruption of lenticular homeostasis. Our data point to the possibility of AQP5 and glucose transporters and cotransporters as potential therapeutic targets for senile and diabetic cataracts.
Highlights.
-
►
First report on the role of AQP5 in maintaining lens transparency and homeostasis
-
►
AQP5 regulates lens osmotic balance
-
►
AQP5 knockout lens exhibits osmotic swelling and develops hyperglycemic cataract
-
►
Glucose transporters and cotransporters cause hyperglycemia-induced water influx
-
►
AQP5 is critical for protecting the lens from diabetic and senile cataracts
Acknowledgments
Research support: NIH - NEI grant R01: EY20506.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Brian G, Taylor H. Cataract blindness--challenges for the 21st century. Bull. World Health Organ. 2001;79:249–256. [PMC free article] [PubMed] [Google Scholar]
- 3.Vinson JA. Oxidative stress in cataracts. Pathophysiology. 2006;13:151–162. doi: 10.1016/j.pathophys.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Robinson KR, Patterson JW. Localization of steady currents in the lens. Curr. Eye Res. 1983;2:843–847. doi: 10.3109/02713688209020020. [DOI] [PubMed] [Google Scholar]
- 5.Mathias RT, Rae JL, Baldo GL. Physiological properties of the normal lens. Physiol. Rev. 1997;77:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- 6.Kumari SS, Varadaraj M, Yerramilli VS, Menon AG, Varadaraj K. Spatial expression of aquaporin 5 in mammalian cornea and lens and regulation of its localization by phosphokinase A. Mol. Vis. 2012;18:957–967. [PMC free article] [PubMed] [Google Scholar]
- 7.Grey AC, Walker KL, Petrova RS, Han J, Wilmarth PA, David LL, Donaldson PJ, Schey KL. Verification and spatial localization of aquaporin-5 in the ocular lens. Exp. Eye Res. 2013;108:94–102. doi: 10.1016/j.exer.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patil RV, Saito I, Yang X, Wax MB. Expression of aquaporins in the rat ocular tissue. Exp. Eye Res. 1997;64:203–209. doi: 10.1006/exer.1996.0196. [DOI] [PubMed] [Google Scholar]
- 9.Bassnett S, Wilmarth PA, David LL. The membrane proteome of the mouse lens fiber cell. Mol. Vis. 2009;15:2448–2463. [PMC free article] [PubMed] [Google Scholar]
- 10.Stamer WD, Snyder RW, Smith BL, Agre P, Regan JW. Localization of aquaporin CHIP in the human eye: implications in the pathogenesis of glaucoma and other disorders of ocular fluid balance. Invest. Ophthalmol. Vis. Sci. 1994;35:3867–3872. [PubMed] [Google Scholar]
- 11.Varadaraj K, Kushmerick C, Baldo GJ, Bassnett S, Shiels A, Mathias RT. The role of MIP in lens fiber cell membrane transport. J. Membr. Biol. 1999;170:191–203. doi: 10.1007/s002329900549. [DOI] [PubMed] [Google Scholar]
- 12.Varadaraj K, Kumari S, Mathias RT. Functional expression of aquaporins in embryonic, postnatal, and adult mouse lenses. Dev. Dyn. 2007;236:1319–1328. doi: 10.1002/dvdy.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz-Ederra J, Verkman AS. Accelerated cataract formation and reduced lens epithelial water permeability in aquaporin-1-deficient mice. Invest. Ophthalmol. Vis. Sci. 2006;47:3960–3967. doi: 10.1167/iovs.06-0229. [DOI] [PubMed] [Google Scholar]
- 14.Mulders SM, Preston GM, Deen PM, Guggino WB, van Os CH, Agre P. Water channel properties of major intrinsic protein of lens. J. Biol. Chem. 1995;270:9010–9016. doi: 10.1074/jbc.270.15.9010. [DOI] [PubMed] [Google Scholar]
- 15.Michea LF, de la Fuente M, Lagos N. Lens major intrinsic protein (MIP) promotes adhesion when reconstituted into large unilamellar liposomes. Biochemistry. 1994;33:7663–7669. doi: 10.1021/bi00190a021. [DOI] [PubMed] [Google Scholar]
- 16.Kumari SS, Varadaraj K. Intact AQP0 Performs Cell-to-Cell Adhesion. Biochem. Biophys. Res. Commun. 2009;390:1034–1039. doi: 10.1016/j.bbrc.2009.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J. Biol. Chem. 1999;274:20071–20074. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 18.Krane CM, Melvin JE, Nguyen HV, Richardson L, Towne JE, Doetschman T, Menon AG. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J. Biol. Chem. 2001;276:23413–23420. doi: 10.1074/jbc.M008760200. [DOI] [PubMed] [Google Scholar]
- 19.Thiagarajah JR, Verkman AS. Aquaporin deletion in mice reduces corneal water permeability and delays restoration of transparency after swelling. J. Biol. Chem. 2002;277:19139–19144. doi: 10.1074/jbc.M202071200. [DOI] [PubMed] [Google Scholar]
- 20.Creighton MO, Stewart-DeHaan PJ, Ross WM, Sanwal M, Trevithick JR. Modelling cortical cataractogenesis. 1. In vitro effects of glucose, sorbitol and fructose on intact rat lenses in medium 199. Can. J. Ophthalmol. 1980;15:183–188. [PubMed] [Google Scholar]
- 21.Varadaraj K, Kumari S, Mathias RT. Transgenic expression of AQP1 in the fiber cells of AQP0 knockout mouse: effects on lens transparency. Exp. Eye Res. 2010;91:393–404. doi: 10.1016/j.exer.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuck J. The formation of fructose in the ocular lens. Arch Ophthalmol. 1961;65:840–846. doi: 10.1001/archopht.1961.01840020842019. [DOI] [PubMed] [Google Scholar]
- 23.Lubek BM, Avaria M, Basu PK, Wells PG. Pharmacological studies on the in vivo cataractogenicity of acetaminophen in mice and rabbits. Fundam. Appl. Toxicol. 1988;10:596–606. doi: 10.1016/0272-0590(88)90186-8. [DOI] [PubMed] [Google Scholar]
- 24.Bettelheim FA, Li L, Zeng FF. Do changes in the hydration of the diabetic human lens preceded cataract formation? Res. Commun. Mol. Pathol. Pharmacol. 1998;102:3–14. [PubMed] [Google Scholar]
- 25.Coulter JB, 3rd, Eaton DK, Marr LK. Effects of diabetes and insulin treatment on sorbitol and water of rat lenses. Ophthalmic, Res. 1986;18:357–362. doi: 10.1159/000265463. [DOI] [PubMed] [Google Scholar]
- 26.Merriman-Smith R, Donaldson P, Kistler J. Differential expression of facilitative glucose transporters GLUT1 and GLUT3 in the lens. Invest. Ophthalmol. Vis. Sci. 1999;40:3224–3230. [PubMed] [Google Scholar]
- 27.Merriman-Smith BR, Krushinsky A, Kistler J, Donaldson PJ. Expression patterns for glucose transporters GLUT1 and GLUT3 in the normal rat lens and in models of diabetic cataract. Invest. Ophthalmol. Vis. Sci. 2003;44:3458–3466. doi: 10.1167/iovs.02-1235. [DOI] [PubMed] [Google Scholar]
- 28.Varadaraj K, Merriman-Smith R, Kumari SS, Philip F, Donaldson P, Kistler J, Mathias RT. Glucose transport by lens epithelial and fiber cells. Invest. Ophthalmol. Vis. Sci. 2001;42:S876. [Google Scholar]
- 29.Smith BR, Varadaraj K, Krushinski A, Donaldson P, Mathias R, Kistler J. Glucose transport in the lens. Invest. Ophthalmol. Vis. Sci. 2002;43:4646. [Google Scholar]
- 30.Fischbarg J, Kuang KY, Vera JC, Arant S, Silverstein SC, Loike J, Rosen OM. Glucose transporters serve as water channels. Proc. Natl. Acad. Sci. U S A. 1990;87:3244–3247. doi: 10.1073/pnas.87.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomioka S. Water transport by glucose transporter type 3 expressed in Xenopus oocytes. Neuroreport. 2012;23:21–25. doi: 10.1097/WNR.0b013e32834da877. [DOI] [PubMed] [Google Scholar]
- 32.Zeuthen T, Belhage B, Zeuthen E. Water transport by Na+-coupled cotransporters of glucose (SGLT1) and of iodide (NIS). The dependence of substrate size studied at high resolution. J. Physiol. 2006;570:485–499. doi: 10.1113/jphysiol.2005.100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moseley AE, Dean WL, Delamere NA. Isoforms of Na,K-ATPase in rat lens epithelium and fiber cells. Invest. Ophthalmol. Vis. Sci. 1996;37:1502–1508. [PubMed] [Google Scholar]
- 34.Kinoshita JH, Nishimura C. The involvement of aldose reductase in diabetic complications. Diabetes Metab. Rev. 1988;4:323–337. doi: 10.1002/dmr.5610040403. [DOI] [PubMed] [Google Scholar]
- 35.Varma SD, Kinoshita JH. The absence of cataracts in mice with congenital hyperglycemia. Exp. Eye Res. 1974;19:577–582. doi: 10.1016/0014-4835(74)90095-5. [DOI] [PubMed] [Google Scholar]
- 36.Hegde KR, Henein MG, Varma SD. Establishment of the mouse as a model animal for the study of diabetic cataracts. Ophthalmic. Res. 2003;35:12–18. doi: 10.1159/000068193. [DOI] [PubMed] [Google Scholar]
- 37.Kumari SS, Eswaramoorthy S, Mathias RT, Varadaraj K. Unique and analogous functions of aquaporin 0 for fiber cell architecture and ocular lens transparency. Biochim. Biophys. Acta. 2011;1812:1089–1097. doi: 10.1016/j.bbadis.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preisser L, Teillet L, Aliotti S, Gobin R, Berthonaud V, Chevalier J, Corman B, Verbavatz JM. Downregulation of aquaporin-2 and-3 in aging kidney is independent of V2 vasopressin receptor. Am. J. Physiol. 2000;279:F144–F152. doi: 10.1152/ajprenal.2000.279.1.F144. [DOI] [PubMed] [Google Scholar]
- 39.Zhang YW, Bi LT, Hou SP, Zhao XL, Song YL, Ma TH. Reduced lung water transport rate associated with downregulation of aquaporin-1 and aquaporin-5 in aged mice. Clin. Exp. Pharmacol. Physiol. 2009;36:734–738. doi: 10.1111/j.1440-1681.2009.05156.x. [DOI] [PubMed] [Google Scholar]
- 40.SiebingI I, Vrensen GF, De Mul FF, Greve J. Age-related changes in local water and protein content of human eye lenses measured by Raman microspectroscopy. Exp. Eye Res. 1991;53:233–239. doi: 10.1016/0014-4835(91)90079-t. [DOI] [PubMed] [Google Scholar]
- 41.Hollborn M, Dukic-Stefanovic S, Pannicke T, Ulbricht E, Reichenbach A, Wiedemann P, Bringmann A, Kohen L. Expression of aquaporins in the retina of diabetic rats. Curr. Eye Res. 2011;36:850–856. doi: 10.3109/02713683.2011.593108. [DOI] [PubMed] [Google Scholar]