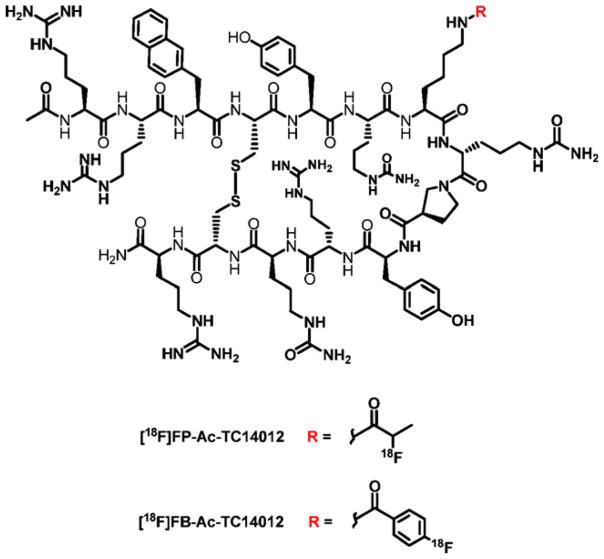
Abstract
Purpose
CXCR4 is overexpressed on tumor cells from many types of human cancers. A high level of CXCR4 expression often correlates with poor prognosis, chemotherapy resistance, and metastasis. The development of CXCR4-specific radiotracers for positron emission tomography (PET) imaging will allow in vivo evaluation of receptor expression level for diagnosis or therapeutic evaluation.
Procedures
Two new 18F-labeled radiotracers based on an Ac-TC14012 peptide, [18F]FP-Ac-TC14012 and [18F]FB-Ac-TC14012, were synthesized and characterized. The affinities of the 2-fluoropropionate (FP)-conjugated or 4-fluorobenzoate (FB)-conjugated peptides to CXCR4-transfected Chinese hamster ovarian (CHO) cells were evaluated in a competitive binding assay with [125I]CXCL12 radioligand. The cell uptake and retention of [18F]FP-labeled and [18F]FB-labeled peptides were measured. The tumor targetability and pharmacokinetics of these two tracers were also evaluated by microPET imaging and biodistribution studies.
Results
The labeled peptides retained high binding affinity to CXCR4 and showed much higher uptake in CXCR4-positive CHO cells than in CXCR4-negative cells in vitro. The smaller and more hydrophilic [18F]FP prosthetic group resulted in higher affinity and lower nonspecific cell uptake compared to the [18F]FB-labeled peptide. Both radiotracers showed much higher accumulation in CXCR4-positive than CXCR4-negative tumor xenografts in mice and allowed clear visualization of CXCR4 expression by PET. Among the two, [18F]FP-Ac-TC14012 showed higher tumor uptake and better tumor-to-background contrast. Unlike their N-terminal 4-F-benzoate analogs, these two tracers had minimal blood retention, likely due to reduced red blood cell binding. Metabolic organs, such as the liver and kidney, also showed high uptake. When blocked with low-dose cold peptide (10 μg), the tumor uptake was significantly increased, most likely due to the increased concentration in blood circulation, as evidenced by decreased liver uptake.
Conclusion
These results demonstrate that the [18F]FP-labeled Ac-TC14012 peptide with high tumor uptake, low nonspecific binding, and good tumor-to-background contrast promises [18F]FP-Ac-TC14012 as a PET tracer for in vivo PET imaging of CXCR4 expression.
Keywords: CXCR4, PET imaging, CXCR4 antagonist peptides, 18F-labeling
Introduction
Chemokine receptors are cytokine receptors found on the surface of certain cells that interact with a chemokine. There have been 19 distinct chemokine receptors described in mammalian cells. Each chemokine receptor has a seven-transmembrane structure and couples to G protein for signal transduction within a cell. CXCR4, belonging to the family of CXC chemokine receptors, is overexpressed on tumor cells from at least 23 different types of human cancers, including epithelial, mesenchymal, and hematopoietic origins [1]. CXCL12 (or SDF1-α) is the only known natural ligand for CXCR4.
CXCR4 is found in primary tumor sites in lymphoma, glioma, ovarian, and pancreatic cancers [2–5] and at the sites of metastasis in breast, lung, thyroid, neuroblastoma, hematological cancers, and inflammatory diseases [6–10]. It is also constitutively expressed in many normal organs, including the spleen, pancreas, and bone marrow [11,12]. Research on the role of CXCR4 in cancer has revealed a correlation between high level of CXCR4 expression and poor prognosis [13,14], chemotherapy resistance [15–17], and metastasis [18,19] in CXCL12-expressing organs. Therefore, the CXCR4 ligand or antagonist could be an effective strategy for both therapeutic and imaging purposes. Indeed, Plerixafor (AMD3100, a small molecular CXCR4 antagonist) has been used in the clinic as an immunostimulant to mobilize hematopoietic stem cells for transplantation.
Development of CXCR4-specific radiotracers for positron emission tomography (PET) imaging will allow in vivo evaluation of receptor expression level for diagnostic or therapeutic evaluation. A few CXCR4 ligands have been radiolabeled for PET imaging, including small molecules [20–23] and peptides [24–29], although an optimal imaging agent is still yet to be found. The extensive research by Tamamura and coworkers has led to the finding and optimization of a 14-amino-acid CXCR4 inhibitor T140 peptide and its derivatives [30–33]. Previously in our group, a TN14003 peptide [33] has been labeled with 4-[18F]-fluorobenzoate at the N terminus for CXCR4 imaging in vivo [25]. Although this radiotracer possesses excellent CXCR4 binding affinity, it shows very high red blood cell (RBC) binding as well. The RBC binding resulted in low tumor-to-background contrast in vivo. Also, the side chains of Lys7 and Lys8 need to be protected before labeling, which adds a deprotection step. When labeled with 64Cu through chelators at the side chains of Lys7 and Lys8 while keeping the N-fluorobenzoate group [26], the high CXCR4 binding affinity remains but the high RBC binding is still similar. Furthermore, when 64Cu-labeling is done at the N terminus [27], although the binding affinity was decreased by 1–2 orders of magnitude, the RBC binding is greatly diminished, which provides a better imaging contrast in vivo.
In this manuscript, we developed two new 18F-labeled radiotracers based on an Ac-TC14012 peptide (Fig. 1). The Ac-TC14012 peptide has shown very low EC50 (29 nM) in anti-HIV activity [33]. There is only one Lys7 ε-amine group in the peptide sequence for labeling, which could avoid the labeling at multiple sites. Moreover, labeling at the Lys7 position should not affect its CXCR4 affinity significantly, as it is not essential for CXCR4 binding [30,33]. Also, labeling at the Lys7 side chain results in very low RBC binding without reducing CXCR4 binding affinity significantly, comparing to N-terminal 4-F-fluorobenzoate-conjugated analogs [25,26]. Their behaviors for in vivo PET imaging of CXCR4 were evaluated and discussed.
Fig. 1.
Structures of [18F]FP-Ac-TC14012 and [18F]FB-Ac-TC14012.
Materials and Methods
All solvents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Fisher Scientific (Waltham, MA, USA) and used as received. Ac-TC14012 (sequence Ac-Arg-Arg-NaI-Cys-Tyr-Cit-Lys-DCit-Pro-Tyr-Arg-Cit-Cys-Arg-NH2 Cys4-Cys13 disulfide) was purchased from C.S. Bio Co. (Menlo Park, CA, USA). Mass spectra were obtained with a Waters LC-MS system (Waters, Milford, MA, USA) that included an Acquity UPLC system coupled to a Waters Q-T of Premier high-resolution mass spectrometer.
High-performance liquid chromatography (HPLC) was performed on a system with a variable wavelength detector and with a radioactivity detector containing a NaI crystal. Analytical HPLC used a Phenomenex Luna 5 μm C18 column (5 μm, 4.60× 150 mm). Elution, at 1 ml/min, used a gradient system, starting from 95 % of solvent A (0.1 % trifluoroacetic acid [TFA] in water) and 5 % of solvent B (0.1 % TFA in acetonitrile) and changing to 50 % solvent A and 50 % solvent B at 30 min. The semi-preparative HPLC system used a Phenomenex Luna 5 μm C18 column (5 μm, 10×250 mm). The flow was set at 5 ml/min using a gradient system, starting from 95 % of solvent A (0.1 % TFA in water) and 5 % of solvent B (0.1 % TFA in acetonitrile) for 5 min and changing to 35 % solvent A and 65 % solvent B at 35 min.
C18 cartridges (Waters Corporation, Milford, MA, USA) were each activated with 5 ml of EtOH and 10 ml of water. After trapping, the cartridges were washed with 5 ml H2O before the desired products were eluted out using 10 mM HCl in ethanol.
Synthesis of 2-Fluoropropionate-Ac-TC14012 (FP-Ac-TC14012)
Three milligrams of Ac-TC14012 peptide was dissolved in 400 μl of dimethyl sulfoxide (DMSO). 4-Nitrophenyl 2-fluoropropionate (1.1 eq) and 5 μl of diisopropylethylamine was added and reacted at room temperature (RT) for 20 min. The reaction was quenched with 10 μl TFA and loaded on semi-preparative HPLC (Beckman, Brea, CA, USA; Ultrasphere™ C18 column, 5 μm, 10×250 mm). The desired product was collected at 27 min and lyophilized to afford a white powder with a yield of 56 %. HRMS Calcd for C95H146FN34O21S2 [M+H]+= 2,182.0827 (m/z). Found=2,182.4392.
Synthesis of 4-Fluorobenzoate-Ac-TC14012 (FB-Ac-TC14012)
Three milligrams of Ac-TC14012 peptide was dissolved in 500 μl of 100 mM Na2HPO4 pH 8.5 buffer. N-succinimidyl 4-fluorobenzoate in 100 μl CH3CN (1.5 eq) was added and reacted at RT for 20 min. The reaction was quenched with 20 μl TFA and loaded on semi-preparative HPLC (Beckman, Brea, CA, USA; Ultrasphere™ C18 column, 5 μm, 10×250 mm). The desired product was collected at 31 min and lyophilized to afford a white powder with a yield of 42 %. HRMS Calcd for C99H146FN34O21S2 [M+H]+=2,230.0827 (m/z). Found=2,230.1590.
Synthesis of 4-Nitrophenyl 2-[18F]-fluoropropionate ([18F]FP)
4-Nitrophenyl 2-[18F]-fluoropropionate ([18F]FP) was prepared on a GE TRACERLab FXN module according to a published procedure [34]. For a typical run, from 160 mCi of F-18, 20 mCi of [18F]FP was obtained following HPLC purification and trapped onto a C18 cartridge. The total synthesis time for [18F]FP was ~100 min with uncorrected yield of 12.4±3.4 % (mean±standard deviation (SD), n=4).
Synthesis of [18F]FP-Ac-TC14012
The activity trapped on the C18 cartridge as described previously was eluted manually with 1 ml of methylene chloride into a 1.5-ml polypropylene tube, and the water layer on top of the methylene chloride was removed by using a syringe. The methylene chloride in the tube was evaporated under argon flow at RT, and 200 μg of Ac-TC14012 in 0.1 ml of DMSO containing 5 μl of diisopropylethylamine was added to the tube and heated at 80 °C for 10 min. At the end of the reaction, the reaction mixture was cooled and diluted with 0.5 ml of water containing 0.1 % of TFA and loaded onto a semi-preparative HPLC column. The desired product was collected at a retention time of 18.9 min and diluted with 10 ml water and trapped on a Varian Bond Elute C18 column (100 mg). The radioactivity trapped on the C18 column was eluted with 0.15 ml of 10 mM HCl–ethanol solution, the ethanol was removed with argon flow, and the final product was then dissolved in phosphate-buffered saline (PBS). In a typical experiment, 2.4 mCi of product could be obtained starting from 9.4 mCi of [18F]-FP with a total synthesis time of ~50 min. The radiochemical yield for coupling of [18F]FP with Ac-TC14012 was 38.1±10.5 % (mean±SD, n=4, decay corrected). The quality control on an analytical HPLC showed tR =17.5 min and radiochemical purities≥95 %.
Synthesis of N-succinimidyl 4-[18F]-fluorobenzoate ([18F]FB)
N-succinimidyl 4-[18F]-fluorobenzoate ([18F]FB) was prepared on an Eckert and Ziegler (Eurotope GmbH) module with the same setup as previous reported [35]. The synthetic method is similar according to a published procedure [25]. For a typical run, from 160 mCi of F-18, 20 mCi of [18F]-FB was obtained following HPLC purification and trapped onto a C18 cartridge. The total synthesis time for [18F]-FB was ~100 min with uncorrected yield of 17.4±5.7 % (mean±SD, n=4).
Synthesis of [18F]FB-Ac-TC14012
The activity trapped on the C18 cartridge as described previously was eluted manually with 1 ml of methylene chloride into a 1.5-ml polypropylene tube, and the water layer on top of the methylene chloride was removed by using a syringe. The methylene chloride in the tube was evaporated under argon flow at RT, and 200 μg of Ac-TC14012 in 0.1 ml of 100 mM pH 8.5 Na2HPO4 buffer was added to the tube and reacted at RT for 20 min. At the end of the reaction, the reaction mixture was diluted with 0.5 ml of water containing 0.1 % of TFA and loaded onto a semi-preparative HPLC column. The desired product was collected at a retention time of 20.5 min and diluted with 10 ml water and trapped on a Varian Bond Elute C18 column (100 mg). The radioactivity trapped on the C18 column was eluted with 0.15 ml of 10 mM HCl–ethanol solution. The ethanol was removed with argon flow, and the final product was then dissolved in PBS. In a typical experiment, 2.0 mCi of product could be obtained starting from 10.7 mCi of [18F]-FB with a total synthesis time of ~60 min. The radiochemical yield for coupling of [18F]-FB with Ac-TC14012 was 31.6±7.0 % (mean±SD, n=4, decay corrected). The quality control on an analytical HPLC showed tR =19.5 min and radiochemical purities ≥ 98 %.
Cell Culture and Flow Cytometry
Wild-type Chinese hamster ovarian (CHO) cells and CHO cells that were stably transfected with CXCR4 (CHO-CXCR4) were a kind gift from Dr. David McDermott (NIAID, NIH, Bethesda, MD, USA). The cells were cultured following a previously reported procedure [25]. The CXCR4 expression level was confirmed by flow cytometry by using PE-conjugated antihuman CXCR4 (R&D, Minneapolis, MN, USA) and an LSR-II cytometer (Becton Dickinson, San Jose, CA, USA). The flow data were analyzed using FlowJo (Tree Star, Ashland, OR, USA).
Competitive Binding Assay
For receptor binding assay, CHO-CXCR4 cells (105 cells per well) were incubated with 200 μl of binding buffer (PBS containing 50 mM HEPES, 1 mM CaCl2, 5 mM MgCl2, 0.5 % (w/v) BSA, and 0.3 mM NaN3)), 250 nCi (9.25 KBq) of [125I]CXCL12, and 0–1,000 nM of Ac-TC14012, FP-Ac-TC14012, or FB-Ac-TC14012 peptide for 45 min on a shaker at RT, respectively. The cell-bound radioactivity was measured using a gamma counter and expressed as percent of total counts. The IC50 values were calculated using Prism software (GraphPad, La Jolla, CA, USA).
Cell Uptake and Efflux Studies
For cell uptake studies, CHO-CXCR4 or CHO cells were seeded into a 24-well plate (105 cells per well) and incubated with 18F-labeled TC14012 derivatives (25 μCi/well, 925 kBq/well) at 37 °C. Cells were then washed twice with cold PBS at different time points and harvested by addition of 250 μl of 0.1 M NaOH. Internalization studies were conducted in a similar fashion. After incubation of CHO-CXCR4 cells with radiotracers at 37 °C for different amounts of time, the cells were washed twice with cold PBS and then incubated with acid washing buffer (50 mM glycine, 0.1 M NaCl, pH 2.8) for 1 min to remove surface-bound radioligand. Thereafter, the cells were further washed with cold PBS and harvested by adding 250 μl of 0.1 M NaOH. For efflux studies, radiotracers (25 μCi/well, 925 KBq/well) were added to the cells in a 24-well plate and incubated for 60 min at 37 °C. Then, the cells were washed with cold PBS and incubated with F-12K medium for different amounts of time. After washing twice with PBS, cells were harvested by addition of 250 μl of 0.1 M NaOH. The collected radioactivity from each well was measured in a gamma counter. Each data point is an average of triplicate wells.
Animals
All animal studies were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Animals using protocols approved by the NIH Institutional Animal Care and Use Committee. Athymic nude mice (4–6 weeks old from Taconic, Germantown, NY, USA) were injected subcutaneously at two sites on the shoulder with 107 CXCR4-positive or CXCR4-negative CHO tumor cells per site. The imaging or biodistribution studies were performed 2 weeks after tumor inoculation.
PET Studies
For PET scans, each mouse was injected via the tail vein with 100 μCi (3.7 MBq) of radiotracers in a volume of 100 μl PBS under isoflurane anesthesia. The images were acquired using an Inveon scanner (Siemens Medical Solutions) at 0.5 and 1 h postinjection (p.i.). For blocking experiments, 100 μCi (3.7 MBq) of radiotracers were co-injected with 10, 50, or 200 μg of Ac-TC14012 peptide. The images were reconstructed by a three-dimensional ordered subsets expectation maximization algorithm with no attenuation or scattering correction. Image analysis was done using ASI Pro VMTM software. Tumor and tissue uptake values derived from coronal PET images were obtained by analyzing regions of interest and converting the values to percent injected dose per gram tissue (n=4–6/group).
Biodistribution
For biodistribution, each animal was injected with the appropriate PET tracer (50 μCi, 1.85 MBq) in 100 μl PBS. For the experiment, 50 μg of Ac-TC14012 peptide were co-injected with the labeled peptide. At 2 h p.i., the animals were sacrificed, tumor and major organs and tissues were collected, wet weighed, and the radioactivity was measured by a gamma counter. The results were calculated as percent injected dose per gram tissue (n=4–6/group).
Statistical Analysis
Two-tailed paired and unpaired Student’s t tests were used to test differences between groups. Comparisons are made between CHO-CXCR4 and CHO tumors and between unblocked and blocked experiments. P value <0.05 was considered statistically significant.
Results and Discussion
Synthesis and Radiochemistry
Nonradioactive FP-Ac-TC14012 and FB-Ac-TC14012 were synthesized as standards for confirming the identity of radiolabeled compounds and for cell binding assays. The chemical yields were 56 % for FP-Ac-TC14012 and 42 % for FB-Ac-TC14012. The retention times of unconjugated peptide, FP-conjugated peptide, and FB-conjugated peptide are 14.6, 17.5, and 19.2 min, respectively, on a C18 HPLC column, which indicates the expected change in relative lipophilicity of the various peptide analogs. During the synthesis of FB-Ac-TC14012, two other peptide components were observed with HPLC retention times of 23 and 27 min. HRMS suggested that both are peptides containing two FB moieties. The peptide contains two phenolic and three guanidine function groups that could potentially react. We did not attempt to determine which positions may have reacted. Modification of the reaction conditions (change in base, molar equivalents, and solvents) were not successful in preventing these side reactions.
The radiosynthesis of 4-nitrophenyl 2-[18F]-fluoropropionate ([18F]FP) utilized three-step procedures and were performed using automated procedures [34]. The total synthesis time for [18F]FP was ~100 min with uncorrected yield of 12.4±3.4 % (mean±SD, n=4). The radiolabeling of Ac-TC14012 with [18F]FP was carried out in DMSO using DIPEA as a base. After reaction at 80 °C for 10 min, the labeled peptide was isolated by semi-preparative HPLC. The radiochemical yield of labeling was 38.1± 10.5 % (mean±SD, n=4, decay corrected), with radiochemical purities ≥ 95 % (for the HPLC chromatogram, see Supplemental Materials Fig. S1). The specific activity ranged from 13.6 to 17.6 GBq/μmol at the time of measurement (prior to animal studies).
The radiosynthesis of N-succinimidyl 4-[18F]-fluorobenzoate ([18F]FB) also utilized three-step procedures and were performed using automated procedures [35]. The total synthesis time for [18F]FB was ~110 min with uncorrected yield of 17.4±5.7 % (mean±SD, n=4). When labeling with [18F]FB, a preliminary test of reaction buffers was first performed. At pH 8.5 (100 mM sodium bicarbonate, 100 mM sodium phosphate, or 100 mM sodium borate buffer, pH adjusted using 5 M NaOH and HCl), the coupling reaction at RT for 30 min provided similar radiolabeling yields of 59–75 % (calculated from HPLC peak integration), while higher or lower pH (9.20 or 8.20, NaHCO3 buffer) resulted in significantly lower yields (11–28 %); 100 mM pH 8.5 Na2HPO4 buffer was used thereafter. In radiolabeling, when peptide was in large excess, the two side products seen in nonradioactive synthesis were much less, with labeling yield of desired product 55–65 % (calculated from HPLC peak integration). The radiochemical yield for coupling of [18F]SFB with Ac-TC14012 was 31.6± 7.0 % (mean±SD, n=4, decay corrected) with radiochemical purities ≥98 % (for the HPLC chromatogram, see Supplemental Materials Fig. S2). The specific activity ranged from 18.7 to 31.6 GBq/μmol at the time of measurement (prior to animal studies).
Competitive Binding Assay with [125I]CXCL12 Radioligand
CHO cells were stably transfected with human CXCR4 (CHO-CXCR4). Flow cytometry showed that transfected CHO-CXCR4 cells had 30 times higher fluorescence than the parent CHO cells (Fig. 2a). The affinities of the FPA-conjugated or SFB-conjugated peptides to CXCR4-transfected CHO cells were evaluated in a competitive binding assay with [125I]CXCL12 radioligand, which has been widely used for CXCR4 ligand binding affinity measurement since CXCL12 (SDF1-α) is a natural ligand to CXCR4 with high affinity. As shown in Fig. 2b, the IC50 of the unconjugated Ac-TC14012 peptide was found to be 2.47±0.53 nM (mean±SD), while the IC50s of FP-Ac-TC14012 and FB-Ac-TC14012 were 7.52±1.62 and 25.1± 5.3 nM, respectively. These IC50 values are lower compared to N-terminal chelator-conjugated TN14003 peptides [27], demonstrating the minor role of Lys7 in receptor binding. Also, the larger and more hydrophobic FB group has a larger effect on the binding affinity than the FP group.
Fig. 2.
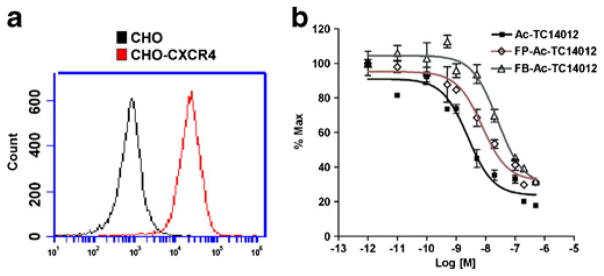
a CXCR4 expression of the CHO-CXCR4 and CHO cell lines analyzed by flow cytometry. b Competitive binding assay of FP-Ac-TC14012 and FB-Ac-TC14012 with [125I]CXCL12 in CHO-CXCR4 cells.
Cell Uptake and Retention
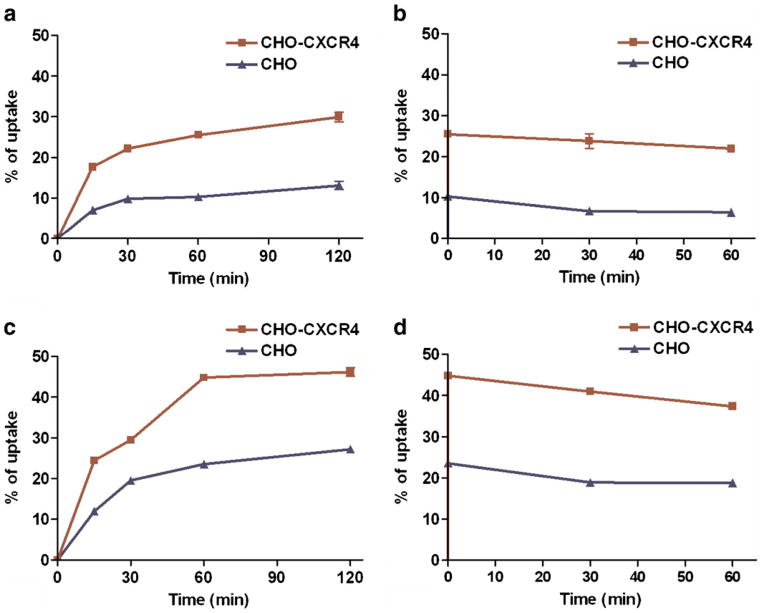
The cell uptake and retention of [18F]FP-labeled and [18F]FB-labeled peptides were measured in CHO-CXCR4 cells and compared with CHO cells (not transfected) as well. As shown in Fig. 3a, the uptake of [18F]FP-Ac-TC14012 in CHO-CXCR4 reached 29.94±1.16 % (of total added dose, same as in the succeeding sentences) at 2 h, compared to only 13.17±1.04 % in CHO cells, indicating a receptor-specific uptake. The retention is also higher in CHO-CXCR4 cells than in CHO cells (Fig. 3b), with 21.97±0.73 and 6.47 ±0.47 % left, respectively, after 1 h. Thus, 73 % of the uptaken dose was retained in CHO-CXCR4 cells, while only 49 % was retained in CHO cells. A similar trend was also observed for [18F]FB-Ac-TC14012 uptake, with 46.18±1.05 % in CHO-CXCR4 cells at 2 h and 27.23±0.46 % in CHO cells (Fig. 3c); the retention after 1 h was 37.38±0.77 and 18.76± 0.26 % for CHO-CXCR4 and CHO cells, respectively (Fig. 3d). Eighty-one percent of the uptaken dose was retained in CHO-CXCR4 cells, and 68 % was retained in CHO cells. The positively charged nature of the peptide and the modification with hydrophobic moieties may have contributed to the nonspecific binding to CHO-CXCR4 and CHO cells. The higher uptake of the more hydrophobic [18F]FB-Ac-TC14012 in both cells implies higher nonspecific interactions with the cells.
Fig. 3.
Cellular uptake and retention (of total added dose) of [18F]FP-Ac-TC14012 (a uptake; b retention) and [18F]FB-Ac-TC14012 (c uptake; d retention) in CHO-CXCR4 and CHO cells.
PET Imaging
In vivo imaging of CXCR4 expression was evaluated by static microPET scan using mice bearing subcutaneous CHO-CXCR4 and CHO tumors. The radiotracers were injected intravenously and the representative decay-corrected coronal images at 30 and 60 min after injection of the radiotracers are shown in Figs. 4 and 5. The CHO-CXCR4 tumors were clearly visualized with good tumor-to-background contrast with both tracers at 30 min p.i., while the CHO tumors, with low CXCR4 expression, showed much lower uptake. Unlike previously reported N-fluorobenzoate TN140003 peptides [25,26], these radiotracers showed much less blood retention, likely due to minimal RBC binding. This implies the role of the N-fluorobenzoate group on RBC binding. Besides CXCR4-expressing tumor, metabolic organs, such as the liver and kidneys, also showed high uptake. Co-injection of radiotracers with unconjugated peptide Ac-TC14012 (10, 50, or 200 μg) into mice was also performed. It has been shown that CXCR4 is expressed in the brain in a variety of cell types, including microglia, astrocytes, neurons, and vascular endothelial cells [36–38]. At the 50- and 200-μg blocking doses, the liver uptake was blocked; hence, there was more radiotracer available in the blood circulation, and some brain uptake was observed 30 min p.i.
Fig. 4.
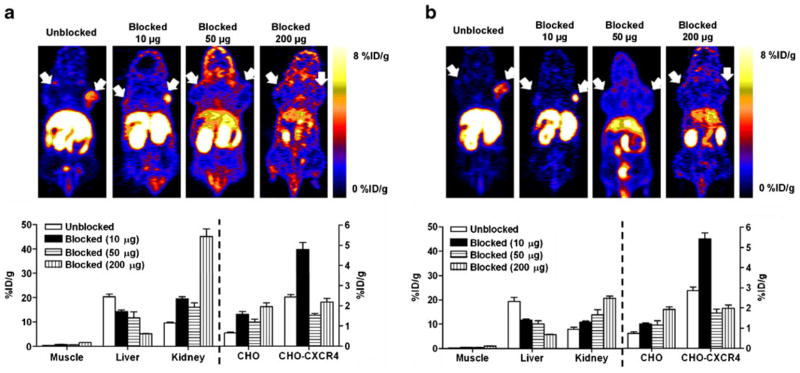
Representative coronal PET images (upper panel) and uptake calculation (lower panel) of athymic nude mice bearing CHO-CXCR4 (right shoulder) and CHO (left shoulder) tumors after being injected with 100 μCi (3.7 MBq) of [18F]FP-Ac-TC14012. a 30 min; b 60 min p.i. Results are shown as the average±SE of four to six mice.
Fig. 5.
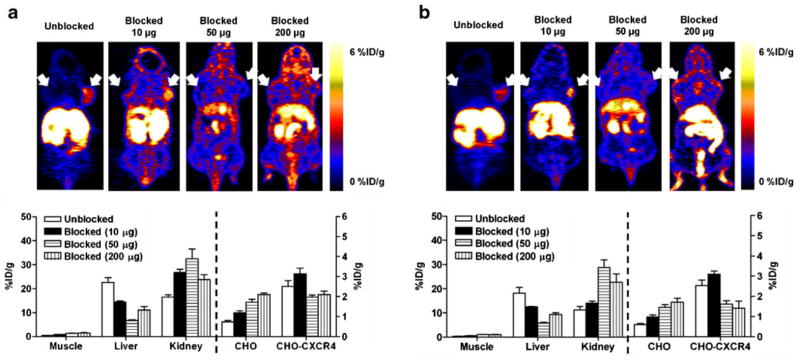
Representative coronal PET images (upper panel) and uptake calculation (lower panel) of athymic nude mice bearing CHO-CXCR4 (right shoulder) and CHO (left shoulder) tumors after being injected with 100 μCi (3.7 MBq) of [18F]FB-Ac-TC14012. a 30 min; b 60 min p.i. Results are shown as the average±SE of four to six mice.
For [18F]FP-Ac-TC14012, co-injection with 10 μg (~10-fold excess) cold peptide resulted in significantly higher CHO-CXCR4 tumor accumulation than the tracer alone (30 min, 4.81±0.33 vs. 2.43±0.13 %ID/g; 60 min, 4.52± 0.24 vs. 2.37±0.16 %ID/g; P<0.01), possibly due to the blockage of liver uptake (30 min, 14.27±0.66 vs. 20.36± 1.15 %ID/g; 60 min, 11.66±0.46 vs. 19.37±1.56 %ID/g; P<0.01) in the presence of 10 μg cold peptide. After co-injection with higher doses (50 or 200 μg, ~50-fold or ~200-fold excess) of cold peptide, radiotracer accumulation in CHO-CXCR4 tumor was significantly deceased (30 min, 1.53±0.09 and 2.20±0.17 %ID/g; 60 min, 1.45±0.17 and 1.65±0.14 %ID/g; P<0.01 compared to co-injection with 10 μg cold peptide); and the uptake was not significantly different than the CXCR4-negative CHO tumor (P>0.05). The co-injection of unlabeled peptide also resulted in decreased liver uptake and increased kidney uptake, indicating that the liver may have specific uptake and the kidney is a major metabolism and/or excretion organ. For [18F]FB-Ac-TC14012, the uptake patterns in CHO-CXCR4 and CHO tumors were similar as [18F]FP-Ac-TC14012, albeit the uptake levels at 0 and 10 μg blocking doses were lower. This could be explained by the lower binding affinity of [18F]FB-Ac-TC14012. Moreover, at the highest blocking doses (200 μg), liver uptake of [18F]FB-Ac-TC14012 is higher than that of [18F]FP-Ac-TC14012 (P<0.05). The higher nonspecific binding is consistent with the cell uptake results shown in Fig. 3.
Biodistribution
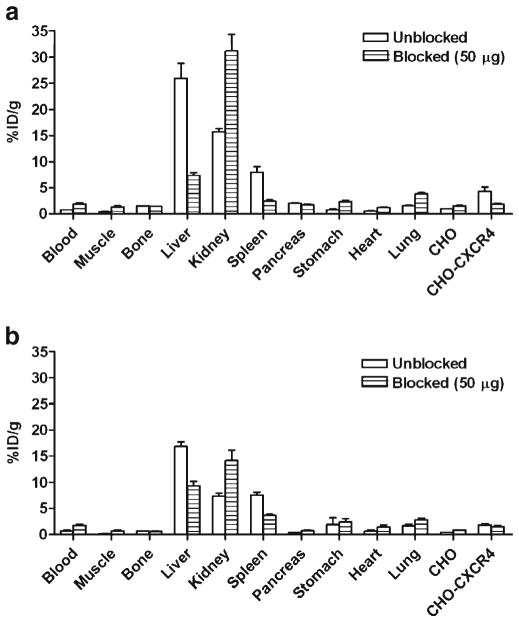
Biodistribution of [18F]FP-Ac-TC14012 and [18F]FB-Ac-TC14012 was analyzed by organ dissection with gamma counting in athymic nude mice that had been inoculated subcutaneously with CHO-CXCR4 and CHO tumors (Fig. 6). Data were obtained at 2 h p.i.. Both radiotracers showed higher uptake in CHO-CXCR4 tumor than in CHO tumor ([18F]FP-Ac-TC14012, 4.30±0.86 vs. 0.93±0.06 %ID/g, P<0.01; [18F]FB-Ac-TC14012, 1.86±0.19 vs. 0.44± 0.02 %ID/g, P<0.01). At blocking doses of 50 μg cold peptide (~50-fold excess), CHO-CXCR4 tumor uptake decreased; the uptake was not significantly different between CHO-CXCR4 and CHO tumors (P>0.05) for both radiotracers. Although the uptake in CXCR4-negative CHO tumors also increased, the change was not significant (P>0.05).
Fig. 6.
Biodistribution of [18F]FP-Ac-TC14012 (a) and [18F]FB-Ac-TC14012 (b) in athymic nude mice bearing CHO-CXCR4 and CHO tumors at 2 h p.i. in the presence of different amounts of unlabeled Ac-TC14012 peptide (0 and 50 μg). Results are shown as the average±SE of four to six mice.
For the N-terminal 4-F-benzoate analogs [25,26], the radiotracers showed very high blood retentions (12–15 %ID/g). In comparison, the radiotracers reported here only showed 0.7 and 0.8 %ID/g in the blood for [18F]FP-labeled and [18F]FB-labeled peptides, respectively. These results demonstrated that these radiotracers have much less blood retention than their N-terminal 4-F-benzoate analogs. Both radiotracers showed spleen uptake (8.00±1.07 and 7.53± 0.58 %ID/g for [18F]FP-Ac-TC14012 and [18F]FB-Ac-TC14012, respectively), which is a CXCR4-expressing organ. This specific uptake was blocked by co-injection of unlabeled peptide in approximately the same proportion (50 %) as observed in the CXCR4-transfected tumor. In addition to the spleen, high uptake in the liver and kidney was similar as previously observed for other T140-based imaging agents [26,27,29,39]. For both radiotracers, after co-injection with nonradioactive peptide, the liver uptake decreased significantly, implying some proportion of specific uptake, as it was reported that mouse liver expresses high levels of CXCR4 messenger RNA [40]. Meanwhile, the kidney uptake increased as the blocking dose increased, consistent with the microPET imaging results. The uptake in the blood, muscle, and all other major organs were very low and not affected by the co-injection of unlabeled peptide.
Several T140 peptide derivatives have been labeled by different methods for SPECT or PET imaging of CXCR4. Each derivative has its own advantages and disadvantages. For example, labeling of an Ac-TZ14011 peptide with [111In]DTPA resulted in only 0.51 %ID/g uptake in a pancreatic AsPC-1 tumor model, despite similar IC50 values of the labeled and unlabeled peptides [41]. The N-fluorobenzoate TN14003 peptide has very high binding affinity regardless of labeling, but showed high RBC binding in vivo, which resulted in low imaging contrast [25,26]. The small-molecule CXCR4 antagonists AMD3100/AMD3465 have been labeled with 64Cu for PET imaging as well [20–23]. These radiotracers showed similar uptake patterns: high liver and kidney uptake were also observed; the liver uptake can be blocked by cold compounds, while metal transchelation was also present. For the study that used a similar animal model, high radiotracer accumulation (12 %ID/g) was seen in CXCR4-positive tumor, but only at 6 h p.i.. The two new radiotracers reported here had high tumor uptake with minimal blood retention, which provided good tumor-to-background contrast. Meanwhile, they showed high uptake in metabolic organs, similar as that seen for other T140 peptide analogs, which may make imaging of the abdominal region problematic.
Conclusions
In this manuscript, Ac-TC14012 was labeled with two different 18F prosthetic groups for PET imaging of CXCR4 expression. The peptide was labeled on Lys7 using 4-nitrophenyl 2-[18F]-fluoropropionate ([18F]FP) or N-succinimidyl 4-[18F]-fluorobenzoate ([18F]FB). The radio-chemical yields were reasonable and radiolabeled products were obtained in high radiochemical purity. When imaging athymic nude mice bearing CHO-CXCR4 and CHO tumors, the CHO-CXCR4 tumors were clearly visualized with good tumor-to-background contrast for both radiotracers, while the uptake in CXCR4-negative CHO tumor was much lower. Co-injection with low dose (10 μg) of unlabeled peptide can further increase the uptake in the CHO-CXCR4 tumor, probably through the blocking of liver uptake. The CHO-CXCR4 tumor uptake can be blocked by higher blocking doses (50 and 200 μg). Among the two radiotracers, the [18F]FP-labeled peptide showed higher tumor uptake, lower nonspecific binding, and better tumor-to-background contrast, which suggests that the smaller size and lower hydrophobicity of the prosthetic group improves in vivo imaging. The work in this manuscript showed the potential of using [18F]FP-Ac-TC14012 for in vivo PET imaging of CXCR4 expression.
Supplementary Material
Acknowledgments
We want to thank the Intramural Research Program of the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH) for the funding support. This work was performed while X-X Zhang held a National Research Council Research Associateship Award at NIH/NIBIB.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11307-013-0640-0) contains supplementary material, which is available to authorized users.
Conflict of Interest. The authors declare that they have no con3ict of interest.
References
- 1.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14:171–179. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y, Larsen PH, Hao C, Yong VW. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem. 2002;277:49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- 3.Corcione A, Ottonello L, Tortolina G, et al. Stromal cell-derived factor-1 as a chemoattractant for follicular center lymphoma B cells. J Natl Cancer Inst. 2000;92:628–635. doi: 10.1093/jnci/92.8.628. [DOI] [PubMed] [Google Scholar]
- 4.Koshiba T, Hosotani R, Miyamoto Y, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6:3530–3535. [PubMed] [Google Scholar]
- 5.Scotton CJ, Wilson JL, Scott K, et al. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 2002;62:5930–5938. [PubMed] [Google Scholar]
- 6.Hwang JH, Hwang JH, Chung HK, et al. CXC chemokine receptor 4 expression and function in human anaplastic thyroid cancer cells. J Clin Endocrinol Metab. 2003;88:408–416. doi: 10.1210/jc.2002-021381. [DOI] [PubMed] [Google Scholar]
- 7.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 8.Geminder H, Sagi-Assif O, Goldberg L, et al. A possible role for CXCR4 and its ligand, the CXC chemokine stromal cell-derived factor-1, in the development of bone marrow metastases in neuroblastoma. J Immunol. 2001;167:4747–4757. doi: 10.4049/jimmunol.167.8.4747. [DOI] [PubMed] [Google Scholar]
- 9.Wald O, Shapira OM, Izhar U. CXCR4/CXCL12 axis in non-small cell lung cancer (NSCLC) pathologic roles and therapeutic potential. Theranostics. 2013;3:26–33. doi: 10.7150/thno.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werner L, Guzner-Gur H, Dotan I. Involvement of CXCR4/CXCR7/CXCL12 interactions in inflammatory bowel disease. Theranostics. 2013;3:40–46. doi: 10.7150/thno.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratajczak MZ, Serwin K, Schneider G. Innate immunity derived factors as external modulators of the CXCL12–CXCR4 axis and their role in stem cell homing and mobilization. Theranostics. 2013;3:3–10. doi: 10.7150/thno.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsumoto K, Kume S. The role of CXCL12–CXCR4 signaling pathway in pancreatic development. Theranostics. 2013;3:11–17. doi: 10.7150/thno.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel–Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 14.Helbig G, Christopherson KW, Bhat-Nakshatri P, et al. NF-κB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278:21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 15.Azab AK, Runnels JM, Pitsillides C, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–4351. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurtova AV, Tamayo AT, Ford RJ, Burger JA. Mantle cell lymphoma cells express high levels of CXCR4, CXCR5, and VLA-4 (CD49d): importance for interactions with the stromal microenvironment and specific targeting. Blood. 2009;113:4604–4613. doi: 10.1182/blood-2008-10-185827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peled A, Tavor S. Role of CXCR4 in the pathogenesis of acute myeloid leukemia. Theranostics. 2013;3:34–39. doi: 10.7150/thno.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Qi L, Li M, et al. Chemokine CXCL12 and its receptor CXCR4 expression are associated with perineural invasion of prostate cancer. J Exp Clin Cancer Res. 2008;27:62. doi: 10.1186/1756-9966-27-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon Y, Liang Z, Zhang X, et al. CXC chemokine receptor-4 antagonist blocks both growth of primary tumor and metastasis of head and neck cancer in xenograft mouse models. Cancer Res. 2007;67:7518–7524. doi: 10.1158/0008-5472.CAN-06-2263. [DOI] [PubMed] [Google Scholar]
- 20.Weiss I, Jacobson O, Kiesewetter D, et al. Positron emission tomography imaging of tumors expressing the human chemokine receptor CXCR4 in mice with the use of 64Cu-AMD3100. Mol Imaging Biol. 2012;14:106–114. doi: 10.1007/s11307-010-0466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Silva RA, Peyre K, Pullambhatla M, Fox JJ, Pomper MG, Nimmagadda S. Imaging CXCR4 expression in human cancer xenografts: evaluation of monocyclam 64Cu-AMD3465. J Nucl Med. 2011;52:986–993. doi: 10.2967/jnumed.110.085613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson O, Weiss ID, Szajek L, Farber JM, Kiesewetter DO. 64Cu-AMD3100—a novel imaging agent for targeting chemokine receptor CXCR4. Biorg Med Chem. 2009;17:1486–1493. doi: 10.1016/j.bmc.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nimmagadda S, Pullambhatla M, Stone K, Green G, Bhujwalla ZM, Pomper MG. Molecular imaging of CXCR4 receptor expression in human cancer xenografts with [64Cu]AMD3100 positron emission tomography. Cancer Res. 2010;70:3935–3944. doi: 10.1158/0008-5472.CAN-09-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hennrich U, Seyler L, Schäfer M, et al. Synthesis and in vitro evaluation of 68Ga-DOTA-4-FBn-TN14003, a novel tracer for the imaging of CXCR4 expression. Bioorg Med Chem. 2012;20:1502–1510. doi: 10.1016/j.bmc.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson O, Weiss ID, Kiesewetter DO, Farber JM, Chen X. PET of tumor CXCR4 expression with 4-18F-T140. J Nucl Med. 2010;51:1796–1804. doi: 10.2967/jnumed.110.079418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson OWI, Szajek LP, Niu G, Ma Y, Kiesewetter DO, Farber JM, Chen X. PET imaging of CXCR4 using copper-64 labeled peptide antagonist. Theranostics. 2011;1:251–262. doi: 10.7150/thno/v01p0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson O, Weiss ID, Szajek LP, et al. Improvement of CXCR4 tracer specificity for PET imaging. J Control Release. 2012;157:216–223. doi: 10.1016/j.jconrel.2011.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demmer O, Gourni E, Schumacher U, Kessler H, Wester H-J. PET Imaging of CXCR4 receptors in cancer by a new optimized ligand. ChemMedChem. 2011;6:1789–1791. doi: 10.1002/cmdc.201100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demmer O, Dijkgraaf I, Schumacher U, et al. Design, synthesis, and functionalization of dimeric peptides targeting chemokine receptor CXCR4. J Med Chem. 2011;54:7648–7662. doi: 10.1021/jm2009716. [DOI] [PubMed] [Google Scholar]
- 30.Tamamura H, Omagari A, Oishi S, et al. Pharmacophore identification of a specific CXCR4 inhibitor, T140, leads to development of effective anti-HIV agents with very high selectivity indexes. Bioorg Med Chem Lett. 2000;10:2633–2637. doi: 10.1016/s0960-894x(00)00535-7. [DOI] [PubMed] [Google Scholar]
- 31.Tamamura H, Omagari A, Hiramatsu K, et al. Development of specific CXCR4 inhibitors possessing high selectivity indexes as well as complete stability in serum based on an anti-HIV peptide T140. Bioorg Med Chem Lett. 2001;11:1897–1902. doi: 10.1016/s0960-894x(01)00323-7. [DOI] [PubMed] [Google Scholar]
- 32.Tamamura H, Hiramatsu K, Mizumoto M, et al. Enhancement of the T140-based pharmacophores leads to the development of more potent and bio-stable CXCR4 antagonists. Org Biomol Chem. 2003;1:3663–3669. doi: 10.1039/b306613b. [DOI] [PubMed] [Google Scholar]
- 33.Tamamura H, Hiramatsu K, Kusano S, et al. Synthesis of potent CXCR4 inhibitors possessing low cytotoxicity and improved biostability based on T140 derivatives. Org Biomol Chem. 2003;1:3656–3662. doi: 10.1039/b306473p. [DOI] [PubMed] [Google Scholar]
- 34.Lang L, Li W, Guo N, et al. Comparison study of [18F]FAl-NOTA-PRGD2, [18F]FPPRGD2, and [68Ga]Ga-NOTA-PRGD2 for PET imaging of U87MG tumors in mice. Bioconjug Chem. 2011;22:2415–2422. doi: 10.1021/bc200197h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiesewetter DO, Jacobson O, Lang L, Chen X. Automated radiochemical synthesis of [18F]FBEM: a thiol reactive synthon for radiofluorination of peptides and proteins. Appl Radiat Isot. 2011;69:410–414. doi: 10.1016/j.apradiso.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong ML, Xin WW, Duman RS. Rat LCR1: cloning and cellular distribution of a putative chemokine receptor in brain. Mol Psychiatr. 1996;1:133–140. [PubMed] [Google Scholar]
- 37.Lazarini F, Casanova P, Tham TN, et al. Differential signalling of the chemokine receptor CXCR4 by stromal cell-derived factor 1 and the HIV glycoprotein in rat neurons and astrocytes. Eur J Neurosci. 2000;12:117–125. doi: 10.1046/j.1460-9568.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- 38.Lavi E, Strizki JM, Ulrich AM, et al. CXCR-4 (fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol. 1997;151:1035–1042. [PMC free article] [PubMed] [Google Scholar]
- 39.Kuil J, Buckle T, Yuan H, et al. Synthesis and evaluation of a bimodal CXCR4 antagonistic peptide. Bioconjug Chem. 2011;22:859–864. doi: 10.1021/bc2000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moepps B, Frodl R, Rodewald H-R, Baggiolini M, Gierschik P. Two murine homologues of the human chemokine receptor CXCR4 mediating stromal cell-derived factor 1α activation of Gi2 are differentially expressed in vivo. Eur J Immunol. 1997;27:2102–2112. doi: 10.1002/eji.1830270839. [DOI] [PubMed] [Google Scholar]
- 41.Hanaoka H, Mukai T, Tamamura H, et al. Development of a 111In-labeled peptide derivative targeting a chemokine receptor, CXCR4, for imaging tumors. Nucl Med Biol. 2006;33:489–494. doi: 10.1016/j.nucmedbio.2006.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.