Abstract
Regions of transcription initiation and termination in kinetoplastid protists lack known eukaryotic promoter and terminator elements, although epigenetic marks such as histone variants and the modified DNA base J have been localized to these regions in Trypanosoma brucei, Trypanosoma cruzi, and/or Leishmania major. Phenotypes of base J mutants vary significantly across trypanosomatids, implying divergence in the epigenetic networks governing transcription during evolution. Here, we demonstrate that the histone variants H2A.Z and H2B.V are essential in L. major using a powerful quantitative plasmid segregation-based test. In contrast, H3.V is not essential for viability or normal growth in Leishmania. Steady-state transcript levels and the efficiency of transcription termination at convergent strand switch regions (SSRs) in H3V-null parasites were comparable to WT parasites. Our genetic tests show a conservation of histone variant phenotypes between L. major and T. brucei, unlike the diversity of phenotypes associated with genetic manipulation of the DNA base J modification.
Keywords: epigenetics, histone variants, transcriptional read-through, chromatin, trypanosomatid protozoa, genetics
The generation of mature messenger RNAs (mRNAs) in Leishmania and other kinetoplastid parasites involves a bipartite mechanism of transcription by RNA polymerase II (RNAP II), unlike the majority of eukaryotes studied to date. All protein-coding genes are transcribed as pre-mRNAs arising from long head-to-tail arrays called polycistronic gene clusters (PGCs), while the RNAs encoding the capped 5′ ends of mature transcripts are transcribed separately from the spliced leader (SL) RNA array (reviewed in [1]. Polycistronic pre-mRNAs are then processed by 5′ trans-splicing of the SL RNA to generate the capped 5′ end of the mRNA and cleavage and polyadenylylation to generate the 3′ end. In this system, individual transcription units are mostly defined by the boundaries of PGCs: transcription primarily initiates within divergent strand-switch regions (dSSRs), where two PGCs are oriented head-to-head, and terminates in convergent strand-switch regions (cSSRs), where two PGCs meet tail-to-tail. These regions lack known eukaryotic promoter and terminator elements [2,3,4], and trypanosomatid genomes reveal the presence of general but not sequence-specific RNAP II transcription factors [5]. However, a number of epigenetic marks have been identified in trypanosomatids, including histone variants, histone modifications, and the trypanosomatid-specific DNA modification β-d-glucopyranosyloxymethyluracil (base J), many of which have been mapped to dSSRs or cSSRs in one or more trypanosomatid species [2,6,7,8,9,10]. However, transcription termination and re-initiation may also potentially occur within a PGC, since these chromatin signatures have been found within PGCs [2,6,11].
Despite the apparent conservation of epigenetic marks and their genomic localization amongst trypanosomatid species, recent data suggest that their functions may differ greatly. This is most clearly seen in studies of DNA base J, perturbations of which show widely varying consequences in the three lineages examined thus far. In T. brucei, T. cruzi, L. tarentolae, and L. major, base J has been localized to convergent and divergent SSRs as well as telomeres, including the inactive subtelomeric variant surface glycoprotein genes in T. brucei [7,8,9,12]. In T. brucei, deletion of the genes encoding the thymidine hydroxylases JBP1 and JBP2, which catalyze the first step in base J biosynthesis, generates viable parasites lacking J with no other observable phenotypes or changes in gene expression [13]. In T. cruzi, the JBP1-/JBP2- double null mutant was not viable, while individual JBP1- or JBP2 mutants showed altered transcriptional rates and polymerase occupancy near dSSRs, but normal transcription termination at the cSSRs examined [9]. In Leishmania tarentolae, JBP1 is essential [14], and JBP2-null mutants showed massive transcriptional read-through at cSSRs in addition to increased antisense transcription and use of alternative transcription start sites in dSSRs [7].
The evolutionary diversity evident from base J perturbations prompted us to ask whether other epigenetic marks might show functional divergence as well. In T. brucei, chromatin immunoprecipitation studies localized H2A.Z and H2B.V to dSSRs and H3.V and H4.V to cSSRs [2], implicating these proteins in regulation of transcription initiation and termination, respectively. Both H2AZ and H2BV are essential in T. brucei, while H3V and H4V are not [2]. The histone variants H2A.Z, H2B.V, and H3.V are readily identified in L. major, although a clear ortholog of H4.V has not been identified yet [5]. Here we focus on the Leishmania histone variants H2A.Z, H2B.V, and H3.V, and explore the functional consequences of their genetic inactivation on viability and transcription.
In anticipation that H2AZ and H2BV would be essential in Leishmania, we employed a definitive test for gene essentiality which relies on segregational loss of an episomal complementation vector [15]. In addition to separating the test of gene function from the process of transfection and allelic replacement, this technique allows for screening high number of events rapidly, improving the chances of isolating null mutants whose fitness may be compromised and mutants from loci where homologous recombination is less efficient. Furthermore, when null mutants are not obtained, one has a higher confidence in conclusions concerning the essentiality of the gene of interest [15,16]. We generated heterozygous (H2AZ/HYG [pXNG-H2AZ] and H2BV/SAT [pXNG-H2BV]) and chromosomal-null lines Δh2az [pXNG-H2AZ] and Δh2bv [pXNG-H2BV] bearing an episomal complementation vector expressing either H2AZ or H2BV along with GFP (see Supplemental Data 1 for methods and primer sequences). These lines were grown without selection to permit loss of the episomal complementation vector. We observed that only 0.2% of the Δh2az [pXNG-H2AZ] were ‘GFP-dim’ compared to the 33.7% of the H2AZ/HYG [pXNG-H2AZ] heterozygote line, potentially heralding that this gene is essential (Fig. 1A). Similar results were obtained for Δh2bv [pXNG-H2BV] (0.1% ‘GFP-dim’) and H2BV/PAC [pXNG-H2BV] (10.4% ‘GFP-dim’) (Supplemental Fig. S1A).
Figure 1. H2AZ is essential in L. major.
(A) Quantitation of pXNG-H2AZ levels by GFP flow cytometry following removal of nourseothricin selection. The dark gray shaded regions represent GFP fluorescence of the experimental lines, and the dotted line shows GFP fluorescence values from WT L. major FV1. Light gray shaded regions represent FACS gates used for recovery of ‘GFP-dim’ (left shaded region) and ‘GFP-bright’ (right shaded region) cells; parasites with a GFP fluorescence signal of 1 or less were not included in the ‘GFP-dim’ gate. The lines H2AZ/HYG[pXNG-H2AZ] (left panel) and Δh2az[pXNG-H2AZ] (right panel) were grown for 48 hours (~12 cell doublings) in the absence of nourseothricin to allow loss of the episome before GFP fluorescence was analyzed. Boxes show percent of parasites classified as ‘GFP-bright’ or ‘GFP-dim’. (B) Single cells from ‘GFP-dim’ and ‘GFP-bright’ H2AZ/HYG[pXNG-H2AZ] and Δh2az[pXNG-H2AZ] were sorted into 96-well plates containing supplemented Schneiders’ medium (see Materials and Methods). Boxes show the percentage of wells scored for robust growth after two weeks of incubation at 26°C; numbers in parentheses represent the total number of cells sorted (total from two independent experiments). For these, retention of pXNG-H2AZ was tested by growth in the presence of nourseothricin, conferred by the plasmid SAT marker. Boxes show the percentage of cells demonstrating nourseothricin resistance; numbers in parentheses represent the total number of wells subjected to nourseothricin resistance testing.
Single cells from the ‘GFP-dim’ and ‘GFP-bright’ populations of the chromosomal-null and heterozygote lines were sorted into 96-well microtiter plates and incubated with media until robust growth was seen in control wells. Sorting of the heterozygous lines bearing the episome (H2AZ/HYG [pXNG-H2AZ] and H2BV/SAT [pXNG-H2BV]) yielded growth in 70–80% of wells for both the ‘GFP-dim’ and ‘GFP-bright’ populations (Fig. 1B and Supplemental Fig. S1B, respectively); this provides a basal measure of cell survival and growth following sorting. As expected, all of the ‘GFP-bright’ clones retained episomes containing the streptothricin acetyltranferase (SAT) or hygromycin B phosphotransferase (HYG) markers, while most (80–100%) cells arising from the ‘GFP-dim’ populations completely lost the episome and became sensitive to the selective antibiotics (Fig. 1B, Supplemental Fig. S1B). Sorting of the ‘GFP-bright’ cells from the Δh2az [pXNG-H2AZ] and Δh2bv [pXNG-H2BV] populations yielded 70–85% growth, comparable to that of the heterozygous control populations. In contrast, only a small fraction (0.6–0.8%) of the ‘GFP-dim’ cells showed growth following sorting; none of these cells had lost the episome bearing H2AZ or H2BV as judged by retention of the selectable marker from the episome (Fig. 1B, Supplemental Fig. S1B). From the plating efficiency and numbers of wells tested, we estimated that approximately 610 events were scored in this assay for H2AZ and 740 events for H2BV, many more than typically screened by traditional non-segregational methods. Thus, we conclude from these experiments that both H2AZ and H2BV are essential in L. major.
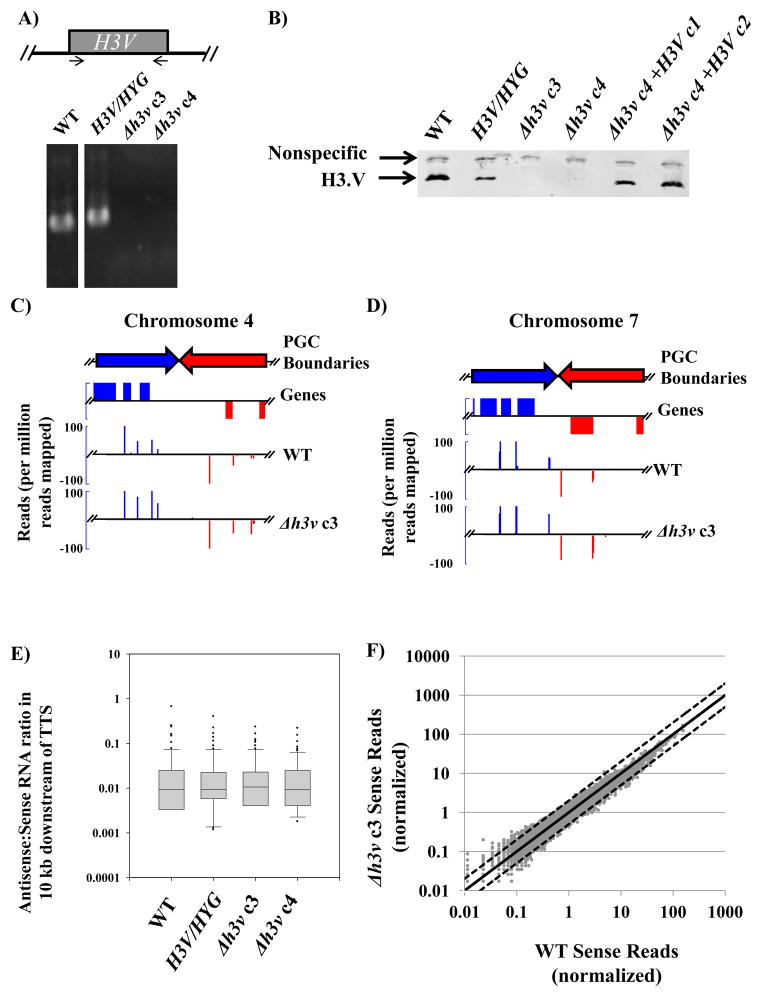
In contrast to H2A.Z and H2B.V, we were able to delete both H3V alleles by the standard method of two rounds of allelic replacement, yielding homozygous null mutants (Δh3v). Colonies were readily obtained from the second round of allelic replacement, and out of six colonies screened five had lost the H3V gene. This was shown by the presence of the planned replacements as revealed by PCR using primers flanking and internal to the targeting fragment (data not shown), and the absence of the H3V ORF by PCR using primers within the H3V ORF (Fig. 2A). The absence of H3.V protein was shown by Western blotting with H3.V-specific antisera (Fig. 2B; see Supplemental Fig. S2 for characterization of antisera). Complemented lines were generated by transfection of an H3V-containing episome and showed restoration of H3.V protein levels to levels comparable to WT (Fig. 2B). Since typically episomes are present in multiple copies leading to overexpression of encoded genes, these data suggest the possibility that H3.V levels are regulated at the protein level. Analysis of several clonal Δh3v lines showed that they were phenotypically normal, showing WT growth in vitro (Fig. S2C). Although we observed a significant increase in the fraction of metacyclic parasites in both Δh3v clones, a similar increase was observed in the complementing lines (Fig. S2D). While it is possible that the episomally-encoded H3V is regulated differently than chromosomal copies of the gene, the WT H3.V levels in the complemented lines lead us to believe that this phenotype is unrelated to loss of H3.V expression. To identify defects in parasite virulence we inoculated BALB/c mice in the footpad with 107 stationary phase parasites from six independent clones. All lines generated lesions within one month, similar to WT L. major (data not shown). Together these data demonstrate that deletion of H3V does not alter viability of L. major in the promastigote stage or significantly impair the infectivity of amastigote stages in murine infections.
Figure 2. Deletion of H3.V does not increase read-through transcription as observed by SL-RNA-seq.
(A) Deletion of H3V was shown by PCR analysis using H3V ORF primers located as depicted in the upper figure in this panel. (B) Loss of H3.V expression shown by western blotting using anti-H3.V antisera. The migration position of H3.V is shown, as is a nonspecific band evident in all samples. The nonspecific band does not arise from cross-reactivity with H3 (Fig. S1). (C–D) Integrative Genomics Viewer [IGV; 24] screenshots demonstrating SL-RNA-seq coverage across ‘simple’ cSSRs. The Y-axes represent normalized read counts (per million reads mapped) and the X-axis represents physical location on each chromosome; a 20 kb window showing 10 kb flanking the transcription termination site (TTS) is shown (C: Chromosome 4, 118,903–138,903 bp; D: Chromosome 7, 49,636–69,636 bp). Unlike random RNA-seq reads, SL-RNA-seq results in clustering of reads on a limited number of splice acceptor sites (regardless of whether they are ‘normal’ or ‘cryptic’ [7]). (E) Quantitative analysis of transcription termination assessed by SL-RNA-seq. Following previous studies [7], TTS within cSSRs were defined using the midpoint of base J ‘peaks’ associated with TTS; reads mapping to the ‘sense’ and ‘antisense’ strand within 10 kb of the TTS were quantitated and the ratio of antisense to sense reads is shown by a box plot. The middle line represents the median, while the box represents the 25th through 75th percentiles. Whiskers represent the 10th through 90th percentiles, and dots represent individual cSSRs which lie below the 10th or above the 90th percentile. (F) Total mRNA levels quantitated by SL-RNA-seq are unchanged in Δh3v parasites. Read counts were normalized to the median number of reads mapped to each gene (see [17] for methods used). The X- and Y-axes shows sense strand read counts for genes from WT and Δh3v clone 3, respectively. The solid line shows the slope (1) expected for no changes in transcript levels; dotted lines represent 2-fold higher and lower boundaries. The correlation coefficient (R2) comparing WT and Δh3v clone 3 was 0.9646. Comparable results were obtained in comparisons of WT with H3V/HYG (R2 = 0.9905) or Δh3v clone 4 (R2 = 0.9918).
To elucidate potential roles for H3.V in transcription in Leishmania, we analyzed mRNA levels in WT and Δh3v parasites by high-throughput sequencing of spliced leader (SL)-primed cDNA libraries. This method quantifies steady-state RNA levels in a population of cells by specifically amplifying only transcripts with an SL sequence at their 5′ end [7,11,17,18]. Importantly, studies in L. tarentolae demonstrate that this approach is also a sensitive method for detecting read-through transcription arising from defects in transcription termination, as these abnormal RNAs can give rise to stable RNAs after processing using cryptic splice acceptor sites (see Fig. 2 in reference [7] for an example arising from base J deficiency in L. tarentolae). The sensitivity of detection of both normal ‘sense’ and cryptic ‘antisense’ splice acceptors is very high, with ranges in the hundreds of reads per million reads mapped for both ‘normal’ and ‘cryptic’ splice acceptors [7].
We focus first on transcriptional read-through, a hallmark of defects in transcriptional termination. As in previous studies in L. tarentolae and T. brucei [7,11,18], in WT L. major the vast majority of SL-containing reads map to the coding strand, with very few mapping to antisense regions beyond cSSRs (see Fig. 2C–D, Supplemental Figs. S3, S4). Remarkably, this pattern was unchanged in Δh3v parasites across the parasite genome (Supplemental Fig. S4). This included ‘simple’ cSSRs (Fig. 2C C, D show two representative examples), cSSRs containing RNA polymerase III-transcribed genes (which are known to suppress transcriptional read-through in the absence of base J in L. tarentolae [7]; Supplemental Fig. S3A,B), or the single cSSR known to lack base J in L. major (located on chromosome 28; Supplemental Fig. S3C). Quantitative measurement of transcriptional read-through (the antisense-to-sense ratio of reads mapping within 10 kb of a cSSR) shows a very similar distribution in the WT, heterozygous, and Δh3v lines (Fig. 2E). These findings are in stark contrast to the results seen in L. tarentolae by SL-primed RNA sequencing, where perturbations of base J synthesis in JBP2dKO parasites led to high levels of transcriptional read-through [7].
Lastly, we compared mRNA levels by plotting the normalized number of reads mapping to the sense strand of individual L. major genes for WT against Δh3v parasites. Again, normalized sense transcript levels were remarkably similar between WT and Δh3v parasites, with R2 values >0.96 for two independent Δh3v clonal transfectants (Fig. 2F). Examination of all genes containing at least 50 mapped reads in the WT and/or Δh3v datasets showed that only two genes showed greater than two-fold differences, occurring in both independent Δh3v clonal lines. These genes are located in the middle of PGCs and would appear unlikely candidates to be unaffected by any potential alterations in regulation of transcription initiation or termination. The P27 protein (Lmj28.0980), a component of the cytochrome c oxidase complex [19,20], was up-regulated 2.3-fold in both Δh3v lines tested relative to WT. In addition, a protein tyrosine phosphatase 1-like protein (LmjF36.2180) was up-regulated 2.2–2.3-fold in these lines. This protein has not been characterized to date in Leishmania but is an important regulator of cell differentiation in T. brucei [21]. Given the absence of detectable phenotypes in Δh3v mutants, the significance of these small changes or whether they even result in changes in protein levels is uncertain. Together, these data suggest that H3.V is not required for defining transcriptional stops in Leishmania and likely does not play a critical role in controlling mRNA abundance.
While it is often standard practice to translate the functional aspects of epigenetic networks from one system to another based on localization patterns, studies of the hypermodified DNA base J in kinetoplastids demonstrates that assumptions of conserved function based on conserved localization patterns may be incorrect [7,8,9,22]. In our survey of histone variants in Leishmania, we found that H2AZ and H2BV were essential (much like in T. brucei), suggesting that their functions are likely conserved. Given their genomic distribution in T. brucei and the high degree of H2A.Z conservation among all eukaryotes, we suspect that these proteins are integral components of the epigenetic networks controlling transcription initiation. However, the dSSR-associated epigenetic network could differ in T. cruzi, as base J mutants show a transcription initiation-related phenotype [9,22] that is not replicated in in base J mutants in T. brucei [8] or L. tarentolae [7]. In such a case, H2A.Z and/or H2B.V may play functionally different roles which could differ significantly from eukaryotes studied to date. Elucidation of the effects of H2A.Z/H2B.V incorporation on chromatin compaction and characterization of histone variant incorporation during parasite differentiation will allow us to more specifically define the roles of these proteins in kinetoplastids.
In contrast to H2A.Z and H2B.V, we found that H3V-null L. major were viable, morphologically normal, and infectious; moreover, they behaved as WT parasites with respect to transcriptional regulation and most interestingly, transcription termination. These data, when interpreted in the light of recent work demonstrating the deleterious effects of perturbation of transcription termination-associated epigenetic networks in Leishmania mediated by base J [7], suggests that H3.V is not an essential component of this epigenetic network. Transcription termination-associated phenotypes were not examined in H3V-null T. brucei [23] and no data exist regarding the essentiality of this protein in T. cruzi, so it remains unclear whether this protein is functioning redundantly with other components in the epigenetic network of these parasite species. Although H3.V may not be a critical component of the transcription termination-associated epigenetic networks, chromatin-based studies of H3.V mutants may further define the roles of this conserved, kinetoplastid-specific histone variant.
Supplementary Material
Supplemental Data 1. Extended methods and primer sequences used for generation of deletion constructs for H2AZ, H2BV, and H3V, amplification of histone variant ORFs for protein expression and expisomal complementation vectors, and demonstration of Δh3v planned replacements.
Supplemental Figure S1. H2BV is essential in L. major. (A) Quantitation of pXNG(HYG)-H2BV levels by GFP flow cytometry following removal of hygromycin selection. GFP fluorescence panels and boxes are defined as in Fig. 1A. H2BV/PAC[pXNG-H2BV] (left panel) and Δh2bv[pXNG-H2BV] (right panel) cells were grown for 48 hours (~12 cell doublings) in the absence of hygromycin to allow loss of the episome. (B) Single cells from ‘GFP-dim’ and ‘GFP-bright’ H2BV/PAC[pXNG-H2BV] and Δh2bv[pXNG-H2BV] were sorted and scored as described in Fig. 1. Boxes are defined as in Fig. 1B. Plasmid retention was tested using hygromycin resistance of cells from robustly growing wells and is presented as described in Fig. 1B.
Demonstration of anti-H3 (A, B) and anti-H3.V specificity by western blotting and characterization of Δh3v phenotypes in vitro. (A, C) Antisera were tested using the recombinant proteins used as immunogens (A, H3-N; C, H3.V-N). (B, D) Antisera were tested against a purified acid-soluble fraction from L. major chromatin (B, anti-H3-N; D, anti-H3.V-N). The migration of molecular weight markers is shown; the expected MW are 14.6 kDa for H3 and 16.3 kDa for H3.V. (E–F) Deletion of H3V in L. major does not alter growth or metacyclogenesis. (E) WT and Δh3v mutants grow comparably in vitro. (F) Metacyclogenesis was quantitated after 3 d in stationary phase using the density gradient method [25]. Error bars represent standard deviation of three biological replicates.
Transcription termination is unaltered in cSSRs containing RNA polymerase III-transcribed genes, or lacking base J, in Δh3v parasites. (A–B) IGV screenshots demonstrating SL-RNA-seq coverage across convergent SSRs containing one (A) or multiple (B) RNA polymerase III- transcribed genes. Y-axes represent normalized read counts (per million reads mapped) and X-axes represents physical location on the chromosome; 20 kb windows are shown as described in Fig. 4. (C) IGV screenshot demonstrating SL-RNA-seq coverage across the sole cSSR lacking base J in L. major, located on chromosome 28 [7]. Despite the absence of both base J and H3.V, transcription termination is not altered.
Transcription termination is unaltered genome-wide in Δh3v parasites. (A–L) IGV screenshots are shown for L. major chromosomes 1–36, displaying SL-RNA-seq mappings as described in Fig. 2. Y-axes represent normalized read counts (per million reads mapped) and are scaled to 1000 reads per million reads mapped, and X-axes represent physical location on the chromosome. Unlike random RNA-seq reads, SL-RNA-seq results in clustering of reads on a limited number of splice acceptor sites.
Histone variants H2A.Z and H2B.V are essential in Leishmania major
Histone variant H3.V is not essential in L. major
H3.V loss does not affect read-through transcription, gene expression, viability nor virulence in a mouse model
Genetic tests suggest that the roles of epigenetic networks can be conserved (histone variants) or can diverge (DNA base J) during evolution
Acknowledgments
We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Mo., for the use of the Siteman Flow Cytometry Core, which provided the single-cell sorting service. The Siteman Cancer Center is supported in part by NCI Cancer Center Support Grant #P30 CA91842. This work was funded by the National Institutes of Health (R0129646 to SMB and ILKW, T32 GM007067 to BAA) and the Washington University Berg-Morse and Schlesinger Graduate Fellowships to BAA. We thank Andrew Haydock and members of our laboratories for discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gunzl A, Vanhamme L, Myler P. Transcription in trypanosomes: a different means to the end. In: Barry J, Mottram J, McCulloch R, Acosta-Serrano A, editors. Trypanosomes - After the Genome. Horizon Bioscience. Wymondham; Norfolk, UK: 2007. pp. 177–208. [Google Scholar]
- 2.Siegel TN, Hekstra DR, Kemp LE, Figueiredo LM, Lowell JE, Fenyo D, et al. Four histone variants mark the boundaries of polycistronic transcription units in Trypanosoma brucei. Genes Dev. 2009;23:1063–76. doi: 10.1101/gad.1790409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martínez-Calvillo S, Nguyen D, Stuart K, Myler PJ. Transcription Initiation and Termination on Leishmania major Chromosome 3. Eukaryot Cell. 2004;3:506–17. doi: 10.1128/EC.3.2.506-517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martínez-Calvillo S, Yan S, Nguyen D, Fox M, Stuart K, Myler PJ. Transcription of Leishmania major Friedlin chromosome 1 initiates in both directions within a single region. Mol Cell. 2003;11:1291–9. doi: 10.1016/s1097-2765(03)00143-6. [DOI] [PubMed] [Google Scholar]
- 5.Ivens AC, Peacock CS, Worthey EA, Murphy L, Aggarwal G, Berriman M, et al. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309:436–42. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas S, Green A, Sturm NR, Campbell DA, Myler PJ. Histone acetylations mark origins of polycistronic transcription in Leishmania major. BMC Genomics. 2009;10:152. doi: 10.1186/1471-2164-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Luenen HGAM, Farris C, Jan S, Genest P-A, Tripathi P, Velds A, et al. Glucosylated hydroxymethyluracil, DNA base J, prevents transcriptional readthrough in Leishmania. Cell. 2012;150:909–21. doi: 10.1016/j.cell.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cliffe LJ, Siegel TN, Marshall M, Cross GAM, Sabatini R. Two thymidine hydroxylases differentially regulate the formation of glucosylated DNA at regions flanking polymerase II polycistronic transcription units throughout the genome of Trypanosoma brucei. Nucleic Acids Res. 2010;38:3923–35. doi: 10.1093/nar/gkq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekanayake DK, Sabatini R. Epigenetic regulation of polymerase II transcription initiation in Trypanosoma cruzi: modulation of nucleosome abundance, histone modification, and polymerase occupancy by O-linked thymine DNA glucosylation. Eukaryot Cell. 2011;10:1465–72. doi: 10.1128/EC.05185-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright JR, Siegel TN, Cross GAM. Histone H3 trimethylated at lysine 4 is enriched at probable transcription start sites in Trypanosoma brucei. Mol. Biochem. Parasitol. 2010;172:141–4. doi: 10.1016/j.molbiopara.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolev NG, Franklin JB, Carmi S, Shi H, Michaeli S, Tschudi C. The transcriptome of the human pathogen Trypanosoma brucei at single-nucleotide resolution. PLoS Pathog. 2010;6:e1001090. doi: 10.1371/journal.ppat.1001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Leeuwen F, Wijsman ER, Kieft R, Van Der Marel GA, Van Boom JH, Borst P. Localization of the modified base J in telomeric VSG gene expression sites of Trypanosoma brucei. Genes Dev. 1997;11:3232–41. doi: 10.1101/gad.11.23.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cliffe LJ, Kieft R, Southern T, Birkeland SR, Marshall M, Sweeney K, et al. JBP1 and JBP2 are two distinct thymidine hydroxylases involved in J biosynthesis in genomic DNA of African trypanosomes. Nucleic Acids Res. 2009;37:1452–62. doi: 10.1093/nar/gkn1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genest P-A, ter Riet B, Dumas C, Papadopoulou B, van Luenen HGAM, Borst P. Formation of linear inverted repeat amplicons following targeting of an essential gene in Leishmania. Nucleic Acids Research. 2005;33:1699–709. doi: 10.1093/nar/gki304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murta SMF, Vickers TJ, Scott DA, Beverley SM. Methylene tetrahydrofolate dehydrogenase/cyclohydrolase and the synthesis of 10-CHO-THF are essential in Leishmania major. Molecular Microbiology. 2009;71:1386–401. doi: 10.1111/j.1365-2958.2009.06610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng X, Rodriguez-Contreras D, Polley T, Lye L-F, Scott D, Burchmore RJS, et al. ‘Transient’ genetic suppression facilitates generation of hexose transporter null mutants in Leishmania mexicana. Molecular microbiology. 2013;87:412–29. doi: 10.1111/mmi.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittra B, Cortez M, Haydock A, Ramasamy G, PJM, Andrews N. Iron uptake controls the generation of Leishmania infective forms through regulation of ROS levels. J Exp. Med. 2013;210:401–16. doi: 10.1084/jem.20121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nilsson D, Gunasekara K, Mani J, Osteras M, Farinelli L, Baerlocher L, et al. Spliced leader trapping reveals widespread alternative splicing patterns in the highly dynamic transcriptome of Trypanosoma brucei. PLoS Pathog. 2010;6:e1001037. doi: 10.1371/journal.ppat.1001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dey R, Dagur PK, Selvapandiyan A, McCoy JP, Salotra P, Duncan R, et al. Live attenuated Leishmania donovani p27 gene knockout parasites are nonpathogenic and elicit long-term protective immunity in BALB/c mice. J. Immunol. 2013;190:2138–49. doi: 10.4049/jimmunol.1202801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dey R, Meneses C, Salotra P, Kamhawi S, Nakhasi HL, Duncan R. Characterization of a Leishmania stage-specific mitochondrial membrane protein that enhances the activity of cytochrome c oxidase and its role in virulence. Mol. Microbiol. 2010;77:399–414. doi: 10.1111/j.1365-2958.2010.07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szöőr B, Wilson J, McElhinney H, Tabernero L, Matthews KR. Protein tyrosine phosphatase TbPTP1: a molecular switch controlling life cycle differentiation in trypanosomes. J Cell Biol. 2006;175:293–303. doi: 10.1083/jcb.200605090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ekanayake DK, Minning T, Weatherly B, Gunasekera K, Nilsson D, Tarleton R, et al. Epigenetic regulation of transcription and virulence in Trypanosoma cruzi by O-linked thymine glucosylation of DNA. Mol. Cell. Biol. 2011;31:1690–1700. doi: 10.1128/MCB.01277-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowell JE, Cross GAM. A variant histone H3 is enriched at telomeres in Trypanosoma brucei. J. Cell Sci. 2004;117:5937–47. doi: 10.1242/jcs.01515. [DOI] [PubMed] [Google Scholar]
- 24.Thorvaldsdottir H, Robinson J, Mesirov J. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14:178–92. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Späth GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Experimental parasitology. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data 1. Extended methods and primer sequences used for generation of deletion constructs for H2AZ, H2BV, and H3V, amplification of histone variant ORFs for protein expression and expisomal complementation vectors, and demonstration of Δh3v planned replacements.
Supplemental Figure S1. H2BV is essential in L. major. (A) Quantitation of pXNG(HYG)-H2BV levels by GFP flow cytometry following removal of hygromycin selection. GFP fluorescence panels and boxes are defined as in Fig. 1A. H2BV/PAC[pXNG-H2BV] (left panel) and Δh2bv[pXNG-H2BV] (right panel) cells were grown for 48 hours (~12 cell doublings) in the absence of hygromycin to allow loss of the episome. (B) Single cells from ‘GFP-dim’ and ‘GFP-bright’ H2BV/PAC[pXNG-H2BV] and Δh2bv[pXNG-H2BV] were sorted and scored as described in Fig. 1. Boxes are defined as in Fig. 1B. Plasmid retention was tested using hygromycin resistance of cells from robustly growing wells and is presented as described in Fig. 1B.
Demonstration of anti-H3 (A, B) and anti-H3.V specificity by western blotting and characterization of Δh3v phenotypes in vitro. (A, C) Antisera were tested using the recombinant proteins used as immunogens (A, H3-N; C, H3.V-N). (B, D) Antisera were tested against a purified acid-soluble fraction from L. major chromatin (B, anti-H3-N; D, anti-H3.V-N). The migration of molecular weight markers is shown; the expected MW are 14.6 kDa for H3 and 16.3 kDa for H3.V. (E–F) Deletion of H3V in L. major does not alter growth or metacyclogenesis. (E) WT and Δh3v mutants grow comparably in vitro. (F) Metacyclogenesis was quantitated after 3 d in stationary phase using the density gradient method [25]. Error bars represent standard deviation of three biological replicates.
Transcription termination is unaltered in cSSRs containing RNA polymerase III-transcribed genes, or lacking base J, in Δh3v parasites. (A–B) IGV screenshots demonstrating SL-RNA-seq coverage across convergent SSRs containing one (A) or multiple (B) RNA polymerase III- transcribed genes. Y-axes represent normalized read counts (per million reads mapped) and X-axes represents physical location on the chromosome; 20 kb windows are shown as described in Fig. 4. (C) IGV screenshot demonstrating SL-RNA-seq coverage across the sole cSSR lacking base J in L. major, located on chromosome 28 [7]. Despite the absence of both base J and H3.V, transcription termination is not altered.
Transcription termination is unaltered genome-wide in Δh3v parasites. (A–L) IGV screenshots are shown for L. major chromosomes 1–36, displaying SL-RNA-seq mappings as described in Fig. 2. Y-axes represent normalized read counts (per million reads mapped) and are scaled to 1000 reads per million reads mapped, and X-axes represent physical location on the chromosome. Unlike random RNA-seq reads, SL-RNA-seq results in clustering of reads on a limited number of splice acceptor sites.