Abstract
Background/Purpose
Wound healing is a complex process that involves multiple intercellular and intracellular processes and extracellular interactions. Explanted human skin has been used as a model for the re-epithelialization phase of human wound healing. The currently used standard technique employs a circular punch biopsy tool to make the initial wound. Despite its wide use, the geometry of round wounds makes them difficult to measure reliably.
Methods
Our group has designed a linear wounding tool, and compared the variability in ex vivo human linear and circular wounds.
Results
An F test for differences in variances demonstrated that the linear wounds provided a population of wound size measurements that was fifty percent less variable than that obtained from a group of matched circular wounds. This reduction in variability would provide substantial advantages for the linear wound technique over the circular wound punch technique, by reducing the sample sizes required for comparative studies of factors that alter healing.
Conclusion
This linear wounding tool thus provides method for wounding that is standardized, provides minimal error in wound gap measurements, and is easily reproducible. We demonstrate its utility in an ex vivo model for the controlled investigation of human skin wounds.
INTRODUCTION
“In 2003, the age-adjusted discharge rate for diabetic foot ulcer was 6.9 per 1,000 diabetic population”1. Furthermore, ulcers are the most frequent lower extremity complication of diabetic patients; greater than peripheral arterial disease and neuropathy1. In addition, venous stasis is another condition accounting for 40-70% of lower extremity chronic wounds 2-4. These kinds of chronic wounds incur not only a substantial economic burden of morbidity and mortality, but also a loss in the quality of life. Wound healing studies wherein chronic wounds are modeled, created, and repeated experimentally, provide a critical translational approach for development of new wound treatments.
Some of the earliest studies of wounds were done on human volunteers 5. Eventually due to ethical concerns and impracticality, mammalian in-vivo and ex-vivo models became the standard approach for wound healing studies. Animal models for chronic wounds have utilized a wide array of species, including diabetic mice, rates and pigs 6-9. Animal models offer the ability to examine living tissue wounds under reproducible, controlled conditions. Rodent models, in particular, offer the advantage of being able to examine the effect of a single gene on healing, given the relative ease of generating transgenic animals in those species. However wound healing proceeds differently in different species: rodent skin wounds heal primarily by contraction, while human skin wounds heal primarily by granulation 10-12. Thus there is a need for a human skin-based model for chronic wounds. An ex-vivo organotypic human skin wound model developed by Kratz and colleagues describes culture conditions under which a mid-dermal round excisional wound does not re-epithelialize, despite the sustained viability of the peri-wound cells. In this model, once the culture conditions are optimized by addition of serum, previously unhealed wound phenotypes are reversed to fully epithelialized wounds 13. Certainly, this model is limited in mimicking a human chronic wound since multiple factors such as inflammatory cells, and an intact circulation, are missing. Nevertheless, by limiting the amount of serum supplied to the organotypically cultured wounds, the suboptimal wound healing response created in the model allows for quantitative analysis of the contribution of exogenously added growth factors or other biological modifiers to the wound healing process 14, 15.
The complexity inherent to wound healing research is due to a variety of key regulators and mediators within the skin network, including intercellular communications with other skin cells, a concomitant reconstruction and tissue remodeling by immune cells and enzymatic proteinases, and the overall coordination of contractile and migratory forces produced from these events. Damage to tissue and skin epithelium results in a series of cellular and molecular responses that form ordered processes to prevent infection and initiate the repair of the damaged skin. The mechanism of reepithelialization involves both the migration and proliferation of keratinocytes. There are many important molecules such as growth factors, integrins, extra-cellular matrices, and metallo-proteases that are involved in the proliferation and migration of human skin keratinocytes (reviewed in 16-18. Given the multitude of environmental and cellular conditions involved in wound healing, a consistent, reliable, and easily reproducible technique of wounding and for measuring the effects of interventions on healing is critically needed for efficient study design.
The standard technique of wounding in almost all of the experimental models (including the explant cultured human skin model) has been with a circular punch biopsy tool. Circular wound biopsies are limited by the challenge of obtaining reliable and reproducible wound size estimates based on histological sections; the sections are frequently asymmetric, due to sectioning plane effects 19. We have constructed a novel yet simple linear incision tool that creates ex-vivo wounds in a reproducible and consistent manner. Here, we compared the variances of wound-size measurements created using human ex-vivo wound models that mimic the non-healing, chronic wound environment. The first model uses a circular punch biopsy tool (as originally described 13) to create the wound, and the second model uses a linear, rectangular biopsy tool to create the wound: in both models the width of the opening at a cross section through the widest part of the wound, the “wound gap” is measured at various times after wounding. In summary, we hypothesized that the linear excision wounding technique would produce minimal variance in wound gap measurements compared to the commonly used circular biopsy punch wounds.
MATERIALS AND METHODS
Linear excision tool fabrication
The two metal scalpel blade handles were immobilized and the removable steel blades (15 mm) were spaced 2 mm apart, and each blade positioned at a 45 degree angle (approximately) facing each other (Figure 1a). The angle of the blade is important in order to prevent infolding of the epidermis of the resultant wound edges after excision. Prior to the cementing of the two handles, opposite (inward facing) sides of each scalpel blade handle were scored with a file, creating a rough surface for better cement adhesion. Weld-it cement (Devcon 18245) was applied between the two scored surface handles and allowed to dry overnight. Excess cement was removed with a file. The linear tool was sterilized in an autoclave and with fresh, sterile blades attached, could be used repeatedly.
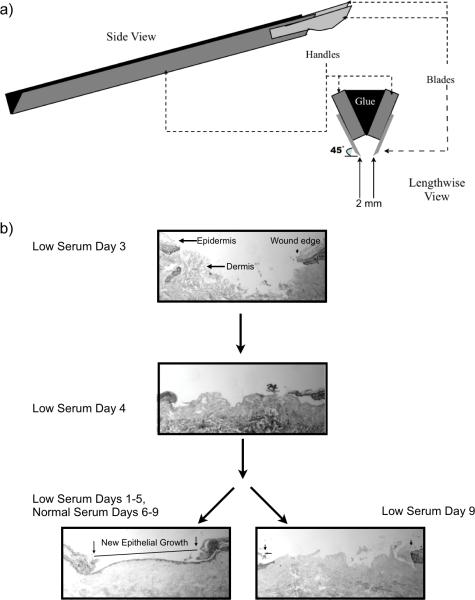
Figure 1. Linear wounds in explanted human skin.
(a) Schematic representation of the linear excision tool. Schematic representation of the linear excision tool used for creating ex-vivo linear wounds examined in this study. Two scalpel blade handles were approximated and bonded to each other with cement, blades positioned at 45° angles, and blade-to-blade width set at 2 mm. (b) Human ex-vivo linear wounds in low serum conditions maintain cell viability and re-epithelialize after the replacement of low serum media with normal serum media. Both linear and circular wounds were incubated in low serum containing media for 4 days. No evidence of reepithelialization was found in day 4 samples. Samples were then split, one group continued to receive low serum media while the other group received normal serum media up until day 9. Wounds that received normal containing media were able to fully re-epithelialize while those in low serum media remained unhealed. The wound gap was determined as the measured distance between the originally cut epithelial borders of the wound (arrows, lower left image), as visualized with H&E staining under light microscopy. The new epithelial growth is easily differentiated from the original wound margin by the absence of fully stratified epithelium and absence of the stratum corneum (outlined and easily visualized) at the wound.
Ex-Vivo Wound Healing Assay
We adapted a human skin, chronic wound healing model developed by Kratz 13. Normal human skin was obtained from elective breast reductions and abdominoplasties under an approved exemption granted by the Internal Review Board at University of California, Davis. Using sterile technique, skin samples were rinsed three times with sterile PBS containing antibiotic/antimycotic, subcutaneous fat trimmed, and 6” × 3” skin section strips cut and pinned onto sterile corkboard. A 3 mm punch tool (Sklar Instruments, West Chester, PA) was used to make wounds in the epidermis and into the superficial dermis and the 3 mm circular discs of skin were excised using sterile scissors. 6 mm skin discs, with the 3 mm epidermal wound in the center, were excised using a 6 mm biopsy punch tool (SMS Inc., Columbia, MD). To emulate a chronic, non-healing wound environment, the skin samples were immediately transferred to a 12 well dish and submerged in 2 ml of “low serum medium” (DMEM (Dulbecco's Modified Eagle's Medium, Gibco, Grand Island, NY) containing 5% fetal bovine serum (Tissue Culture Biologicals, Tulare, CA) and antibiotics). The 12 well dishes were incubated at 37°C in a humidified atmosphere of 5% CO2. The medium was changed every day. The alternate linear wounding method was accomplished using the 2 mm linear excision tool (Figure 1a). The strip of tissue containing the linear wound was divided into sections each measuring 8 by 5 mm, and each piece containing an identical linear wound was then cultured, treated and processed in the same manner as the circularly wounded tissue. The wounded tissue samples were cultured in low serum medium ex-vivo for the noted number of days and then fixed in 4% neutral buffered formaldehyde overnight. To confirm that wound tissues retained its cell viability, after culturing 5 days in low serum medium, the culture medium was changed to a 10% serum (“normal serum”) containing medium for another 4 days prior to fixation and histological processing. At day 9 analysis of histologic sections demonstrate that the wound had fully re-epithelialized (Figure 1b), confirming that the keratinocytes in the non-healed tissue maintained in low serum medium for 5 days had indeed retained their viability.
Histology
The formaldehyde fixed tissue biopsies were dehydrated through an ethanolxylene series and embedded in paraffin. Cross sections, 5 um, taken from the wound gap, and stained using the hematoxylin-eosin technique. For the circular wounds, the wound gap sample was chosen as the widest one from a series of wound sections, which were made in what was judged to be the center and the widest part of each wound. The wound gap was determined as the measured distance between the originally cut epithelial borders of the wound, as visualized with H&E staining under light microscopy (Figure 1b, lower left image). Wound gap measurements were taken under light microscopy. Any new epithelial growth into the wound could be differentiated from the original epithelial wound margin by the presence of a fully stratified epithelium and fully formed stratum corneum in the latter. Slides were viewed on an inverted Nikon Diaphot microscope using a 10× objective. Images were captured using Q-imaging Retiga-EX cameras (Burnaby, BC, Canada). Specimens that were damaged in the histologic process or otherwise non interpretable were excluded from the study. Measurements were made by an observer blinded to the wounding technique used.
Statistics/Measurement Analysis
The wound gap was measured in eleven independent skin samples wounded either with the circular punch biopsy tool or using the linear excision technique. Circular and linear wound samples were matched for experimental days in culture, the day of collection and histologic processing, and treatment conditions.
To compare sample variability in wound gap measurements between the two wounding techniques, we first tested the variances of the two sample populations using an F test. Values were considered significant when p < 0.01. To compare wound gap mean values between the two wounding techniques a two-sample t test for independent samples with unequal variances was used. Measured values were considered significant when p < 0.01.
RESULTS
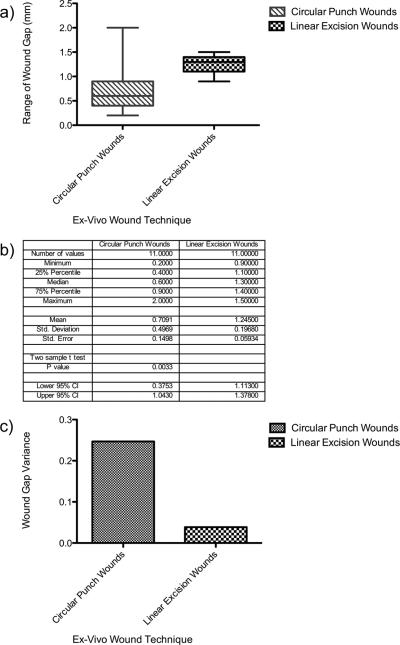
For these experiments, 11 independently sampled wounds each for the circular and linear wounding techniques were generated, and analyzed at various times between days 3-8 after wounding. Wound gap measurements were taken from the initial wound margins and did not include any new re-epithelialization growth. The circular punch biopsy that was used had a 3 mm cutting diameter, and the eleven resulting wounds (collected at days 3-8) ranged in size from a minimum of 0.2 mm to a maximum of 2 mm in wound gap size (Figure 2a and 2b). The linear excision tool had a linear 2 mm cutting aperture width, and the eleven wounds generated with this tool (in explants matched to experimental day of the circular wound group) ranged from a minimum of 0.9 mm to a maximum of 1.4 mm in wound gap size (Figure 2a and 2b). The variance of samples from circular wounds was 0.247, a value almost six times larger than the variance of the linear wounds 0.0387 (Figure 2c). An F test of equality of variances showed that this ratio as large as 6.375 was highly unlikely to occur by chance alone if the two techniques created wounds of equal variance at biopsy (F11, 11 = 6.375 P=0.0072). The linear excision technique thus yields significantly more consistent, stable measurements of wound size under identical experimental conditions.
Figure 2. Linear ex-vivo wounds have less variability in wound gap measurements compared to circular punch wounds.
Linear and circular wounds were compared by analyzing wound gap mean values, variance, and statistical analysis. (a) Box plot whiskers illustrate the range of wound gap measurements in millimeters. Cross bar in the boxes represents the mean wound gap values in the sample population. (b) Numerical values of wound gap minimum, maximum, mean, standard deviation of the two wounding techniques, and a two-sample t test with unequal variances. Statistical significance for the two-sample t test with unequal variances comparing wound gap means was at a p value = 0.0033. (c) Variance for each sample population (linear and circular wounds) was measured. An F test of equality of variances was employed for statistical analysis. Statistical significance for the F test was at a p value = 0.0072.
Wound gap size was also larger for the linear excision tool than for the circular biopsy punch, despite the fact that the biopsy punch has a 3 mm diameter while the linear excision tool has a 2 mm width (Figure 2a and 2b). The mean wound gap size for circular punch wounds was 0.71 mm (SD=0.497), compared to a mean of 1.25 mm (SD=0.197) for the linear excision wounds. The increase of 0.54 mm (95% confidence interval 1.11 to 1.37) for linear wounds compared to circular wounds was statistically significant (two-sample t test with unequal variances, P=0.0033) (Figure 2c). Thus despite the larger diameter of the circular punch, the wounds generated by that tool are not only more variable than those generated by the smaller width linear wounding tool, but also smaller in diameter, again possibly making consistent sampling difficult.
DISCUSSION
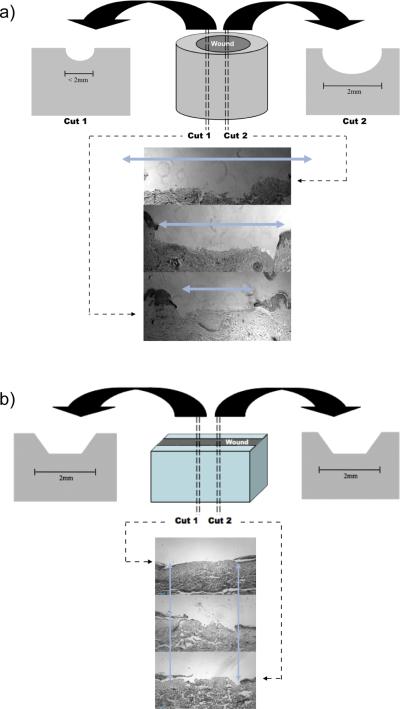
This study has demonstrated in a controlled experimental setting that linear excisional wounds in ex-vivo cultured human skin yield a strikingly more consistent, reproducible wound size than do wounds generated by a circular punch tool. This may be because the geometry of a circle and different corresponding dimensions of the vertical plane width that can transect it for sampling (Figure 3a) make it technically difficult to take a biopsy slice in the same position and angle relative to the circle in every wound. To compensate, this circular punch biopsy technique necessitates the complete sectioning of the entire tissue, processing all the planes and comparing wound gap sizes to estimate the largest wound gap that is then interpreted to be the center of the circular wound. This approach is inefficient, tedious, and difficult to replicate. Even with these estimations the variability in wound size is still significant as reported here. On the other hand, the rectangular geometry of the linear excision wound creates very uniform sides and wound gaps along the entire length of all the vertical sections (Figure 3b). The circular biopsy tool-created wounds leave a great deal of guessing to the location of the “center” wound gap.
Figure 3. Potential for variation in circular vs linear wounds.
. (a) Circular punch biopsy wounds can vary greatly in wound gap after histological sectioning. Based on a horizontal circular wound, vertical planes sectioned across the wound can generate different wound gaps values. (b) Linear excision wounds are consistently less variable in wound gap after histological sectioning compared to circular punch biopsies. The rectangular geometry of the linear excision wound creates very uniform sides and wound gaps along the entire length of all the vertical sections and results in more consistent and reproducible samples. Unlike a horizontal circle, a horizontal rectangular wound and resulting vertical planes will be equal in wound gap value.
Problems with variation in wound size lead to difficulties in measuring wound healing in studies designed to compare the effect of different treatments. Previous researchers have tried to develop mathematical approaches to adjust for this added source of substantial variation in wound gap size measurements. For example, it is common to report wound measurements as percentage of reepithelialization of the total wound size. This method of calculating wound sizes can lead to a misinterpretation of reported results. For example in a small wound, given a certain amount of epithelial growth from the wound edge can translate into a large “percentage” of wound reepithelialization, but in a larger wound this new epithelial growth would translate into a small “percentage” of re-epithelialization when, in fact, the degree of keratinocyte migration from the wound edge is equal in both scenarios. Indeed Gorin and colleagues have noted this problem when describing healing of chronic wounds in patients and therefore recommend measuring the linear distance across a wound by a formula, called the “linear wound size” 20.
A second key finding of our study is that the mean width of wounds created by the 2 mm linear excision tool was about 0.5 mm greater than the wounds created with the 3 mm diameter circular punch biopsy tool. This second finding was unexpected, given that the width of circular biopsy tool is 50% than that of the linear excision tool. One possible explanation is that circular biopsy punched wounds have more irregular contraction likely due to contraction along the different axial tension lines of the skin, as has been reported 21, 22. While using the linear excision technique, wounds are created parallel to the line of axis of skin tension, perhaps minimizing the amount of contraction after excision.
Although multiple blade scalpels are readily available commercially as tools for harvesting strips of hair bearing scalp for hair transplantation, these tools set the blades parallel to one another. Our preliminary studies have determined that the angle of the blades relative to another (45°) is important in order to prevent infolding of the epidermis of the resultant wound edges after excision. Wounds generated with parallel-set blades resulted in inward collapse of the wound edges, culminating in an uneven wound margins, and inability to reproduce consistent wound gaps.
Our findings have substantial implications for design of comparative translational studies of wound healing. A six-fold greater variance that could not be addressed by statistical adjustment would require a six-fold greater sample size to achieve the same power to detect treatment effects. Studies using the circular biopsy punch run the risk of either requiring much larger sample sizes than those using a linear excision technique, or having inadequate power and thus potentially failing to detect clinically meaningful differences in wound healing. In addition, the ability to use a simpler technique for biopsy sampling and analysis of the data reduces the potential for errors. Human models for wound healing have continued to the advance the study of the role of keratinocyte migration in the re-epithelialization of chronic wounds. Here we report that wounds generated with a linear instrument can be used to replicate human wounds in an ex-vivo culture with substantial improvements in terms of reduced variability in size and increased ease of reproducibility, as compared to the currently used circular punch tool- generated wound.
ACKNOWLEDGMENTS
AER is a T32 fellow, supported by the Clinical Translational Science Center at the University of California, Davis, funded by grant UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, as was the biostatistical support (LAB). Grants from Shriners Hospital (8550), NIH (R01AR044518 and R21AI080604), and Department of Veterans Affairs (Merit Award 104679) to RRI provided partial support for these studies. We also thank Drs. Stevenson and Wong (Department of Plastic Surgery, University of California, Davis) for their help in providing discarded skin for the experiments described herein.
LITERATURE CITED
- 1.Data and Trends Centers for Disease Control and Prevention. 2007 Mar 26; ( http://www.cdc.gov/diabetes/statistics/hosplea/diabetes_complications/fig2_ulcer.htm)
- 2.Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol. 2002;46:381–386. doi: 10.1067/mjd.2002.121739. [DOI] [PubMed] [Google Scholar]
- 3.Phillips TJ. Chronic cutaneous ulcers: etiology and epidemiology. J Invest Dermatol. 1994;102:38S–41S. doi: 10.1111/1523-1747.ep12388556. [DOI] [PubMed] [Google Scholar]
- 4.Lees TA, Lambert D. Prevalence of lower limb ulceration in an urban health district. Br J Surg. 1992;79:1032–1034. doi: 10.1002/bjs.1800791015. [DOI] [PubMed] [Google Scholar]
- 5.Odland G, Ross R. Human wound repair. I. Epidermal regeneration. J Cell Biol. 1968;39:135–151. doi: 10.1083/jcb.39.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salcido R, Popescu A. Ahn C: Animal models in pressure ulcer research. J Spinal Cord Med. 2007;30:107–116. doi: 10.1080/10790268.2007.11753921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoekstra MJ, Hupkens P, Dutrieux RP, Bosch MM, Brans TA, Kreis RW. A comparative burn wound model in the New Yorkshire pig for the histopathological evaluation of local therapeutic regimens: silver sulfadiazine cream as a standard. Br J Plast Surg. 1993;46:585–589. doi: 10.1016/0007-1226(93)90111-n. [DOI] [PubMed] [Google Scholar]
- 8.Rigal C, Pieraggi MT, Serre G, Bouissou H. Optimization of a model of full-thickness epidermal burns in the pig and immunohistochemical study of epidermodermal junction regeneration during burn healing. Dermatology. 1992;184:103–110. doi: 10.1159/000247514. [DOI] [PubMed] [Google Scholar]
- 9.Stein HD, Keiser HR. Collagen metabolism in granulating wounds. J Surg Res. 1971;11:277–283. doi: 10.1016/0022-4804(71)90101-6. [DOI] [PubMed] [Google Scholar]
- 10.Cohen IK, Moore CD, Diegelmann RF. Onset and localization of collagen synthesis during wound healing in open rat skin wounds. Proc Soc Exp Biol Med. 1979;160:458–462. doi: 10.3181/00379727-160-40470. [DOI] [PubMed] [Google Scholar]
- 11.Emanuelsson P, Kratz G. Characterization of a new in vitro burn wound model. Burns. 1997;23:32–36. doi: 10.1016/s0305-4179(96)00073-3. [DOI] [PubMed] [Google Scholar]
- 12.Kratz G, Lake M, Gidlund M. Insulin like growth factor-1 and -2 and their role in the reepithelialisation of wounds; interactions with insulin like growth factor binding protein type 1. Scand J Plast Reconstr Surg Hand Surg. 1994;28:107–112. doi: 10.3109/02844319409071187. [DOI] [PubMed] [Google Scholar]
- 13.Kratz G. Modeling of wound healing processes in human skin using tissue culture. Microsc Res Tech. 1998;42:345–350. doi: 10.1002/(SICI)1097-0029(19980901)42:5<345::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 14.Heilborn JD, Nilsson MF, Kratz G, Weber G, Sorensen O, Borregaard N, Stahle-Backdahl M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 15.Pullar CE, Rizzo A, Isseroff RR. beta-Adrenergic receptor antagonists accelerate skin wound healing: evidence for a catecholamine synthesis network in the epidermis. J Biol Chem. 2006;281:21225–21235. doi: 10.1074/jbc.M601007200. [DOI] [PubMed] [Google Scholar]
- 16.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 17.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 18.Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122:3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coulombe PA. Wound epithelialization: accelerating the pace of discovery. J Invest Dermatol. 2003;121:219–230. doi: 10.1046/j.1523-1747.2003.12387.x. [DOI] [PubMed] [Google Scholar]
- 20.Gorin DR, Cordts PR, LaMorte WW, Manzoian JO. The influence of wound geometry on the measurement of wound healing rates in clinical trials. J Vasc Surg. 1996;23:524–528. doi: 10.1016/s0741-5214(96)80021-8. [DOI] [PubMed] [Google Scholar]
- 21.Smith DA, Barker IK. Healing of cutaneous wounds in the common garter snake (Thamnophis sirtalis). Can J Vet Res. 1988;52:111–119. [PMC free article] [PubMed] [Google Scholar]
- 22.Smith DA, Barker IK, Allen OB. The effect of ambient temperature and type of wound on healing of cutaneous wounds in the common garter snake (Thamnophis sirtalis). Can J Vet Res. 1988;52:120–128. [PMC free article] [PubMed] [Google Scholar]