Abstract
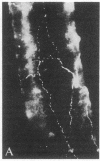
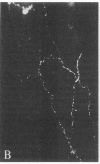
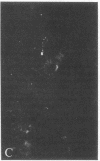
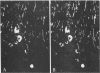
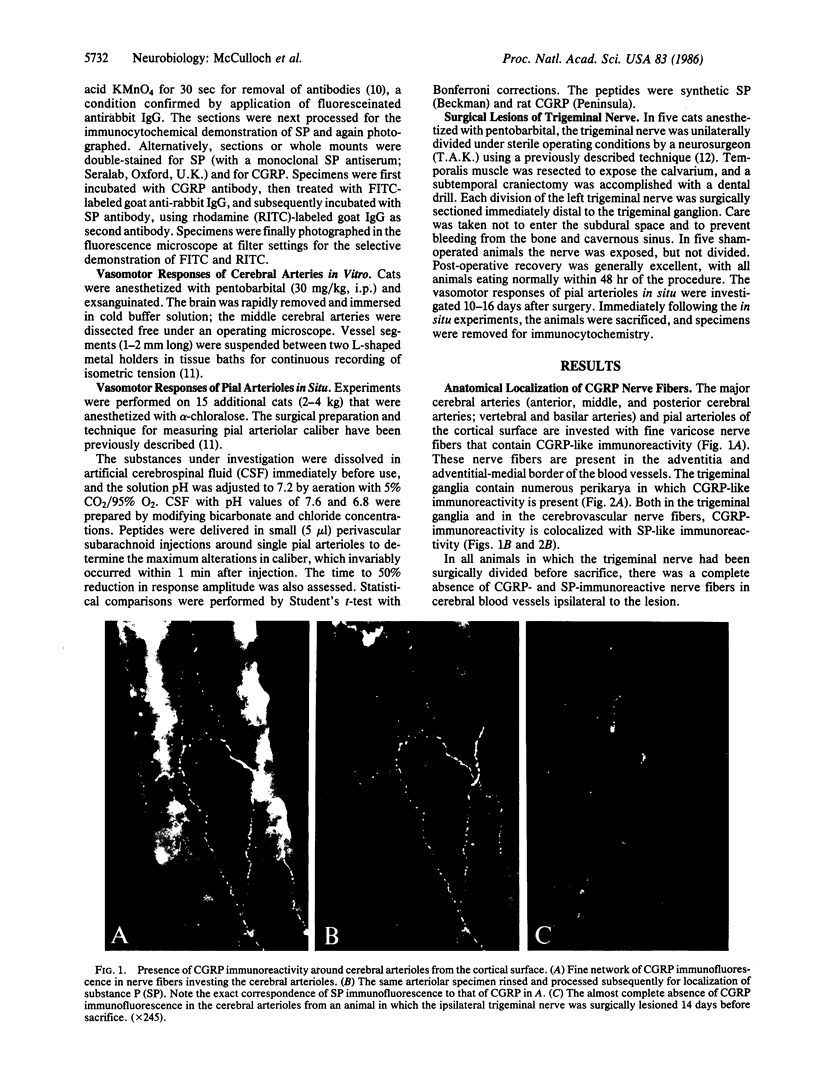
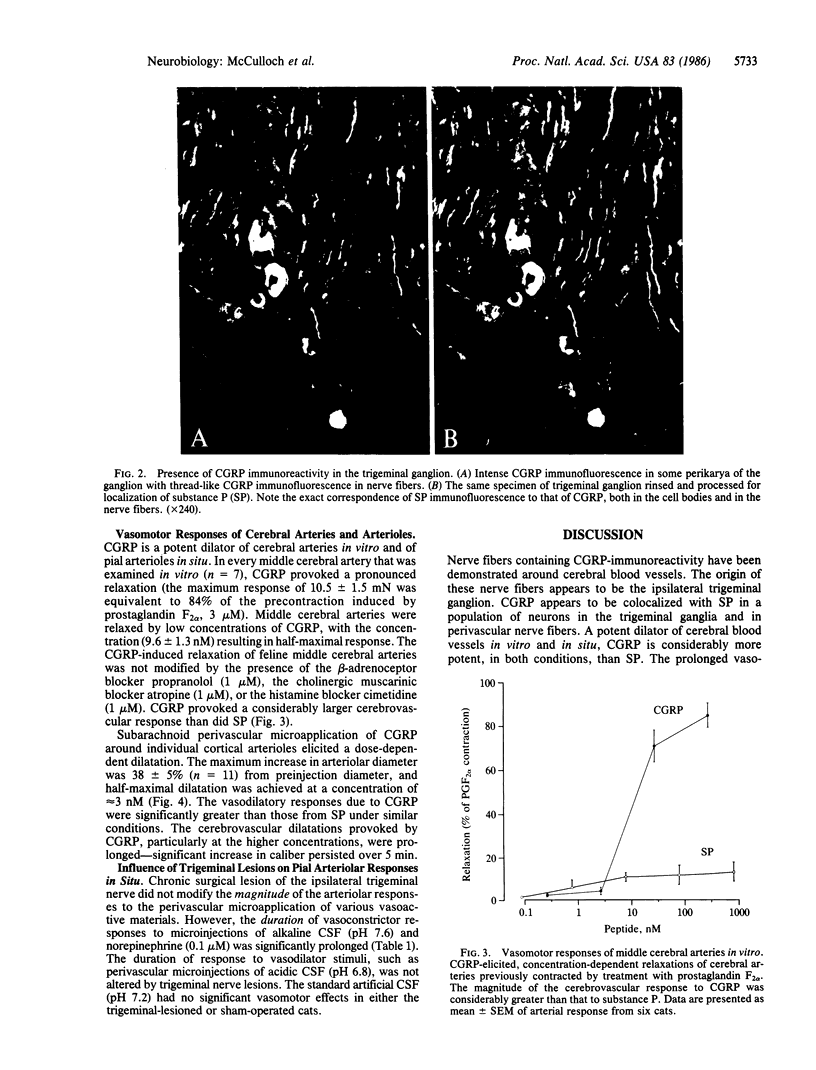
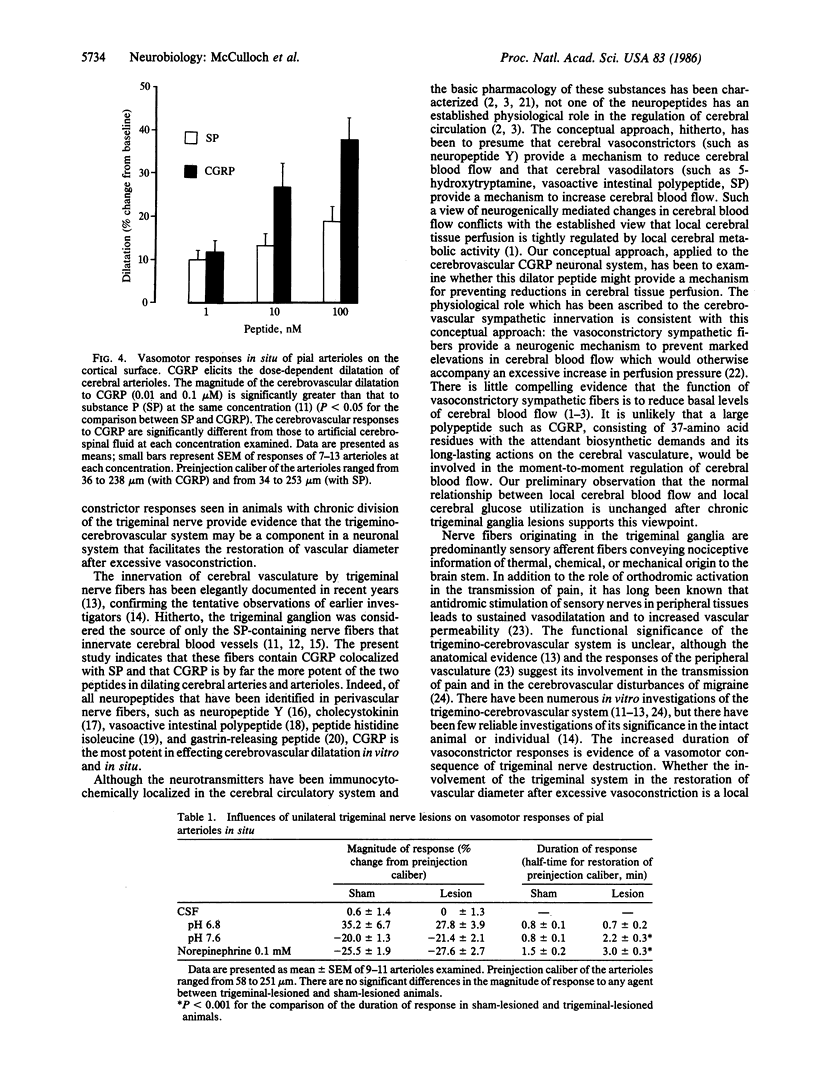
Distribution studies disclosed that all major cerebral arteries and cortical arterioles of the cat were invested with fine varicose nerve fibers that contained calcitonin gene-related peptide (CGRP)-like immunoreactivity; the trigeminal ganglia likewise contained CGRP immunoreactivity. Sequential immunostaining with antibodies to CGRP and to substance P (SP) revealed identical distributions of these two peptides in trigeminal ganglia and cerebrovascular nerve fibers, suggesting that CGRP and SP are colocalized in these nerves. CGRP completely disappeared from ipsilateral blood vessels after unilateral section of the trigeminal nerve. Exogenous CGRP was a potent relaxant of feline middle cerebral arteries in vitro (maximum relaxation, 10.5 +/- 1.5 mN; concentration eliciting half-maximal response, 9.6 +/- 1.3 nM). Perivascular microapplication of CGRP to individual cortical arterioles of chloralose-anesthetized cats provoked dose-dependent dilatations (maximum increase in diameter, 38 +/- 5%; concentration eliciting half-maximal response, approximately equal to 3 nM). CGRP was significantly more potent than SP as a cerebrovascular dilator, both in vitro and in situ. Chronic division of the ipsilateral trigeminal nerve in cats did not modify the magnitude of arteriolar responses to perivascular microapplication of either vasoconstrictor or vasodilator agents, but the duration of vasoconstrictor responses to norepinephrine (0.1 mM) or alkaline solutions (pH 7.6) was significantly increased. The cerebrovascular trigeminal neuronal system, in which CGRP is the most potent vasoactive constituent, may participate in a reflex or local response to excessive cerebral vasoconstriction that restores normal vascular diameter.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Bill A., Linder J. Sympathetic control of cerebral blood flow in acute arterial hypertension. Acta Physiol Scand. 1976 Jan;96(1):114–121. doi: 10.1111/j.1748-1716.1976.tb10176.x. [DOI] [PubMed] [Google Scholar]
- Brain S. D., Williams T. J., Tippins J. R., Morris H. R., MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985 Jan 3;313(5997):54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- COONS A. H., LEDUC E. H., CONNOLLY J. M. Studies on antibody production. I. A method for the histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J Exp Med. 1955 Jul 1;102(1):49–60. doi: 10.1084/jem.102.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L., Emson P., McCulloch J., Tatemoto K., Uddman R. Neuropeptide Y: immunocytochemical localization to and effect upon feline pial arteries and veins in vitro and in situ. Acta Physiol Scand. 1984 Oct;122(2):155–163. doi: 10.1111/j.1748-1716.1984.tb07493.x. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., McCulloch J. Distribution and vasomotor effects of peptide HI (PHI) in feline cerebral blood vessels in vitro and in situ. Regul Pept. 1985 Apr;10(4):345–356. doi: 10.1016/0167-0115(85)90047-3. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., McCulloch J., Uddman R. Substance P: immunohistochemical localization and effect upon cat pial arteries in vitro and in situ. J Physiol. 1981 Sep;318:251–258. doi: 10.1113/jphysiol.1981.sp013862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L. A., Kikkawa D. O., Rivier J. E., Amara S. G., Evans R. M., Rosenfeld M. G., Vale W. W., Brown M. R. Stimulation of noradrenergic sympathetic outflow by calcitonin gene-related peptide. Nature. 1983 Oct 6;305(5934):534–536. doi: 10.1038/305534a0. [DOI] [PubMed] [Google Scholar]
- Hendry S. H., Jones E. G., Beinfeld M. C. Cholecystokinin-immunoreactive neurons in rat and monkey cerebral cortex make symmetric synapses and have intimate associations with blood vessels. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2400–2404. doi: 10.1073/pnas.80.8.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler J. A., Adler J. E., Black I. B. Substance P and somatostatin regulate sympathetic noradrenergic function. Science. 1983 Sep 9;221(4615):1059–1061. doi: 10.1126/science.6192502. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Bell W. O., Black I. B. Interactions between the sympathetic and sensory innervation of the iris. J Neurosci. 1983 Jun;3(6):1301–1307. doi: 10.1523/JNEUROSCI.03-06-01301.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuschinsky W., Wahl M. Local chemical and neurogenic regulation of cerebral vascular resistance. Physiol Rev. 1978 Jul;58(3):656–689. doi: 10.1152/physrev.1978.58.3.656. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Edvinsson L., Fahrenkrug J., Håkanson R., Owman C., Schaffalitzky de Muckadell O., Sundler F. Immunohistochemical localization of a vasodilatory polypeptide (VIP) in cerebrovascular nerves. Brain Res. 1976 Aug 27;113(2):400–404. doi: 10.1016/0006-8993(76)90951-3. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Gamse R. Substance P in peripheral sensory processes. Ciba Found Symp. 1982;(91):35–54. doi: 10.1002/9780470720738.ch4. [DOI] [PubMed] [Google Scholar]
- Liu-Chen L. Y., Han D. H., Moskowitz M. A. Pia arachnoid contains substance P originating from trigeminal neurons. Neuroscience. 1983 Aug;9(4):803–808. doi: 10.1016/0306-4522(83)90268-3. [DOI] [PubMed] [Google Scholar]
- Mayberg M. R., Zervas N. T., Moskowitz M. A. Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. J Comp Neurol. 1984 Feb 10;223(1):46–56. doi: 10.1002/cne.902230105. [DOI] [PubMed] [Google Scholar]
- McCulloch J., Edvinsson L. Cerebrovascular smooth muscle reactivity: a critical appraisal of in vitro and in situ techniques. J Cereb Blood Flow Metab. 1984 Jun;4(2):129–139. doi: 10.1038/jcbfm.1984.21. [DOI] [PubMed] [Google Scholar]
- Moskowitz M. A. The neurobiology of vascular head pain. Ann Neurol. 1984 Aug;16(2):157–168. doi: 10.1002/ana.410160202. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Mermod J. J., Amara S. G., Swanson L. W., Sawchenko P. E., Rivier J., Vale W. W., Evans R. M. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983 Jul 14;304(5922):129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Schon F., Ghatei M., Allen J. M., Mulderry P. K., Kelly J. S., Bloom S. R. The effect of sympathectomy on calcitonin gene-related peptide levels in the rat trigeminovascular system. Brain Res. 1985 Nov 25;348(1):197–200. doi: 10.1016/0006-8993(85)90380-4. [DOI] [PubMed] [Google Scholar]
- Tramu G., Pillez A., Leonardelli J. An efficient method of antibody elution for the successive or simultaneous localization of two antigens by immunocytochemistry. J Histochem Cytochem. 1978 Apr;26(4):322–324. doi: 10.1177/26.4.207771. [DOI] [PubMed] [Google Scholar]
- Uddman R., Edvinsson L., Owman C., Sundler F. Nerve fibres containing gastrin-releasing peptide around pial vessels. J Cereb Blood Flow Metab. 1983 Sep;3(3):386–390. doi: 10.1038/jcbfm.1983.56. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Matsuyama T., Shiosaka S., Inagaki S., Senba E., Shimizu Y., Ishimoto I., Hayakawa T., Matsumoto M., Tohyama M. Overall distribution of substance P-containing nerves in the wall of the cerebral arteries of the guinea pig and its origins. J Comp Neurol. 1983 Apr 20;215(4):421–426. doi: 10.1002/cne.902150406. [DOI] [PubMed] [Google Scholar]