Abstract
Invasion of microbial DNA into the cytoplasm of animal cells triggers a cascade of host immune reactions that help clear the infection; however, self DNA in the cytoplasm can cause autoimmune diseases. Biochemical approaches led to the identification of cyclic GMP-AMP (cGAMP) synthase (cGAS) as a cytosolic DNA sensor that triggers innate immune responses. Here we show that cells from cGAS-deficient (cGas−/−) mice, including fibroblasts, macrophages and dendritic cells, failed to produce type-I interferons and other cytokines in response to DNA transfection or DNA virus infection. cGas−/− mice were more susceptible to lethal infection with herpes simplex virus-1 (HSV1) than wild type mice. We also show that cGAMP is an adjuvant that boosts antigen-specific T cell activation and antibody production in mice.
The detection of foreign DNA invasion is a fundamental mechanism of host defense. In mammalian cells, the presence of foreign or self DNA in the cytoplasm is a danger signal that triggers the host innate immune responses(1). Through biochemical studies, we have recently identified cyclic GMP-AMP (cGAMP) synthase (cGAS) as an innate immune sensor of cytosolic DNA that triggers the production of type-I interferons and other inflammatory cytokines(2, 3). cGAS binds to DNA independently of its sequence; this binding activates cGAS to catalyze the synthesis of a unique cGAMP isomer, which contains both 2’–5’ and 3’–5’ phosphodiester linkages(4–7). This molecule, termed 2’3’cGAMP, functions as a second messenger that binds and activates the adaptor protein STING(3, 7). STING then activates the protein kinases IKK and TBK1, which in turn activate the transcription factors NF-κB and IRF3 to induce interferons and cytokines(8).
To investigate the function of cGAS in vivo, we generated a cGas knockout mouse strain, in which the first exon is spliced into a LacZ cassette, thus abrogating the expression of the endogenous locus (fig. S1A and S1B)(9). The cGas−/− mice were born at the Mendelian ratio, and did not display any overt developmental abnormality (fig. S1C and S1D). Quantitative reverse transcription PCR (q-RT-PCR) analyses of RNA from lung fibroblasts and bone marrow derived macrophages (BMDM) confirmed that the cGas−/− cells were defective in producing cGas RNA, whereas cGas+/− cells produced intermediate levels of cGas RNA (fig. S1E and S1F).
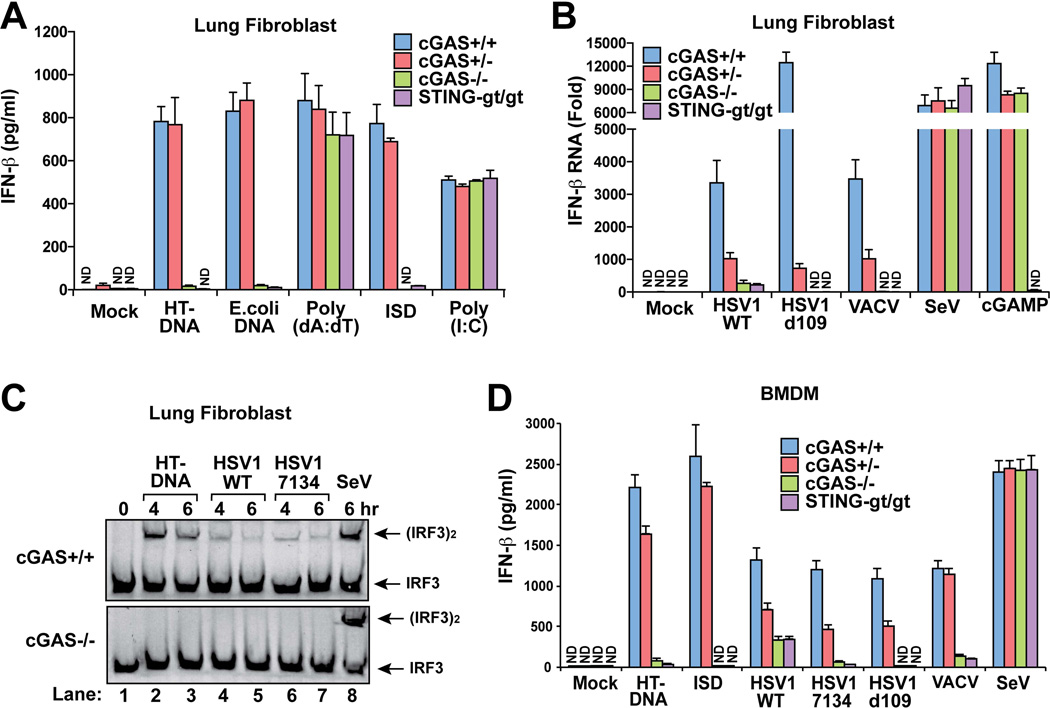
We obtained lung fibroblasts from WT, cGas+/− and cGAS−/− mice as well as the goldenticket (gt/gt) mouse, which has a point mutation that results in the loss of expression of STING(10). Transfection of different types of DNA, including herring testis DNA (HT-DNA), E. coli DNA and interferon stimulatory DNA (ISD; a 45bp double-stranded DNA)(11), into the lung fibroblasts from WT and cGas+/− mice led to robust production of IFNβ protein, as measured by ELISA (Fig.1A). In contrast, the cGas−/− and Stinggt/gt cells failed to produce any detectable level of IFNβ. Poly[I:C], a double-stranded RNA analogue known to induce IFNβ through the RIG-I like-receptor (RLR) pathway(12), induced IFNβ normally in the absence of cGas or Sting. Interestingly, poly[dA:dT], which was previously shown to induce type-I interferons through the RNA polymerase III – RIG-I – MAVS pathway(13, 14), induced IFNβ normally in the cGas−/− and Stinggt/gt cells. q-RT-PCR analyses further confirmed that cGAS is essential for IFNβ RNA induction by different types of synthetic or bacterial DNA, except poly[dA:dT] (fig. S2A). Time course experiments show that IFNβ induction by ISD was completely abolished in cGas−/− lung fibroblasts even at early time points (2–8 hr) after the DNA transfection (fig. S2B and S2C), indicating that cGAS is indispensable for IFNβ induction by cytosolic DNA.
Figure 1. cGAS is essential for IRF3 activation and IFN-β induction by DNA transfection and DNA virus infection in fibroblasts and macrophages.
(A) Mouse lung fibroblasts were transfected with different forms of DNA or poly[I:C] (2 µg/ml) for 24 h followed by measurement of IFN-β protein by ELISA. Unless indicated otherwise, lipofectamine 2000 was used in all transfection experiments. (B) Lung fibroblasts were infected with the indicated viruses or stimulated with 2’3’cGAMP (200 nM) for 9 h followed by measurement of IFN-β RNA by q-RT-PCR. (C) Lung fibroblasts were transfected with HT-DNA or infected with HSV1 or Sendai virus for the indicated times. Cell extracts were analyzed for IRF3 dimerization by native gel electrophoresis. (D) BMDMs were transfected with HT-DNA, ISD or infected with the indicated virus for 20 h, then IFN-β levels were measured by ELISA. For the q-RT-PCR results in this and other figures, the error bars represent standard deviations of triplicate measurements. Unless otherwise indicated, the ELISA results in this and other figures represent variation ranges of duplicate measurements. ‘ND’ in this and other figures indicates ‘not detected’.
To measure cGAMP production in WT and cGas−/− cells, we performed a bioassay that measures the cGAMP activity in cytoplasmic extracts from ISD-transfected cells. The extracts were heated at 95°C to denature most proteins, which were removed by centrifugation. The supernatants that might contain cGAMP were delivered to the human monocytic cell line THP1, which had been permeabilized with the bacterial toxin perfringolysin-O (PFO). Dimerization of IRF3, a hallmark of its activation, was then measured by native gel electrophoresis (fig. S2D). This assay showed that the extracts of ISD-transfected lung fibroblasts from WT but not cGas−/− mice contained the cGAMP activity, demonstrating that cGAS has a non-redundant role in catalyzing cGAMP synthesis in these cells in response to cytosolic DNA.
Next, we infected the lung fibroblasts with the DNA viruses herpes simplex virus-1 (HSV1), vaccinia virus (VACV) and a mutant strain of HSV1 called d109, which has a deletion of viral proteins such as ICP0 that is known to antagonize immune responses(15). IFNβ induction by each of these viruses was largely abolished in cGas−/− and Stinggt/gt cells, and partially inhibited in cGas+/− cells (Fig. 1B). In contrast, IFNβ induction by Sendai virus, an RNA virus known to activate the RIG-I pathway, was not affected by the deficiency in cGas or Sting. Delivery of cGAMP into the cytoplasm rescued IFNβ induction in cGas−/− cells but not Stinggt/gt cells (Fig. 1B). Similarly, induction of the chemokine CXCL10 by the DNA viruses was dependent on cGas and Sting (fig. S2E). Measurement of IRF3 dimerization showed that cGas−/− cells failed to activate IRF3 in response to transfection of HT-DNA or infection by WT HSV1 or the HSV1 strain 7134, which also lacks the interferon antagonist ICP0 (Fig. 1C)(16). The cGas deficiency did not impair IRF3 activation by Sendai virus. Thus, cGAS is required for IRF3 activation and cytokine induction by DNA viruses but not RNA viruses in mouse lung fibroblasts.
BMDM from cGas−/− and Stinggt/gt mice were defective in producing IFNβ in response to transfection with HT-DNA or ISD (Fig. 1D). Similarly, IFNβ induction by VACV and the HSV1 strains d109 and 7134 was largely abolished in cGas−/− and Stinggt/gt BMDM. However, IFNβ induction by WT HSV1 was severely but not completely blocked in either cGas−/− or Stinggt/gt BMDM, suggesting that these cells possess another pathway that could partially compensate for the loss of the cGAS-STING pathway to detect WT HSV1 infection. The loss of cGAS or STING in BMDM did not affect IFNβ induction by Sendai virus. Kinetic experiments show that IFNβ induction by ISD and HSV1-d109 was abolished in cGas−/− BMDM throughout the time course of stimulation (fig. S3A and S3B). Similarly to IFNβ, the induction of TNFα by HT-DNA or ISD was abolished in cGas−/− or Stinggt/gt BMDM (fig. S3C). q-RT-PCR analyses showed that the induction of IFNβ, interleukin-6 (IL6) and CXCL10 RNA by transfection of HT-DNA or ISD or infection with HSV1-d109 was completely dependent on cGas and Sting (fig. S3D–F). In contrast, the RNA levels of these cytokines induced by poly[I:C] or Sendai virus were not affected by the deficiency in cGas or Sting.
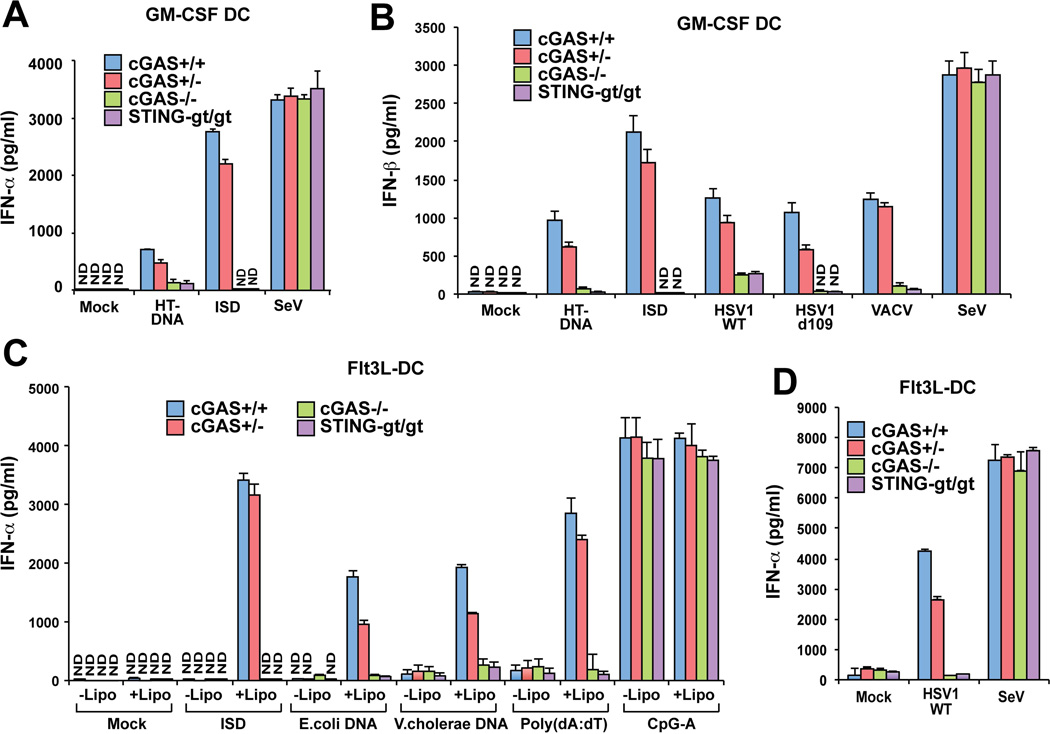
We obtained conventional dendritic cells (cDC) and plasmacytoid DCs (pDC) by culturing bone marrows in conditioned media containing GM-CSF and Flt3 ligand (Flt3L), respectively. The GM-CSF DCs, which contains largely cDC, from the cGas−/− and Stinggt/gt mice failed to induce IFNα or IFNβ in response to transfection of HT-DNA or ISD (Fig. 2A and 2B). The loss of cGAS or STING in GM-CSF DCs abolished IFNβ induction by HSV1-d109 and VACV, and partially inhibited IFNβ induction by WT HSV1. In contrast, the deficiency in cGAS or STING did not impair IFNα or IFNβ induction by Sendai virus. q-RT-PCR experiments further confirmed that cGAS and STING were essential for the induction of IFNβ, IL6 and CXCL10 RNA by transfection with HT-DNA or ISD or infection with HSV1-d109, whereas the induction of these cytokines by poly[I:C] or Sendai virus was independent of cGAS or STING (fig. S4A–C)
Figure 2. cGAS is essential for type-I interferon induction by DNA transfection and DNA virus infection in dendritic cells.
(A) GM-CSF-induced dendritic cells (GM-CSF DCs) were transfected with HT-DNA or ISD or infected with Sendai virus for 20 h followed by measurement of IFN-α by ELISA. (B) GM-CSF DCs were transfected with the indicated DNA or viruses for 20 h, and then IFN-β was measured by ELISA. (C & D) Flt3L-induced dendritic cells (Flt3L-DCs) were incubated with the indicated DNA for 16 h in the absence or presence of lipofectamine 2000 (C), or infected with HSV1 or Sendai virus (D) for 16 h, followed by measurement of IFN-α by ELISA. Lipo: lipofectamine 2000.
pDCs are known to express TLR9 that is responsible for the induction of type-I interferons by synthetic CpG DNA containing phosphorothioate bonds (17). When the CpG DNA was used to stimulate Flt3L-DCs, which contains largely pDCs, in the presence or absence of liposome (lipofectamine 2000), it induced robust production of IFNα and IFNβ even in the cGas−/− and Stinggt/gt cells (Fig. 2C and fig. S4D). In contrast, other forms of DNA, including ISD, poly[dA:dT] and genomic DNA from E. coli and Vibrio cholerae, induced IFNα in Flt3L-DCs only in the presence of liposome, and this induction by each DNA was abolished in the absence of cGAS or STING. The strong dependency of IFNα induction by poly[dA:dT] on cGAS and STING in pDCs suggests that the cGAS-STING pathway, but not the Pol-III – RIG-I pathway, plays a major role in sensing the DNA in these cells. The Flt3L-DC from the cGas−/− and Stinggt/gt mice induced IFNα and IFNβ in response to infection by Sendai virus, but not HSV1 (Fig. 2D and fig. S4D). Together, these results demonstrate that cGAS is responsible for detecting natural DNA (e.g., bacterial DNA) and DNA virus infections in dendritic cells.
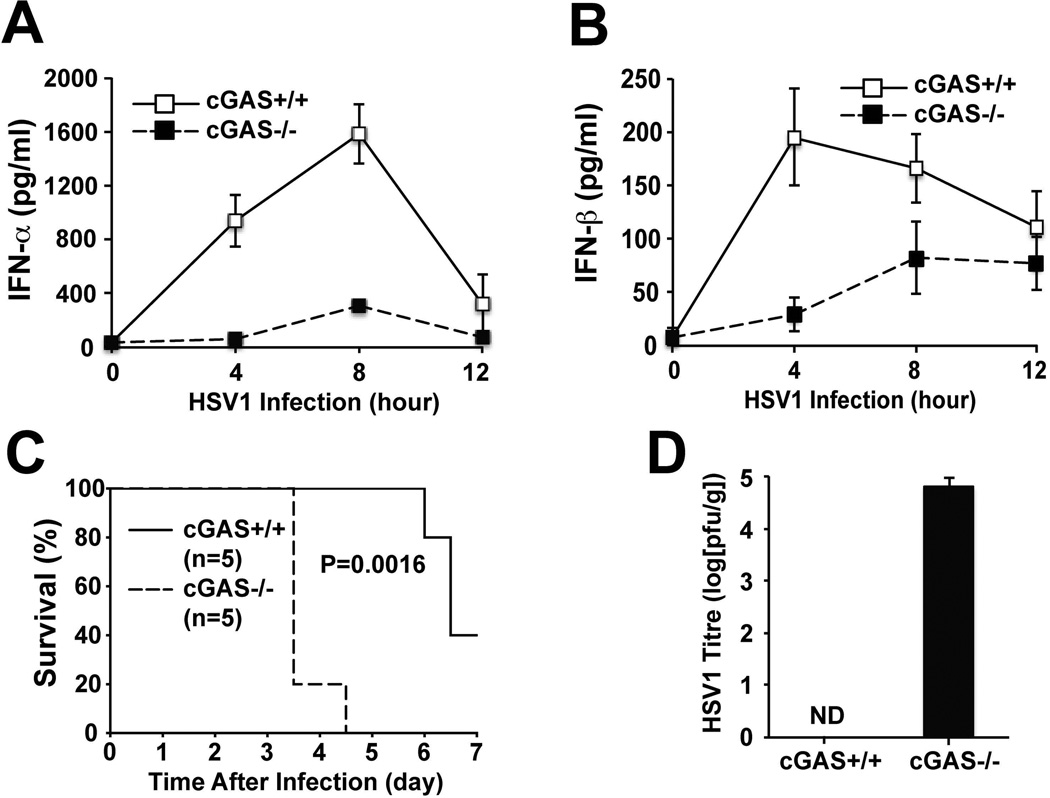
To determine the role of cGAS in immune defense against DNA viruses in vivo, we infected WT and cGas−/− mice with HSV1 via the intravenous (i.v) route (Fig. 3). ELISA analyses showed that the sera of WT mice contained elevated levels of IFNα and IFNβ, which peaked at 8 and 4 hours, respectively, after HSV1 infection (1×107 pfu/mouse). The levels of IFNα and IFNβ were severely attenuated in the cGas−/− mice infected with the same infectious dose of HSV1 (Fig. 3A and 3B). In an independent experiment in which the mice were monitored for their survival after infection with HSV1 at the infectious dose of 1×106 pfu/mouse, four out of the five cGas−/− mice developed ataxia and paralysis in 3 days after the virus infection and died a few hours after these symptoms appeared (Fig. 3C). The fifth cGas−/− mouse died on day 4 after infection. Three out of five WT mice developed these symptoms on day 6 and died shortly afterwards. When the brains of WT and cGas−/− mice were extracted to measure viral titers on day 3 after infection, high levels of HSV1 were detected in all five cGas−/− mice, whereas none of the WT mice had detectable levels of HSV1 in the brains (Fig. 3D). Similar survival curves were observed and similar viral titers in the brains were detected in independent experiments where the infectious dose of HSV1 was increased to 1×107 pfu per mouse (fig. S5A and S5B). The susceptibility of cGas−/− mice to HSV1 infection was similar to that of Stinggt/gt mice, which also had marked reduction of IFNα and IFNβ in the sera, and died within 3–4 days after HSV1 infection (fig. S5C–S5E)(18).
Figure 3. cGAS is essential for immune defense against HSV1 infection in vivo.
(A & B) WT and cGas−/− mice (n=5 each) were infected i.v. with HSV1 at 1× 107 pfu per mouse, and then sera were collected at different time points as indicated. The levels of IFNα and IFNβ were measured by ELISA. The error bars indicate standard deviations. (C) WT and cGas−/− mice (n=5 each) were infected i.v. with HSV1 at 1×106 pfu per mouse. The survival of each mouse was monitored for 7 days. (D) Brains of the HSV1 infected mice were excised on day 3 to prepare homogenates, which were used to measure viral titers by the plaque assay. The viral titer was recorded as pfu per gram brain (pfu/g). The experiments shown in C and D were repeated in an independent set of experiments, except that the HSV1 infectious dose was increased to 1×107 pfu per mouse (fig. S5A and S5B).
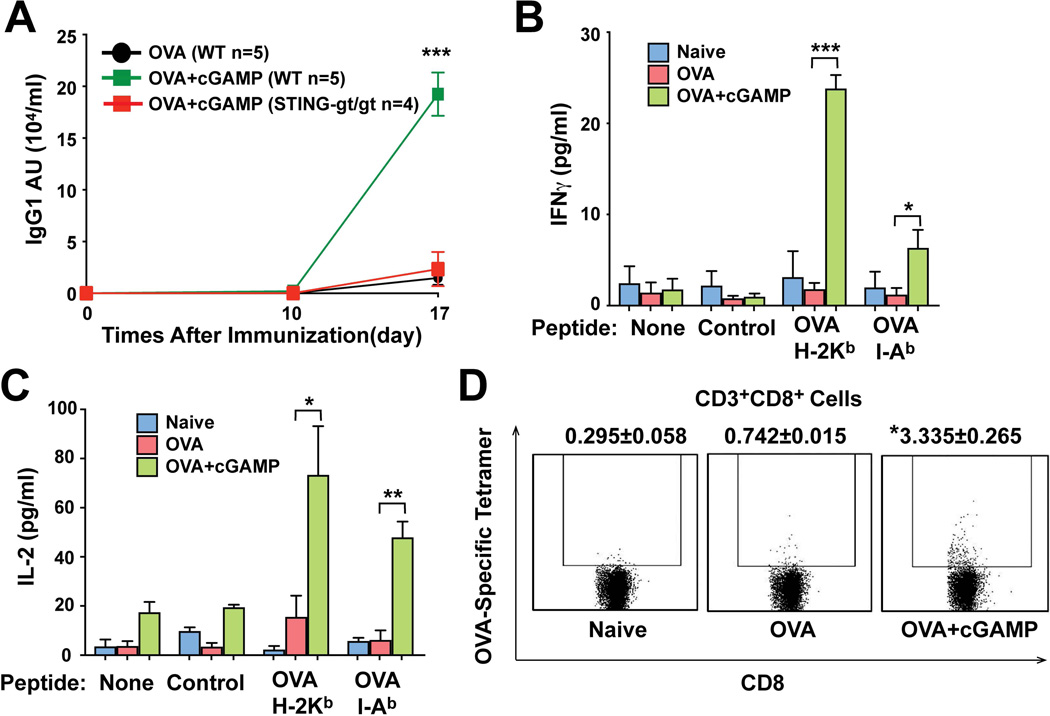
Our results that cGAS is essential for the induction of type-I interferons by cytosolic DNA in multiple cell types, including antigen presenting cells, suggest that the cGAS product, 2’3’cGAMP, may be used to substitute for the immune stimulatory effect of DNA, including the adjuvant effect of DNA vaccines (19). To explore the potential adjuvant effect of 2’3’cGAMP, we injected the model protein antigen ovalbumin (OVA) in the absence or presence of 2’3’cGAMP into WT or Stinggt/gt mice via the intramuscular (i.m) route. The mice were boosted once on day 10 with the same antigen formulation. ELISA analyses showed that 2’3’cGAMP strongly enhanced the production of OVA-specific antibodies on day 17 in the WT, but not Stinggt/gt mice (Fig. 4A). This adjuvant effect of 2’3’cGAMP was also not observed in type-I interferon receptor deficient mice (Ifnar−/−; fig. S6). To investigate the effect of 2’3’cGAMP on T cell activation, splenic leukocytes isolated from the WT mice, which had been immunized with OVA or OVA + 2’3’cGAMP for 7 days, were cultured with an OVA peptide known to stimulate CD4 T cells through the MHC class II molecule I-Ab or another OVA peptide that stimulates CD8 T cells through the MHC class I molecule H-2Kb. Both CD4 and CD8 T cells from the mice immunized with OVA + 2’3’cGAMP, but not OVA alone, produced elevated levels of IFNγ and IL-2 after stimulation with the cognate peptides (Fig. 4B and 4C). Flow cytometry analysis using a tetramer composed of an OVA peptide in complex with H-2Kb showed a marked increase in the percentage of the tetramer-positive CD8 T cells in the mice immunized with OVA + 2’3’cGAMP, indicating that 2’3’cGAMP stimulated the expansion of CD8 T cells bearing the OVA-specific T cell receptor (Fig. 4D). Taken together, these results indicate that 2’3’cGAMP functions as an immune adjuvant to stimulate antigen-specific T cell and B cell responses.
Figure 4. cGAMP is an adjuvant that stimulates T cell activation and antibody production in a STING-dependent manner.
(A) WT and Stinggt/gt mice were injected intramuscularly with OVA alone or OVA + 2’3’cGAMP on day 1 and day 10. Sera were collected at the indicated time points to measure OVA-specific IgG1 by ELISA. The error bar indicates SEM. Data is representative of three independent experiments, each with 5 mice per group. (B & C) WT mice were immunized with OVA or OVA + 2’3’cGAMP as above. Spleen leukocytes were collected on day 7, and stimulated with OVA peptides known to complex with MHC class I (H-2Kb) or class II (I-Ab). Secretion of IFNγ (B) and IL-2 (C) by T cells was measured by ELISA. *P < 0.05, **P < 0.01, and ***P < 0.001; Student’s t-test. (D) Similar to (B), except that FACS analysis was performed to measure the percentage of CD8 T cells bearing the T cell receptor specific for an OVA-H-2Kb tetramer. *P < 0.05, Student’s t-test. Data shown in (B–D) are representative of two independent experiments, each with 3 mice per group.
Here we provide genetic evidence that cGAS is essential for the induction of type-I interferons and other inflammatory cytokines by DNA transfection and DNA virus infection. With the exception of poly[dA:dT] and CpG DNA, most DNA molecules, especially those found in nature (e.g, bacterial and viral DNA), stimulate type-I interferons exclusively through the cGAScGAMP-STING pathway. In multiple cell types, including fibroblasts, macrophages and dendritic cells, the induction of type-I interferons by vaccinia viruses and several strains of HSV1 is completely dependent on cGAS and STING. Notably, however, IFNβ induction by wild type HSV1 is severely but not completely abolished in BMDM and GM-CSF DCs from cGas−/− or Stinggt/gt mice. It is possible that other putative DNA sensors, such as IFI16 or DDX41, may be involved in this residual induction of IFNβ by WT HSV1 (20, 21). However, the roles of these other putative DNA sensors in vivo have yet to be investigated through genetic experiments. In the case of cGAS, the phenotypes of cGas−/− mice are strikingly similar to those of Sting−/− mice (this study and ref. 18). These results, together with our biochemical data showing that cGAS is a cytosolic enzyme activated by its binding to generic DNA (2, 3), formally demonstrate that cGAS is a non-redundant and general cytosolic DNA sensor that activates STING.
We present evidence that 2’3’cGAMP is an effective adjuvant that boosts the production of antigen-specific antibodies and T cell responses in mice. Although the bacterial second messengers cyclic di-GMP and cyclic di-AMP are being developed as potential vaccine adjuvants(22), 2’3’cGAMP is a much more potent ligand of STING than any of the bacterial cyclic di-nucleotides(7). Thus, 2’3’cGAMP may be developed as an adjuvant for next generation vaccines to prevent or treat human diseases, including infectious diseases and cancer.
Supplementary Material
Acknowledgements
We thank Dr. R. Hammer and the transgenic core facility at UT Southwestern Medical Center for performing in vitro fertilization, Mr. S. Murray for technical assistance in HSV1 infection in mice, and Dr. N. DeLuca and Dr. D. Knipe for providing HSV1 strains d109 and 7134, respectively. This work was supported by grants from NIH (AI-093967) and Cancer Prevention and Research Institute of Texas (RP110430). H. W. was supported by an NIH postdoctoral training grant (5T32AI070116). Z. J. C is an investigator of Howard Hughes Medical Institute.
Footnotes
The University of Texas has filed a patent on using cGAMP and its derivatives as vaccine adjuvants (US patents 61/829,251 and 61/739.072; pharmaceutical targeting of a mammalian cyclic di-nucleotide signaling pathway). Materials including the cGas−/− mice may be requested upon signing of a materials transfer agreement. The data presented in this manuscript are tabulated in the main paper and the supplementary materials.
References and Notes
- 1.O'Neill LA. Immunology. Sensing the dark side of DNA. Science. 2013 Feb 15;339:763. doi: 10.1126/science.1234724. [DOI] [PubMed] [Google Scholar]
- 2.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013 Feb 15;339:786. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013 Feb 15;339:826. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ablasser A, et al. cGAS produces a 2'–5'-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013 Jun 20;498:380. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diner EJ, et al. The Innate Immune DNA Sensor cGAS Produces a Noncanonical Cyclic Dinucleotide that Activates Human STING. Cell Rep. 2013 May 30;3:1355. doi: 10.1016/j.celrep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao P, et al. Cyclic [G(2',5')pA(3',5')p] Is the Metazoan Second Messenger Produced by DNA-Activated Cyclic GMP-AMP Synthase. Cell. 2013 May 23;153:1094. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, et al. Cyclic GMP-AMP Containing Mixed Phosphodiester Linkages Is An Endogenous High-Affinity Ligand for STING. Molecular cell. 2013 Jun 3; doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishikawa H, Barber GN. The STING pathway and regulation of innate immune signaling in response to DNA pathogens. Cellular and molecular life sciences : CMLS. 2011 Apr;68:1157. doi: 10.1007/s00018-010-0605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.cGas−/− mice were generated by in vitro fertilization using sperms harboring a targeted insertion at the cGas/Mb21d1 locus. For details, see Materials and Methods in Supplemental Materials
- 10.Sauer JD, et al. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect Immun. 2011 Feb;79:688. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006 Jan;24:93. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004 Jul;5:730. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 13.Ablasser A, et al. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009 Jul 16; doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009 Aug 7;138:576. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samaniego LA, Neiderhiser L, DeLuca NA. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. Journal of virology. 1998 Apr;72:3307. doi: 10.1128/jvi.72.4.3307-3320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melroe GT, DeLuca NA, Knipe DM. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. Journal of virology. 2004 Aug;78:8411. doi: 10.1128/JVI.78.16.8411-8420.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010 Mar 19;140:805. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009 Oct 8;461:788. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nature reviews. Immunology. 2012 Jul;12:479. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, et al. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nature immunology. 2011 Oct;12:959. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unterholzner L, et al. IFI16 is an innate immune sensor for intracellular DNA. Nature immunology. 2010 Nov;11:997. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen W, Kuolee R, Yan H. The potential of 3',5'-cyclic diguanylic acid (c-di-GMP) as an effective vaccine adjuvant. Vaccine. 2010 Apr 19;28:3080. doi: 10.1016/j.vaccine.2010.02.081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.