Abstract
Assessment of mitochondrial ADP-stimulated respiratory kinetics in permeabilized skeletal myofibres (PmFB) is increasingly used in clinical diagnostic and basic research settings. However, estimates of the Km for ADP vary considerably (∼20-300 μM) and tend to overestimate respiration at rest. Noting PmFBs spontaneously contract during respiration experiments, we systematically determined the impact of contraction, temperature and oxygenation on ADP-stimulated respiratory kinetics. Blebbistatin (BLEB), a myosin II ATPase inhibitor, blocked contraction under all conditions and yielded high Km values for ADP of >∼250 and ∼80 μM in red and white rat PmFB, respectively. In the absence of BLEB, PmFB contracted and the Km for ADP decreased by ∼2 to 10-fold in a temperature-dependent manner. PmFB were sensitive to hyperoxia (increased Km) in the absence of BLEB (contracted) at 30°C but not 37°C. In PmFB from humans, contraction elicited high sensitivity to ADP (m <100 μM) whereas blocking contraction (+BLEB) and including PCr:Cr = 2 to mimic the resting energetic state yielded a Km for ADP = ∼1560 μM, consistent with estimates of in vivo resting respiratory rates of <1% maximum. These results demonstrate the sensitivity of muscle to ADP varies over a wide range in relation to contractile state and cellular energy charge, providing evidence that enzymatic coupling of energy transfer within skeletal muscle becomes more efficient in the working state.
Keywords: bioenergetics, creatine kinase, blebbistatin, N-benzyltoluene sulphonamide, metabolism, skeletal muscle contraction
Introduction
Determining the sensitivity and responsiveness of the respiratory system to ADP is commonly performed in the diagnosis of mitochondrial diseases as well as in a variety of basic and applied research settings [1, 2]. Respiratory sensitivity to ADP also influences a number of cellular processes dependent on mitochondrial membrane potential, including reactive oxygen species generation, sub-cellular calcium distribution, and specific mitochondrial membrane transport mechanisms. However, in mitochondria isolated from rodent skeletal muscle, the apparent Km for ADP [3-5] is similar to the resting cellular concentration of free ADP [6-8], implying in vivo respiratory rates at rest are ∼50% of maximal respiratory capacity – obviously an erroneous conclusion. The apparent Km for ADP is ∼10-to ∼15- fold higher in permeabilized fibre bundles (PmFB) than in isolated mitochondria from rodent cardiac and slow twitch muscles (∼300 vs ∼20 to 30 μM, respectively) [3, 4, 9-12], suggesting an additional level of regulation is preserved in fibre bundles. However, PmFB from fast-twitch muscle (red and white gastrocnemius) are characterized by a high sensitivity to ADP (Km <50-100 μM), similar to isolated mitochondria from the same muscles [12]. In human skeletal muscle, which is a mixture of fast- and slow-twitch fibres, the Km for ADP is higher in PmFB (∼60-120 μM ADP [13, 14]) than isolated mitochondria [5], but still predictive of much higher rates of respiration (10-20% of max) than at rest in vivo (<1% of max). The factors responsible for the obvious discrepancy in accurately assessing mitochondrial respiratory control by ADP have remained elusive.
During the course of respiration experiments, we have consistently observed that PmFB spontaneously contract in a Ca2+-independent manner (Supplemental Video). To determine whether contractile activity alters ADP-stimulated respiratory kinetics, we compared the effect of two recently developed myosin II-specific inhibitors that block contractile activity: N-benzyltoluene sulfonamide (BTS) and blebbistatin (BLEB). BTS inhibits the Ca2+-stimulated ATPase activity of myosin II subfragment 1 (S1), weakening myosin's interaction with F-actin [15]. BLEB preferentially binds to the active site of the S1 ATPase when ADP and phosphate are bound, stabilizing the intermediate state [16]. We report spontaneous contraction of PmFB increases mitochondrial respiratory sensitivity by several-fold in a fiber-type-specific manner that is both temperature and tissue oxygenation-dependent. We also report a Km for ADP in resting human skeletal muscle that is several-fold higher than previously determined, consistent with an estimated in vivo rate of respiration of <1% of max. Importantly, this Km is only obtained by performing analyses with myosin-ATPase inhibition at 37°C with phosphocreatine and creatine concentrations reflective of resting muscle in vivo.
Experimental
Animals and reagents
Male Sprague-Dawley rats were purchased from Charles River Laboratory (Wilmington, MA). Rats were housed in a temperature (22°C) and light controlled room and given free access to food and water. At the time of the experiments, rats were 8-10 wk old and weighed 250-350 g. Skeletal muscle was obtained from anesthetized animals (100 mg/kg i.p. ketamine-xylazine). After surgery, animals were sacrificed by cervical dislocation while anesthetized. All procedures were approved by the Institutional Review Board of East Carolina University. All chemicals were purchased from Sigma-Aldrich. Both BLEB and BTS were dissolved in DMSO and added to the oxygraph chamber at a final concentration of 25 μM and 100 μM, respectively.
Human subjects and tissue biopsy
Ten healthy but sedentary men were recruited to participate in this investigation. Their mean age, weight, height and BMI were 23.2 ± 1.7 y, 1.76 ± 0.05 m, 74.2 ± 4.9 kg and 23.9 ± 0.8 kg·m-2, respectively. All participants were non-smokers. None of the subjects had any diseases or were taking any medications known to alter skeletal muscle metabolism. Subjects were given both oral and written information about the experimental procedures before giving their informed consent. These experiments were approved by the Institutional Review Board of East Carolina University and conducted in accordance with the Declaration of Helsinki.
On the day of the experiment, subjects reported to the clinical laboratory following an overnight fast (∼12 h). A single skeletal muscle sample was obtained from the lateral aspect of vastus lateralis by percutaneous needle biopsy technique with suction under local subcutaneous anesthesia (1% lidocaine). A portion of the biopsy sample was immediately placed into ice-cold buffer X (described below) and used to prepare permeabilized fibre bundles.
Preparation of permeabilized muscle fibres
The technique is partially adapted from previous methods [12, 17] and has been described previously [18, 19]. Briefly, small portions (∼25 mg) of muscle were dissected and placed in ice-cold buffer X, containing (in mM) 50 MES, 7.23 K2EGTA, 2.77 CaK2EGTA, 20 imidazole, 0.5 DTT, 20 taurine, 5.7 ATP, 14.3 PCr, and 6.56 MgCl2-6 H2O (pH 7.1, 290 mOsm). The muscle was trimmed of connective tissue and fat. Four small muscle bundles (∼2-7 mm, 1.0-2.5 mg wet weight) of either red or white gastrocnemius were prepared from each rat. Each bundle was gently separated along the longitudinal axis with a pair of needle-tipped forceps under magnification (MX6 Stereoscope, Leica Microsystems, Inc., Wetzlar, DE). Rat bundles were then treated with 50 μg/ml saponin in ice-cold buffer X, and incubated on a rotor for 30 min at 4°C. Human muscle fibre bundles were treated with 30 μg/ml saponin, as separate experiments determined optimal respiration with this saponin concentration in humans [20]. Saponin is a mild, cholesterol-specific detergent that selectively permeabilizes the sarcolemmal membranes while keeping mitochondrial membranes, which contain little cholesterol, completely intact [21, 22]. Following permeabilization, the PmFB were placed in buffer Z containing (in mM) 105 K-MES, 30 KCl, 1 EGTA, 10 K2HPO4, and 5 MgCl2-6 H2O, 0.005 glutamate, and 0.002 malate with 5.0 mg/ml BSA (pH 7.4, 290 mOsm). PmFB remained in buffer Z on a rotator at 4°C until analysis (< 30 min).
Mitochondrial respiration in permeabilized fibres
High-resolution O2 consumption measurements were conducted in 2 mL of buffer Z using the OROBOROS Oxygraph-2k (OROBOROS INSTRUMENTS, Corp., Innsbruck, AT) with stirring at 750 rpm. Buffer Z contained 20 mM creatine hydrate to saturate creatine kinase, which facilitates mitochondrial ADP transport [4, 10, 23-25], with the exception of specific experiments on human PmFBs which were conducted in the presence of 24 mM phosphocreatine and 12 mM creatine hydrate (described below). 5 mM pyruvate and 2 mM malate were added as complex I substrates. ADP was titrated in step-wise increments and all experiments were completed before oxygraph chamber [O2] reached 150 μM. At the conclusion of each experiment, PmFBs were washed in double-distilled H2O to remove salts, frozen at -20°C, and dried via lyophilization (Labconco Corp., Kansas City, MO). Polarographic oxygen measurements were acquired in 2-second intervals, with the rate of respiration derived from 40 data points, and expressed as pmol • s-1 • mg-1 dry weight. Dry and wet bundle weights were consistently between 0.2 - 0.6 mg and ∼1.0 to 2.5 mg, respectively. Cytochrome c was added to test for mitochondrial membrane integrity as partial loss of cytochrome c during sample preparation may limit active respiration. A cytochrome c response was dectected in <5% of all experiments and no response generated >10% increase in respiration. No relationship was observed between the relative cytochrome c response and Km when grouping all human and rodent data (R2 = 0.013, p>0.05). Additionally, no significant relationship was observed in humans when using a paired t-test to compare the Km for those experiments showing 0-5% cytochrome c response relative to those few samples exhibiting a 5-10% cytochrome c response. Four PmFBs from each rat or human were run simultaneously in four separate oxygraph chambers. Two of the chambers contained either 100 μM BTS or 25 μM BLEB. A third chamber contained 1.25% DMSO (vehicle, +V) to match the content of DMSO added in the BTS and BLEB conditions with the remaining chamber serving as the control (minus vehicle) condition.
The Km for ADP was determined through the Michaelis-Menten enzyme kinetics - fitting model (Y = Vmax*X/(Km + X)), where X = [free ADP; ADPf] and Y = JO2 at [ADPf], using Prism (GraphPad Software, Inc., La Jolla, CA). This equation was also used to calculate the fraction of maximal mitochondrial respiration in resting human skeletal muscle in vivo. This calculation was performed using the experimentally determined Km values assuming resting [ADPf] to be ∼14.6 μM in human skeletal muscle [6].
Microscopic imaging of PmFB conformation
Images of PmFB conformation were captured immediately upon exposure to 25°C, 30°C or 37°C before visible contraction occurred, with further images taken throughout the contraction process (Leica Microsystems, Inc.). Separate images were taken of PmFB before and after the addition of 2 mM ADP (with 5 mM pyruvate and 2 mM malate). Media temperature was maintained by a temperature-controlled base (heat-retaining gel pack and metal block).
Statistics
Data are presented as means ± SEM. Statistical analyses were performed using paired t-tests, 1-way or 2-way ANOVA (as appropriate) with Student-Newman-Keuls method for analysis of significance using Statistica software (StatSoft Inc., Tulsa, OK). DMSO (+V) did not affect respiratory responses under any of the experimental conditions tested. Statistical analyses were performed including both -V and +V, but only +V data are presented for clarity. The alpha level of significance was set at P < 0.05.
Results and Discussion
Inhibiting spontaneous contraction yields higher Km for ADP-stimulated respiration
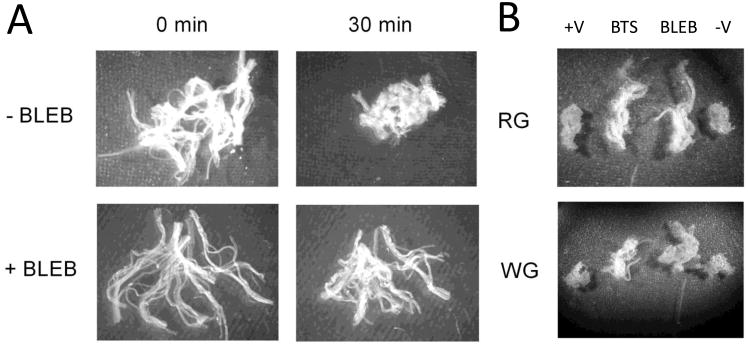
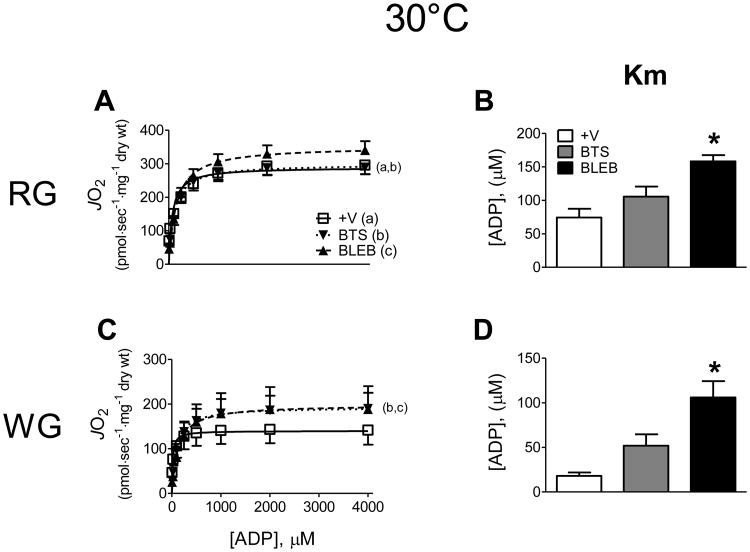
We first tested the efficacy of inhibiting myosin ATPase to prevent PmFB spontaneous contraction (Supplemental Video). Visually, both BTS (data not shown) and BLEB (Fig. 1A) substantially reduced the spontaneous contraction occurring in PmFB from both red and white rat skeletal muscle in the absence of substrates, or during routine glutamate/malate-supported ADP-stimulated respiration experiments (Fig. 1B). Preventing contraction in rat PmFB with BLEB increased the Km for ADP from ∼75 to >150 μM in red muscle, and from ∼15 to >100 μM in white muscle (Fig. 2A-D). In general, BTS was less effective than BLEB in red muscle, but tended to exert similar changes in Km in white muscle (P = 0.059-0.087 vs -/+V). Combining BLEB and BTS had no additional effect on Km or Vmax in either muscle (data not shown). These findings demonstrate that PmFB spontaneously contract and that the inclusion of contraction inhibitors, particularly BLEB, lowers the respiratory sensitivity to ADP, yielding higher Km values that are more reflective of rates of oxygen consumption at rest in vivo. Contraction of PmFB therefore likely accounts for the higher sensitivities to ADP reported previously [9, 12, 26].
Figure 1.
Effect of spontaneous contraction on rat PmFB respiratory kinetics. PmFB contraction in red (RG) and white (WG) gastrocnemius following exposure to 30°C for 30 min is prevented by myosin inhibitors blebbistatin (BLEB) and N-benzyltoluene sulfonamide (BTS) (A; RG) alone (1× amplification) and (B) following ∼30-45 min of ADP-stimulated respiration (0.6× amplification).
Figure 2.
Effect of contraction on ADP-stimulated mitochondrial respiratory kinetics at 30°C in red (RG; A-B) and white gastrocnemius (WG; C-D) PmFB under air-saturated media (∼150-220 μM O2). Data represent means ± SEM; n = 4-5; *P < 0.05 vs all other treatments at same temperature.
Temperature- and oxygen-dependent effects of spontaneous contraction on mitochondrial respiration in rat PmFBs
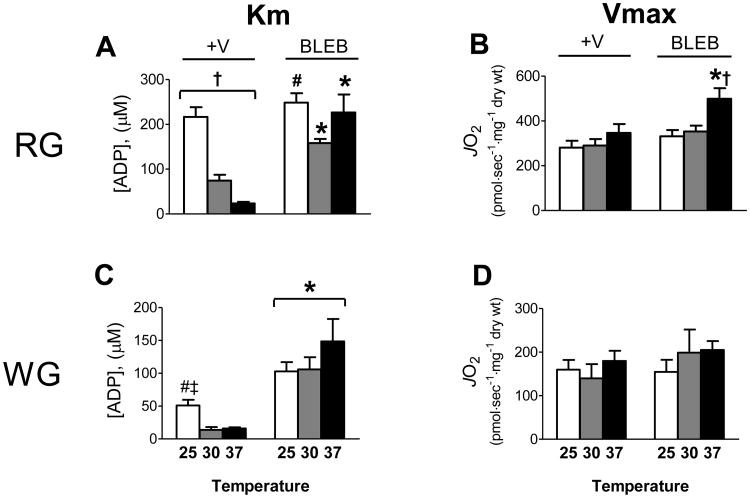
PmFB remain relaxed and maintain respiratory function for as long as ∼6 h when kept at 4°C in substrate-free buffer [26] but will begin to contract when transferred to the same buffer at 30°C (Fig. 1A). A number of previous studies assessing respiratory kinetics have been performed at 25°C [9, 12, 26] where spontaneous contraction is not as evident in the absence of ADP but occurs readily upon ADP addition (Supplementary Fig. 1). To determine the influence of temperature on ADP-stimulated respiratory kinetics, rat PmFB from both red and white muscle were subjected to ADP-titrations during complex I (glutamate/malate) - supported respiration in the absence or presence of BLEB at 25°C or 37°C (Supplementary Fig. 2A-D), and compared with data obtained at 30°C. All experiments were conducted under air-saturated conditions (∼150-220 μM O2) except in specific cases noted later. In RG PmFB in the absence of BLEB (i.e., +V), the apparent Km for ADP decreased markedly from >200 μM at 25°C to ∼75 μM at 30°C, and to <25 μM at 37°C (Fig. 3A). In WG PmFB, Km dropped from ∼50 μM at 25°C to ∼15 μM at 30 and 37°C (Fig 3C). By contrast, when contraction was inhibited by BLEB, the apparent Km for ADP was near or >200 μM in RG and >100 μM in WG PmFB at all three temperatures. In general, higher reaction temperatures did not affect Vmax for ADP-stimulated respiration in RG or WG, except in the presence of BLEB at 37°C in RG (Fig. 3B,D). These findings demonstrate that, in the absence of a contraction inhibitor, the apparent Km for ADP is markedly lower at higher temperatures. The effect is not mediated by temperature per se, but by an apparent acceleration of fibre contraction. Thus, the more accurate Km for ADP in resting muscle is obtained in contraction-inhibited PmFB at 37°C. Importantly however, contraction clearly increases the sensitivity of the respiratory system to ADP (up to ∼10-fold) [24], indicating that the Km for ADP in vivo is fluid depending on the level of contractile activity.
Figure 3.
Respiratory sensitivity to ADP and Vmax are influenced by contraction in a temperature-dependent manner in rat red (RG; A-B) and white (WG; C-D) gastrocnemius PmFB under air-saturated media (∼150-220 μM O2). Data represent means ± SEM; n = 4-5; *P < 0.05 vs all other treatments at same temperature; †P < 0.05 significant differences between all temperatures within same treatment; #P < 0.05 vs 30°C, or ‡ vs 37°C within same treatment.
Temperature coefficients have been applied to respiratory flux data at 25°C and 30°C to convert to predicted respiration at 37°C (2.30 and 1.62 respectively, assuming a Q10 effect whereby flux doubles for a 10°C increase [27]). Using data from Fig. 3A-D, the calculated ratios for 25-37°C conversion are ∼1.1-1.3 in a contracted state and ∼1.3-1.5 during relaxation (BLEB), with similar ratios for 30-37°C conversion (∼1.2-1.3 and ∼1.0-1.4 during contraction and relaxation respectively, data not shown). The reason for the disparity from expected ratios is unclear. However, these results raise the possibility that factors present in PmFB limit the effect of increased temperature on respiratory flux, and that the Q10 principle, while relevant to in vitro analyses of enzymatic activity, may not be applicable for converting respiratory flux in PmFB where the intracellular organization of metabolic and respiratory systems is preserved.
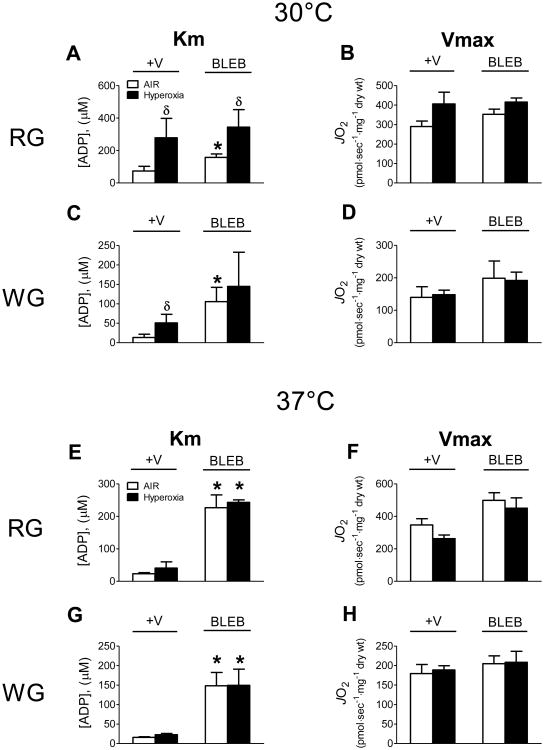
Oxygen concentrations as low as 20 μM do not pose a limitation to respiration in isolated mitochondria; however, in PmFB the sensitivity to low oxygen supply is reportedly increased ∼100-fold due to diffusion restrictions limiting oxygen supply to the core of the fibre bundle [28]. To offset this limitation, hyperoxygenation has been employed to maintain oxygen concentrations above air saturation (∼500-200 μM). To determine whether hyperoxia affects ADP respiratory kinetics when contraction is prevented, PmFB from red and white muscle were studied in air-saturated (∼150-200 μM) and hyperoxic (∼275-450 μM) conditions in the absence or presence of BLEB at 30°C and 37°C. Interestingly, at 30°C hyperoxia increased the apparent Km for ADP by 2- to 3-fold in both RG and WG (Fig. 4A,C). In RG, the higher apparent Km for ADP was evident regardless of whether BLEB was present. This sharp decrease in the sensitivity to ADP suggests that hyperoxia may alter the molecular components of oxidative phosphorylation, perhaps via oxidation of redox-sensitive proteins within the respiratory system [29]. Hyperoxia also increased Vmax for ADP-stimulated respiration in RG in the absence of BLEB (Fig. 4B), consistent with the notion of oxygen diffusion limitations in PmFB under room air-saturated conditions (<200 μM O2) described previously [28]. However, the effect of hyperoxia on Vmax was not seen in RG when contraction was prevented or in WG in the absence or presence of the contraction inhibitor (Fig. 4D).
Figure 4.
Respiratory sensitivity to ADP and Vmax are influenced by hyperoxic conditions (Hyperoxia, ∼275-450 μM O2) compared to air-saturated media (AIR, ∼150-220 μM O2) in a temperature-dependent manner in rat red (RG) and white gastrocnemius (WG) PmFB (A-H). Data represent means ± SEM; n = 4-5; *P < 0.05 vs all other treatments at same temperature; δP < 0.05 vs AIR within same treatment.
In contrast to PmFB at 30°C, hyperoxia at 37°C had no effect on the apparent Km or Vmax for ADP-stimulated respiration in RG or WG (Fig. 4E-H). As in earlier experiments conducted at 37°C (Fig. 3A,C), preventing contraction with BLEB generated a markedly higher apparent Km for ADP compared with PmFB that were allowed to contract, but no difference was seen between air-saturated versus hyperoxic conditions (Fig. 4E,G). It is important to note that in RG in the absence of BLEB, hyperoxia at 30°C raised the apparent Km for ADP to >250 μM (Fig. 4A) whereas at 37°C the Km remained below 40 μM regardless of whether hyperoxia was employed (Fig. 4E,G). Moreover, the apparent Km only increased to >250 μM when contraction was inhibited (+BLEB; Fig. 4E,G). These findings indicate that a 37°C reaction temperature is sufficient to prevent any effect of hyperoxia on ADP-stimulated respiratory kinetics, regardless of whether a contraction inhibitor is employed. Methodologically, this is important as initial hyperoxygenation of the respiratory buffer prevents oxygen from becoming rate limiting during experiments with longer protocols. These findings further emphasize the importance of properly defining experimental conditions (i.e., contraction inhibitors, reaction temperature, and oxygen concentrations) relative to the experimental questions being addressed when assessing respiratory function.
Temperature-dependent effects of spontaneous contraction on mitochondrial respiration in human PmFBs
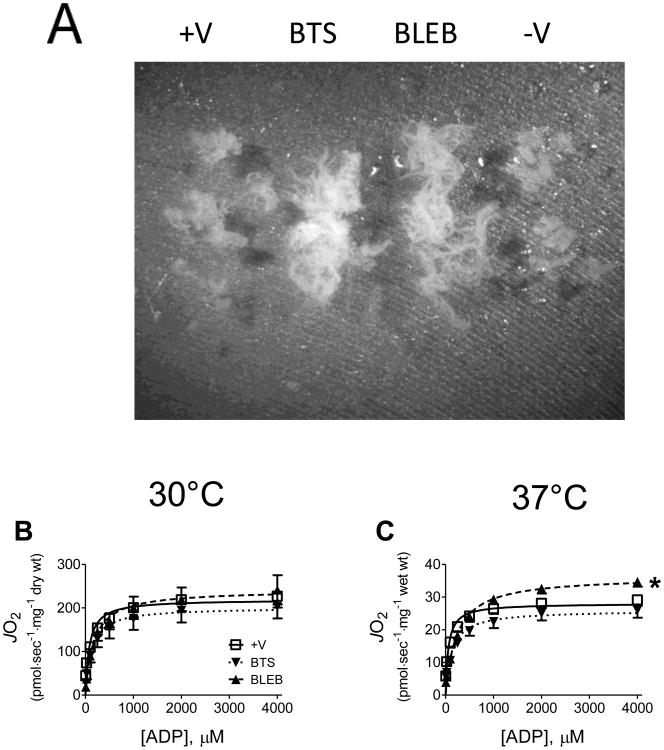
To determine whether contraction and/or temperature influence respiratory kinetics similarly in human skeletal muscle, ADP titrations were performed on PmFB from vastus lateralis muscle in the absence or presence of BTS or BLEB at 30 or 37°C under air-saturated conditions (Fig 5B,C). At 37°C human PmFB consistently broke apart in the absence of contraction inhibitors, making it impossible to express mitochondrial oxygen consumption to PmFB dry weight. In the presence of BTS or BLEB however, PmFB remained intact (Fig 5A). At 30°C, PmFB remained intact independent of whether contraction inhibitor was included. Compared to rodent muscle, human fibre bundles contain less connective tissue which may account for their fragility at 37°C, but this is only speculation. Regardless, oxygen consumption data at 37°C was normalized to PmFB wet weight to permit comparisons across all treatments (Fig 5C, 6C). Similar to observations in rats, preventing contraction with BLEB in human PmFB increased the apparent Km for ADP from ∼80 μM (+V) to ∼175 μM at 30°C and ∼240 μM at 37°C (Fig. 6A). BTS had a similar but less pronounced effect. Vmax was not different between treatments at 30°C and only slightly elevated in BLEB-treated fibres at 37°C (Fig. 6B,C)
Figure 5.
Effects of spontaneous contraction on human PmFB respiratory kinetics. (A) Conformation of PmFB from human male vastus lateralis following ADP-stimulated respiration at 30°C without (+/-V) or with myosin inhibitors BLEB and BTS (0.6× amplification). (B-C) Effect of contraction on ADP-stimulated respiratory kinetics 30°C and 37°C under air-saturated media (∼150-220 μM O2). Data represent means ± SEM; n = 5; *P < 0.05 vs all other treatments at same temperature.
Figure 6.
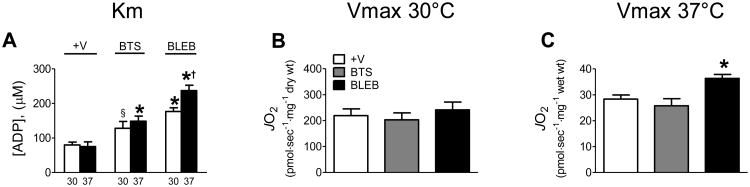
Temperature-dependent effects of contraction on respiratory sensitivity (Km) to ADP and Vmax in human PmFB under air-saturated media (∼150-220 μM O2) (A-C). Data represent means ± SEM; n = 4; *P < 0.05 vs all other treatments at same temperature; †P < 0.05 significant differences between both temperatures within same treatment; §P < 0.05 vs +V within same temperature.
The contribution of phosphocreatine/creatine to ADP-stimulated mitochondrial respiratory kinetics
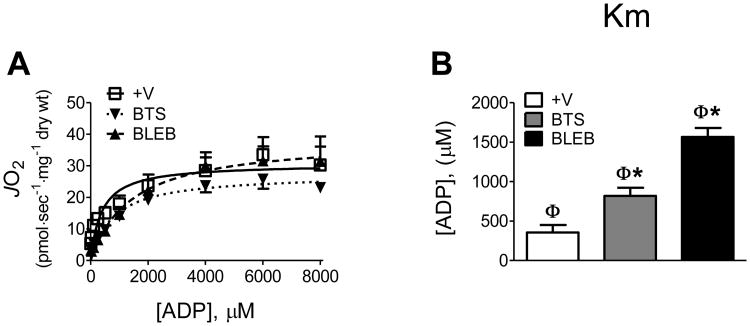
All of the experiments in PmFB from rats and humans described above were performed with 20 mM creatine (Cr) included in the respiration buffer. Cr has been shown to markedly decrease the apparent Km for ADP in PmFB of rats and humans to <50-100 μM. The effect is evident only in slow (or cardiac) fibres and has been interpreted to reflect the likely organization of intracellular energetic units utilizing Cr and phosphocreatine (PCr) via creatine kinase (CK) to facilitate energy transfer between mitochondria and intracellular ATPases [30]. However, while a higher sensitivity to ADP might appear advantageous for the myofibre, as mentioned earlier this is difficult to reconcile with the fact that a Km of <50-100 μM in resting muscle predicts an in vivo respiration rate of ∼10-20% of Vmax (see methods for calculation). The ratio of PCr:Cr in vivo fluctuates according to the energy turnover rate. One report has shown that a higher Km (∼300 μM) is obtained with a ratio of PCr:Cr more typical of resting muscle (2:1, 24 mM PCr:12 mM Cr) during experiments conducted at 25°C [25]. To determine whether a PCr:Cr typical of resting muscle affects ADP respiratory kinetics in the presence of contraction inhibitors at 37°C, we repeated experiments in human PmFB in the presence of 24 mM PCr and 12 mM Cr under air-saturated conditions (Fig. 7A). In the absence of contraction inhibitors (+V), the apparent Kms were similar to previous reports (∼350 μM) [25]. However, in the presence of BTS or BLEB, the apparent Km for ADP increased to ∼820 μM and ∼1560 μM, respectively (Fig. 7B). This value predicts resting rates of ADP-stimulated respiration in vivo to be <1% of Vmax, nearly 50 times lower than previous estimates from isolated human mitochondria [5] and ∼5 times lower than previous estimates from human PmFB [25]. Thus, assessing respiratory function in the presence of a contraction inhibitor at 37°C using a PCr:Cr ratio present in resting muscle yields kinetic data that is consistent with predicted rates of respiration in myofibres at rest.
Figure 7.
ADP-stimulated respiratory kinetics in the presence of 24 mM phosphocreatine and 12 mM creatine at 37°C (A-B), representative of “resting” in vivo conditions. Data represent means ± SEM; n = 4; *P < 0.05 vs all other treatments at same temperature; φP < 0.05 vs same treatment with 20mM Creatine at 37°C (Fig. 6). Experiments were conducted under air-saturated media (∼150-220 μM O2).
Perspectives and Conclusions
Collectively, these studies demonstrate the sensitivity of the mitochondrial respiratory system to ADP varies over a wide range and is directly related to the contractile state of the fibre, thereby optimizing the ability of skeletal muscle to respond efficiently and appropriately to large perturbations in energy demand. Why would skeletal muscle have evolved a system where the sensitivity of the mitochondrial respiratory system to ADP varies over such a wide range? In resting muscle, the lower sensitivity to ADP presumably reduces mitochondrial efficiency (i.e., the rate of ATP regeneration per rate of oxygen consumption (P/O ratio)) when energy demand is low which, together with proton leak, will tend to favour heat production. Conversely, when energy demand is elevated as a consequence of contractile activity, the increase in ADP sensitivity increases mitochondrial efficiency (P/O ratio) reflecting the priority given to energy regeneration during exercise.
The mechanism by which contraction modulates mitochondrial ADP sensitivity is unknown, but may be due to regulatory influences of force transmission from the cytoskeleton to the mitochondria itself [4, 12]. ADP/ATP flux in and out of the mitochondria is mediated primarily by the voltage-dependent anion channel (VDAC) at the outer membrane and the adenine nucleotide translocator (ANT) at the inner mitochondrial membrane. The cytoskeletal protein tubulin has recently been shown to bind to and induce voltage-sensitive closure of VDAC in reconstituted systems [31]. When added to isolated mitochondria, tubulin partially restores the apparent high Km for ADP evident in PmFB, presumably by restoring a limitation on VDAC-mediated permeability of the mitochondrial outer membrane. Further work will be required to establish whether reversible closure of VDAC by tubulin is responsible for regulating mitochondrial ADP sensitivity under different contractile/metabolic states. If true, this mechanism may explain how mitochondria are able to rapidly sense changes in energy demand, particularly at the onset of contraction before excessive energy deficits are created.
A number of factors that could be responsible for triggering the spontaneous contraction in PmFBs were tested, including the presence of residual ADP, redox mediated control of SERCA, and cytosolic Ca2+ accumulation. However, neither inhibition of CK by iodoacetamide to prevent possible residual ADP-ATP cycling between CK and myosin ATPase, inclusion of the superoxide scavenger Tempol or TBQ to prevent redox-triggered calcium flux through SERCA [32], nor preventing endogenous Ca2+ accumulation by EGTA (up to 5 mM) prevented the spontaneous contraction (data not shown). Thus, the mechanism triggering spontaneous contraction in PmFB remains unclear but does appear to be independent of CK-mediated cycling of endogenous adenine nucleotides or Ca2+.
To conclude, these findings report the novel observation that spontaneous contraction occurs inPmFB which imparts significant regulation on ADP-stimulated respiratory kinetics in a temperature-dependent manner. We report the highest mitochondrial Km for ADP to date by identifying andcontrolling critical regulators to be more reflective of in vivo conditions for relaxed skeletal muscle(contraction inhibition, 37°C, PCr:Cr concentrations of 24mM:12mM). This suggests in vivo mitochondrial respiratory sensitivity at rest is lower than previously reported. Of equal significance, our findings also reveal contraction increases the sensitivity to ADP markedly in skeletal muscle, providing a more efficient coupling of ATP-producing to ATP-consuming systems at a time when energy turnover can rapidly and dramatically increase. From a methodological standpoint, these findings also highlight the importance of properly defining the objectives, analytical protocols, and interpretive boundaries within these different parameters when designing clinical diagnostic tests and basic research experiments. Such considerations are critical in view of the rapidly expanding interests in mitochondrial processes regulating basic cell function and disease etiology.
Acknowledgments
We thank the subjects for their participation as well as Rita Bowden and Angela Clarke for their expert medical and technical assistance.
Funding: This study was supported by National Institutes of Health Grants RO1 DK073488 and DK074825.
Abbreviations used
- ADP
adenosine diphosphate
- PmFB
permeabilized fibre bundles
- BTS
N-benzyltoluene sulphonamide
- BLEB
blebbistatin
- RG
red gastrocnemius
- WG
white gastrocnemius
- CK
creatine kinase
- PCr
phosphocreatine
- Cr
creatine
- VDAC
voltage-dependent anion channel
- ANT
adenine nucleotide translocator
- ATP
adenosine triphosphate
- SERCA
sarco/endoplasmic reticulum calcium ATPase
- TBQ
2,5-di(tert-butyl)-1,4-hydroquinone
Footnotes
Author Contributions: G.R.P., D.A.K., E.J.A., P.D.N.; study design and conception. C.G.R.P., D.A.K., C.T.L., B.L.C.; conducted rodent experiments. C.G.R.P., R.K., B.L.C., D.S.L., C.L.K., P.B., T.P.G.; conducted human tissue experiments. C.G.R.P., D.A.K., P.D.N were the primary writers of the manuscript while all authors participated in its preparation. P.D.N.; project director.
References
- 1.Sperl W, Skladal D, Gnaiger E, Wyss M, Mayr U, Hager J, Gellerich FN. High resolution respirometry of permeabilized skeletal muscle fibers in the diagnosis of neuromuscular disorders. Mol Cell Biochem. 1997;174:71–78. [PubMed] [Google Scholar]
- 2.N'Guessan B, Zoll J, Ribera F, Ponsot E, Lampert E, Ventura-Clapier R, Veksler V, Mettauer B. Evaluation of quantitative and qualitative aspects of mitochondrial function in human skeletal and cardiac muscles. Mol Cell Biochem. 2004;256-257:267–280. doi: 10.1023/b:mcbi.0000009874.14649.ca. [DOI] [PubMed] [Google Scholar]
- 3.Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955;217:383–393. [PubMed] [Google Scholar]
- 4.Saks VA, Kuznetsov AV, Khuchua ZA, Vasilyeva EV, Belikova JO, Kesvatera T, Tiivel T. Control of cellular respiration in vivo by mitochondrial outer membrane and by creatine kinase. A new speculative hypothesis: possible involvement of mitochondrial-cytoskeleton interactions. J Mol Cell Cardiol. 1995;27:625–645. doi: 10.1016/s0022-2828(08)80056-9. [DOI] [PubMed] [Google Scholar]
- 5.Tonkonogi M, Sahlin K. Rate of oxidative phosphorylation in isolated mitochondria from human skeletal muscle: effect of training status. Acta Physiol Scand. 1997;161:345–353. doi: 10.1046/j.1365-201X.1997.00222.x. [DOI] [PubMed] [Google Scholar]
- 6.Perry CG, Heigenhauser GJ, Bonen A, Spriet LL. High-intensity aerobic interval training increases fat and carbohydrate metabolic capacities in human skeletal muscle. Appl Physiol Nutr Metab. 2008;33:1112–1123. doi: 10.1139/H08-097. [DOI] [PubMed] [Google Scholar]
- 7.Radda GK. Control of bioenergetics: from cells to man by phosphorus nuclear-magnetic-resonance spectroscopy. Eighteenth CIBA medal lecture. Biochem Soc Trans. 1986;14:517–525. doi: 10.1042/bst0140517. [DOI] [PubMed] [Google Scholar]
- 8.Sahlin K, Soderlund K, Tonkonogi M, Hirakoba K. Phosphocreatine content in single fibers of human muscle after sustained submaximal exercise. Am J Physiol. 1997;273:C172–178. doi: 10.1152/ajpcell.1997.273.1.C172. [DOI] [PubMed] [Google Scholar]
- 9.Veksler VI, Kuznetsov AV, Anflous K, Mateo P, van Deursen J, Wieringa B, Ventura-Clapier R. Muscle creatine kinase-deficient mice. II. Cardiac and skeletal muscles exhibit tissue-specific adaptation of the mitochondrial function. J Biol Chem. 1995;270:19921–19929. doi: 10.1074/jbc.270.34.19921. [DOI] [PubMed] [Google Scholar]
- 10.Saks VA, Belikova YO, Kuznetsov AV, Khuchua ZA, Branishte TH, Semenovsky ML, Naumov VG. Phosphocreatine pathway for energy transport: ADP diffusion and cardiomyopathy. Am J Physiol. 1991;261:30–38. doi: 10.1152/ajplung.1991.261.4.L30. [DOI] [PubMed] [Google Scholar]
- 11.Bygrave FL, Lehninger AL. The affinity of mitochondrial oxidative phosphorylation mechanisms for phosphate and adenosine diphosphate. Proc Natl Acad Sci U S A. 1967;57:1409–1415. doi: 10.1073/pnas.57.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuznetsov AV, Tiivel T, Sikk P, Kaambre T, Kay L, Daneshrad Z, Rossi A, Kadaja L, Peet N, Seppet E, Saks VA. Striking differences between the kinetics of regulation of respiration by ADP in slow-twitch and fast-twitch muscles in vivo. Eur J Biochem. 1996;241:909–915. doi: 10.1111/j.1432-1033.1996.00909.x. [DOI] [PubMed] [Google Scholar]
- 13.Tonkonogi M, Harris B, Sahlin K. Mitochondrial oxidative function in human saponin-skinned muscle fibres: effects of prolonged exercise. J Physiol. 1998;510(Pt 1):279–286. doi: 10.1111/j.1469-7793.1998.279bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh B, Tonkonogi M, Sahlin K. Effect of endurance training on oxidative and antioxidative function in human permeabilized muscle fibres. Pflugers Arch. 2001;442:420–425. doi: 10.1007/s004240100538. [DOI] [PubMed] [Google Scholar]
- 15.Cheung A, Dantzig JA, Hollingworth S, Baylor SM, Goldman YE, Mitchison TJ, Straight AF. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol. 2002;4:83–88. doi: 10.1038/ncb734. [DOI] [PubMed] [Google Scholar]
- 16.Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 17.Tonkonogi M, Fernstrom M, Walsh B, Ji LL, Rooyackers O, Hammarqvist F, Wernerman J, Sahlin K. Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflugers Arch. 2003;446:261–269. doi: 10.1007/s00424-003-1044-9. [DOI] [PubMed] [Google Scholar]
- 18.Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H(2)O(2) generation. Am J Physiol. 2006;290:C844–851. doi: 10.1152/ajpcell.00402.2005. [DOI] [PubMed] [Google Scholar]
- 19.Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J Biol Chem. 2007;282:31257–31266. doi: 10.1074/jbc.M706129200. [DOI] [PubMed] [Google Scholar]
- 20.Kane DA, Lin CT, Anderson EJ, Kwak HB, Cox JH, Brophy PM, Hickner RC, Neufer PD, Cortright RN. Progesterone Increases Skeletal Muscle Mitochondrial H2O2 Emission in Non-Menopausal Women. Am J Physiol Endocrinol Metab. 2011;300:E528–E535. doi: 10.1152/ajpendo.00389.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veksler VI, Kuznetsov AV, Sharov VG, Kapelko VI, Saks VA. Mitochondrial respiratory parameters in cardiac tissue: a novel method of assessment by using saponin-skinned fibers. Biochim Biophys Acta. 1987;892:191–196. doi: 10.1016/0005-2728(87)90174-5. [DOI] [PubMed] [Google Scholar]
- 22.Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc. 2008;3:965–976. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- 23.Saks VA, Khuchua ZA, Vasilyeva EV, Belikova O, Kuznetsov AV. Metabolic compartmentation and substrate channelling in muscle cells. Role of coupled creatine kinases in in vivo regulation of cellular respiration--a synthesis. Mol Cell Biochem. 1994;133-134:155–192. doi: 10.1007/BF01267954. [DOI] [PubMed] [Google Scholar]
- 24.Anmann T, Eimre M, Kuznetsov AV, Andrienko T, Kaambre T, Sikk P, Seppet E, Tiivel T, Vendelin M, Seppet E, Saks VA. Calcium-induced contraction of sarcomeres changes the regulation of mitochondrial respiration in permeabilized cardiac cells. Febs J. 2005;272:3145–3161. doi: 10.1111/j.1742-4658.2005.04734.x. [DOI] [PubMed] [Google Scholar]
- 25.Walsh B, Tonkonogi M, Soderlund K, Hultman E, Saks V, Sahlin K. The role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J Physiol. 2001;537:971–978. doi: 10.1111/j.1469-7793.2001.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burelle Y, Hochachka PW. Endurance training induces muscle-specific changes in mitochondrial function in skinned muscle fibers. J Appl Physiol. 2002;92:2429–2438. doi: 10.1152/japplphysiol.01024.2001. [DOI] [PubMed] [Google Scholar]
- 27.Pesta D, Gnaiger E. High-resolution respirometry OXPHOS protocols for human cell cultures and permeabilized fibres from small biopsies of human muscle. In: Palmeira C, Moreno A, editors. Mitochondrial bioenergetics: methods and protocols. 2011. pp. 1–24. In Press. [Google Scholar]
- 28.Gnaiger E. Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int J Biochem Cell Biol. 2009;41:1837–1845. doi: 10.1016/j.biocel.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem. 2004;279:4127–4135. doi: 10.1074/jbc.M310341200. [DOI] [PubMed] [Google Scholar]
- 30.Seppet EK, Eimre M, Andrienko T, Kaambre T, Sikk P, Kuznetsov AV, Saks V. Studies of mitochondrial respiration in muscle cells in situ: use and misuse of experimental evidence in mathematical modelling. Mol Cell Biochem. 2004;256-257:219–227. doi: 10.1023/b:mcbi.0000009870.24814.1c. [DOI] [PubMed] [Google Scholar]
- 31.Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, Sackett DL. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc Natl Acad Sci U S A. 2008;105:18746–18751. doi: 10.1073/pnas.0806303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van der Poel C, Stephenson DG. Effects of elevated physiological temperatures on sarcoplasmic reticulum function in mechanically skinned muscle fibers of the rat. Am J Physiol Cell Physiol. 2007;293:C133–141. doi: 10.1152/ajpcell.00052.2007. [DOI] [PubMed] [Google Scholar]