Abstract
We reported previously that pre-programming mesenchymal stem cells with the GATA-4 gene increases significantly cell survival in an ischemic environment. In this study, we tested whether regulation of microRNAs and their target proteins was associated with the cytoprotective effects of GATA-4.
Methods and Results
Mesenchymal stem cells were harvested from adult rat bone marrow and transduced with GATA-4 (MSCGATA-4) using the murine stem cell virus retroviral expression system. Cells transfected with empty vector (MSCNull) were used as controls. Quantitative real-time PCR data showed that the expression levels of miR-15 family members (miR-15b, miR-16, and miR-195) were significantly down-regulated in MSCGATA-4. The protein expression of Bcl-w (Bcl-2-like-2), an anti-apoptotic Bcl-2 family protein, was increased in MSCGATA-4. Hypoxic culture (low glucose and low oxygen) induced the release of lactate dehydrogenase from mesenchymal stem cells and reduced cell survival. Compared to MSCNull, MSCGATA-4 showed less lactate dehydrogenase release and greater cell survival following 72 hour hypoxia exposure. The mitochondrial membrane potential, detected with the dye JC-1, was well maintained, and mitochondrial membrane permeability, expressed as caspase 3 and 7 activities in response to the ischemic environment was lower in MSCGATA-4. Moreover, transfection with miR-195 significantly down-regulated Bcl-w expression in mesenchymal stem cells through a binding site in the 3’-UTR of the Bcl-w mRNA and reduced mesenchymal stem cell resistance to ischemic injury.
Conclusions
The overexpression of GATA-4 in mesenchymal stem cells down-regulates miR-15 family members, causing increased resistance to ischemia through the up-regulation of anti-apoptotic proteins in the Bcl-2 family.
Keywords: miR-15 Family, Bcl-w, Mesenchymal Stem Cells, Cytoprotection, GATA-4
1. Introduction
Stem cell-based tissue repair has been suggested as a clinically translatable strategy for treatment after acute myocardial infarction (AMI). Cell therapy inhibits tissue degeneration in the acute phase following AMI (Assmus et al., 2010, Medicetty et al., 2012, Wollert et al., 2004, Yousef et al., 2009) and regenerates cardiac muscle in the failing heart (Ahmed et al., 2011, Beltrami et al., 2003, Boonbaichaiyapruck et al., 2010, Dawn et al., 2005, Taylor et al., 1998). Various cell types, including cardiac stem cells (Beltrami, Barlucchi, 2003, Dawn, Stein, 2005, Tokunaga et al., 2010), embryonic stem cells (Christoforou et al., 2010, Xu et al., 2002), skeletal myoblasts (Taylor, Atkins, 1998), inducible pluripotent stem cells (Gu et al., 2012, Kawamura et al., 2012), and bone marrow–derived mesenchymal stem cells (MSCs) (Quevedo et al., 2009, Uemura et al., 2006), are under current consideration for use as ischemic myocardium treatments. However, the poor survival of transplanted stem cells in the acidotic and ischemic microenvironment of the infarcted myocardium is the major impediment to clinical stem cell-based therapy.
To increase cell survival, stem cell preconditioning and reprogramming strategies have been developed. One emergent technology is genetic engineering of stem cells to express survival signaling molecules (Gnecchi et al., 2006, Li et al., 2010b, Li et al., 2007). We transduced the GATA-4 gene into MSCs and found that hearts transplanted with these MSCs showed the greater improvement in left ventricle function and reduction in infarct size. Further study indicated that the effect of GATA-4 was associated with the improved survival of the GATA-4-expressing MSCs and their offspring (Li et al. 2010b). The zinc finger transcription factor GATA-4 is an important regulator of heart development and an essential survival factor in postnatal cardiomyocytes (CM) (Kelley et al., 1993, Peterkin et al., 2005, Suzuki et al., 2004). Inhibition of GATA-4 DNA-binding activity or decrease of GATA-4 expression induces CM apoptosis and restoration of GATA-4 activity protects CM from anthracycline-induced apoptosis and cardiomyopathy (Kim et al., 2003, Li et al., 2006).
microRNAs (miRs) are noncoding, single-stranded RNAs of approximately 22 nucleotides that negatively regulate gene expression at the posttranscriptional level (Lim et al., 2005). miRs play critical roles in biological processes, including development, cell differentiation, proliferation, and apoptosis. The miR-15 family members (i.e., miR-15a, 15b, 16, 195, 427, and 497) are consistently up-regulated in mouse and human heart failure (Nishi et al., 2010, Topkara and Mann, 2011). The heart-specific overexpression of miR-195 results in cardiac dysfunction (van Rooij et al., 2006), suggesting that miR-15 family members may mediate the development of heart failure. Moreover, miR-15 family members are involved in different aspects of apoptosis (Guo et al., 2009, Yang et al., 2012). While GATA-4 can regulate miR expression (Zhang et al., 2010), it is unclear whether GATA-4-mediated cytoprotection is associated with the regulation of the miR-15 family in MSCs. In this study, we not only investigated the effects of GATA-4 on the expression of miRs in MSCs but also elucidated the roles of related miR-15 family members in MSC survival.
2. Methods
Experiments using animal subjects or animal-derived materials were performed in accordance with the guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication No. 85-23, Revised 1996). Protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
2.1. MSC Culture and Transduction with the GATA-4 Plasmid
Primary cultured MSCs were obtained from bone marrow cells flushed from femurs and tibias of euthanized male Sprague-Dawley (SD) rats (2~4 months) (Uemura et al., 2006). Cells were cultured in Iscove’s Modified Dulbecco’s Medium (IMDM) (Gibco) containing 15% fetal bovine serum (FBS) and penicillin (100 U/mL)/ streptomycin (100 μg/mL) at 37°C in humid air with 5% CO2. Medium was changed every 3 days. MSCs adhered to the bottom of culture plates following three medium changes.
A retrovirus expressing GATA-4 was constructed using a murine stem cell virus retroviral (pMSCV) expression system (Clontech). IRES-EGFP, which contains the internal ribosome entry site (IRES) of the encephalomyocarditis virus (ECMV) between the MCS and the EGFP (enhanced green fluorescent protein) coding region, was cloned into pMSCV vectors at XhoI and EcoRI sites. GATA-4 was excised from pcDNA-GATA-4 with HindIII and XhoI restriction enzymes and cloned into pMSCV-IRES-EGFP at BglII and SalI sites. GP2-293 cells (Clontech) were maintained in DMEM supplemented with 10% FBS and 2 mM glutamine and cotransfected with pMSCV-GATA-4-IRES-EGFP and pVSVG. Vector pMSCV-IRES-EGFP and pVSVG were cotransfected into GP2-293 cells as an empty vector control. After 48 hours, the medium containing retroviral particles were collected and filtered through 0.45 μm syringe filters. Second passage MSCs were selected for transduction with recombinant GATA-4 (Li et al., 2010b). MSCs were incubated with the supernatant obtained from GP2-293 cells for 12 hours in polybrene (10 μg/ml) (Sigma). The expression of GATA-4 in MSCs was verified by immunostaining and western blot after 5 days of selection with puromycin (3 μg/ml) (Sigma).
2.2. Microarray and Real Time PCR for miRs
Total RNA was extracted from MSCs using the mirVana™ miR isolation kit (Ambion) according to the manufacturer’s protocol. cDNAs corresponding to different miRs were synthesized using the miScript Reverse Transcription Kit (Qiagen).
RNA from 3 samples of MSCGATA-4, and 3 samples of MSCNull (each MSCGATA-4 and MSCNull was paired to transfection) was isolated for miR microarray analysis. Profiling of miR expression was analyzed by LC Sciences on their microarray platform Sanger miR-Base Release 15.0 (http://www.sanger.ac.uk/Software/Rfam/mirna/). miR profiles were directly read from Cy3 and Cy5 images of chips. In the Cy3 and Cy5 intensity images, as signal intensity increases from 1 to 65,535 the corresponding color changes from blue to green, to yellow, and to red. Differential expressions between the corresponding samples was obtained from ratio images.
Quantitative real-time PCR was carried out with miR-specific primers on an iQ5 real-time PCR system (Bio-Rad) with the miScript SYBR Green PCR Kit (Qiagen). U6 snRNA was used as an internal control. miR expression was calculated based on the threshold cycle (CT) as r = 2−Δ(ΔCT), where ΔCT = CT target − CT GAPDH and Δ(ΔCT) = ΔCT experimental − ΔCT control and modified with the specific efficiency of each primer.
2.3. miR Transfection
A lentiviral expression system was used to achieve the effective overexpression of the miRs in MSCs. A lentivector-based pre-miR 195-copGFP construct and scramble-copGFP control construct were purchased from System Biosciences (SBI). The expression vector or control vector and pPACK H1 packaging plasmid were co-transfected into 293TN cells using the PureFection transfection reagent (SBI). After 72 hours, the supernatant was collected and concentrated with PEG-it™ virus precipitation solution (SBI). The titers of the pseudo-viral particles were determined using the Global Ultra Rapid Lentiviral Titer Kit (SBI). Second passage MSCs at 50-70% confluence were infected with the generated viral particles at an MOI (multiplicity of infections) of 5-10. A transduction efficiency of 70-90% was achieved 72 hours later, as determined by the percentage of copGFP green fluorescence-positive cells.
2.4. Luciferase Assay
TargetScan was used to identify potential miR target sites. The miTarget™ dual luciferase reporter vector (pEZX-MT01) containing the full-length 3’ UTR sequence of the Bcl-2l2 (Bcl-w) mRNA (NM_021850.2) was purchased from GeneCopoeia. 293TN cells were seeded at a density of 5×104/well in 6-well plates 24 hours prior to transfection. Cells were then co-transfected with 1 μg of miR-195 expression plasmid (pre-miR-195) or negative control plasmid (pre-miR-NC) (SBI) and 1 μg of the dual luciferase reporter vector containing the full-length 3’UTR of Bcl-w using Lipofectamine 2000 (Invitrogen). After 48 hours, cells were lysed, and the luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega) on a luminometer (HIDEX, Plate CHAMELEON). The results were normalized and expressed as relative luciferase activity.
2.5. Immunocytochemistry
Cells cultured on glass coverslips were fixed in 4% paraformaldehyde and incubated with rabbit polyclonal anti-GATA-4 (Abcam). After thorough washing, cells were incubated with fluorescent-labeled secondary antibodies (Invitrogen). Nuclei were stained with 4′,6-diamino-2-phenylindole (DAPI). Images were obtained with an Olympus fluorescence microscope.
2.6. Western Blotting
Proteins were extracted using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Scientific) according to the supplier’s protocol and quantified with DC protein assay reagent (Bio-Rad). Denatured proteins (30 μg cytosolic protein for Bcl-w, 60 μg nuclear protein for GATA-4) were then analyzed using 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). After electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane. The membrane was incubated with the following primary antibodies: monoclonal rabbit anti-Bcl- w, or anti-β-actin (Cell Signaling), or polyclonal rabbit anti-GATA-4 (Santa Crus), at 4°C overnight. After thorough washing, the membrane was incubated with HRP conjugated goat anti-rabbit secondary antibody (Cell Signaling) and developed with the ECL Plus kit (GE Healthcare). Densitometric analysis of the blots was performed with FluoChem SP software (Alpha Innotech).
2.7. Cell Ischemic Injury
An in vitro hypoxic model (1% oxygen) was used to mimic in vivo ischemic injury. The medium was replaced with low glucose (1 g/L) MEM and MSCs were placed into a hypoxic chamber (CO2/O2 incubator, MCO-18M, Sanyo) with 1% O2, 5% CO2, and 94% N2. Cell injury was evaluated based on the release of lactate dehydrogenase (LDH) measured with a commercially available kit (Promega). The number of surviving cells was estimated using a tetrazolium compound, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS), in the CellTiter 96® AQueous One Solution Cell Proliferation Assay kit (Promega).
Mitochondrial membrane permeability was assessed by the activity of caspases 3 and 7 with the Caspase-Glo® 3/7 Assay Kit (Promega). Mitochondrial membrane potential (ΔΨm) was monitored with 5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethyl-benzamidazolocarbocyanin iodide (JC-1). In brief, MSCs were incubated with JC-1 (5 μmol) at 37°C for 15 min. JC-1 monomer (green) fluorescence was measured using excitation at 488 nm and emission from 505 to 530 nm. JC-1 aggregate (red) fluorescence was detected using excitation at 543 nm and emission at wavelengths over 560 nm. Images were taken using an Olympus fluorescence microscope. The intensities of both fluorescence ranges were read using a microplate M3 spectrophotometer (Molecular Devices). The ratio of hyper- (red) to hypo- (green) polarized mitochondria in MSCs was interpreted as ΔΨm. A decrease in the ratio was interpreted as loss of ΔΨm (Troyan et al., 1997).
2.8. Statistical Analysis
Group data were presented as the mean ± SEM. All data were obtained from at least three independent experiments and analyzed using GraphPad Prism (GraphPad Software). Differences between groups were evaluated by Student’s t-test or one-way ANOVA with Bonferroni post hoc test and/or Holm-Sidak method. Differences were considered significant at probability values <0.05.
3. Results
3.1. Transduction of GATA-4 into MSCs
There was no cellular morphology difference between MSCGATA-4 and MSCNull. Both MSCNull and MSCGATA-4 were GFP positive, although not all MSCs expressed GFP (Figure 1A, 1B, white arrows) prior to puromycin selection. GATA-4 was expressed only in MSCGATA-4, which was consistent with the expression of the GFP marker (Figure 1B). After puromycin selection, all MSCGATA-4 were both GFP and GATA-4 immunopositive (Figure 1C). Semi-quantitative data obtained from western blot analyses showed that the GATA-4 protein in MSCGATA-4 was approximately at 10-fold higher than that in MSCNull (Figure 1D).
Figure 1.
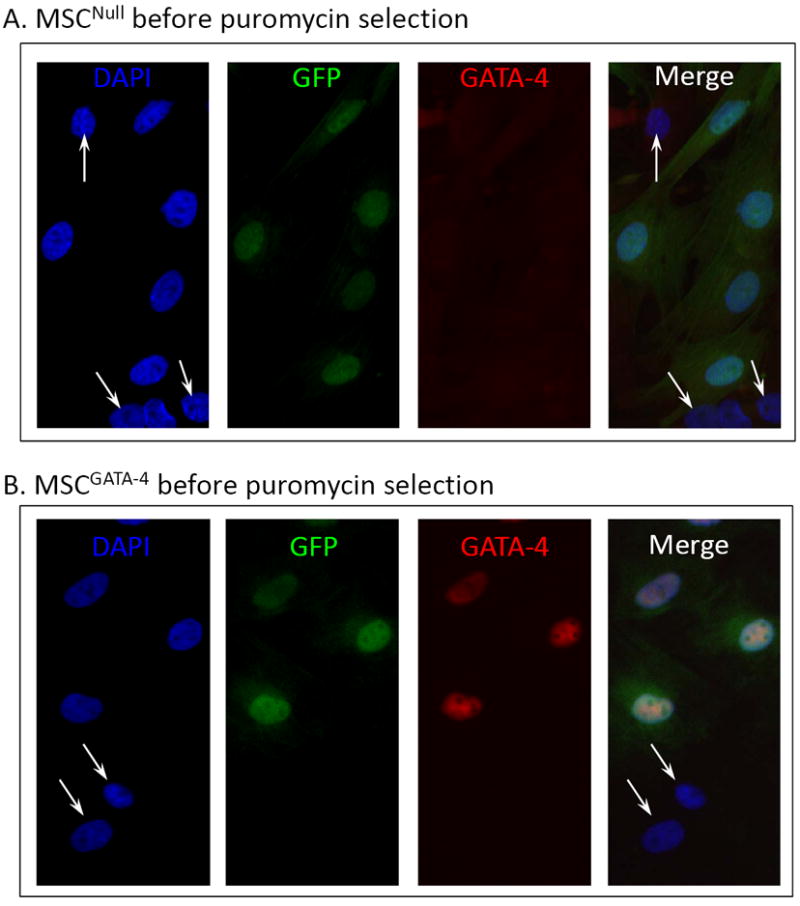
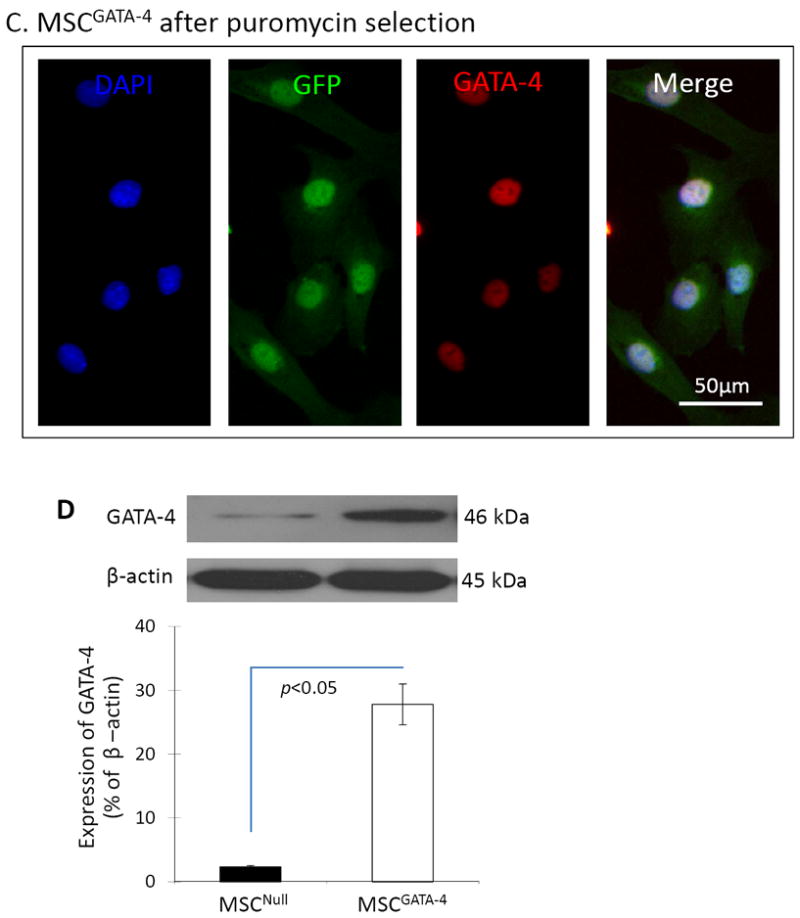
Characterization of MSCGATA-4. Panels A and B: Immunostaining of MSCNull (A) and MSCGATA-4 (B) prior to puromycin selection. GFP was expressed in both MSCNull and MSCGATA-4, but GATA-4 was only expressed in MSCGATA-4, which is consistent with the GFP expression. A subset of MSCs expressed neither GFP nor GATA-4 (white arrows). Panel C: All MSCGATA-4 were both GFP- and GATA-4-positive after puromycin selection. Panel D: Western blot of GATA-4 in MSCGATA-4 and MSCNull, and corresponding semi-quantitative data.
3.2. MSC Culture under Hypoxic Conditions
Basal MSCs (MSCbas) were exposed to hypoxia for 24 ~ 96 hours to identify the time courses of hypoxia-induced injury. Following culture under hypoxic conditions for 24 hours, MSCs exhibited a less density but no significant changes in cell morphology compared with cells in normoxic conditions. However, cells became shrunken, lost their normal structures, and dead cells (red arrows) appeared after they exposed to hypoxic condition longer than 48 hours (Figure 2A). The number of viable MSCs calculated using MTS uptake, was significantly reduced after culture under hypoxic conditions (Figure 2B). The cell numbers after 24, 48, 72, and 96 hours under hypoxic conditions were approximately 73%, 48%, 42%, and 35% of that in normoxic culture, respectively. The concentration of LDH in culture medium was increased significantly in MSCs exposed to hypoxic culture (Figure 2C). To exclude any potential effects of cell proliferation, we calculated the LDH released from hypoxic MSCs (104 cells) and found that it was increased significantly compared to control MSCs exposed to normoxia (Figure 2D). The LDH release from cells at 24, 48, 72, and 96 hours of culture in hypoxic condition was approximately 179%, 334%, 382%, and 463% of that in normoxic culture, respectively. Taken together, these results indicated that MSCs started to be injured at 24 hours of exposure to hypoxia and suffered severe injury beginning at 48 hours, and that most cell death was induced when cells were exposed to hypoxia for longer than 72 hours.
Figure 2.
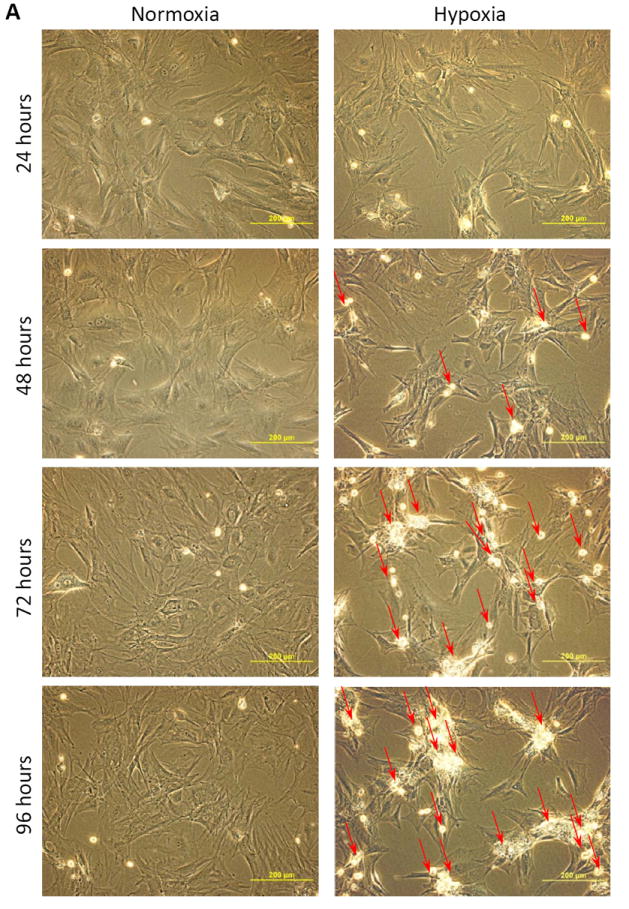
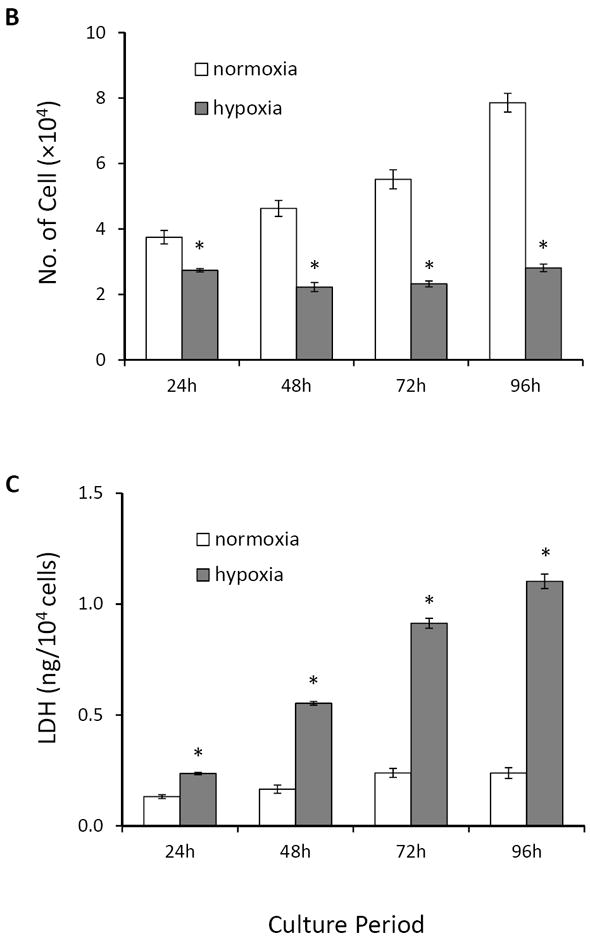
MSCbas injury after exposure to hypoxia. Panel A. Morphological changes in MSCs. Very few dead or dying cells (bright dots) were observed after normoxic culture for 24 ~ 96 hours. The number of dying cells (red arrows) increased with exposure to the hypoxic environment. Panel B: The number of MSCbas in cultures under normoxic and hypoxic conditions for 24 ~ 96 hours. Panel C: LDH concentration in culture medium. Panel D: LDH released from MSCs per 1×104 cells under normoxic and hypoxic conditions. *, p<0.05 vs normoxic culture, respectively.
3.3. GATA-4 Overexpression Increases MSC Survival and Maintains Mitochondrial Membrane Potential and Integrity
To investigate the cytoprotective effect of GATA-4, morphological changes, cell number, and LDH release were compared between MSCGATA-4 and MSCNull after exposure to 72 hours of hypoxia. MSCGATA-4 showed fewer dying cells (bright cells) compared to MSCNull. The number of surviving cells was significantly higher in MSCGATA-4 (83.9 ± 5.1% of normoxic control) than in MSCNull (64.1 ± 7.5% of normoxic control, p<0.05) (Figure 3B). LDH release from MSCGATA-4 was significantly lower than that in MSCNull (Figure 3C). However, there was no significant difference between MSCNull and MSCbas. These data suggest that engineering stem cells to express GATA-4 increased MSC tolerance to ischemic injury.
Figure 3.
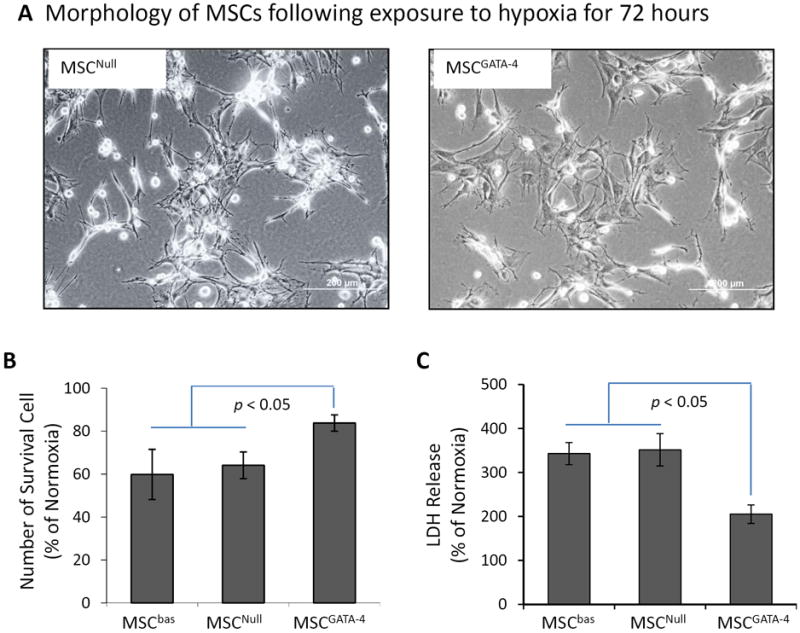
The injury of MSCGATA-4 and MSCNull subjected to hypoxic culture for 72 hours. Panel A. Morphology of MSCGATA-4 and MSCNull. The bright cells are damaged MSCs. Panel B: Number of surviving cells evaluated by MTS uptake. Panel C: LDH released from different MSCs. The data are expressed as the percentage of normal culture.
Mitochondrial dysfunction was observed before cell severe injury occurred in MSCs exposed to the hypoxic environment. Therefore, mitochondrial membrane potential and permeability were evaluated at 48 hours. The ΔΨm was assayed by a unique fluorescent cationic dye, JC-1. Cells exhibited a heterogeneous distribution of hypo- (green fluorescence of monomer) and hyper- (red fluorescence of J-aggregate) polarized mitochondria when JC-1 was loaded. Green fluorescent mitochondria (hypo-polarized) were localized near the nucleus, whereas the red fluorescent mitochondria (hyper-polarized) were confined to the cell periphery (Figure 4A). The ratio of JC-1 J-aggregate (red) to monomer (green) fluorescence was 0.997 ± 0.066 in MSCNull and 0.950 ± 0.083 in MSCGATA-4 under normoxic culture. After exposure to hypoxia for 48 hours, the JC-1 ratio was reduced (ΔΨm decreased). However, the JC-1 ratio in MSCGATA-4 (0.643 ± 0.043) was significantly higher than that in MSCNull (0.427 ± 0.025). The activity of caspase 3/7 in MSC under normal culture condition was 517.0 ± 19.1 (Intensity of Luminescence) without significant difference between MSCGATA-4 and MSCNull. Caspase 3/7 activity was significantly increased in MSCs after exposure to hypoxia for 48 hours. However, caspase 3/7 activity was significantly lower in MSCGATA-4 than that in MSCNull (Figure 4C). These results indicate that the cytoprotective effect of GATA-4 may be associated with the maintenance of mitochondrial membrane stability.
Figure 4.
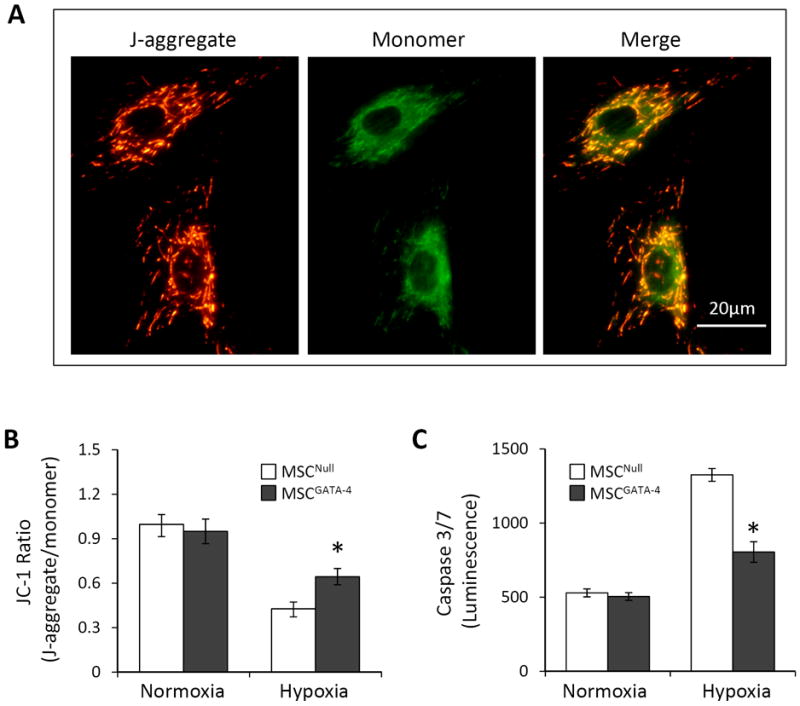
Mitochondrial membrane potential (ΔΨm) and the activity of caspases 3/7 in MSCGATA-4 and MSCNull following exposure to hypoxia for 48 hours. Panel A: Representative JC-1 fluorescence imaging of mitochondria. Green and red fluorescence indicate depolarized (monomeric form of JC-1) and hyperpolarized (J-aggregate form of JC-1) mitochondria, respectively. Panel B: Quantification of ΔΨm expressed as the ratio of J-aggregate to monomer fluorescence. Panel C: Caspases 3/7 activity expressed as luminescence. *, p<0.05 vs MSCNull, respectively.
3.4. GATA-4 Downregulates miR-15 Family Members and Increases Bcl-w in MSC
Increasing evidence indicates that miRs are among the most important, even pivotal, factors controlling cell survival-related gene expression. We assessed miR expression using comparative miR arrays to elucidate whether the cytoprotective effect of GATA-4 is associated with the regulation of miRs. The results revealed that a number of miRs were differently modulated in MSCGATA-4. Notably, the expression levels of miR-15 family members (miR-15b, miR-16, and miR-195) were consistently lower in MSCGATA-4 than in MSCNull (Figure 5A). The changes in the expression levels of these miRs were further validated using quantitative real-time PCR. The expression of miR-15 family was significantly down-regulated in MSCGATA-4 compared to that in MSCNull, irrespective of MSCs exposure to hypoxia (Figure 5B1 and B2). The expression of miR-15 family members were significantly up-regulated in MSCbas exposed to hypoxia for 72 hours comparing to the cells cultured in normoxia (Figure 5B3).
Figure 5.
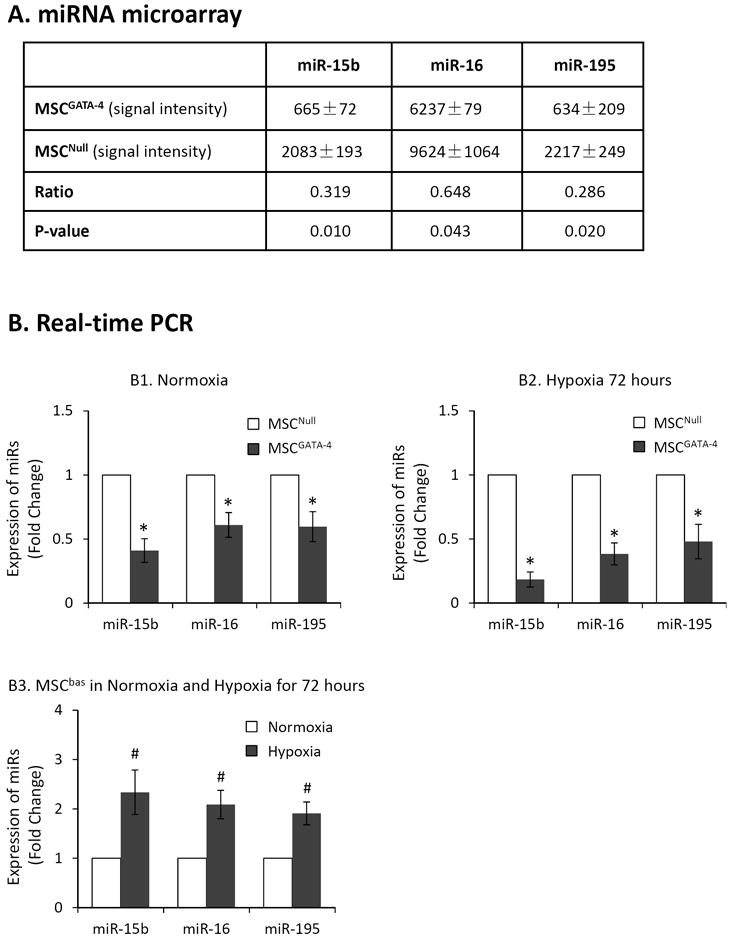
The expression of miR-15 family members in MSCGATA-4 and MSCNull evaluated by using miR microarray and quantitative real-time PCR. Panel A: miR-microarray data expressed as signal intensity. Panel B: real-time PCR expressed as fold change. MSCGATA-4 and MSCNull were cultured under normoxia (B1) and hypoxia (B2), respectively. B3: Comparison the expression of miR-15 family members in MSCbas under normal and hypoxic condition for 72 hours. *, p<0.05 vs MSCNull. #, p<0.05 vs normal culture, respectively.
We identified Bcl-w as a target protein of the miR-15 family using TargetScan (Figure 6A). The 3′-UTR of the Bcl-w gene is perfectly complementary to the seed region of miR-195, miR-15b, and miR-16 at positions 1105 – 1111, 1370 – 1376, and 1892 – 1898. To confirm the effects of miR-15 family members on the 3’UTR of Bcl-w, one member of the miR-15 family, miR-195, was selected for transfection into 293TN cells. The pre-miR-195 vector and Bcl-w 3’UTR luciferase reporter vector were transiently transfected into 293TN cells. Transfection with miR-195 resulted in a significant inhibition of the basal level of Bcl-w related luciferase activity compared to transfection with negative control miR (miR-NC) (Figure 6B, 6C). These data indicated that the miR-15 family members have highly conserved binding sites in the 3′-UTR region of Bcl-w. We evaluated Bcl-w protein expression in MSCs to investigate whether Bcl-w plays a role in GATA-4-mediated cytoprotection. Bcl-w protein expression in MSCGATA-4 was significantly higher than that in MSCNull (Figure 6D). Although the expression of Bcl-w in MSCs was decreased significantly following 72 hours hypoxia exposure (Figure 6E), the decrease of Bcl-w in MSCGATA-4 was less than that in MSCNull. After 72 hours hypoxia exposure, the level of Bcl-w was 47.5% in MSCGATA-4 and 32.6 % in MSCNull, compared to the MSCs cultured in normal condition, respectively. We then introduced pre-miR-195-copGFP and pre-miR-NC-copGFP into MSCGATA-4 to ascertain whether Bcl-w expression in MSCs was regulated by miR-15 family members. Western blot analyses indicated that MSCGATA-4 transfection with miR-195 (MSCGATA-4-miR-195) decreased significantly Bcl-w protein levels compared with those in MSCGATA-4 transfected with miR-NC (MSCGATA-4-miR-NC) (Figure 6F). Bcl-w protein expression in MSCGATA-4-miR-195 was only 67% of that in MSCGATA-4-miR-NC. This result indicates that the up-regulation of Bcl-w in MSCGATA-4 is partially related to the down-regulation of miR-15 family members.
Figure 6.
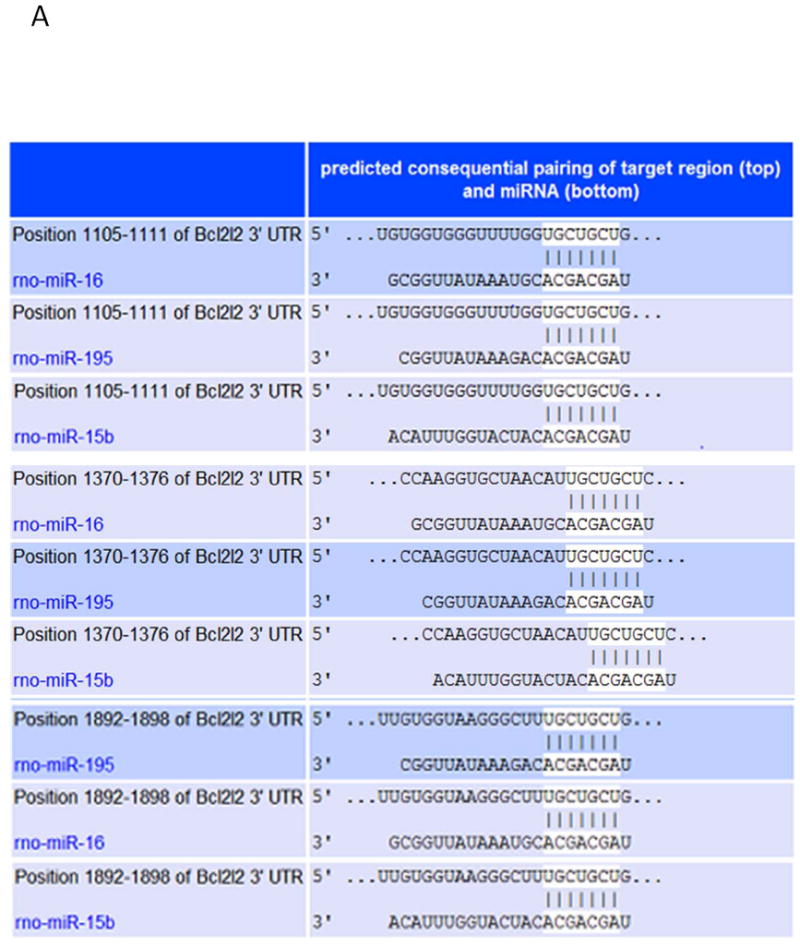
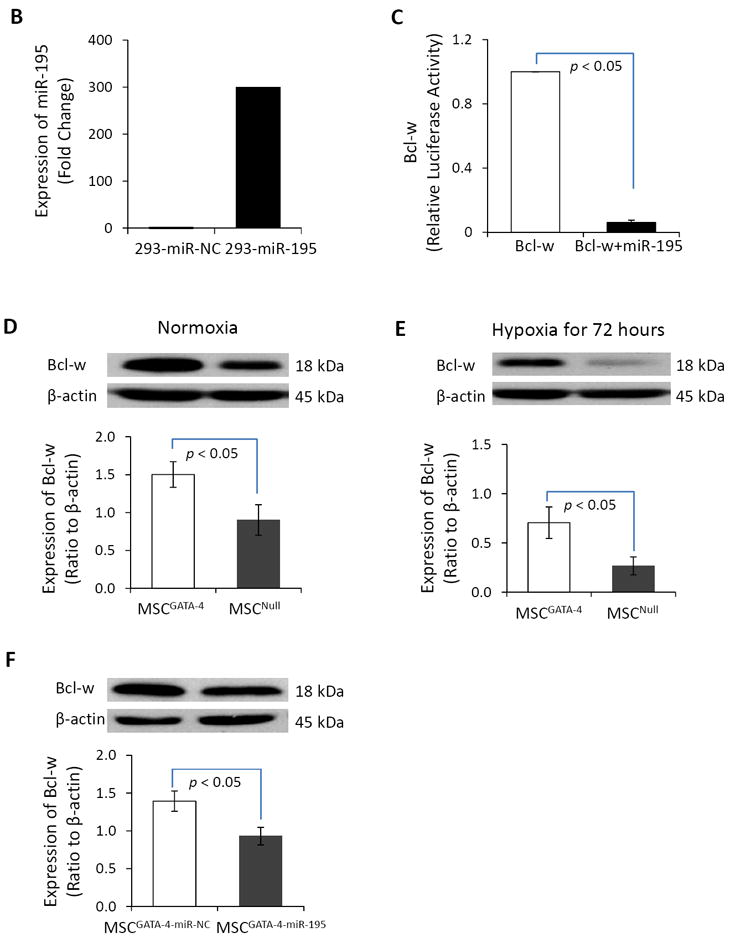
The miR-15 family regulates the expression of Bcl-w in MSC. Panel A. TargetScan shows that 3’ UTR of Bcl-w contains conserved miR-15 family binding sites. Panel B. The expression of miR-195 in the cells of 293TN transfected with miR-195 and miR-NC. Panel B: Relative luciferase activity of Bcl-w in 293TN cells after transfection with miR-195 and miR-NC, respectively. Panels D and E: Bcl-w protein in MSCGATA-4 and MSCNull, as measured by semi-quantitative western blot in normal (D) and hypoxic (E) conditions for 72 hours. Panel F: Bcl-w protein expression in MSCGATA-4 transfected with miR-195 or miR-NC.
3.5. miR-195 Reduces the Resistance of MSCs to Ischemic Injury
We transfected MSCs with miR-195 (MSCmiR-195) and cultured these cells under hypoxic conditions to further investigate whether GATA-4-mediated cytoprotection was associated with down-regulation of miR-15 family members. The morphology of MSCmiR-195 was similar to that of MSCs transfected with miR-NC (MSCmiR-NC) (Figure 7A). However, after cells were 72 hours hypoxia exposure, the cell number was decreased significantly in MSCmiR-195 compared to MSCmiR-NC (Figure 7B). LDH release from MSCmiR-195 was significantly higher than that from MSCmiR-NC (Figure 7C). Moreover, caspase 3/7 activity was significantly higher in MSCmiR-195 (Figure 7D) and ΔΨm of MSCmiR-195 (0.508 ± 0.043) was significantly lower than MSCmiR-NC (0.641 ± 0.029) (Figure 7E) after the cells were exposed to hypoxia for 48 hours. These data suggested that the overexpression of miR-195 reduced MSC resistance to hypoxic injury.
Figure 7.
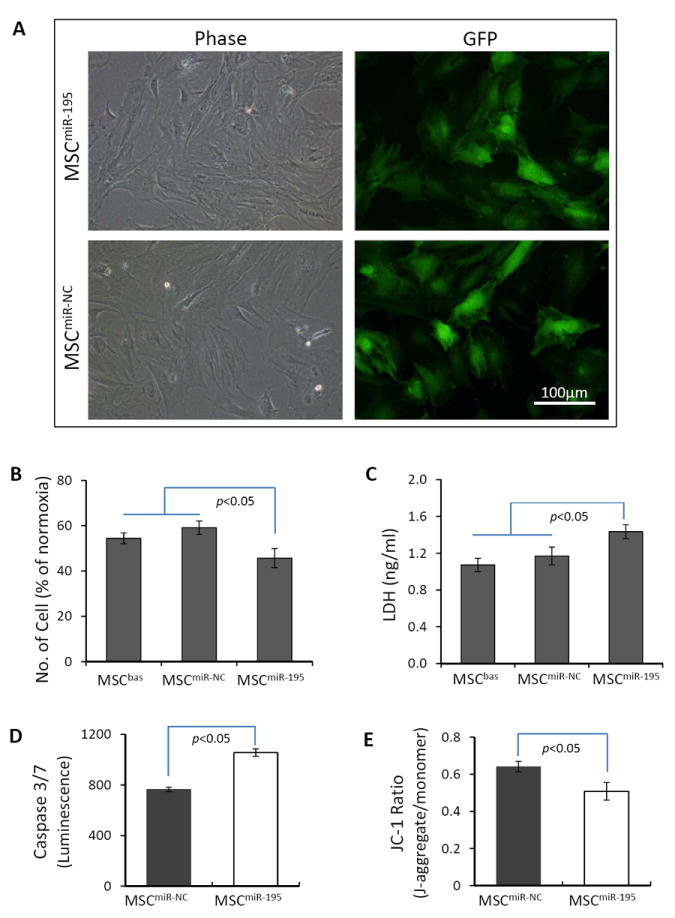
The overexpression of miR-195 reduces MSC resistance to hypoxic injury. Panel A: The morphology of MSCs transfected with miR-195 and with miR-NC under normoxic culture conditions. Panels B and C: Survived cell number (B) and LDH release (C) from MSCbas, MSCmiR-195, and MSCmiR-NC after exposure to hypoxia for 72 hours. Panels D and E: Activity of caspase 3/7 (D) and ΔΨm (E) in MSCmiR-195 and MSCmiR-NC after exposure to hypoxia for 48 hours.
4. Discussion
This study reveals that GATA-4 mediated cytoprotection involves the regulation of miR-15 family member expression. This study reports three key findings: 1) GATA-4 overexpression increases the tolerance of MSCs to hypoxic injury and preserves mitochondrial membrane potential and integrity; 2) GATA-4 regulates the expression of many miRs that are related to cell survival in MSCs, specifically, down-regulating miR-15 family members; 3) The miR-15 family members regulate the anti-apoptotic protein Bcl-w, which is responsible for GATA-4 mediated cytoprotection. The present study opens a new avenue for promoting stem cell survival in an ischemic microenvironment through genetic engineering and regulating miRs.
4.1. GATA-4 increases MSC survival and preserves mitochondria
Bone marrow is an easily accessible source of autologous adult stem cells. Clinical studies have shown that patients exhibited a significant improvement in the global left ventricular ejection fraction after treatment with autologous bone marrow stem cells (Assmus, Rolf, 2010, Boonbaichaiyapruck, Pienvichit, 2010, Medicetty, Wiktor, 2012, Wollert, Meyer, 2004, Yousef, Schannwell, 2009). Stem cell loss due to the hostility of the host-tissue microenvironment has the potential to diminish the overall efficacy of cell therapy.
MSC injury was mostly depended on the period which cells were exposed to hypoxia. Cells were no severe damage or only slight damage when they exposed to very brief hypoxia (e.g. 24 hours). Apoptotic cells showed up when cells were exposed to hypoxia for 48 hours. Cells went necrosis and dying when MSC were exposed to hypoxia for 72 hours. Therefore, we selected 48 hours as a time point to observe the mitochondrial membrane potential and permeability, and 72 hours as a time point to investigate the cell survival. The present study suggests that overexpression of GATA-4 not only can increase MSC survival but also can preserve mitochondria in ischemic environments. It is recognized that suppression of GATA-4 activity induces damage in cardiomyocytes and that restoration or increase of GATA activity can attenuate apoptosis (Aries et al., 2004, Kim, Ma, 2003, Li, Takemura, 2006, Shan et al., 2009). Hypoxia-induced apoptosis and cell injury often depend on the activation of proapoptotic mitochondrial pathways, which promote the depolarization of ΔΨm and mitochondrial outer membrane permeabilization (MOMP). ΔΨm is an important parameter of mitochondrial membrane barrier function. The loss of ΔΨm results in the release of mitochondrial apoptogenic factors and caspase-9, which activates mitochondrial apoptosis pathways. A reduction in ΔΨm increases the likelihood of MOMP and induces the release of mitochondrial factors including cytochrome c. Cytosolic cytochrome c triggers caspase cascades to initiate apoptosis. In this study, we measured the activities of caspase 3 and 7, the key executioner caspases in the apoptotic program, to evaluate MOMP in MSCs and used JC-1 to assess ΔΨm of MSCs. Our results indicate that overexpression of GATA-4 maintains ΔΨm and reduces mitochondrial membrane permeabilization.
4.2. GATA-4 regulates the proteins of Bcl-2 family
Bcl-2 family proteins are the key regulators of mitochondria-dependent apoptosis in nucleated cells. It is well known that the Bcl-2 family includes both antiapoptotic (e.g., Bcl-XL, Bcl-2, Bcl-w, A1, Mcl-1) and proapoptotic (e.g., Bak, Bax, Bid, Bim, Bad, Bik, Bmf, Noxa, PUMA) members. Bcl-2, Bcl-xL, and Bcl-w promote cell survival, while Bax and Bak facilitate cell death. The up-regulation of anti-apoptotic members of Bcl-2 family (Gibson et al., 1996, Tran et al., 2005) and/or the loss of the pro-apoptotic members (Lindsten et al., 2000) increase cells resistant to many apoptotic stimuli. The overexpression of Bcl-2 prevents a cytokine-induced decrease in cell viability, and the ΔΨm of these cells is increased significantly (Barbu et al., 2002). Several mechanisms have been proposed to explain how Bcl-2 might increase ΔΨm, for example, by directly or indirectly enhancing proton efflux (Shimizu et al., 1998) or by inhibiting the endogenous activity of the permeability transition pore (Dispersyn et al., 1999, Kowaltowski et al., 2000). The critical role of GATA-4 as a survival factor may be explained in part by its function as an upstream activator of the Bcl-2 gene family (Aries, Paradis, 2004, Kobayashi et al., 2006). Gain- and loss-of-function approaches suggest that Bcl-2 and Bcl-xL are potential GATA-4 target genes (Aries, Paradis, 2004, Kitta et al., 2003, Li, Zuo, 2010b). In this study, we demonstrated that the expression of Bcl-w was increased significantly in MSCs transduced with GATA-4. Bcl-w overexpression protects cells against apoptosis induced by cytokine withdrawal or drug treatment (Gibson, Holmgreen, 1996). GATA-4 not only up-regulates anti-apoptotic members of the Bcl-2 family but also down-regulates pro-apoptotic members of the Bcl-2 family. We found that the expression of P53 up-regulated modulator of apoptosis (PUMA) was significantly lower in MSCs transduced with GATA-4 than in MSCs transduced with vector control (unpublished data).
4.3. miRs play an important role in the GATA-4 mediated regulation of Bcl-2 family members
The expression of Bcl-2 family proteins in MSCs may be regulated by various miRs. Data obtained from miR microarray and real-time PCR assays indicated that GATA-4 regulates the expression of many miRs in MSCs. The expression of miR-15 family members was down-regulated in MSCGATA-4. Our study indicates that the overexpression of miR-195 in MSCs reduces the resistance of these cells to hypoxia; MSC survival and ΔΨm was reduced, and the activity of caspases 3/7 increased, consistent with previous reports demonstrating that the overexpression of miR-15 family members enhances apoptosis (Chung et al., 2010, Guo, Pan, 2009).
The miR-15 family members (i.e., miR-15a, 15b, 16, 195, 427, and 497) possess the same seed sequence and have the same target genes (Nishi, Ono, 2010). TargetScan results indicate that Bcl-w is one of the target proteins of the miR-15 family. We employed 293TN cells to investigate the effects of one member of miR-15 family, miR-195 on the regulation of Bcl-w. Our work reveals that miR-195 directly down-regulates Bcl-w expression through binding sites in the 3’-UTR of the Bcl-w mRNA, thereby modulating the susceptibility of cells to oxidative stress-induced apoptosis. miR-15 family members increase cell apoptosis at least in part by targeting Bcl-w (Chung, Yoon, 2010, Yang, Yin, 2012). The down-regulation of miR-15 family members was shown to increase Bcl-2 expression, and transfection with miR-15 family members reduced Bcl-2 protein levels (Guo, Pan, 2009, Li et al., 2010a). These results indicated that miR-15 family members participate in a miR–gene regulatory network that is likely essential for apoptosis by targeting Bcl-w. Bcl-w is well acknowledged as a critical regulator of the mitochondrial pathway, diminishing cytochrome c release, which leads to the inhibition of apoptosis (Murphy et al., 2007, Rodust et al., 2012, Yao et al., 2007). In addition to its effects on Bcl-w, miR-15 family may also play a pro-apoptotic role through the regulation of Arl2 (Nishi, Ono, 2010) or Sirt1 (Zhu et al., 2011).
The cytoprotective effect of GATA-4 is associated with its regulation of the expression of not only the miR-15 family but also other miRs. We have also found that the overexpression of GATA-4 significantly up-regulated miR-221/222 in MSCs (unpublished data). The transfer of pre-miR-221 into MSCs significantly increased their survival under hypoxic conditions. Others have reported that GATA-4 regulates the miR-144/451 cluster expression, both miR-144 and miR-451 protect against simulated ischemia/reperfusion-induced cell death (Zhang, Wang, 2010). Taken together, these data show that GATA-4 overexpression regulates many miRs, which results in the up-regulation of anti-apoptotic proteins and the down-regulation of pro-apoptotic proteins. Therefore, the transduction of GATA-4 into MSCs can significantly increase MSC survival in an ischemic microenvironment. However, it is unclear how GATA-4 regulates the expression of various miRs. Fan et al. (Zhang, Wang, 2010) suggested that some miRs, e.g., miR-144 and miR-451, share a GATA-4 target promoter and are processed from a single polycistronic precursor transcript.
We have suggested earlier that paracrine factors are responsible for the increased resistance of cells mediated by GATA-4 against ischemic injury (Li, Zuo, 2010b), which are consistent with those previously reported by Kawaguchi et al. (2010) where the concentration of IGF-1 was increased significantly in the c-kitpos GATA-4 high cardiac stem cells/cardiomyocyte co-cultures. There was a positive correlation between IGF-1 concentration and cardiomyocyte survival. Further study is required to illustrate whether these miRs related to GATA-4 regulate the secretion of paracrine factors.
The results obtained in the present study demonstrate that GATA-4 transduction increased MSC survival in an ischemic environment by stabilizing ΔΨm and reducing MOMP, which may be associated with the regulation of the expression of many miRs, especially the down-regulation of miR-15 family members, resulting in increased levels of anti-apoptotic Bcl-2 family proteins. In conclusion, the cytoprotective effect of GATA-4 is associated with the maintenance or increase of anti-apoptotic Bcl-w in MSCs via the regulation of miR-15 family member expression.
Acknowledgments
This work was supported by National Institutes of Health grants HL105176 and HL114654 (M. Xu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed RP, Haider HK, Buccini S, Li L, Jiang S, Ashraf M. Reprogramming of skeletal myoblasts for induction of pluripotency for tumor-free cardiomyogenesis in the infarcted heart. Circulation research. 2011;109:60–70. doi: 10.1161/CIRCRESAHA.110.240010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aries A, Paradis P, Lefebvre C, Schwartz RJ, Nemer M. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6975–80. doi: 10.1073/pnas.0401833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmus B, Rolf A, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, et al. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circulation Heart failure. 2010;3:89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]
- Barbu A, Welsh N, Saldeen J. Cytokine-induced apoptosis and necrosis are preceded by disruption of the mitochondrial membrane potential (Deltapsi(m)) in pancreatic RINm5F cells: prevention by Bcl-2. Mol Cell Endocrinol. 2002;190:75–82. doi: 10.1016/s0303-7207(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- Boonbaichaiyapruck S, Pienvichit P, Limpijarnkij T, Rerkpattanapipat P, Pongpatananurak A, Saelee R, et al. Transcoronary infusion of bone marrow derived multipotent stem cells to preserve left ventricular geometry and function after myocardial infarction. Clinical cardiology. 2010;33:E10–5. doi: 10.1002/clc.20545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforou N, Oskouei BN, Esteso P, Hill CM, Zimmet JM, Bian W, et al. Implantation of mouse embryonic stem cell-derived cardiac progenitor cells preserves function of infarcted murine hearts. PloS one. 2010;5:e11536. doi: 10.1371/journal.pone.0011536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung GE, Yoon JH, Myung SJ, Lee JH, Lee SH, Lee SM, et al. High expression of microRNA-15b predicts a low risk of tumor recurrence following curative resection of hepatocellular carcinoma. Oncology reports. 2010;23:113–9. [PubMed] [Google Scholar]
- Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3766–71. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dispersyn G, Nuydens R, Connors R, Borgers M, Geerts H. Bcl-2 protects against FCCP-induced apoptosis and mitochondrial membrane potential depolarization in PC12 cells. Biochim Biophys Acta. 1999;1428:357–71. doi: 10.1016/s0304-4165(99)00073-2. [DOI] [PubMed] [Google Scholar]
- Gibson L, Holmgreen SP, Huang DC, Bernard O, Copeland NG, Jenkins NA, et al. bcl-w, a novel member of the bcl-2 family, promotes cell survival. Oncogene. 1996;13:665–75. [PubMed] [Google Scholar]
- Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb J. 2006;20:661–9. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- Gu M, Nguyen PK, Lee AS, Xu D, Hu S, Plews JR, et al. Microfluidic single-cell analysis shows that porcine induced pluripotent stem cell-derived endothelial cells improve myocardial function by paracrine activation. Circulation research. 2012;111:882–93. doi: 10.1161/CIRCRESAHA.112.269001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CJ, Pan Q, Li DG, Sun H, Liu BW. miR-15b and miR-16 are implicated in activation of the rat hepatic stellate cell: An essential role for apoptosis. J Hepatol. 2009;50:766–78. doi: 10.1016/j.jhep.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126:S29–37. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- Kelley C, Blumberg H, Zon LI, Evans T. GATA-4 is a novel transcription factor expressed in endocardium of the developing heart. Development. 1993;118:817–27. doi: 10.1242/dev.118.3.817. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ma AG, Kitta K, Fitch SN, Ikeda T, Ihara Y, et al. Anthracycline-induced suppression of GATA-4 transcription factor: implication in the regulation of cardiac myocyte apoptosis. Mol Pharmacol. 2003;63:368–77. doi: 10.1124/mol.63.2.368. [DOI] [PubMed] [Google Scholar]
- Kitta K, Day RM, Kim Y, Torregroza I, Evans T, Suzuki YJ. Hepatocyte growth factor induces GATA-4 phosphorylation and cell survival in cardiac muscle cells. The Journal of biological chemistry. 2003;278:4705–12. doi: 10.1074/jbc.M211616200. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Lackey T, Huang Y, Bisping E, Pu WT, Boxer LM, et al. Transcription factor gata4 regulates cardiac BCL2 gene expression in vitro and in vivo. Faseb J. 2006;20:800–2. doi: 10.1096/fj.05-5426fje. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Smaili SS, Russell JT, Fiskum G. Elevation of resting mitochondrial membrane potential of neural cells by cyclosporin A, BAPTA-AM, and bcl-2. Am J Physiol Cell Physiol. 2000;279:C852–9. doi: 10.1152/ajpcell.2000.279.3.C852. [DOI] [PubMed] [Google Scholar]
- Li G, Miskimen KL, Wang Z, Xie XY, Brenzovich J, Ryan JJ, et al. STAT5 requires the N-domain for suppression of miR15/16, induction of bcl-2, and survival signaling in myeloproliferative disease. Blood. 2010a;115:1416–24. doi: 10.1182/blood-2009-07-234963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zuo S, He Z, Yang Y, Pasha Z, Wang Y, et al. Paracrine factors released by GATA-4 overexpressed mesenchymal stem cells increase angiogenesis and cell survival. Am J Physiol Heart Circ Physiol. 2010b;299:H1772–81. doi: 10.1152/ajpheart.00557.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Takemura G, Li Y, Miyata S, Esaki M, Okada H, et al. Preventive effect of erythropoietin on cardiac dysfunction in doxorubicin-induced cardiomyopathy. Circulation. 2006;113:535–43. doi: 10.1161/CIRCULATIONAHA.105.568402. [DOI] [PubMed] [Google Scholar]
- Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem cells. 2007;25:2118–27. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–73. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Molecular cell. 2000;6:1389–99. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicetty S, Wiktor D, Lehman N, Raber A, Popovic ZB, Deans R, et al. Percutaneous adventitial delivery of allogeneic bone marrow-derived stem cells via infarct-related artery improves long-term ventricular function in acute myocardial infarction. Cell transplantation. 2012;21:1109–20. doi: 10.3727/096368911X603657. [DOI] [PubMed] [Google Scholar]
- Murphy B, Dunleavy M, Shinoda S, Schindler C, Meller R, Bellver-Estelles C, et al. Bcl-w protects hippocampus during experimental status epilepticus. The American journal of pathology. 2007;171:1258–68. doi: 10.2353/ajpath.2007.070269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi H, Ono K, Iwanaga Y, Horie T, Nagao K, Takemura G, et al. MicroRNA-15b modulates cellular ATP levels and degenerates mitochondria via Arl2 in neonatal rat cardiac myocytes. The Journal of biological chemistry. 2010;285:4920–30. doi: 10.1074/jbc.M109.082610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkin T, Gibson A, Loose M, Patient R. The roles of GATA-4, -5 and -6 in vertebrate heart development. Seminars in cell & developmental biology. 2005;16:83–94. doi: 10.1016/j.semcdb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14022–7. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodust PM, Fecker LF, Stockfleth E, Eberle J. Activation of mitochondrial apoptosis pathways in cutaneous squamous cell carcinoma cells by diclofenac/hyaluronic acid is related to upregulation of Bad as well as downregulation of Mcl-1 and Bcl-w. Exp Dermatol. 2012;21:520–5. doi: 10.1111/j.1600-0625.2012.01516.x. [DOI] [PubMed] [Google Scholar]
- Shan X, Xu X, Cao B, Wang Y, Guo L, Zhu Q, et al. Transcription factor GATA-4 is involved in erythropoietin-induced cardioprotection against myocardial ischemia/reperfusion injury. International journal of cardiology. 2009;134:384–92. doi: 10.1016/j.ijcard.2008.03.043. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Eguchi Y, Kamiike W, Funahashi Y, Mignon A, Lacronique V, et al. Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1455–9. doi: 10.1073/pnas.95.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki YJ, Nagase H, Day RM, Das DK. GATA-4 regulation of myocardial survival in the preconditioned heart. Journal of molecular and cellular cardiology. 2004;37:1195–203. doi: 10.1016/j.yjmcc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nature medicine. 1998;4:929–33. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- Tokunaga M, Liu ML, Nagai T, Iwanaga K, Matsuura K, Takahashi T, et al. Implantation of cardiac progenitor cells using self-assembling peptide improves cardiac function after myocardial infarction. Journal of molecular and cellular cardiology. 2010;49:972–83. doi: 10.1016/j.yjmcc.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Topkara VK, Mann DL. Role of MicroRNAs in Cardiac Remodeling and Heart Failure. Cardiovasc Drugs Ther. 2011 doi: 10.1007/s10557-011-6289-5. [DOI] [PubMed] [Google Scholar]
- Tran NL, McDonough WS, Savitch BA, Sawyer TF, Winkles JA, Berens ME. The tumor necrosis factor-like weak inducer of apoptosis (TWEAK)-fibroblast growth factor-inducible 14 (Fn14) signaling system regulates glioma cell survival via NFkappaB pathway activation and BCL-XL/BCL-W expression. The Journal of biological chemistry. 2005;280:3483–92. doi: 10.1074/jbc.M409906200. [DOI] [PubMed] [Google Scholar]
- Troyan MB, Gilman VR, Gay CV. Mitochondrial membrane potential changes in osteoblasts treated with parathyroid hormone and estradiol. Experimental cell research. 1997;233:274–80. doi: 10.1006/excr.1997.3570. [DOI] [PubMed] [Google Scholar]
- Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circulation research. 2006;98:1414–21. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18255–60. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circulation research. 2002;91:501–8. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- Yang X, Yin J, Yu J, Xiang Q, Liu Y, Tang S, et al. miRNA-195 sensitizes human hepatocellular carcinoma cells to 5-FU by targeting BCL-w. Oncology reports. 2012;27:250–7. doi: 10.3892/or.2011.1472. [DOI] [PubMed] [Google Scholar]
- Yao M, Nguyen TV, Pike CJ. Estrogen regulates Bcl-w and Bim expression: role in protection against beta-amyloid peptide-induced neuronal death. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:1422–33. doi: 10.1523/JNEUROSCI.2382-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef M, Schannwell CM, Kostering M, Zeus T, Brehm M, Strauer BE. The BALANCE Study: clinical benefit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infarction. Journal of the American College of Cardiology. 2009;53:2262–9. doi: 10.1016/j.jacc.2009.02.051. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang X, Zhu H, Zhu C, Wang Y, Pu WT, et al. Synergistic effects of the GATA-4-mediated miR-144/451 cluster in protection against simulated ischemia/reperfusion-induced cardiomyocyte death. Journal of molecular and cellular cardiology. 2010;49:841–50. doi: 10.1016/j.yjmcc.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Yang Y, Wang Y, Li J, Schiller PW, Peng T. MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc Res. 2011;92:75–84. doi: 10.1093/cvr/cvr145. [DOI] [PubMed] [Google Scholar]


