Abstract
As the major structural component of the extracellular matrix, collagen plays a crucial role in tissue development and regeneration. Since structural and metabolic abnormalities of collagen are associated with numerous debilitating diseases and pathologic conditions, the ability to target collagens of diseased tissues could lead to new diagnostics and therapeutics. Collagen is also a natural biomaterial widely used in drug delivery and tissue engineering, and construction of synthetic collagen-like materials is gaining interests in the biomaterials community. The unique triple helical structure of collagen has been explored for targeting collagen strands, and for engineering collagen-like functional assemblies and conjugates. This review focuses on the forefront of research activities in the use of the collagen mimetic peptide for both targeting and mimicking collagens via its triple helix mediated strand hybridization and higher order assembly.
Introduction
Collagen, the most abundant protein in mammals, plays a critical role in tissue development and regeneration. It is a major structural component of the extracellular matrix (ECM), where cells proliferate and differentiate. While fibrous collagens (e.g., type I, II) provide mechanical strength to connective tissues, network-like collagens (e.g., type IV) form the basic scaffold of the basement membrane where cells attach and grow into organized tissues. Abnormal collagen remodeling activities are typically seen during wound healing response and in chronic pathological conditions such as cancer, osteoporosis, arthritis, and fibrosis. Therefore, the ability to target remodeling collagens could help understand the progression of such diseases, as well as provide new diagnostic and therapeutic opportunities. Collagen is one of the most widely used natural biomaterials for medical applications in biocompatible coatings, drug delivery and tissue engineering. However, in order to overcome the structural and compositional complexity of animal-derived collagen, researchers are constructing artificial collagen-like scaffolds with tunable physico-chemical properties and unique biological functions. Collagen mimetic peptide (CMP) is a family of small synthetic peptides that mimic natural collagens: they share the collagen's hallmark structural motif – the triple helix, as well as the Gly-Xaa-Yaa triplet repeat sequence, where Xaa and Yaa are largely populated by proline and 4(R)-hydroxylproline, respectively [1]. These peptides were traditionally used as synthetic models to study the structure and folding behaviors of collagens [1]. In this review, we will discuss recent progress in two distinct research areas in the biomedical application of CMPs: i) targeting collagens in pathological tissues [2–5], and ii) creating self-assembled collagen-like biomaterials and molecular constructs [6–8], both of which are based on the CMP's unique triple helical structure. With our eyes set on identifying applications in bioimaging, drug delivery, and tissue engineering, we will review recent progress in CMP-based collagen/gelatin targeting in the context of other collagen-targeting molecules, as well as highlight various CMP derivatives, and collagen mimetic assemblies and conjugates that are inspired by the structure and function of natural collagen.
Collagen-targeting molecules
Among many collagen binding molecules, only a few have been explored in the context of collagen targeting. One example is CNA35, a 35 kDa collagen-binding domain in the adhesin protein found on the surface of bacterium Staphylococcus aureus, which hugs the triple helical collagen molecule with its two subdomains through hydrophobic interactions [9]. Merkx group and others developed fluorescently labeled recombinant CNA35 and CNA35-functionalized micelles for visualization of collagens in various biological samples including cell culture (e.g., collagen-producing myofibroblasts), engineered tissue constructs, and animal tissues (e.g., blood vessels and kidney) [10–12]. They found out that fluorescent CNA35 administered in vivo shows high uptake in atherosclerotic arteries, particularly in the areas of atherosclerotic plaque that are rich in collagen networks [13]. Using the phage display method, Caravan and coworkers developed a type I collagen-binding cyclic peptide which was used as MRI contrast agent for myocardial scar [14]. During the phage display efforts to find cartilage binding peptides, Hubbell's research group identified a type II collagen binding peptide of sequence WYRGRL. This peptide supported local delivery and immobilization of polymeric nanoparticles in knee cartilages after intra-articular injection [15]. A peptide mimetic of glycoprotein VI, the main platelet receptor of type I and III collagens, was used for in vivo radioactive imaging of lung fibrosis and scars in healed myocardial infarction [16]. A peptide derived from the collagen-binding proteoglycan, decorin, was also used to target collagens to modulate collagen fibrillogenesis [17], and to reduce collagen degradation and dermal scarring [18].
Since collagen is present ubiquitously in the body, collagen-binding molecules discussed above face the challenge of distinguishing collagens in the diseased tissue from those in the healthy tissue, if they are to be used for targeted drug delivery and molecular imaging [13]. Some strategies have been taken to design or select collagen targeting molecules that are more specific to collagens in diseased tissues. Recombinant CNA35 constructs whose collagen-binding is activated by matrix metalloproteinases (MMPs) showed improved selectivity for collagens undergoing MMP-mediated remodeling. [19,20]. Library approaches have been used to identify monoclonal antibody [21,22] and peptides [23] that specifically bind to cryptic sites in collagen strands that become exposed after denaturation. For example, humanized monoclonal antibody (D93) that recognizes the GPO repeating sequence in denatured collagen strands was developed by Baeuerle and coworkers [22,24]. Studies showed that D93 specifically adheres to vascular basement membrane in tumors but not to blood vessels in normal tissues [22]. The peptide sequence, TLTYTWS, which was selected by phage display for binding to MMP2-modified type IV collagen was found to accumulate in tumors and inhibit angiogenesis in vivo [23].
CMP-collagen hybridization
The triple helix, which is the hallmark structure of collagen, provides a unique mechanism for targeting denatured collagen strands. The triple helical tertiary structure is nearly exclusively seen in collagens except as small sub-domains in a few non-collagen proteins [1]. During tissue remodeling, the collagen molecules within the collagen fibers and networks are degraded by proteases (e.g., MMPs or cathepsin) and become denatured at body temperature. We recently discovered that the collagen mimetic peptide [sequence: (GPO)n, n = 6–10] with its strong triple helix folding propensity can specifically bind to such denatured collagen strands both in vitro and in vivo (Figure 1) [2,3,25]. This binding is primarily driven by the triple helix hybridization between monomeric CMPs and the denatured collagen strands, which is similar to DNA fragments binding to complimentary DNA strands. Because homotrimeric CMPs have little driving force for collagen hybridization, CMPs had to be thermally dissociated to the monomeric state before binding to collagen substrates (Figure 1a, left panel). Such thermally induced CMP hybridization allowed us to i) directly detect collagenous proteins in SDS-PAGE gel [3] (Figure 1b), ii) image collagens in tissue sections [4,26], and iii) immobilize angiogenic signals to collagen scaffolds [27,28].
Figure 1.
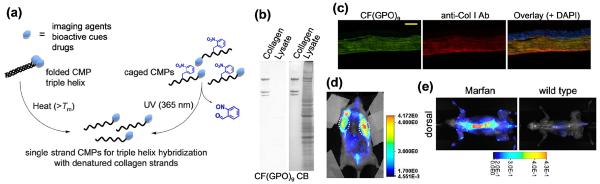
(a) Schematic illustration of two approaches (heat and UV activation) to generate single strand CMPs that hybridize with denatured collagen strands. (b) Fluorescent images of SDS-PAGE gel loaded with type I collagen and endothelial cell lysate stained by carboxyfluorescein-labeled CMP [CF(GPO)9] (left) in comparison to the same gel stained by coomassie blue (CB) (right) showing high specificity of CMP-collagen hybridization (adapted from Ref. [3]). (c) Fluorescent micrographs of fixed mouse cornea sections stained by photo-triggered caged carboxyfluorescein-labeled CMP (green), and co-stained with anti-collagen I antibody (red) and DAPI (blue). The CMP staining clearly reveals the parallel organization of collagen fibrils in the corneal stroma (adapted from Ref. [3]). (d) Near infrared (NIR) fluorescence image of a mouse bearing PC-3 prostate tumors at forward flanks (circled) administered with UV-activated caged and NIR fluorophore labeled CMPs, indicating stable and tumor specific CMP uptake (adapted from Ref. [2]). (e) Comparative NIR fluorescence images of mouse model with Marfan syndrome showing high CMP uptake in the skeleton of the diseased mouse (adapted from Ref. [2]).
Since addition of hot CMP solution can damage tissues, non-thermal means to control the triple helix folding and collagen binding have been developed. The Raines group synthesized a 4-fluoroproline containing CMP analog that is incapable of self-trimerizing due to inter-strand steric hindrance, but capable of hybridizing with natural collagen strands in vitro [5]. Recently, our group developed a caged CMP with a photo-cleavable nitrobenzyl (NB) group attached to the central glycine [sequence: (GPO)4NBGPO(GPO)4]. The NB cage group sterically prevented the CMP from folding into triple helix; yet removal of the cage group by UV irradiation immediately triggered the triple helical folding of the peptide and its hybridization with unfolded collagen strands [2] (Figure 1a, right panel). Systemically delivered freshly de-caged CMPs were able to bind to denatured collagens in tissues undergoing normal (e.g., in bone and cartilage) and pathological remodeling (e.g., in tumors, Marfan syndrome) even up to 7 days (Figure 1d) [2]. The CMP-collagen hybridization is a revolutionary new way to target collagens of abnormal tissues which complements the conventional library approaches, and could lead to new opportunities for management of numerous pathologic conditions associated with collagen remodeling (e.g., cancer, osteoporosis, arthritis, and fibrosis).
Functional collagen-like assemblies and conjugates
From surface coatings to tissue engineering, natural collagens are used extensively in biomedical applications, due to their biocompatibility and natural abundance. However, animal-derived collagens have complex physico-chemical properties that are difficult to control, and can pose potential problems of immunogenicity and pathogen transmission. Therefore, bioengineers have been developing a variety of assemblies and conjugates based on synthetic CMPs, aimed to recapitulate the structure and function of collagens as well as to generate new collagen-like scaffold materials that are tunable and conducive to functionalization.
Collagen mimetic peptide assemblies
Almost all CMP-based materials utilize the CMP's triple helical structure [29], largely because it is extremely stable under physiological condition. Conjugation of CMPs [(POG)8 or 9] to multiarm poly(ethylene glycol) (PEG) polymers led to a formation of hydrogels via triple helix mediated crosslinks (Figure 2a) [30,31], which was used to encapsulate human mesenchymal stem cells [30]. Kojima and coworkers prepared PAMAM dendrimers with CMP [(GPP)5 or 10] end groups [32,33], which were also shown to form hydrogels with potential application in controlled drug release [32]. Because the triple helix folding of CMPs is thermally reversible, these hydrogels [30–33] can be cycled through melting and gelation processes by heating and cooling, similar to gelatin (i.e., thermally denatured collagen).
Figure 2.
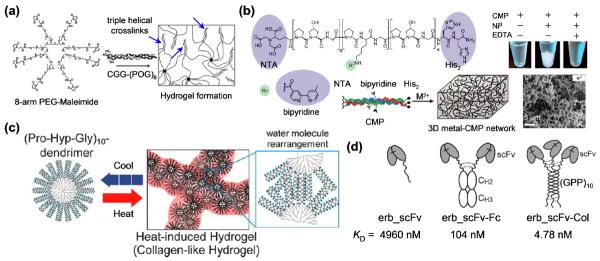
(a) Schematic illustration of hydrogel formation mediated by physical crosslinks of CMP triple helices (arrows) which are conjugated to an 8-arm PEG-maleimide star polymer (adapted from Ref. [30]). (b) Structure of a CMP conjugated to a nitrilotriacetic acid (NTA), two histidine residues (His2), and a bipyridine group, and a schematic representation of this triple helical CMP assembling into 3D scaffold by metal ion supported crosslinks. Precipitation of the assembled CMPs can be seen in the presence of NiII ions while addition of EDTA dissolves the precipitates. The network-like microstructure of the NiII crosslinked CMP assembly can be observed in the scanning electron micrograph (adapted from Ref. [38]). (c) (POG)10-conjugated PAMAM dendrimers form heat-induced hydrogels which are crosslinked by triple helix-triple helix association mediated by rearrangement of water molecules on the surface of the dendrimers (adapted from Ref. [42]). (d) Three single-chain variable fragments (scFv) against extracellular domain of epidermal growth factor receptor are assembled into a trimer by triple helical folding of a CMP domain that is fused to the protein. The trimeric compound shows an antigen-binding affinity that is orders of magnitude higher than those of the mono- and bi-valent analogues (adapted from Ref. [8]).
Many types of natural collagens form supramolecular structures of fibers and networks. Therefore, researchers have been creating new CMP designs that could also self-assemble into higher order architecture beyond the triple helix. Brodsky group first reported that triple helical CMP of simple POG repeats, (POG)10, can aggregate into branched filamentous structures by lateral hydrogen bonds mediated by hydroxyprolines [34]. Others have recently developed CMPs that can be triggered to form high order assemblies. Chmielewski group attached metal biding ligands (e.g., histidines, nitrilotriacetic acid) to the CMPs at residues within the sequence [35,36], and/or at the termini [7,37,38] (Figure 2b). Similarly, CMP sequences containing multiple histidine residues [e.g., HG(PPG)4PHG(PPG)4GH] were developed by Horng and coworkers [39]. In the presence of divalent metal ions, these triple helical CMPs quickly formed microscale assemblies with distinct morphologies that correlated with the type of the metal ions and location of the metal binding ligands, while treatment with metal chelators (e.g., EDTA) resulted in instant dissolution of the assemblies [7,40]. It was determined that both the lateral CMP association and the metal-mediated crosslinking were critical to the assembly process. Surprisingly, these metal-supported hydrogels were able to encapsulate and support growth of human endothelial cells with no observable cytotoxicity [38]. In addition, His-tagged proteins (e.g., GFP) were incorporated into the hydrogels by binding to unoccupied metal binding sites of the metal-ligand complexes[41]. These unique properties of the metal-CMP hydrogels could be utilized for cell delivery and controlled release of therapeutic agents.
Several approaches have been taken to generate artificial CMP assemblies that resemble the structure or thermal behaviors of native collagens. Collagen-like nanofibers were created by self-assembling amphiphiles composed of a hydrophilic CMP domain and a hydrophobic tail [6]. In contrast to gelatin and gelatin-like hydrogels [30–33] which melt when heated, native collagens (e.g., type I) assemble from individual molecules to fibrils when heated (e.g., from 25 to 37 °C). To mimic such thermal behavior, Kojima and coworkers synthesized a (POG)10-conjugated PAMAM dendrimer that forms a gel upon heating [42]. The gelation is thought to be mediated by association of the triple helical (POG)10 molecules on the surface of the dendrimers (Figure 2c), which is a entropy-driven process facilitated by heat [34,42]. More interestingly, Kiick group discovered that a conjugate of CMPs and thermoresponsive polymers can change its assembly structure from microspheres to fibrils upon thermal unfolding of the CMP triple helices [43]. These new CMP-based designs are revealing interesting possibilities for fabricating synthetic collagen-like materials.
Protein constructs assembled via collagen-like domains
The triple helix structural motif is found in a few non-collagenous proteins (e.g., complement factor C1q), typically as a spacer or oligomerization domain. This has inspired scientists to design multivalent protein constructs assembled via collagen-like sequences. By fusing human single-chain variable fragment (scFv) with a prolyl-hydroxylated (GPP)10 CMP domain, Chou and coworkers produced a so-called “collabody” in mammalian cells, where CMP's triple helical folding resulted in formation of trivalent antigen-binding fragments (Fab) (Figure 2d) [8]. The collabody showed remarkable serum stability, and an antigen-binding affinity approximately 20- and 1000-fold higher than those of the bivalent and monovalent counterparts, respectively. The Álverz-Vallina group showed that the CMP sequence could be replaced with a trimerizing non-collagenous (NC1) subdomain of type XVIII collagen, which led to another type of recombinant trivalent antibodies for cancer imaging and therapeutic targeting [44,45]. These novel collagen-like domains are becoming a popular multimerizing units that can be conjugated to a variety of biomolecules (e.g., antibodies, growth factors, enzymes, monosaccharides [46]) to improve the binding affinity and ultimately their therapeutic effects.
Functionalized collagen mimetic peptides
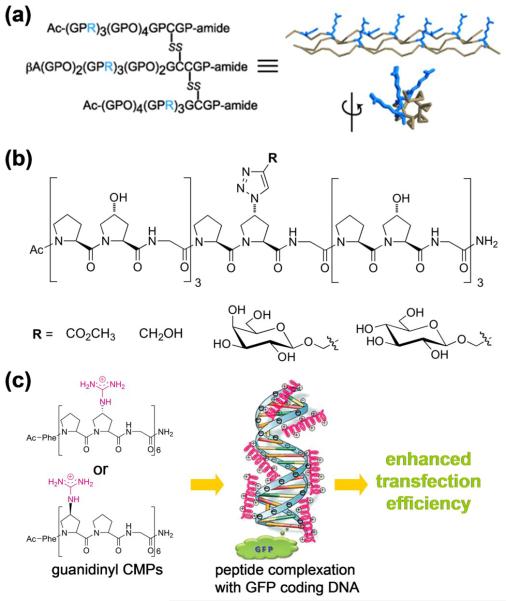
One major advantage of the synthetic CMP is that its sequence or side chain chemical groups can be readily modified for new functions and bioactivities. Insertion of cell adhesion motifs (e.g., GFOGER, GEKGER) into the triple helical CMPs promoted attachment and growth of cells on surfaces modified with these CMPs [6,47,48]. The Koide group recently reported a novel arginine-rich heterotrimeric CMP with cell penetrating capacity (Figure 3a) [49]. The peptide also exhibited high serum stability due to the triple helical structure that is resistant to proteases [49]. Instead of the typical N-terminal modification [50], modified prolines were also used to develop CMPs with exotic functionalities [51,52]. Wennemer and Erdmann introduced azido groups into the prolines of conventional CMPs, which was further linked to various moieties (e.g., sugar molecules) via click chemistry (Figure 3b) [51]. Using this CMP system, they were able to gain insights into the conformational factors of the proline ring that are responsible for triple helix stabilization [53–55]. Nanda and Ganesh reported on-resin synthesis of CMPs containing cationic 4(R/S)-amino- or guanidinyl-proline residues. These CMPs formed complexes with negatively-charged plasmid DNA, which exhibited enhanced transfection efficiency (Figure 3c) [52]. The new chemical modification strategies have dramatically expanded the field of functional CMP family, and are expected to lead to unconventional but potentially high impact applications in biomedicine.
Figure 3.
(a) Sequence of tethered Arg-rich heterotrimeric CMPs, and the spatial orientation of the Arg residues (in blue) on the triple helix which is critical for the peptide's cell penetrating capacity (adapted from Ref. [49]). (b) Functionalization of CMPs through click reaction on the azido-prolines residue (adapted from Ref. [51]). (c) CMPs containing cationic guanidinyl-proline residues can complex with GFP encoding plasmid DNA through electrostatic interactions and enhance gene transfection (adapted from Ref. [52]).
Perspective
As exemplified in this review, the CMP peptides in triple helical or single strand state exhibit distinct properties that can be exploited for different biomedical applications: as a folded triple helix, the CMP is a thermally and enzymatically stable structural motif that can assemble into synthetic ECMs, whereas single strand CMP has a strong triple helix folding propensity suited for hybridization with unfolded collagens in tissues with high ECM turnover rate (Figure 4). By drawing inspiration from both approaches, we envision many new designs and applications of CMPs. For instance, CMP assemblies and conjugates discussed above can be turned into biologically active structures by having them hybridize with CMPs containing bioactive groups [31,56]. One could also generate a family of novel molecules and polymers (e.g., fusion proteins, hydrogels [57], nanoparticles [58,59], dendrimers) displaying monomeric CMPs that can target collagens in remodeling tissues. Moreover, the interactions between folded CMP and collagen molecules need to be further explored. It was recently reported that 4-arm-PEG conjugated with triple helical CMPs [(POG)10] self-assembles into nanoparticles which can be used to create physical crosslinks between collagen fibrils, presumably by formation of hydrogen bonds between the hydroxyprolines of the triple helical CMPs and the collagen fibrils [60]. This interesting discovery suggests that CMPs may be able to interact with collagens in a mechanism that is drastically different from the strand hybridization. Further investigation of this interaction could not only reveal new collagen assembly mechanisms, but also open new directions toward the tissue engineering applications of triple helical CMPs, particularly for tissues that are rich in collagen fibers (e.g., skin, cornea). With so many new possibilities, we believe that the CMP is a promising peptide family that could one day lead to new breakthroughs in many biomedical fields, particularly in molecular imaging, drug delivery, and tissue engineering.
Figure 4.
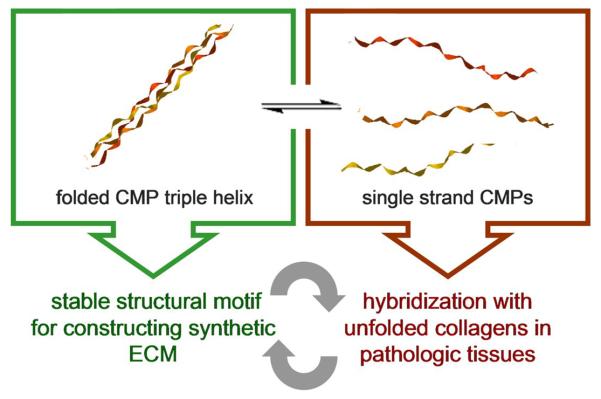
The triple helical (left box) structure and single strand (right box) conformation of CMP exhibit distinctively different physical properties that have been separately explored for various biomedical applications. Combining the two approaches in a synergistic fashion will inspire even more interesting CMP designs for targeting and mimicking collagens which could ultimately lead to a widespread use of CMP derivatives in the biomedical community.
Highlights
Targeting collagens of diseased tissue can lead to new diagnostics and therapeutics.
Collagen mimetic peptide (CMP) can hybridize to denatured collagen strands.
CMP assembles into triple helix and other higher order structures.
Study of CMP assemblies & conjugates may lead to new applications in biomedicine.
Acknowledgements
Part of the work presented in this article was supported by National Institute of Health (R01-AR060484) and US Department of Defense (W81XWH-12-1-0555).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
· of special interest
·· of outstanding interest
- 1.Engel J, Bächinger HP, Brinckmann J, Notbohm H, Müller PK. Collagen. vol 247. Springer; 2005. Structure, stability and folding of the collagen triple helix; pp. 7–33. [Google Scholar]
- 2.Li Y, Foss CA, Summerfield DD, Doyle JJ, Torok CM, Dietz HC, Pomper MG, Yu SM. Targeting collagen strands by photo-triggered triple-helix hybridization. Proc Natl Acad Sci U S A. 2012;109:14767–14772. doi: 10.1073/pnas.1209721109. [DOI] [PMC free article] [PubMed] [Google Scholar]; ·· This paper first demonstrated that single strand CMPs can hybridize with unfolded collagen strands in remodeling tissues in vivo.
- 3.Li Y, Ho D, Meng H, Chan TR, An B, Yu H, Brodsky B, Jun AS, Michael Yu S. Direct detection of collagenous proteins by fluorescently labeled collagen mimetic peptides. Bioconj Chem. 2013;24:9–16. doi: 10.1021/bc3005842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang AY, Foss CA, Leong S, Mo X, Pomper MG, Yu SM. Spatio-temporal modification of collagen scaffolds mediated by triple helical propensity. Biomacromolecules. 2008;9:1755–1763. doi: 10.1021/bm701378k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chattopadhyay S, Murphy CJ, McAnulty JF, Raines RT. Peptides that anneal to natural collagen in vitro and ex vivo. Org Biomol Chem. 2012;10:5892–5897. doi: 10.1039/c2ob25190f. [DOI] [PMC free article] [PubMed] [Google Scholar]; · This paper introduced the fluoroproline containing CMP which does not self-assemble into homotrimers but can hybridize with unfolded natural collagen strands.
- 6.Luo JN, Tong YW. Self-assembly of collagen-mimetic peptide amphiphiles into biofunctional nanofiber. Acs Nano. 2011;5:7739–7747. doi: 10.1021/nn202822f. [DOI] [PubMed] [Google Scholar]
- 7.Pires MM, Przybyla DE, Rubert Pérez CM, Chmielewski J. Metal-mediated tandem coassembly of collagen peptides into banded microstructures. J Am Chem Soc. 2011;133:14469–14471. doi: 10.1021/ja2042645. [DOI] [PubMed] [Google Scholar]
- 8.Fan CY, Huang CC, Chiu WC, Lai CC, Liou GG, Li HC, Chou MY. Production of multivalent protein binders using a self-trimerizing collagen-like peptide scaffold. FASEB J. 2008;22:3795–3804. doi: 10.1096/fj.08-111484. [DOI] [PubMed] [Google Scholar]; ·· This paper showcased a novel strategy to bundle protein domains through triple helical folding of the fused CMPs.
- 9.Zong Y, Xu Y, Liang X, Keene DR, Hook A, Gurusiddappa S, Hook M, Narayana SVL. A `collagen hug' model for staphylococcus aureus CNA binding to collagen. EMBO J. 2005;24:4224–4236. doi: 10.1038/sj.emboj.7600888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reulen SWA, Dankers PYW, Bomans PHH, Meijer EW, Merkx M. Collagen targeting using protein-functionalized micelles: the strength of multiple weak interactions. J Am Chem Soc. 2009;131:7304–7312. doi: 10.1021/ja807723p. [DOI] [PubMed] [Google Scholar]
- 11.Boerboom RA, Krahn KN, Megens RTA, van Zandvoort MAMJ, Merkx M, Bouten CVC. High resolution imaging of collagen organisation and synthesis using a versatile collagen specific probe. J Struct Biol. 2007;159:392–399. doi: 10.1016/j.jsb.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Lee SK, Abd-Elgaliel WR, Liang L, Galende E-Y, Hajjar RJ, Tung C-H. Assessment of Cardiovascular Fibrosis Using Novel Fluorescent Probes. PLoS ONE. 2011;6:e19097. doi: 10.1371/journal.pone.0019097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Megens RTA, oude Egbrink MGA, Cleutjens JPM, Kuijpers MJE, Schiffers PHM, Merkx M, Slaaf DW, van Zandvoort MAMJ. Imaging collagen in intact viable healthy and atherosclerotic arteries using fluorescently labeled CNA35 and two-photon laser scanning microscopy. Molecular Imaging. 2007;6:247–260. [PubMed] [Google Scholar]
- 14.Caravan P, Das B, Dumas S, Epstein FH, Helm PA, Jacques V, Koerner S, Kolodziej A, Shen L, Sun W-C, et al. Collagen-targeted MRI contrast agent for molecular imaging of fibrosis. Angew Chem Int Ed. 2007;46:8171–8173. doi: 10.1002/anie.200700700. [DOI] [PubMed] [Google Scholar]
- 15.Rothenfluh DA, Bermudez H, O'Neil CP, Hubbell JA. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nature Mater. 2008;7:248–254. doi: 10.1038/nmat2116. [DOI] [PubMed] [Google Scholar]
- 16.Muzard J, Sarda-Mantel L, Loyau S, Meulemans A, Louedec L, Bantsimba-Malanda C, Hervatin F, Marchal-Somme J, Michel JB, Le Guludec D, et al. Non-invasive molecular imaging of fibrosis using a collagen-targeted peptidomimetic of the platelet collagen receptor glycoprotein VI. PLoS ONE. 2009;4:e5585. doi: 10.1371/journal.pone.0005585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paderi JE, Panitch A. Design of a synthetic collagen-binding peptidoglycan that modulates collagen fibrillogenesis. Biomacromolecules. 2008;9:2562–2566. doi: 10.1021/bm8006852. [DOI] [PubMed] [Google Scholar]
- 18.Stuart K, Paderi J, Snyder PW, Freeman L, Panitch A. Collagen-binding peptidoglycans inhibit MMP mediated collagen degradation and reduce dermal scarring. PLoS ONE. 2011;6:e22139. doi: 10.1371/journal.pone.0022139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breurken M, Lempens EHM, Merkx M. Protease-activatable collagen targeting based on protein cyclization. ChemBioChem. 2010;11:1665–1668. doi: 10.1002/cbic.201000223. [DOI] [PubMed] [Google Scholar]
- 20.Breurken M, Lempens EHM, Meijer EW, Merkx M. Semi-synthesis of a protease-activatable collagen targeting probe. Chem Commun. 2011;47:7998–8000. doi: 10.1039/c1cc11964h. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Rodriguez D, Kim JJ, Brooks PC. Generation of monoclonal antibodies to cryptic collagen sites by using subtractive immunization. Hybridoma. 2000;19:375–385. doi: 10.1089/02724570050198893. [DOI] [PubMed] [Google Scholar]
- 22.Pernasetti F, Nickel J, Clark D, Baeuerle PA, Van Epps D, Freimark B. Novel anti-denatured collagen humanized antibody D93 inhibits angiogenesis and tumor growth: an extracellular matrix-based therapeutic approach. Int J Oncol. 2006;29:1371–1379. [PubMed] [Google Scholar]
- 23.Mueller J, Gaertner FC, Blechert B, Janssen K-P, Essler M. Targeting of tumor blood vessels: a phage-displayed tumor-homing peptide specifically binds to matrix metalloproteinase-2-processed collagen IV and blocks angiogenesis in vivo. Mol Cancer Res. 2009;7:1078–1085. doi: 10.1158/1541-7786.MCR-08-0538. [DOI] [PubMed] [Google Scholar]
- 24.Freimark B, Clark D, Pernasetti F, Nickel J, Myszka D, Baeuerle PA, Van Epps D. Targeting of humanized antibody D93 to sites of angiogenesis and tumor growth by binding to multiple epitopes on denatured collagens. Mol Immunol. 2007;44:3741–3750. doi: 10.1016/j.molimm.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Wang AY, Mo X, Chen CS, Yu SM. Facile modification of collagen directed by collagen mimetic peptides. J Am Chem Soc. 2005;127:4130–4131. doi: 10.1021/ja0431915. [DOI] [PubMed] [Google Scholar]
- 26.Yu SM, Li Y, Kim D. Collagen mimetic peptides: progress towards functional applications. Soft Matter. 2011;7:7927–7938. doi: 10.1039/C1SM05329A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang AY, Leong S, Liang YC, Huang RCC, Chen CS, Yu SM. Immobilization of growth factors on collagen scaffolds mediated by polyanionic collagen mimetic peptides and its effect on endothelial cell morphogenesis. Biomacromolecules. 2008;9:2929–2936. doi: 10.1021/bm800727z. [DOI] [PubMed] [Google Scholar]
- 28.Chan TR, Stahl PJ, Yu SM. Matrix-bound VEGF mimetic peptides: design and endothelial cell activation in collagen scaffolds. Adv Funct Mater. 2011;21:4252–4262. doi: 10.1002/adfm.201101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akers WJ, Xu B, Lee H, Sudlow GP, Fields GB, Achilefu S, Edwards WB. Detection of MMP-2 and MMP-9 activity in vivo with a triple-helical peptide optical probe. Bioconj Chem. 2012;23:656–663. doi: 10.1021/bc300027y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubert Pérez CM, Panitch A, Chmielewski J. A collagen peptide-based physical hydrogel for cell encapsulation. Macromol Biosci. 2011;11:1426–1431. doi: 10.1002/mabi.201100230. [DOI] [PubMed] [Google Scholar]
- 31.Stahl PJ, Romano NH, Wirtz D, Yu SM. PEG-based hydrogels with collagen mimetic peptide-mediated and tunable physical cross-links. Biomacromolecules. 2010;11:2336–2344. doi: 10.1021/bm100465q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kojima C, Tsumura S, Harada A, Kono K. A collagen-mimic dendrimer capable of controlled release. J Am Chem Soc. 2009;131:6052–6053. doi: 10.1021/ja809639c. [DOI] [PubMed] [Google Scholar]
- 33.Suehiro T, Tada T, Waku T, Tanaka N, Hongo C, Yamamoto S, Nakahira A, Kojima C. Temperature-dependent higher order structures of the (Pro-Pro-Gly)10-modified dendrimer. Biopolymers. 2011;95:270–277. doi: 10.1002/bip.21576. [DOI] [PubMed] [Google Scholar]
- 34.Kar K, Amin P, Bryan MA, Persikov AV, Mohs A, Wang Y-H, Brodsky B. Self-association of collagen triple helic peptides into higher order structures. J Biol Chem. 2006;281:33283–33290. doi: 10.1074/jbc.M605747200. [DOI] [PubMed] [Google Scholar]
- 35.Przybyla DE, Chmielewski J. Metal-triggered collagen peptide disk formation. J Am Chem Soc. 2010;132:7866–7867. doi: 10.1021/ja103148t. [DOI] [PubMed] [Google Scholar]
- 36.Przybyla DE, Rubert Pérez CM, Gleaton J, Nandwana V, Chmielewski J. Hierarchical assembly of collagen peptide triple helices into curved disks and metal ion-promoted hollow spheres. J Am Chem Soc. 2013;135:3418–3422. doi: 10.1021/ja307651e. [DOI] [PubMed] [Google Scholar]
- 37.Pires MM, Lee J, Ernenwein D, Chmielewski J. Controlling the morphology of metal-promoted higher ordered assemblies of collagen peptides with varied core lengths. Langmuir. 2012;28:1993–1997. doi: 10.1021/la203848r. [DOI] [PubMed] [Google Scholar]
- 38.Pires MM, Przybyla DE, Chmielewski J. A metal-collagen peptide framework for three-dimensional cell culture. Angew Chem Int Ed. 2009;48:7813–7817. doi: 10.1002/anie.200902375. [DOI] [PubMed] [Google Scholar]; · This paper demonstrated that metal triggered assemblies of triple helical CMPs can encapsulate and support growth of endothelial cells with no observable cytotoxicity.
- 39.Hsu W, Chen YL, Horng JC. Promoting self-assembly of collagen-related peptides into various higher-order structures by metal-histidine coordination. Langmuir. 2012;28:3194–3199. doi: 10.1021/la204351w. [DOI] [PubMed] [Google Scholar]
- 40.Pires MM, Chmielewski J. Self-assembly of collagen peptides into microflorettes via metal coordination. J Am Chem Soc. 2009;131:2706–2712. doi: 10.1021/ja8088845. [DOI] [PubMed] [Google Scholar]
- 41.Pires MM, Ernenwein D, Chmielewski J. Selective decoration and release of his-tagged proteins from metal-assembled collagen peptide microflorettes. Biomacromolecules. 2011;12:2429–2433. doi: 10.1021/bm2004934. [DOI] [PubMed] [Google Scholar]
- 42.Kojima C, Suehiro T, Tada T, Sakamoto Y, Waku T, Tanaka N. Preparation of heat-induced artificial collagen gels based on collagen-mimetic dendrimers. Soft Matter. 2011;7:8991–8997. [Google Scholar]
- 43.Krishna OD, Wiss KT, Luo T, Pochan DJ, Theato P, Kiick KL. Morphological transformations in a dually thermoresponsive coil-rod-coil bioconjugate. Soft Matter. 2012;8:3832–3840. doi: 10.1039/C2SM07025A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cuesta AM, Sanchez-Martin D, Sanz L, Bonet J, Compte M, Kremer L, Blanco FJ, Oliva B, Alvarez-Vallina L. In vivo tumor targeting and imaging with engineered trivalent antibody fragments containing collagen-derived sequences. Plos One. 2009;4:e5381. doi: 10.1371/journal.pone.0005381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lobo V, Cuesta AM, Sanz L, Compte M, Garcia P, Prieto J, Blanco FJ, Alvarez-Vallina L. Enhanced antiangiogenic therapy with antibody-collagen XVIII NCl domain fusion proteins engineered to exploit matrix remodeling events. Int J Cancer. 2006;119:455–462. doi: 10.1002/ijc.21851. [DOI] [PubMed] [Google Scholar]
- 46.Okada T, Isobe C, Wada T, Ezaki S, Minoura N. Switchable binding affinity of mannose tethered to collagen peptide by temperature-dependent triple-helix formation. Bioconj Chem. 2013;24:841–845. doi: 10.1021/bc3006013. [DOI] [PubMed] [Google Scholar]
- 47.Wojtowicz AM, Shekaran A, Oest ME, Dupont KM, Templeman KL, Hutmacher DW, Guldberg RE, Garcia AJ. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials. 2010;31:2574–2582. doi: 10.1016/j.biomaterials.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krishna OD, Jha AK, Jia X, Kiick KL. Integrin-mediated adhesion and proliferation of human MSCs elicited by a hydroxyproline-lacking, collagen-like peptide. Biomaterials. 2011;32:6412–6424. doi: 10.1016/j.biomaterials.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamazaki CM, Nakase I, Endo H, Kishimoto S, Mashiyama Y, Masuda R, Futaki S, Koide T. Collagen-like cell-penetrating peptides. Angew Chem Int Ed. 2013;52:5497–5500. doi: 10.1002/anie.201301266. [DOI] [PubMed] [Google Scholar]; · This paper introduced an Arg-rich triple helical CMP with cell penetrating capacity and enhanced serum stability.
- 50.Stahl PJ, Cruz JC, Li Y, Yu SM, Hristova K. On-the-resin N-terminal modification of long synthetic peptides. Anal Biochem. 2012;424:137–139. doi: 10.1016/j.ab.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erdmann RS, Wennemers H. Functionalizable collagen model peptides. J Am Chem Soc. 2010;132:13957–13959. doi: 10.1021/ja103392t. [DOI] [PubMed] [Google Scholar]
- 52.Nanda M, Ganesh KN. 4(R/S)-guanidinylprolyl collagen peptides: on-resin synthesis, complexation with plasmid DNA, and the role of peptides in enhancement of transfection. J Org Chem. 2012;77:4131–4135. doi: 10.1021/jo300070p. [DOI] [PubMed] [Google Scholar]
- 53.Erdmann RS, Wennemers H. Importance of ring puckering versus interstrand hydrogen bonds for the conformational stability of collagen. Angew Chem Int Ed. 2011;50:6835–6838. doi: 10.1002/anie.201008118. [DOI] [PubMed] [Google Scholar]
- 54.Erdmann RS, Wennemers H. Conformational stability of collagen triple helices functionalized in the Yaa position by click chemistry. Org Biomol Chem. 2012;10:1982–1986. doi: 10.1039/c2ob06720j. [DOI] [PubMed] [Google Scholar]
- 55.Erdmann RS, Wennemers H. Effect of sterically demanding substituents on the conformational stability of the collagen triple helix. J Am Chem Soc. 2012;134:17117–17124. doi: 10.1021/ja3066418. [DOI] [PubMed] [Google Scholar]
- 56.Stahl PJ, Yu SM. Encoding cell-instructive cues to PEG-based hydrogels via triple helical peptide assembly. Soft Matter. 2012;8:10409–10418. doi: 10.1039/C2SM25903F. [DOI] [PMC free article] [PubMed] [Google Scholar]; · This report showcased a new strategy to add bioactivities to synthetic hydrogels via triple helical strand hybridization of CMPs.
- 57.Lee HJ, Yu C, Chansakul T, Hwang NS, Varghese S, Yu SM, Elisseeff JH. Enhanced chondrogenesis of mesenchymal stem cells in collagen mimetic peptide-mediated microenvironment. Tissue Engineering Part A. 2008;14:1843–1851. doi: 10.1089/ten.tea.2007.0204. [DOI] [PubMed] [Google Scholar]
- 58.Mo X, An YJ, Yun CS, Yu SM. Nanoparticle-assisted visualization of binding interactions between collagen mimetic peptide and collagen fibers. Angew Chem Int Ed. 2006;45:2267–2270. doi: 10.1002/anie.200504529. [DOI] [PubMed] [Google Scholar]
- 59.Ravikumar M, Modery CL, Wong TL, Dzuricky M, Sen Gupta A. Mimicking adhesive functionalities of blood platelets using ligand-decorated liposomes. Bioconj Chem. 2012;23:1266–1275. doi: 10.1021/bc300086d. [DOI] [PubMed] [Google Scholar]
- 60.Matsusaki M, Amekawa R, Matsumoto M, Tanaka Y, Kubota A, Nishida K, Akashi M. Physical and specific crosslinking of collagen fibers by supramolecular nanogelators. Adv Mater. 2011;23:2957–2961. doi: 10.1002/adma.201101284. [DOI] [PubMed] [Google Scholar]; ·· This paper demonstrated that nanoparticles featuring folded triple helical CMPs can form adhesive physical crosslinks with collagen fibers.