Abstract
Objectives
Although T stage is an important prognostic tool for oral tongue cancer, it fails to define the depth of invasion and true three-dimensional volume of primary tumors. The purpose of this paper is to determine the relations between tumor volume and lymph node metastasis and survival in early oral tongue cancer.
Methods
Forty-seven patients with T1-2 tongue cancer were included. Tumor volumes were measured by the computerized segmentation of T2-weighted magnetic resonance imaging.
Results
The overall average tumor volume was 27.7 cm3 (range, 1.4 to 60.1 cm3). A significant positive correlation was found between tumor volume and pathological T stage, depth of invasion, and cervical lymph node metastasis (P<0.001, P<0.001, and P=0.002, respectively). When the tumor volume exceeded 20 cm3, the cervical metastasis rate increased to 69.2%. The overall 5-year disease specific survival rate was 80%. There was a statistically significant association between large tumor volume (≥20 cm3) and the 5-year disease-specific survival (P=0.046).
Conclusion
Tumor volume larger than 20 cm3 was associated with greater risk cervical lymph node metastasis and poor 5-year disease-specific survival rate in early oral tongue cancer patients.
Keywords: Tongue neoplasms, Tumor burden, Lymphatic metastasis, Computer-assisted image processing, Magnetic resonance imaging
INTRODUCTION
Tongue cancer shows various characteristics from relatively benign to highly aggressive with local invasion and distant metastasis, even in early stage disease [1]. This variability in clinical behavior has led to numerous studies being conducted on relationships between the clinical, pathological, and, more recently, between molecular factors and tumor aggressiveness. Tumor-node-metastasis (TNM) staging is extensively used to decide on treatment strategy, assess prognosis, and compare treatment results. TNM staging was devised by Pierre Denoix in 1944, and describes the extent of primary disease based on considerations of maximal diameter and anatomic location [2]. However, although T stage is an important prognostic tool for head and neck cancer, it fails to define the true three-dimensional (3D) volume of primary tumors, and thus, superficial tumors with a favorable prognosis can be allocated the same stages as unfavorable, deeply infiltrating lesions [3]. Furthermore, improvements in imaging techniques enable clinicians to define tumor extent and volume more accurately, and various studies have shown that tumor volumes determined using modern imaging techniques better prediction biological behavior than the T stage system [4-9].
Accordingly, the purpose of this study was to evaluate the potential usefulness of a computer based system to estimate tumor volumes, and to determine the nature of the relations between these and cervical nodal metastases and disease specific survival in patients with early squamous cell carcinoma (SCC) of the tongue.
MATERIALS AND METHODS
Patients and tumor samples
The clinical and pathological data of patients diagnosed with tongue carcinoma, who underwent surgery at the Department of Otolaryngology-Head and Neck Surgery, The Catholic University of Korea, Seoul from November 2003 to October 2009, were reviewed. The study group consisted of 47 patients with previously untreated T1 or T2 SCC of the tongue, whose original pathological specimens were available for evaluation, with complete follow-up records. Mean patient age was 53 years, mean follow-up duration was 35 months (range, 2 to 83 months), and the male-to-female ratio was 30:17. All patients had neck dissection at the time of the primary surgery: a radical neck dissection was performed in 3 cases, a modified radical neck dissection in 19 cases, and a selective (level I-III) neck dissection in 25 cases. Twenty nine patients (63%) had bilateral neck dissections when there was clinical or radiologic involvement of cervical lymph node disease on both sides. For the contralateral cases, selective (level I-III) neck dissection was performed in 25 patients and a modified radical neck dissection in 4. Indications and modalities for adjuvant treatments varied over time: positive or close margins found on the resection, lymphovascular invasion, perineural invasion, multiple nodal metastasis, or extracapsular spread were findings that required adjuvant radiotherapy. The depth of invasion was measured from the surface of mucosa to the maximum depth using an ocular micrometer. When there was exophytic tumor growth, the measurement was made from the height of the surface of the adjacent normal mucosa to the deepest-reaching front of infiltration. The Institutional Review Board of Seoul St. Mary's Hospital (Seoul) approved the retrospective review of medical records and the use of archived tumor specimens.
Imaging technique
All magnetic resonance (MR) studies were performed using a 1.5-T imaging system (Signa Excite, GE Medical System, Milwaukee, WI, USA) and a 4-cm loop coil. Fast spin echo sequences were used to obtain T1-weighted (T1W; repetition time range, 420 to 720 ms; echo time range, 10.2 to 14.5 ms), gadolinium contrast-enhanced, T1-weighted (CET1W) images with fat suppression, and T2-weighted (T2W; repetition time range, 4,000 to 5,400 ms; echo time range, 82.0 to 123.6 ms) images with fat saturation.
Tumor volume measurements
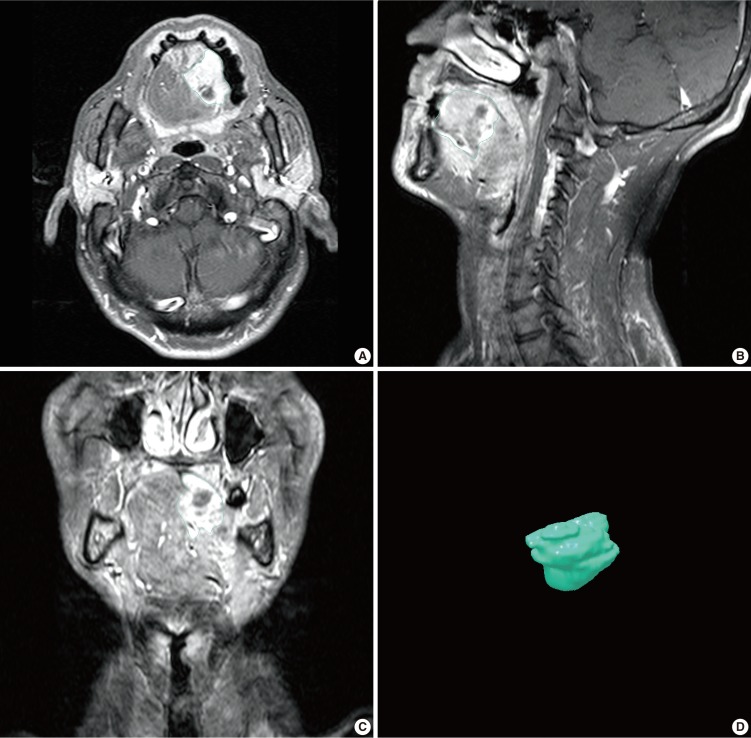
Imaging data were stored in Digital Imaging and Communication in Medicine (DICOM) format and imported to a personal computer. 3D-DOCTOR software (Able Software Co., 5 Appletree Lane, Lexington, MA, USA) was then used to reconstruct 3D images in accordance with anatomic boundaries (Fig. 1). Window widths and centers were varied, depending on the type of tissue being examined.
Fig. 1.
Magnetic resonance images of a patient with tongue cancer and 3-dimensional reconstructed tongue cancer image. The periphery of tumor is outlined on the magnetic resonance images for tumor volume estimation. (A) Axial image, (B) coronal image, (C) sagittal image, (D) reconstructed image.
The maximum diameter of each tongue tumor was determined using T2W MR images and 3D-DOCTOR software. Tumor borders were traced manually on axial images using a mouse-controlled cursor. We excluded the cases with tumors not seen on magnetic resonance imaging (MRI). The tumor volume was determined by 2 experienced head and neck surgeons, who independently traced the tumor outline manually. The software then generated a 3D model and calculated tumor mass volume in each case.
Statistical analysis
Continuous variables (tumor volume, tumor depth of invasion) were recorded as mean±SD, and were analyzed using the t-test. The chi-square test, Fisher exact test, multiple logistic regression analysis, multiple linear regression analysis, and correlation analysis were used, as appropriate, to identify significant relationships between categorical values. Overall survivals were calculated using the Kaplan-Meier method. The Cox proportional hazards model with likelihood ratio statistics was used to identify variables significantly and independently related to tumor volume. Significance was accepted for P<0.05. All calculations were performed using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics
Nineteen patients (40%) had T1 and 28 patients (60%) had T2 carcinoma. In terms of pathologic stage of the cervical lymph nodes, 28 patients (60%) were of N0, 5 patients (11%) of N1, 14 patients (29%) (1N2a, 10N2b, and 3N2c) of N2. Twelve patients (26%) had a positive or close resection margin (<5 mm). Extracapsular spread and lymphovascular invasion were both observed in 17 patients (36%). Postoperative radiotherapy was performed in 15 patients (32%). Depth of invasion averaged 9.2 mm (range, 1 to 23 mm), whereas tumor volumes averaged 27.7 cm3 (range, 1.4 to 60.1 cm3). Patient characteristics are summarized in Table 1.
Table 1.
Patients characteristics
Relationships between tumor volume and pathologic T stage and invasion depth
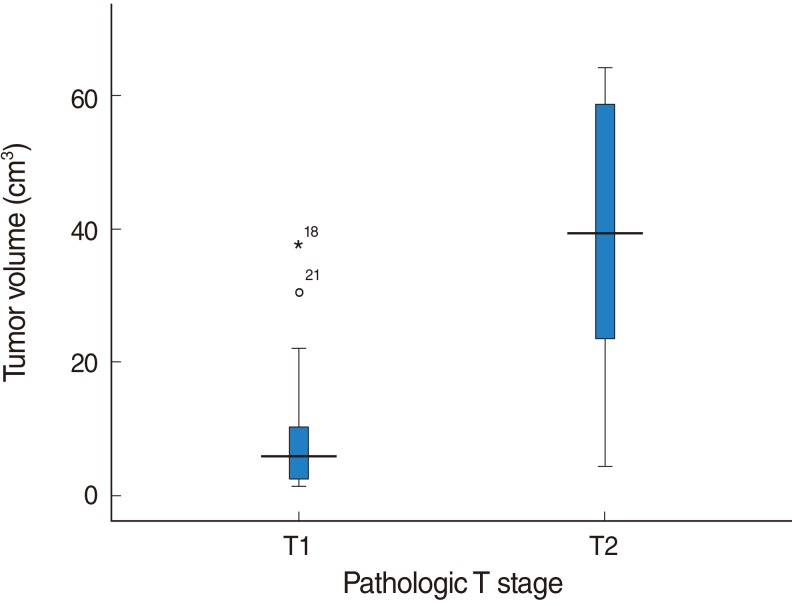
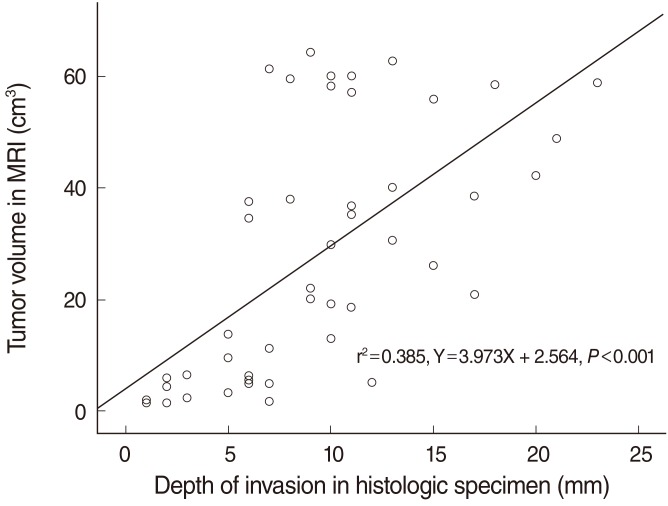
A significant positive correlation was found between tumor volume and T stage (P<0.001) (Fig. 2). Regarding the pathological T stages, the mean tumor volumes of stage T1 and T2 cancers were 9.49±10.46 cm3 and 40.06±19.18 cm3, respectively. A significant positive correlation was found between tumor volume and depth of invasion (P<0.001) (Fig. 3). The Pearson's correlation coefficient for the relation between tumor volume and depth of invasion was 0.385 and the least squares regression equation was Y=3.973X+2.564 (P<0.001).
Fig. 2.
Relationship between tumor volume and pathologic T stage. A significant relation was found between tumor volume and pathologic T stage. T1=9.49±10.46 cm3, T2=40.06±19.18 cm3, P<0.001.
Fig. 3.
Relationship between tumor volume and depth of invasion.
Relationship between tumor volume and cervical lymph node metastasis
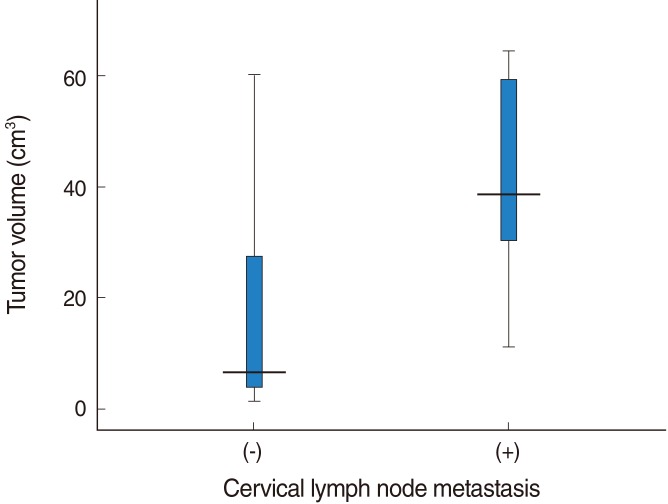
A significant positive correlation was found between tumor volume and the presence of cervical lymph node metastasis (P<0.001) (Fig. 4). The mean tumor volume in cases with cervical lymph node metastasis was 43.61±17.20 cm3, whereas that in cases without cervical lymph node metastasis was 16.91±18.34 cm3. No cervical metastasis was found in the patients with a tumor volume less than 10 cm3. The nodal metastasis rate was 33% (10-15 cm3), 0% (15-20 cm3), 67% (20-25 cm3), and 100% (25-30 cm3), respectively. When the tumor volume exceeded 20 cm3, the cervical metastasis rate increased to 69.2% (18/26).
Fig. 4.
Relationship between tumor volume and cervical lymph node metastasis. A significant relation was found between tumor volume and the presence of cervical lymph node metastasis (tumor volume in cases with cervical lymph node metastasis=43.61±17.20 cm3, tumor volume in cases without cervical lymph node metastasis=16.91±18.34 cm3; P<0.001).
Relationship between tumor volume and survival rate
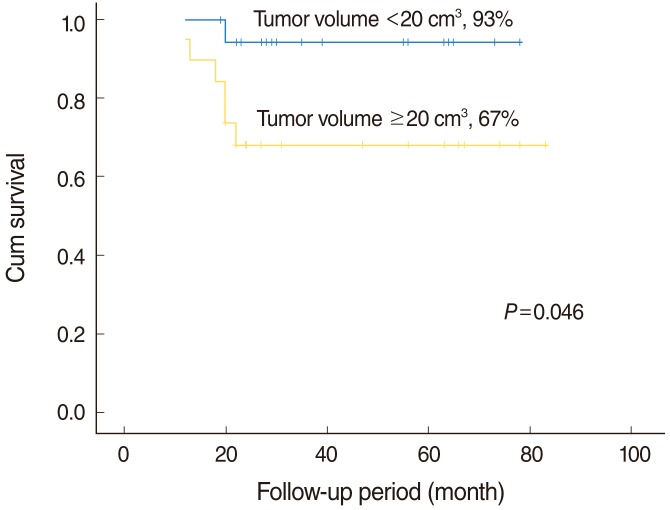
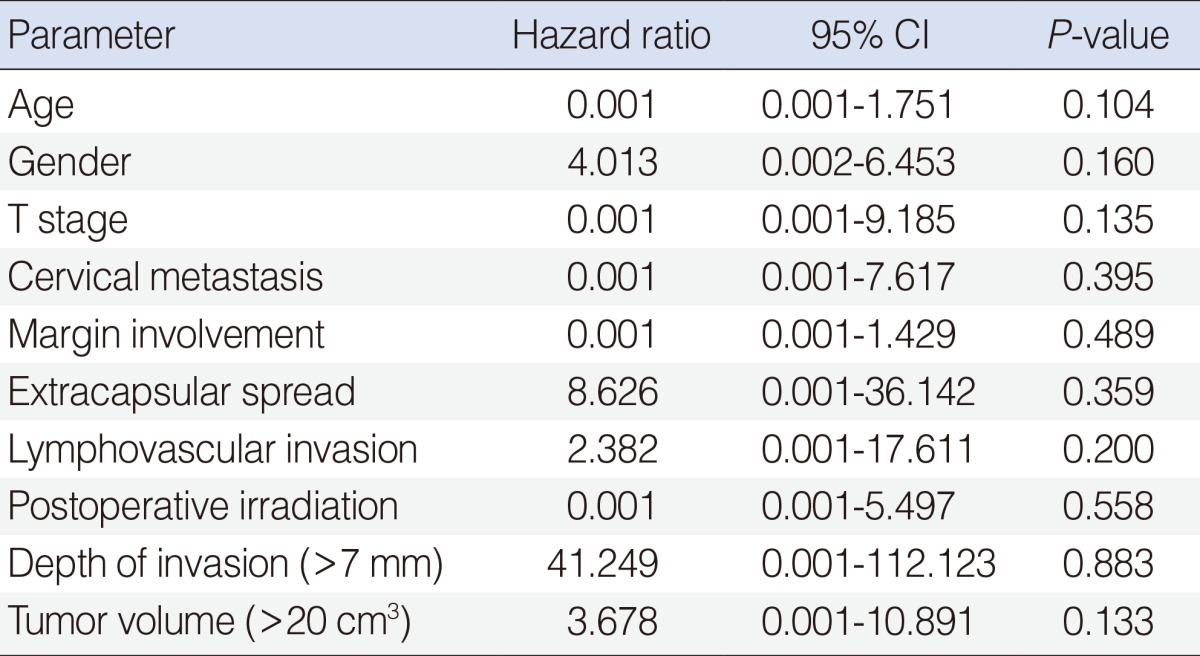
In those 37 patients followed for more than 12 months, the overall 5-year disease specific survival rate was 80%. Tumor volume was found to be significantly associated with disease specific survival (P=0.046). The 5-year disease-specific survival among 19 patients with a large tumor (≥20 cm3) was 67%, whereas for the 18 with a small tumor (<20 cm3) patients it was 93% (Fig. 5). Pathologic T stage (P=0.049), nodal metastasis (P=0.010), depth of tumor invasion (using 7 mm as a cut-off; P=0.023), and extracapsular spread (P=0.038) were found to be associated with 5-year disease specific survival, but gender (P=0.801), age (P=0.238), lymphovascular invasion (P=0.355), postoperative irradiation (P=0.484) and margin involvement (P=0.763) were not by univariate analysis. However, no factor was found to influence 5-year disease specific survival by multivariate analysis (Table 2). After ablative surgery, 8 patients (17%) had recurrences, including three local, three regional, and two distant metastases. Of the three patients who had local recurrences, one patient received surgery and radiotherapy but died of the disease; other two patients underwent surgery and has no evident disease 30 and 83 months, respectively. The one patient with regional recurrence underwent surgery and concurrent chemoradiotherapy and has no evident disease 56 months but other two patients died of the disease. All of the patients with distant metastases died of the disease.
Fig. 5.
Kaplan-Meier 5-year disease specific survival curves according to the tumor volume. There was a statistically significant association between tumor volume and the 5-year disease-specific survival (the 5-year disease-specific survival with a large tumor.
Table 2.
Cox proportional hazards analysis for survival
DISCUSSION
Accurate tumor staging is crucial for the management of tongue cancer. However, staging based on tumor diameter is only marginally related to the risk of subclinical nodal metastasis, local recurrence, and survival, especially for early T1 and T2 cancers. Accordingly, better prognostic indicators have been sought to guide clinical management, particularly in terms of the prediction of lymph node metastasis. Previous studies have shown that tumor depth of invasion and the presence of lymph node metastasis are the most important prognostic factors in patients with tongue cancer [10,11]. Spiro et al. [10] suggested that tumor depth should be incorporated into any staging system and considered when deciding on treatment. Tumor depth of invasion is excluded in the TNM staging system despite its importance in disease prognosis, therefore a new revised staging system has been proposed in the present study. However, we still do not have a tool that is accurate enough to predict the presence of cervical metastasis in patients with clinically negative cervical lymph nodes (N0). Furthermore, some surgeons favor elective neck dissection or elective neck irradiation, even for clinically N0 patients when they believe there is an elevated risk of nodal metastasis after considering factors, such as, T stage and depth of invasion. Concerning tumor volume, in this study, cervical lymph node metastasis was found starting at a tumor volume exceeding 10 cm3 (n=32). The overall cervical metastasis rate in patients with a tumor volume larger than 10 cm3 was 59.4% (19/32). The incidence of cervical metastasis increased when the tumor volume exceeded 20 cm3 (18/26, 69.2%). Based on our results for early tongue cancers exceeding 20 cm3, neck treatment (surgery or irradiation) should be considered.
Solid tumors are 3D structures with unequal rates of tumor spread in different directions and planes, and thus, diameter does not accurately reflect total tumor volume or the total malignant cell burden. However, advanced imaging and data analysis techniques now allow accurate preoperative assessments to be made of the 3D extents of tumors. In particular, MRI provides higher resolution images of the detailed architecture than either computed tomography or sonography, especially with respect to 3D observations of soft-tissue lesions [12]. Furthermore, tongue cancers are hyperintense as compared with normal tongue tissue and tumors and mucosal epithelium, lamina propria, and tongue muscles are well differentiated. In the present study, tumor volumes were assessed objectively using a computerized segmentation technique of T2W MR images.
Tumor volume is a significant prognostic factor in the treatment of malignant tumors [13,14]. Multivariate modeling has shown that tumor volume is a dominant covariate that overwhelms T stage, N stage, and stage group [15-18]. Kuriakose et al. [19] reported 20 tongue cancer and showed that tumor volume correlated well with nodal metastases risk, treatment failure rate, and disease-specific survival. Chew et al. [20], in a series of 17 patients with tongue cancer, showed that larger tumor volume ≥13 mL has poorer disease free survival and overall survival. However, Been et al. [21] reported that tumor volume does not appear to play a significant role in predicting locoregional recurrence for patients with primary squamous cell cancer of the oropharynx treated with primary radiotherapy. In the present study, the mean tumor volume of T2 cancers was significantly larger than that of T1 cancers, and tumor volume was found to be significantly and positively correlated with depth of invasion. In addition, a large tumor volume (≥20 cm3) was found to present a significantly greater risk of cervical lymph node metastases and to have a significantly lower disease specific survival rate than a small tumor volume (<20 cm3), which suggests that tumor volume could be used as a prognostic indicator and as the basis of a new staging method in early tongue cancer.
Some limitations of this study warrant mention. First, the study is inherently limited by its retrospective nature. Second, the cohort size was relatively small, and third, the follow-up was rather short. A further larger-scale study is required to confirm our finding that tumor volume influences prognosis and to probe the reason for differences between our findings and those of previous studies.
In conclusion, in the present study, tumor volume larger than 20 cm3 was found to be significantly related to the presence of cervical lymph node metastasis and disease specific survival in early tongue cancer, which suggests that could be used to predict the presence of lymph node metastasis and to determine neck treatment and prognosis.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Guttman D, Stern Y, Shpitzer T, Ulanovski D, Druzd T, Feinmesser R. Expression of MMP-9, TIMP-1, CD-34 and factor-8 as prognostic markers for squamous cell carcinoma of the tongue. Oral Oncol. 2004 Sep;40(8):798–803. doi: 10.1016/j.oraloncology.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Pameijer FA, Balm A, Hilgers F, Muller SH. Variability of tumor volumes in T3-staged head and neck tumors. Head Neck. 1997 Jan;19(1):6–13. doi: 10.1002/(sici)1097-0347(199701)19:1<6::aid-hed2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Johnson CR, Khandelwal SR, Schmidt-Ullrich RK, Ravalese J, III, Wazer DE. The influence of quantitative tumour volume measurements on local control in advanced head and neck cancer using concomitant boost accelerated superfractionated irradiation. Int J Radiat Oncol Biol Phys. 1995 Jun;32(3):635–641. doi: 10.1016/0360-3016(95)00031-S. [DOI] [PubMed] [Google Scholar]
- 4.Brenner DE, Whitley NO, Houk TL, Aisner J, Wiernik P, Whitley J. Volume determinations in computed tomography. JAMA. 1982 Mar;247(9):1299–1302. [PubMed] [Google Scholar]
- 5.Breiman RS, Beck JW, Korobkin M, Glenny R, Akwari OE, Heaston DK, et al. Volume determinations using computed tomography. AJR Am J Roentgenol. 1982 Feb;138(2):329–333. doi: 10.2214/ajr.138.2.329. [DOI] [PubMed] [Google Scholar]
- 6.Lee RW, Mancuso AA, Saleh EM, Mendenhall WM, Parsons JT, Million RR. Can pretreatment computed tomography findings predict local control in T3 squamous cell carcinoma of the glottic larynx treated with radiotherapy alone? Int J Radiat Oncol Biol Phys. 1993 Mar;25(4):683–687. doi: 10.1016/0360-3016(93)90016-o. [DOI] [PubMed] [Google Scholar]
- 7.Freeman DE, Mancuso AA, Parsons JT, Mendenhall WM, Million RR. Irradiation alone for supraglottic larynx carcinoma: can CT findings predict treatment results? Int J Radiat Oncol Biol Phys. 1990 Aug;19(2):485–490. doi: 10.1016/0360-3016(90)90562-x. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert RW, Birt D, Shulman H, Freeman J, Jenkin D, MacKenzie R, et al. Correlation of tumour volume with local control in laryngeal carcinoma treated by radiation therapy. Ann Otol Rhinol Laryngol. 1987 Sep-Oct;96(5):514–518. doi: 10.1177/000348948709600507. [DOI] [PubMed] [Google Scholar]
- 9.Mukherji SK, Mancuso AA, Mendenhall W, Kotzur IM, Kubilis P. Can pretreatment CT predict local control of T2 glottic carcinomas treated with radiation therapy alone? AJNR Am J Neuroradiol. 1995 Apr;16(4):655–662. [PMC free article] [PubMed] [Google Scholar]
- 10.Spiro RH, Huvos AG, Wong GY, Spiro JD, Gnecco CA, Strong EW. Predictive value of tumor thickness in squamous carcinoma confined to the tongue and floor of the mouth. Am J Surg. 1986 Oct;152(4):345–350. doi: 10.1016/0002-9610(86)90302-8. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Cho NH, Kim K, Lee JS, Koo BS, Kim JH, et al. Correlations of oral tongue cancer invasion with matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF) expression. J Surg Oncol. 2006 Mar;93(4):330–337. doi: 10.1002/jso.20461. [DOI] [PubMed] [Google Scholar]
- 12.Iwai H, Kyomoto R, Ha-Kawa SK, Lee S, Yamashita T. Magnetic resonance determination of tumour thickness as predictive factor of cervical metastasis in oral tongue carcinoma. Laryngoscope. 2002 Mar;112(3):457–461. doi: 10.1097/00005537-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Brenner DJ. Dose, volume and tumor control predictions in radiotherapy. Int J Radiat Oncol Biol Phys. 1993 Apr;26(1):171–179. doi: 10.1016/0360-3016(93)90189-3. [DOI] [PubMed] [Google Scholar]
- 14.Johnson CR, Thames HD, Huang DT, Schmidt-Ullrich RK. The tumor volume and clonogen number relationship: Tumor control predictions based upon tumor volume estimates derived from computed tomography. Int J Radiat Oncol Biol Phys. 1995 Sep;33(2):281–287. doi: 10.1016/0360-3016(95)00119-j. [DOI] [PubMed] [Google Scholar]
- 15.Chua DT, Sham JS, Kwong DL, Tai KS, Wu PM, Lo M, et al. Volumetric analysis of tumor extent in nasopharyngeal carcinoma and correlation with treatment outcome. Int J Radiat Oncol Biol Phys. 1997 Oct;39(3):711–719. doi: 10.1016/s0360-3016(97)00374-x. [DOI] [PubMed] [Google Scholar]
- 16.Willner J, Baier K, Pfreundner L, Flentje M. Tumor volume and local control in primary radiotherapy of nasopharyngeal carcinoma. Acta Oncol. 1999;38(8):1025–1030. doi: 10.1080/028418699432301. [DOI] [PubMed] [Google Scholar]
- 17.Johnson CR, Khandelwal SR, Schmidt-Ullrich RK, Ravalese J, 3rd, Wazer DE. The influence of quantitative tumor volume measurements on local control in advanced head and neck cancer using concomitant boost accelerated superfractionated irradiation. Int J Radiat Oncol Biol Phys. 1995 Jun;32(3):635–641. doi: 10.1016/0360-3016(95)00031-S. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert RW, Birt D, Shulman H, Freeman J, Jenkin D, MacKenzie R, et al. Correlation of tumor volume with local control in laryngeal carcinoma. Ann Otol Rhinol Laryngol. 1987 Sep-Oct;96(5):514–518. doi: 10.1177/000348948709600507. [DOI] [PubMed] [Google Scholar]
- 19.Kuriakose MA, Loree TR, Hicks WL, Welch JJ, Wang H, DeLacure MD. Tumour volume estimated by computed tomography as a predictive factor in carcinoma of the tongue. Br J Oral Maxillofac Surg. 2000 Oct;38(5):460–465. doi: 10.1054/bjom.2000.0316. [DOI] [PubMed] [Google Scholar]
- 20.Chew MH, Khoo JB, Chong VF, Tai BC, Soo KC, Lim DT. Significance of tumour volume measurements in tongue cancer: a novel role in staging. ANZ J Surg. 2007 Aug;77(8):632–637. doi: 10.1111/j.1445-2197.2007.04176.x. [DOI] [PubMed] [Google Scholar]
- 21.Been MJ, Watkins J, Manz RM, Gentry LR, Leverson GE, Harari PM, et al. Tu mor volume as a prognostic factor in oropharyngeal squamous cell carcinoma treated with primary radiotherapy. Laryngoscope. 2008 Aug;118(8):1377–1382. doi: 10.1097/MLG.0b013e318172c82c. [DOI] [PubMed] [Google Scholar]