Abstract
Objectives
To correlate Frey's syndrome with subjective symptoms, Minor's starch iodine test results, and infrared thermography measurements, and to discuss the utility of thermography as a quantitative diagnostic method.
Methods
This study included 59 patients who underwent unilateral parotidectomy. A subjective clinical questionnaire and an objective Minor's starch iodine test were performed to evaluate the incidence of Frey's syndrome. Infrared thermography was performed, and the subjects were divided into seven groups according to the temperature differences between operated and unoperated sites. The thermal differences were correlated with the results from Minor's starch iodine test and the subjective symptoms questionnaire.
Results
Of the 59 patients, 20 patients (33.9%) reported subjective symptoms after eating; 30 patients (50.8%) tested positive for Minor's starch iodine test, 19 patients (63.3%) of which reported subjective symptoms. Of the 29 patients who were negative for the iodine test, 2 patients (6.9%) reported subjective symptoms. Thus, subjective symptoms were well correlated with Minor's starch iodine test (r=0.589, P<0.001). As the thermal differences with infrared thermography increased, the number of patients with subjective symptoms increased (χ2=22.5, P<0.001). Using infrared thermography, the mean temperature difference in the positive group for the iodine test was 0.82℃±0.26℃, and that in the negative group was 0.10℃±0.47℃. With increased thermal differences, more patients showed positivity in the iodine test (χ2=29.9, P<0.001).
Conclusion
Subjective symptoms, Minor's starch iodine test, and infrared thermography are well correlated with one another. Quantitative thermography provides clues for the wide variation in the incidence of Frey's syndrome, and could be a useful method for diagnosing and studying Frey's syndrome.
Keywords: Frey's syndrome, Gustatory, Parotid gland, Sweating, Thermography
INTRODUCTION
Frey's syndrome, also known as gustatory sweating, is one of the most prevalent late complications of parotidectomy. It was first reported in 1923 to describe the aberrant regeneration of sectioned postganglionic parasympathetic cholinergic fibers that were misdirected to innervate the vessels and sweat glands of the skin overlying the parotid [1]. Severed parasympathetic fibers during parotidectomy can damage the secretion activity of the parotid gland. During the regeneration process, parasympathetic fibers may grow along the distal ends of sympathetic fibers to the skin vessels and sweat glands. Thus, a gustatory stimulus produces sweating accompanied by flushing and heating [2]. Symptoms usually crop up 6 weeks after surgery when the injured nerve is regenerated [3].
The incidence of gustatory sweating after parotidectomy varies among reports. Gooden et al. [1] reported that the incidence of Frey's sydrome was 2%-96% depending on subjective symptoms and Minor's starch iodine test, which was thought to be the gold standard of diagnosis [4,5]. Although the test allows visual confirmation of the blue-black reaction in the sweating area, it does not provide quantitative information about Frey's syndrome. Thermography is a method for diagnosing Frey's syndrome with a quantitative thermal difference between operated and unoperated facial regions [6]. In the present study, we compared subjective symptoms, Minor's starch iodine test, and infrared thermography. We discuss the importance of thermography for quantitative diagnoses.
MATERIALS AND METHODS
A total of 59 patients who underwent unilateral parotidectomy from August 2008 to January 2012 were included in this study. Exclusion criteria were: treatment with medication affecting body perspiration; less than 6 weeks of postoperative recuperation time; bilateral parotid disease; bilateral parotidectomy; and previous history of facial trauma, head and neck surgery, or radiation therapy.
Each patient completed the symptoms questionnaire (Appendix) regarding Frey's syndrome during a 12-month follow-up visit after parotidectomy, then took a Minor's starch iodine test. Next, infrared thermography was performed with gustatory stimuli 2 hours after the iodine test.
The symptoms questionnaire queried whether the patients felt facial flushing, pain, or sweating after eating; when the symptoms first appeared after surgery; and how much these symptoms affected daily life. The iodine test was administered by applying an alcohol-iodine-oil solution (3 g iodine, 20 mL castor oil, and 200 mL absolute alcohol) described by Laage-Hellman [7] to facial regions in patients. The solution was applied on the lateral portion of the face that had been surgically treated and the upper region of the neck. The solution was allowed to dry and was covered lightly with starch powder. The patients received lemon candy for a gustatory stimuli for 10 minutes. Blue discoloration of the starch iodine mixture was interpreted as a positive finding for Frey's syndrome.
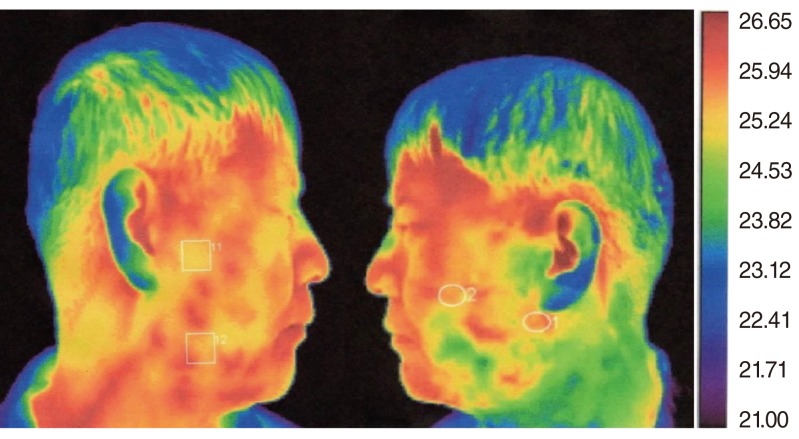
The patients were assessed with infrared thermography (IRIS-500 Digital Infrared Imaging System, Medicore Co., Seoul, Korea) to measure the skin temperature of the operated and unoperated sites (Fig. 1). Patients waited for 15 minutes in a 22℃ temperature-controlled room that did not allow exposure to exterior wind or light. Patients were given a lemon candy for sialogogue. At 3 minutes after gustatory stimulation, the highest temperature point of the preauricular area was recorded and the contralateral side was also measured at the same time, which served as an internal control for each patient. We distinguished seven groups according to the range of temperature differences between operated and unoperated sites (temperature of the operated site temperature - temperature of the unoperated site): group A (-0.9℃ to -0.6℃), group B (-0.6℃ to -0.3℃), group C (-0.3℃ to 0℃), group D (0℃ to 0.3℃), group E (0.3℃ to 0.6℃), group F (0.6℃ to 0.9℃), and group G (0.9℃ to 1.2℃).
Fig. 1.
Infrared thermography. Right face: high temperature at site of parotidectomy. Left face: low temperature at unoperated site.
Paired t-test was used to analyze the thermal difference between the operated and unoperated sites. Fisher exact test was used for calculating the subjective symptoms and Minor's starch iodine test results. Linear by linear association was used to calculate the correlation of infrared thermography with the subjective symptoms and Minor's starch iodine test results. These results were statistically analyzed using the software SPSS ver. 20.0 (IBM Co., Armonk, NY, USA). This study was conducted with the approval of the Institutional Review Board (Hallym IRB No. 2013-I042).
RESULTS
General results
Among 59 patients (26 females, 33 males), 56 patients had a superficial parotidectomy and 3 patients underwent a total parotidectomy. The average age at presentation was 47.3±14.8 years (range, 16 to 88 years). Histopathological results were pleomorphic adenoma in 49 patients (83.1%) and Warthin's tumor in 10 patients (16.9%).
Main results
Of the respondents to the subjective symptoms questionnaire, 20 patients (33.9%) felt facial flushing, pain, or sweating after eating. Among these patients, 6 patients (10.2%) felt symptoms immediately after the operation, 2 patients (3.4%) felt symptoms within 1 month after the operation, 1 patient (1.7%) felt symptoms between 1 and 6 months postoperatively, and 11 patients (18.6%) were not sure exactly when the symptoms began. In total, 17 patients (28.8%) responded that they were not affected by these symptoms in daily life, but 3 patients (5.1%) reported that the symptoms caused a large degree of frustration. While 30 patients (50.8%) were positive in the Minor's starch iodine test. Mean temperature of the operated site was 25.45℃±1.02℃, and that of the unoperated site was 26.07℃±1.26℃ with infrared thermography before the gustatory stimulation. Before the gustatory stimulation, the operated site showed a lower temperature than the unoperated site, but this lower temperature was not statistically significant (P=0.261). However, the mean temperature of the operated site was 26.67℃±1.34℃, and that of the unoperated site was 26.20℃±1.41℃ with infrared thermography 3 minutes after gustatory stimulation. The operated site had a higher temperature than the unoperated site, without statistical significance, after gustatory stimulation (P=0.728). The highest temperature difference (operated site - unoperated site) was 1.16℃, while the lowest difference was -0.84℃. The number of included patients for each group was 3, 2, 8, 9, 7, 18, and 12 for groups A to G, respectively, according to the 0.3℃ temperature differences from -0.9℃ to 1.2℃.
A total of 19 of the 21 patients (90.5%) who had subjective symptoms were positive in Minor's starch iodine test, whereas only 11 of the 38 patients (28.9%) who had not subjective symptoms were positive in the test. There was significant difference of Minor's starch iodine test results between patients who had subjective symptoms and who had not subjective symptoms (P<0.001) (Table 1).
Table 1.
No. of patients according to the subjective symptoms and Minor's starch iodine test
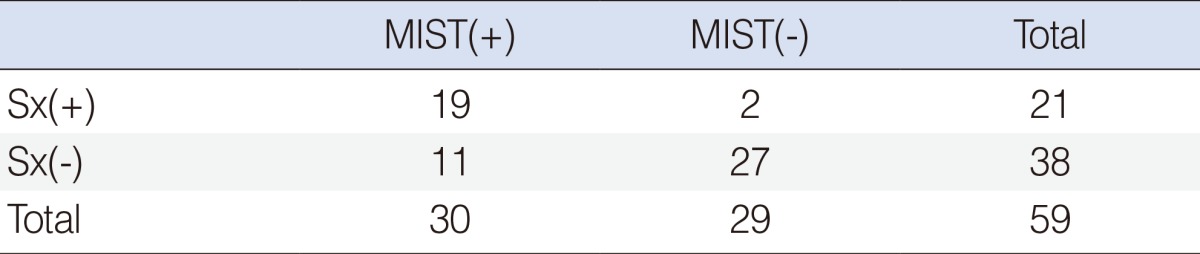
Sx(+), patients who had subjective symptoms; Sx(-), pateints who do not have subjective symptoms; MIST(+), patients who were poisitive in Minor's starch iodine test; MIST(-), patients who were negative in Minor's starch iodine test.
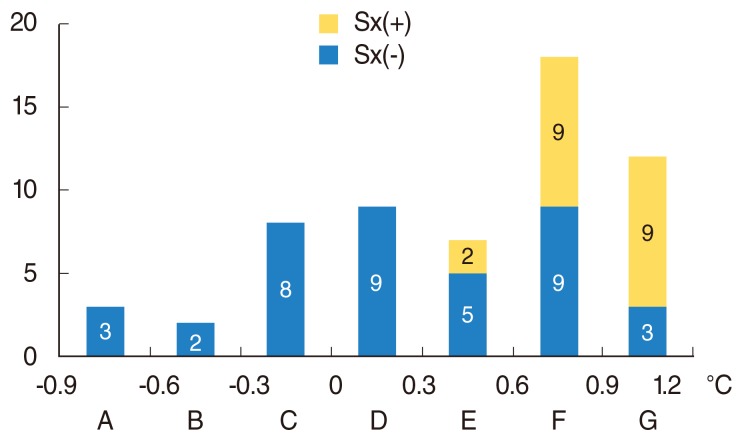
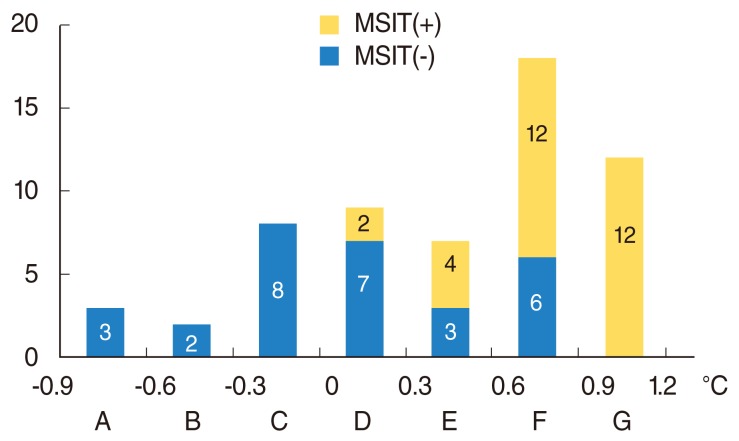
All of the patients in groups A-D, whose thermal differences were less than 0.3℃, had no symptoms. However, 2 of 7 patients (28.6%) in group E, 9 of 18 patients (50%) in group F, and 9 of 12 patients (75%) in group G reported symptoms. As the thermal differences increased, more patients had subjective symptoms (χ2=22.5, P<0.001) (Fig. 2). The mean temperature difference in the group that was positive in the iodine test was 0.82℃±0.26℃, and that in the negative group was 0.10℃±0.47℃ with infrared thermography. All patients in groups A, B, and C, whose thermal differences were less than 0℃, were negative in this test. However, 2 of 9 patients (22.2%) in group D, 4 of 7 patients (57.1%) in group E, 12 of 18 patients (66.7%) in group F, and 12 of 12 patients (100%) in group G tested positive in the test. As the thermal difference with infrared thermography increased, more patients were positive in this test (χ2=29.9, P<0.001) (Fig. 3).
Fig. 2.
The number of patients according to the thermal differences (group A to G) between operated and unoperated sites. Sx(+), patients who had subjective symptoms; Sx(-), pateints who do not have subjective symptoms.
Fig. 3.
The number of patients according to the thermal differences (group A to G) between operated and unoperated sites. MIST(+), patients who were poisitive in Minor's starch iodine test; MIST(-), patients who were negative in Minor's starch iodine test.
DISCUSSION
Infrared thermography is a good diagnostic method for Frey's syndrome [6]. Previous reports recorded the biphasic responses in terms of hot and cold spots; a hot spot resulting from capillary dilatation appeared in the initial stage, and a cold spot due to gustatory sweating was seen in the later stage of Frey's syndrome. In this report, we examined the hot spot 3 minutes after gustatory stimulation and measured the thermal differences between operated and unoperated sites.
Thermography provides quantitative information for Frey's syndrome. This study aimed to correlate the quantitative test of thermography with the reported symptoms and Minor's starch iodine test results. Only the patients in groups E, F, and G reported symptoms, and the incidence of symptoms was higher in the groups with larger thermal differences (Fig. 2). Thus, the patients who showed larger temperature differences had a higher likelihood of feeling symptoms. However, some patients (3/9, 25%) in group G with high thermal differences did not report symptoms, and other patients (2/7, 28.6%) in group E with relatively low thermal differences reported symptoms. It is inferred that a sensitivity difference between patients can explain the varying incidences of feeling subjective symptoms. Only 10% of patients felt symptoms spontaneously, compared to 30%-50% of patients in other reports [5,8]. The patient group that tested positive for Minor's starch iodine test showed larger thermal differences (0.82℃±0.26℃) than the negative group (0.10℃±0.47℃; P=0.003). All of the patients in group G tested positive in that test. However, some patients (6/18, 33.3%) in group F tested negative despite a thermal difference of more than 0.6℃ (Fig. 3). It appears that gustatory sweating does not always follow capillary dilatation. Some patients (2/9, 22.2%) in group D were positive in Minor's starch iodine test with less than 0.3℃ of temperature difference. These results indicate that gustatory sweating in some patients can be stimulated with a limited capillary response.
Previous reports have indicated that the incidence of Frey's syndrome varies widely [1,4,5]. This variation may be due to differences in surgical techniques, methods for evaluating gustatory sweating, timing of the evaluation, duration of the follow-up, and the quantity and quality of the sweat [5,9]. The present study suggests that the sensitivity difference between patients and the different capillary dilatation and gustatory sweating responses to gustatory stimulation contribute to the wide variation in the incidence of Frey's syndrome.
A previous study reported a good correlation between Minor's starch iodine test and clinical complaints [3]. However, this test is qualitative, and does not quantify Frey's syndrome [5]. Thermography shows quantitative differences in temperature. Our results showed that positivity in Minor's starch iodine test was well correlated with symptoms and quantitative temperature differences in affected patients. Thermography has an advantage in that it is a faster test than the iodine test; moreover, it does not require preprocedure skin contact with starch or iodine. However, thermography requires expensive equipment such as the IRIS-500. The price of this equipment is US$40,000, whereas the starch and iodine solution used in Minor's starch iodine test are very inexpensive.
In conclusions, subjective symptoms, Minor's starch iodine test, and infrared thermography were well correlated with one another. Quantitative thermographic measurements provided clues for the wide variation in the incidence of Frey's syndrome. Therefore, thermography could be a useful method for diagnosing and studying Frey's syndrome.
Appendix
Subjective symptoms questionnaire

Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Gooden EA, Gullane PJ, Irish J, Katz M, Carroll C. Role of the sternocleidomastoid muscle flap preventing frey's syndrome and maintaining facial contour following superficial parotidectomy. J Otolaryngol. 2001 Apr;30(2):98–101. doi: 10.2310/7070.2001.19876. [DOI] [PubMed] [Google Scholar]
- 2.Allison GR, Rappaport I. Prevention of frey's syndrome with superficial musculoaponeurotic system interposition. Am J Surg. 1993 Oct;166(4):407–410. doi: 10.1016/s0002-9610(05)80343-5. [DOI] [PubMed] [Google Scholar]
- 3.Santos RC, Chagas JF, Bezerra TF, Baptistella JE, Pagani MA, Melo AR. Frey syndrome prevalence after partial parotidectomy. Braz J Otorhinolaryngol. 2006 Jan-Feb;72(1):112–115. doi: 10.1016/S1808-8694(15)30042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owen ER, Banerjee AK, Kissin M, Kark AE. Complications of parotid surgery: the need for selectivity. Br J Surg. 1989 Oct;76(10):1034–1035. doi: 10.1002/bjs.1800761016. [DOI] [PubMed] [Google Scholar]
- 5.Sood S, Quraishi MS, Bradley PJ. Frey's syndrome and parotid surgery. Clin Otolaryngol Allied Sci. 1998 Aug;23(4):291–301. doi: 10.1046/j.1365-2273.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- 6.Isogai N, Kamiishi H. Application of medical thermography to the diagnosis of frey's syndrome. Head Neck. 1997 Mar;19(2):143–147. doi: 10.1002/(sici)1097-0347(199703)19:2<143::aid-hed10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Laage-Hellman JE. Gustatory sweating and flushing after conservative parotidectomy. Acta Otolaryngol. 1957 Sep;48(3):234–252. doi: 10.3109/00016485709124377. [DOI] [PubMed] [Google Scholar]
- 8.de Bree R, van der Waal I, Leemans CR. Management of frey syndrome. Head Neck. 2007 Aug;29(8):773–778. doi: 10.1002/hed.20568. [DOI] [PubMed] [Google Scholar]
- 9.Laage-Hellman JE. Gustatory sweating and flushing; aetiological implications of latent Period and mode of development after parotidectomy. Acta Otolaryngol. 1958 Jul-Aug;49(4):306–314. doi: 10.3109/00016485809134759. [DOI] [PubMed] [Google Scholar]