Abstract
Background
The American Heart Association recently defined ideal cardiovascular health by simultaneous presence of seven health behaviors and factors. The concept is associated with cardiovascular disease incidence, and cardiovascular disease and all-cause mortality. To effectively promote ideal cardiovascular health already early in life, childhood factors predicting future ideal cardiovascular health should be investigated. Our aim was thus to comprehensively explore childhood determinants of adult ideal cardiovascular health in population based cohorts from three continents.
Methods
The sample comprised a total of 4409 participants aged 3–19 years at baseline from the Cardiovascular Risk in Young Finns Study (YFS; N=1883) from Finland, Childhood Determinants of Adult Health Study (CDAH; N=1803) from Australia and Princeton Follow-up Study (PFS; N=723) from the United States. Participants were re-examined 19–31 years later when aged 30–48 years.
Results
In multivariable analyses, independent childhood predictors of adult ideal cardiovascular health were family socioeconomic status (P<0.01; direct association) and BMI (P<0.001; inverse association) in all cohorts. In addition, blood pressure (P=0.007), LDL-cholesterol (P<0.001) and parental smoking (P=0.006) in the YFS, and own smoking (P=0.001) in CDAH were inversely associated with future ideal cardiovascular health.
Conclusions
Among several lifestyle and clinical indicators studied, higher family socioeconomic status and non-smoking (parental/own) in childhood independently predict ideal cardiovascular health in adulthood. As atherosclerotic cardiovascular diseases are rooted in childhood, our findings suggest that special attention could be paid to children who are from low socioeconomic status families, and who smoke or whose parents smoke, to prevent cardiovascular disease morbidity and mortality.
Keywords: children, cardiovascular diseases, epidemiology, risk factors, prevention
INTRODUCTION
In setting strategic impact goals for 2020, the American Heart Association (AHA) charted a new direction by focusing not only on preventing cardiovascular events but also on promoting cardiovascular health. The goal was to help everyone to move toward ideal cardiovascular health by focusing on seven ideal health metrics that can be used in monitoring cardiovascular health over time.1 Ideal cardiovascular health was defined as the simultaneous presence of four ideal health behaviors (never smoked or quit >12 months ago, body mass index [BMI]<25 kg/m2, physical activity at goal levels, and diet consistent with current guideline recommendations) and three ideal health factors (untreated total cholesterol <5.17 mmol/l [<200mg/dL], untreated blood pressure <120mmHg/<80mmHg and untreated fasting plasma glucose <5.6 mmol/l [<100mg/dL]).
Recent studies have described that the prevalence of ideal cardiovascular health is very low.2–4 Meeting a greater number of ideal cardiovascular health metrics in adulthood is associated with a lower risk of cardiovascular disease (CVD) incidence and mortality of all causes and diseases of the circulatory system.3, 5–7 Recently, the ideal cardiovascular health concept was also inversely associated with incident cancer.8 We have previously shown in the Cardiovascular Risk in Young Finns study that the number of ideal cardiovascular health metrics present in childhood is associated with a lower risk of hypertension, high LDL-cholesterol, metabolic syndrome and high-risk intima-media thickness in adulthood.9 These evidence support the application of the AHA ideal cardiovascular health concept for cardiovascular risk assessment. To effectively promote ideal cardiovascular health already early in life, childhood factors predicting future cardiovascular health should be investigated.
Using data from Finnish, Australian and American cohorts followed-up for 19–31 years since childhood, we examined a comprehensive set of CVD risk factors in childhood as predictors of ideal cardiovascular health in adulthood. Since the initiation of atherosclerotic CVD begins in childhood, these data may have implications in the early prevention of CVDs.
METHODS
The study comprised 4409 participants from three international cohort studies conducted in Finland (YFS), Australia (CDAH), and USA (PFS). Each study was approved by local ethics committees and all participants provided written informed consent. All laboratory measurements were performed using fasting blood samples. For the statistical analyses, we included those participants with data on CVD risk factors in childhood and ideal cardiovascular health metrics in adulthood.
The Cardiovascular Risk in Young Finns study (YFS)
The YFS sample and methods have been described in detail elsewhere.10 In this study, the sample comprised 1883 participants aged 3–18 years at baseline (1980) who provided ideal cardiovascular health metrics data when aged 30–45 years (2007).
Childhood risk factors
All serum lipid level determinations were performed with standard methods.11 Blood pressure was measured using a standard mercury sphygmomanometer. Information on dietary habits, physical activity, participant’s own smoking in childhood, parental smoking and parental socioeconomic status were obtained with questionnaires.11 Children who reported that they have never smoked or have quit smoking were classified as non-smokers and children who reported current smoking as smokers. The length of parent’s education (in years), parental occupation, and family annual income were considered as indicators of socioeconomic status in childhood. Parental occupation was coded from 1 to 5 (1=farmers, 2=lower manual, 3=upper manual, 4=lower non-manual 5=upper non-manual). In the present analyses, we converted the family income in 1980 into its present-day value in euros and formed three income groups: low (<16000 EUR), medium (>16000 EUR to ≤35000 EUR), or high (>35000 EUR). Parental smoking was considered positive if either parent had smoked daily for at least 12 months.
Childhood Determinants of Adult Health Study (CDAH)
The CDAH sample and methods have been described in detail elsewhere.12 In this study, the sample comprised 1803 participants aged 7–15 years at baseline who provided ideal cardiovascular health metrics data at follow-up when aged 26–36 years (2004–2006).
Childhood risk factors
In childhood, blood samples were collected only on those aged 9, 12, and 15 years owning to time and economic constraints. Serum lipid levels were analyzed using standardized methods. 12 Blood pressure measurements were collected using a standard mercury sphygmomanometer. Data on diet, physical activity, participant’s own smoking, parental smoking and family socioeconomic status were acquired through questionnaires.13, 14 Children who reported that they were not smoking regularly were classified as non-smokers and children who reported regular smoking as smokers. Parental smoking was considered positive if at least one parent was smoking.15 Participants retrospectively (at follow-up) reported the highest level of education completed by their parents (low=school only, medium=trade/vocational certificate, high=university) when participants were aged 12 years.11 Participants also retrospectively reported the main occupation of their parents (not in labour force, blue collar, white collar, managers or professionals) until participants turned 12 years.
The Princeton Follow-up Study (PFS)
The PFS sample and methods have been described in detail elsewhere.16 In this study, the sample comprised 723 participants aged 6–19 at baseline who provided ideal cardiovascular health metrics data when aged 30–48.
Childhood risk factors
All serum lipid level determinations were performed with standard methods.16 Blood pressure was measured with a standard sphygmomanometer. Data on smoking in childhood and family socioeconomic status were acquired through questionnaires; physical activity, diet and parental smoking status were not assessed in this cohort in childhood. Children who reported that they have never smoked or have quit smoking were classified as non-smokers and children who reported current smoking as smokers. Educational and occupational classes (range 1 to 7) of the head of the household were considered as indicators of family socioeconomic status.17 For education, completion of less than the seventh grade was coded “1” and completing a graduate or professional degree was coded “7”. For occupation, the code “1” represented unskilled labor while code “7” represented a higher executive, major professional, or proprietor of a large concern.
Assessing the Ideal Cardiovascular Health Metrics
AHA guidelines were used to construct an ideal cardiovascular health index of seven ideal metrics. Ideal health factors were as follows: systolic blood pressure (SBP) <120 mmHg and DBP <80 mmHg); total cholesterol ≤5.17 mmol/l (≤200 mg/dL); fasting glucose <5.6 mmol/l (<100 mg/dL). Ideal health behaviors included: BMI <25 kg/m2; ≥150 min/week moderate or ≥75 min/week vigorous physical activity or combination; and not smoking: either never having smoked or quit smoking >12 months ago. Heterogeneity of data collection among the cohorts required some modifications of the AHA definitions (Table 1). The ideal cardiovascular health index corresponds to the number of ideal health behaviors and factors present at the 2007 follow-up in Young Finns, 2004–2006 follow-up in CDAH or 1998–2003 follow-up in PFS. In analyses we used the ideal cardiovascular health index as a continuous variable (index 0 to 7).
Table 1.
Description of the ideal cardiovascular health metrics.
| Metric | AHA criterion | YFS | CDAH | PFS |
|---|---|---|---|---|
| Blood pressure | 120/80 mmHg | 120/80 mmHg | 120/80 mmHg | 120/80 mmHg |
| Total cholesterol | ≤5.17 mmol/l | ≤5.17 mmol/l | ≤5.17 mmol/l | ≤5.17 mmol/l |
| Glucose | <5.6 mmol/l | <5.6 mmol/l | <5.6 mmol/l | <5.6 mmol/l |
| BMI | <25 kg/m2 | <25 kg/m2 | <25 kg/m2 | <25 kg/m2 |
| Non-smoking | never smoked or quit >1 year ago | never smoked or quit >1 year ago | never smoked or quit >1 year ago | never smoked or quit >1 year ago |
| Physical activity | ≥150 min/wk moderate or ≥75 min/wk vigorous or combination | ≥120 min/wk moderate or combination or ≥60 min/wk vigorous | ≥150 min/wk moderate or combination or ≥75 min/wk vigorous | ≥150 min/wk combination or ≥75 min/wk vigorous |
| Diet | 4 / 5 components expressed for a 2000-kcal diet | 4 / 5 components scaled for caloric intake | 3 / 4 components | 4 / 5 components scaled for caloric intake |
| Fruits and vegetables | ≥4.5 cups per day | ≥450 g/day | ≥4.5 servings/day | ≥4.5 servings/day |
| Fish | ≥two 3.5-oz servings/wk | ≥two servings (100 g)/wk | ≥two fin fish servings/wk | saturated fat intake <7 E% |
| Whole grains | ≥three 1-oz servings/day | ≥three servings (30 g)/day of whole grain rye bread | ≥3 servings/day | ≥3 servings (30 g)/day |
| Sodium | <1500 mg/day | <1500 mg/day | not available | <1500 mg/day |
| Sugared drinks | ≤450 kcal/wk | ≤450 kcal/wk | ≤4 servings/wk | sugar from sweets ≤12.8 E%/day |
Statistical analyses
Associations between individual baseline metrics and ideal cardiovascular health index were examined using age- and sex-adjusted linear regression. The significant determinants were then entered into an age- and sex-adjusted multivariable linear regression model constructed to determine the independent childhood predictors of ideal cardiovascular health index. This model was additionally adjusted for race in PFS cohort. Blood pressure and blood lipids was only available for a subset of CDAH participants (those aged 9, 12, and 15 years at baseline) and blood pressure data for a subset of PFS (N=497). Two models were fitted for these cohorts: the first model maximized the N by only including those childhood variables where data were available for most participants and the second model considered all variables; hence the sample size was reduced. For analysis where the cohorts were pooled, mixed multivariable regression model with cohort as a random effect was used. The statistical tests were performed with SAS version 9.2 (SAS institute, Inc, Cary, NC). Statistical significance was inferred at a 2-tailed P-value <0.05.
RESULTS
Baseline characteristics and the proportion of participants who met individual ideal cardiovascular health metrics in adulthood in each cohort are shown in Table 2. The length of follow-up between measurement time points was 27 years for YFS, from 19 to 21 years for CDAH, and from 22 to 31 years for PFS. Participants in YFS met on average (mean±SD) 3.5±1.5, in CDAH 3.9±1.3, and in PFS 3.8±1.3 of all 7 ideal metrics in adulthood.
Table 2.
Characteristics of participants at baseline and distribution of adult ideal cardiovascular health metrics for YFS, CDAH, and PFS cohorts.
| Characteristic | YFS | CDAH | PFS | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| N | Statistic | N | Statistic | N | Statistic | |
| Age(y) | 1883 | 10.8(5.0) | 1803 | 11.1(2.5) | 723 | 12.5(3.3) |
| Male(%) | 44.2 | 48.5 | 45.2 | |||
| African-American(%) | 1883 | 0 | 1883 | 0 | 723 | 29.1 |
| Socioeconomic status indicators | ||||||
| Family income (low/medium/high)(%) | 1812 | 26/52/22 | - | - | ||
| Parental education* | 1859 | 10.7(3.6) | 1758 | 38.5/31.9/29.7 | 719 | 1.4/9.5/11.3/38.4/19.1/12.1/8.3 |
| Parental occupation, %† | 1865 | 13.6/5.1/22.3/41.8/17.2 | 0.9/23.1/18.3/57.7 | 693 | 3.3/19.3/18.0/16.0/19.3/13.1/10.8 | |
| Smoking indicators | ||||||
| Parental smoking(%) | 1765 | 72.9 | 1409 | 42.5 | - | |
| Smokers(%) | 949 | 21.9 | 1406 | 9.4 | 497 | 11.3 |
| Diet indicators | ||||||
| Vegetable consumption(times/week) | 1869 | 6.2(2.9) | 1128 | 15.7(14.5) | - | |
| Fruit consumption(times/week) | 1866 | 6.9(2.8) | 1128 | 6.2(6.9) | - | |
| Fish consumption(times/week) | 1867 | 1.1(1.1) | 1128 | 0.3(1.6) | - | |
| Low-fat milk consumption(times/week) | - | 1128 | 0.7(3.0) | - | ||
| Milk and milk product consumption(times/week) | - | 1128 | 12.3(10.5) | - | ||
| Physical activity indicators | ||||||
| Physical activity index, 3–6yr¥ | 569 | 16.1(2.4) | - | - | ||
| Physical activity index, 9–18yr¥ | 1256 | 9.0(1.8) | - | - | ||
| Physical activity(hrs/wk) | - | 1417 | 7.3(6.9) | - | ||
| Clinical indicators | ||||||
| BMI(kg/ m2) | 1883 | 17.9(3.1) | 1802 | 18.1(2.7) | 669 | 19.9(4.4) |
| Systolic blood pressure(mmHg) | 1876 | 113(12) | 609 | 109(13) | 497 | 104(13) |
| Diastolic blood pressure(mmHg) | 1606 | 69(9) | 607 | 78(12) | 497 | 62(12) |
| LDL-cholesterol(mmol/l) | 1867 | 3.4(0.8) | 397 | 2.7(0.7) | 706 | 2.8(0.8) |
| HDL-cholesterol(mmol/l) | 1867 | 1.6(0.3) | 399 | 1.5(0.3) | 702 | 1.4(0.3) |
| Triglycerides(mmol/l) | 1868 | 0.7(0.3) | 401 | 0.7(0.4) | 723 | 0.9(0.4) |
|
| ||||||
| Adult ideal cardiovascular health metrics | ||||||
| Ideal health behaviors | ||||||
| Ideal smoking status(%) | 1883 | 72.3 | 1803 | 76.3 | 723 | 70.4 |
| Ideal BMI(%) | 1883 | 47.4 | 1803 | 51.5 | 723 | 33.2 |
| Ideal physical activity(%) | 1883 | 51.1 | 1803 | 46.0 | 723 | 85.3 |
| Ideal diet score(%) | 1883 | 6.3 | 1803 | 4.6 | 723 | 0.7 |
| Ideal health factors | ||||||
| Ideal total cholesterol status(%) | 1883 | 57.0 | 1803 | 64.3 | 723 | 61.6 |
| Ideal blood pressure status(%) | 1883 | 45.9 | 1803 | 54.2 | 723 | 41.5 |
| Ideal fasting plasma glucose(%) | 1883 | 71.5 | 1803 | 92.6 | 723 | 84.2 |
Data are mean (SD) for continuous variables and percent for categorical variables.
Parental education in YFS measured as years (mean(SD)); in CDAH classified from 1 to 3 (3 is highest; shown as %); in PFS classified from 1 to 7 (7 is highest; shown as %)
Parental occupation in YFS classified from 1 to 5 (5 is highest), in CDAH from 1 to 4 (4 is highest), and in PFS from 1 to 7 (7 is highest)
range 9–22 † range 5–14
Associations between childhood risk factors and ideal cardiovascular health index
In YFS, family income, parental education, and childhood vegetable and fruit consumption were directly, and parental smoking and childhood BMI, systolic and diastolic blood pressure, LDL-cholesterol and triglycerides inversely associated with ideal cardiovascular health index (Table 3). In CDAH, parental education and childhood HDL-cholesterol were directly, and parental smoking, participant’s own smoking and childhood BMI, systolic blood pressure, and LDL-cholesterol inversely associated with ideal cardiovascular health index. In PFS, parental education, parental occupation and childhood HDL-cholesterol were directly, and childhood BMI, systolic blood pressure, LDL-cholesterol and triglycerides inversely associated with ideal cardiovascular health index.
Table 3.
Age- and sex-adjusted associations between individual baseline metrics and ideal cardiovascular health index in adulthood in the YFS, CDAH, and PFS cohorts.
| Childhood regressor variable | YFS
|
CDAH
|
PFS
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | β(SE) | p-value | N | β(SE) | p-value | N | β(SE) | p-value | |
| Socioeconomic status indicators | |||||||||
| Family income(low/medium/high) | 1812 | 0.26(0.05) | <0.0001 | - | - | ||||
| Parental education* | 1859 | 0.05(0.01) | <0.0001 | 1758 | 0.1(0.04) | <0.001 | 719 | 0.18(0.03) | <0.0001 |
| Parental occupation† | 1865 | 0.14(0.03) | <0.0001 | 1794 | 0.1(0.03) | <0.001 | 693 | 0.12(0.03) | <0.0001 |
|
| |||||||||
| Smoking indicators | |||||||||
| Parental smoking(no/yes) | 1765 | −0.30(0.07) | <0.0001 | 1406 | −0.43(0.12) | <0.001 | - | - | |
| Smoking(no/yes) | 949 | −0.23(0.12) | 0.06 | 1409 | −0.18(0.07) | 0.006 | 497 | −0.19(0.23) | 0.41 |
|
| |||||||||
| Diet indicators | |||||||||
| Vegetable consumption(times/week) | 1869 | 0.03(0.01) | 0.02 | 1128 | −0.001(0.003) | 0.60 | |||
| Fruit consumption(times/week) | 1866 | 0.04(0.01) | 0.0002 | 1128 | 0.003(0.005) | 0.56 | |||
| Fish consumption(times/week) | 1867 | 0.05(0.03) | 0.09 | 1128 | 0.013(0.023) | 0.57 | |||
| Low-fat milk consumption(times/week) | - - | - | 1128 | 0.004(0.012) | 0.76 | ||||
| Milk and milk product consumption(times/week) | - - | - | 1128 | 0.002(0.004) | 0.58 | ||||
|
| |||||||||
| Physical activity indicators | |||||||||
| Physical activity index | 1810 | 0.01(0.03) | 0.81 | - | - | ||||
| Physical activity(hrs/wk) | - - | - | 1417 | −0.01(0.01) | 0.24 | ||||
|
| |||||||||
| Clinical indicators | |||||||||
| BMI(kg/ m2) | 1883 | −0.10(0.01) | <0.0001 | 1802 | −0.11(0.01) | <0.001 | 669 | −0.09(0.01) | <0.0001 |
| Systolic blood pressure(10mmHg) | 1876 | −0.20(0.03) | <0.0001 | 609 | −0.13(0.04) | 0.001 | 497 | −0.14(0.05) | 0.005 |
| Diastolic blood pressure(10mmHg) | 1606 | −0.10(0.04) | 0.002 | 607 | −0.07(0.04) | 0.07 | 497 | −0.05(0.05) | 0.29 |
| LDL-cholesterol(mmol/l) | 1867 | −0.29(0.04) | <0.0001 | 397 | −0.39(0.09) | <0.001 | 706 | −0.22(0.06) | 0.0002 |
| HDL-cholesterol(mmol/l) | 1867 | 0.11(0.11) | 0.31 | 399 | 0.59(0.21) | 0.005 | 702 | 0.44(0.15) | 0.003 |
| Triglycerides(mmol/l) | 1868 | −0.56(0.11) | <0.0001 | 401 | −0.17(0.15) | 0.26 | 723 | −0.38(0.11) | 0.0006 |
Regression coefficients and standard errors for a 1-unit change in the covariate.
Parental education in YFS measured as years (mean(SD)); in CDAH classified from 1 to 3 (3 is highest); in PFS classified from 1 to 7 (7 is highest).
Parental occupation in YFS classified from 1 to 5 (5 is highest), in CDAH from 1 to 4 (4 is highest), and in PFS from 1 to 7 (7 is highest)
In age-and sex-adjusted multivariable analysis, independent predictors of ideal cardiovascular health (Table 4) in YFS included family income (direct association), parental smoking and childhood BMI, systolic blood pressure, and LDL-cholesterol (inverse association). The results were similar using other indicators of family socioeconomic status (parent’s education or parental occupation, data not shown). In CDAH, independent predictors of ideal cardiovascular health (Table 4) included parental education (direct association), participant’s own smoking in childhood, and childhood BMI (inverse association). The results were similar using parental occupation as a marker of family socioeconomic status. Parental smoking was affected by all variables in the model: adding BMI reduced the regression coefficient for parental smoking by 14%, adding participant’s own smoking reduced the coefficient for parental smoking by 14%, and adding parental education reduced the coefficient for parental smoking by 20%. The model for the CDAH subset that had data on lipids and blood pressure (N=366) showed parental education (direct association), and childhood measures of BMI and LDL-cholesterol (inverse association) to be independent predictors of ideal cardiovascular health (data not shown). In PFS, independent predictors of ideal cardiovascular health included parental education (direct association), and childhood BMI (inverse association). The results were similar using parental occupation as a marker of family socioeconomic status. The results were not altered (coefficient change less than 4% in parental education or childhood BMI) in the PFS subset that had data also on blood pressure. Additionally, the cohorts were pooled and the association of variables available for all subjects with adult ideal cardiovascular health was studied (n=4243). The results in these analyses taking into account cohort variable were similar to those reported in Table 4 showing family socioeconomic status (β±SE 0.18±0.03, p<0.0001) and childhood BMI (β±SE 0.11±0.005, p<0.0001) as independent predictors of adult ideal cardiovascular health.
Table 4.
Multivariable age- and sex-adjusted associations between individual baseline metrics and ideal cardiovascular health index in adulthood in the YFS, CDAH, and PFS cohorts.
| Childhood regressor variable | YFS (N=1668)
|
CDAH (N=1365)
|
PFS (N=659)
|
|||
|---|---|---|---|---|---|---|
| β(SE) | p-value | β(SE) | p-value | β(SE) | p-value | |
| Family socioeconomic status* | 0.21(0.05) | <0.0001 | 0.13(0.04) | 0.002 | 0.12(0.04) | 0.001 |
| Parental smoking (no vs. yes) | −0.26(0.07) | 0.0006 | −0.10(0.07) | 0.12 | - | - |
| Own smoking(no/yes) | - | - | −0.38(0.11) | 0.001 | - | - |
| Vegetable consumption(times/week) | 0.01(0.01) | 0.27 | - | - | N.A. | |
| Fruit consumption(times/week) | 0.01(0.01) | 0.42 | - | - | N.A. | |
| BMI(kg/m2) | −0.08(0.02) | <0.0001 | −0.10(0.01) | <0.001 | −0.07(0.01) | <0.0001 |
| Systolic BP(10mm/Hg) | −0.09(0.03) | 0.007 | - | - | N.A.‡ | |
| LDL-cholesterol(mmol/l) | −0.25(0.05) | <0.0001 | N.A.‡ | −0.10(0.06) | 0.09 | |
| HDL-cholesterol (mmol/l) | - | - | N.A.‡ | 0.27(0.16) | 0.09 | |
| Triglycerides(mmol/l) | −0.17(0.12) | 0.16 | N.A.‡ | −0.09(0.12) | 0.45 | |
| Race(White vs. African-American) | -† | - | -† | - | −0.16(0.11)† | 0.18 |
| Sex(female vs. male) | −1.06(0.07) | <0.0001 | −0.99(0.06) | <0.001 | −0.83(0.09) | <0.0001 |
| Age(years) | −0.004(0.010) | 0.67 | 0.06(0.02) | 0.002 | 0.03(0.01) | 0.09 |
Regression coefficients and standard errors are for a 1-unit change in the covariate;
Indicators of parental socioeconomic status were family income (YFS, 1 to 3) or parental education (CDAH, 1 to 3 and PFS,1 to 7);
Race = 33% African American in Princeton, 0% Young Finns and 0% in CDAH;-Nonsignificant variables (see Table 2) were not included in the analysis;
N.A.=Not Available;
N.A.‡=Available only from a subcohort, hence not included in this analysis.
In an attempt to further delineate the independence of effects for family socioeconomic status in childhood, we performed additional analyses that adjusted for participants own socioeconomic status in adulthood. In YFS, when participant’s own annual income was added to the multivariable model, family income remained as an independent predictor of ideal cardiovascular health index (β±SE 0.18±0.05, p=0.0005). In CDAH, the effect of parental education in childhood was attenuated (p=0.21) when participants’ own education was added to the model. When occupation was used as a marker of participants’ own socioeconomic status in CDAH, parental education remained as an independent predictor of ideal cardiovascular health index (β±SE 0.10±0.04, p=0.02). In PFS, parental education remained as an independent predictor of ideal cardiovascular health index (β±SE 0.08±0.04, p=0.04) when participants own education was added to the model.
Family socioeconomic status, parental smoking and ideal cardiovascular health
Because family socioeconomic status and parental smoking were observed to predict ideal cardiovascular health, we further explored these associations. Family socioeconomic status was directly associated with ideal smoking status, and BMI in all cohorts and additionally with physical activity and blood pressure in YFS, total cholesterol in CDAH, and blood pressure and glucose in PFS (data not shown). Parental smoking was inversely associated with ideal smoking status, BMI, and glucose in YFS, and with ideal smoking status in CDAH (data not shown).
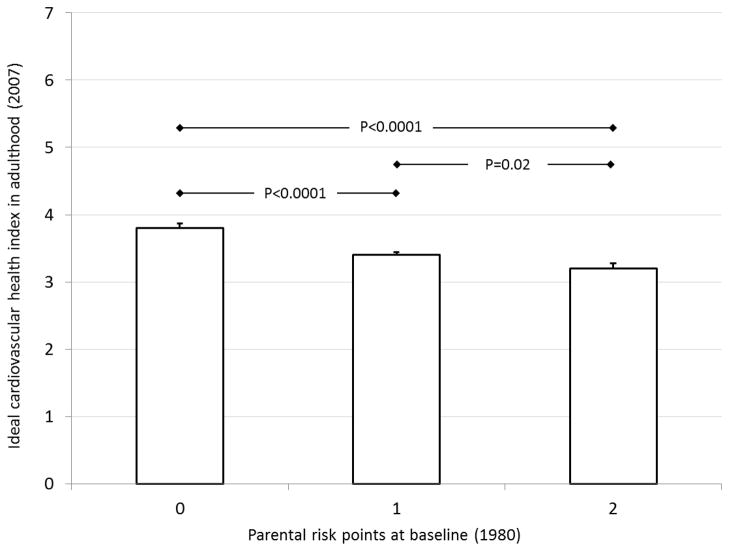
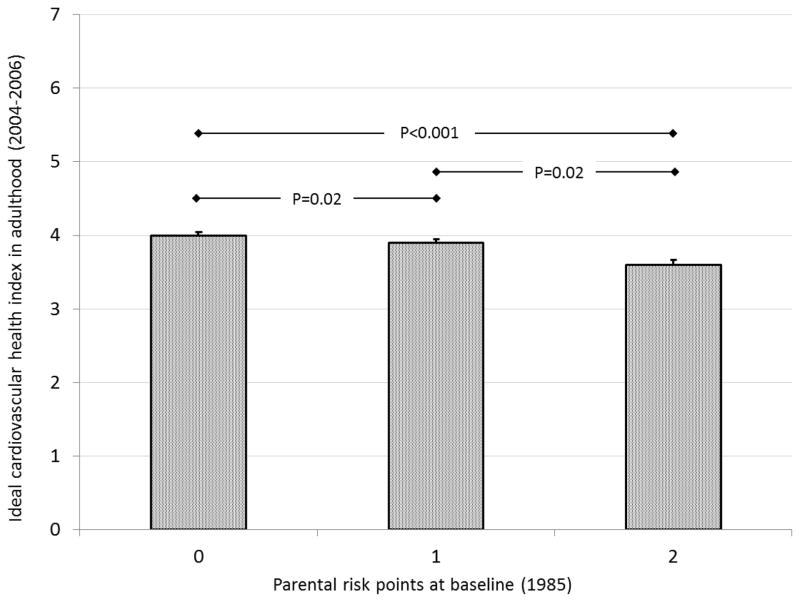
Among cohorts with data on both family socioeconomic status and smoking, we created a parental risk score that assigned a value of 1 for low family income (YFS) or school-only parental education (CDAH) and a value of 1 for parental smoking. Medium or high family income (YFS) and vocational or university education (CDAH), and parental non-smoking were assigned values of 0 (possible score range 0–2). Figures 1a and 1b show age- and sex-adjusted mean values (SEM) of ideal cardiovascular index in the parental risk score groups in YFS and CDAH. In both cohorts, the parental risk score was inversely associated with ideal cardiovascular health index in adulthood (P<0.001). In YFS, participants who had none of the parental risk factors met on average 3.8 (0.07) ideal metrics in adulthood while those with 2 risk factors met 3.2 (0.08) adult ideal metrics. In CDAH, participants who had none of the parental risk factors met on average 4.0 (0.05) ideal metrics in adulthood while those with 2 risk factors met 3.6 (0.07) adult ideal metrics. The association between parental risk score and the number of ideal metrics in adulthood was graded (In YFS, parental risk score group 0 vs. 1: P<0.0001, group 1 vs. 2: p<0.02, and in CDAH parental risk score group 0 vs. 1: P=0.02, group 1 vs. 2: p=0.02).
Figure 1.
Age- and sex-adjusted mean (SEM) of the ideal cardiovascular health index according to childhood parental risk factors (smoking, low socioeconomic status); a) YFS, b) CDAH study.
DISCUSSION
Using data from three independent cohort studies from three continents we studied a comprehensive set of childhood predictors of adult ideal cardiovascular health. Among several clinical and lifestyle indicators studied, we observed that higher family socioeconomic status in all cohorts and non-smoking (parental in YFS, own in CDAH) in childhood were independently associated with ideal cardiovascular health two to three decades later in adulthood.
Already in childhood, passive smoking has been shown to associate with unfavorable lipid profiles18, increase in platelet aggregation19, and carotid and aortic intima-media thickness.20 We have previously reported in the YFS and CDAH cohorts that parental smoking status in childhood is associated with reduced endothelium-dependent vasodilatation measured over 20 years later.15 However, there are no previous studies concerning the association between parental smoking in childhood and clustered cardiovascular health factors and behaviors in adulthood. In this study among the YFS cohort, we showed that children whose parents did not smoke met a greater number of ideal cardiovascular health metrics in adulthood.
In previous studies, childhood socioeconomic disadvantage has been shown to associate with individual cardiovascular risk factor levels in adulthood.21 In addition, childhood socioeconomic circumstance and intergenerational education mobility is associated with health-related behaviours in adulthood.22, 23 In line with these studies, we report novel data using as an endpoint a cluster of AHA-defined ideal cardiovascular health factors and behaviours associated with CVD morbidity and mortality. Cardiometabolic risk factors tend to occur together more frequently than expected by chance alone 24,25,26 and clustering of risk factors is thought to be a better measure of cardiovascular risk, instead of studying individual risk factors24 Importantly, we observed that children with higher family socioeconomic status exhibited a greater number of ideal cardiovascular health metrics in adulthood independent of several other childhood risk factors. In addition, in YFS and PFS this association was independent of participants own socioeconomic status in adulthood. In CDAH, the results were similar when occupational status was used as a marker of participants own socioeconomic status. However, the effect of parental education in childhood was attenuated when participant’s own education was used as a marker of socioeconomic status, consistently with previous reports from the CDAH study.22, 23 These data also support the recent AHA Scientific Statement27, which encourages to implement community-wide interventions that are socially and culturally appropriate to reduce disparities and inequities in the cardiovascular health of socioeconomically disadvantaged subgroups.
Recent studies have shown that meeting a greater number of the ideal cardiovascular health metrics in adulthood is associated with a lower risk for CVD incidence and mortality.3, 5–7 In the present study, children whose parents were smokers and who had a low family socioeconomic status, had 0.4–0.6 adult ideal metrics less than those participants without these risk factors. In the ARIC study, Folsom et al. reported that white adults with 3 ideal health metrics in adulthood had 33% higher CVD incidence rate (12.4/1,000 person-years vs. 9.3/1,000 person years) than those with 4 ideal health metrics.3 In addition, Yang et al. reported that adults with 3 ideal health metrics had 37% higher risk for CVD death and 42% higher risk for all-cause death compared to those with 4 ideal metrics.7 Based on these data, the difference of 0.6 ideal cardiovascular health metrics observed in this study suggests that participants who had a low socioeconomic status and were exposed to parental smoking in childhood may have 15–25% higher CVD incidence than participants who did not have these exposures.
The mechanisms by which passive smoking increases the risk of CVDs are multiple and interact with each other. Exposure to cigarette smoke increases inflammation, platelet aggregation, endothelial dysfunction, oxidation of LDL-cholesterol, oxidative stress, and atherosclerosis.28 Because of their partially developed or compromised cardiovascular, endocrine, and immune systems, the physiologically immature children may be more susceptible to damage from the toxic effects of passive smoking, particularly given the fact that the effects of passive smoking may occur at very low levels of exposure.28, 29 This may be one reason why the effect of parental smoking found in our study seems to remain long-term, and independent of potential confounders (socioeconomic status) or other mediators such as traditional CV risk factors. Likewise, differences in risk factor levels related to socioeconomic status have been observed in the life-course already in children and adolescents.17 In addition, we have found parental occupation related differences in children’s lifestyle choices, particularly in diet and smoking.30 Thus, children with higher parental socioeconomic status may have adopted a healthier lifestyle than those with lower socioeconomic background.
Study limitations
Limitations of the present study include the capture of environmental tobacco smoke exposure using questionnaires of parental smoking without cross-validation against a biomarker of passive smoke exposure. However, it has been shown that questionnaire data on parental smoking habits correlates well with serum cotinine levels.31 A potential source of bias is that those continuing in the study differ from those lost to follow-up. However, we previously reported that baseline characteristics were similar among participants and non-participants in the YFS32 such that the cohort seems to be representative of the original study population. For CDAH, however, we have noted differential loss to follow-up in area-level socioeconomic status, overweight and regular smoking.23 In sensitivity analyses that applied inverse proportional weights to those that attended follow-up to account for differential loss to follow-up among the exposures, we found the results to be essentially similar (no coefficient changed by more than 5%). The major strengths of this study include the longitudinal study design and the extensive follow-up of the participants who were very well phenotyped both in childhood and adulthood among three large cohorts.
Conclusions
Higher family socioeconomic status in all cohorts and non-smoking (parental in YFS, own in CDAH) in childhood predicted ideal cardiovascular health, as defined by the AHA, 19–31 years later in adulthood. These data thus emphasize that special attention could be paid to children who smoke or are exposed to parental smoking or are from low socioeconomic status families. From both the children’s and their parents’ perspective, these families provide one of the primary targets for interventions to promote ideal cardiovascular health and subsequently prevent CVD morbidity and mortality.
Acknowledgments
Acknowledgement of grant support
The YFS has been financially supported by the Academ0y of Finland (126925, 121584, 124282, 129378, 117797, and 41071), the Social Insurance Institution of Finland, Kuopio, Tampere, and Turku University Hospital Medical Funds, Juho Vainio Foundation, Paavo Nurmi Foundation, Finnish Foundation of Cardiovascular Research and Finnish Cultural Foundation, Sigrid Juselius Foundation, Orion-Farmos Research Foundation, Tampere Tuberculosis Foundation, and Emil Aaltonen Foundation.
The CDAH study was funded by grants from the Australian National Health and Medical Research Council, the Australian National Heart Foundation, the Tasmanian Community Fund and Veolia Environmental Services. We gratefully thank the CDAH study sponsors (Sanitarium Health Food Company, ASICS Oceania and Target Australia). A.V receives a National Health and Medical Research Council Career Development Fellowship (APP 1008299). C.G.M. receives a National Health and Medical Research Council Early Career Fellowship(APP 1037559).
The PFS was supported by the American Heart Association (National) 9750129N; National Institutes of Health (NIH), National Heart, Lung and Blood Institute (NHLBI) contract N01-2-HV-2914L; and NIH/NHLBI grants HL62394, HL55025, and HL48941.
Biostatisticians Ville Aalto and Irina Lisinen are acknowledged for statistical advice.
Footnotes
Conflicts of interest
This study has no conflics of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 2.Bambs C, Kip KE, Dinga A, Mulukutla SR, Aiyer AN, Reis SE. Low prevalence of “ideal cardiovascular health” in a community-based population: the heart strategies concentrating on risk evaluation (Heart SCORE) study. Circulation. 2011;123:850–857. doi: 10.1161/CIRCULATIONAHA.110.980151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD ARIC Study Investigators. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu HY, Sun ZH, Cao DP, Wu LX, Zeng Q. Cardiovascular health status in Chinese adults in urban areas: Analysis of the Chinese Health Examination Database 2010. Int J Cardiol. 2012 Oct 23; doi: 10.1016/j.ijcard.2012.09.235. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125:987–995. doi: 10.1161/CIRCULATIONAHA.111.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan study. Circulation. 2012;125:2975–2984. doi: 10.1161/CIRCULATIONAHA.111.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen-Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the atherosclerosis risk in communities study. Circulation. 2013;127:1270–1275. doi: 10.1161/CIRCULATIONAHA.112.001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laitinen TT, Pahkala K, Magnussen CG, Viikari JS, Oikonen M, Taittonen L, Mikkilä V, Jokinen E, Hutri-Kähönen N, Laitinen T, Kähönen M, Lehtimäki T, Raitakari OT, Juonala M. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2012;125:1971–1978. doi: 10.1161/CIRCULATIONAHA.111.073585. [DOI] [PubMed] [Google Scholar]
- 10.Raitakari OT, Juonala M, Rönnemaa T, Keltikangas-Järvinen L, Räsänen L, Pietikäinen M, Hutri-Kähönen N, Taittonen L, Jokinen E, Marniemi J, Jula A, Telama R, Kähönen M, Lehtimäki T, Åkerblom HK, Viikari JS. Cohort profile: The Cardiovascular Risk in Young Finns Study. Int J Epidemiol. 2008;37:1220–1226. doi: 10.1093/ije/dym225. [DOI] [PubMed] [Google Scholar]
- 11.Juonala M, Viikari JS, Kähönen M, Taittonen L, Laitinen T, Hutri-Kähönen N, Lehtimäki T, Jula A, Pietikäinen M, Jokinen E, Telama R, Räsänen L, Mikkilä V, Helenius H, Kivimäki M, Raitakari OT. Life-time risk factors and progression of carotid atherosclerosis in young adults: The Cardiovascular Risk in Young Finns study. Eur Heart J. 2010;31:1745–1751. doi: 10.1093/eurheartj/ehq141. [DOI] [PubMed] [Google Scholar]
- 12.Magnussen CG, Raitakari OT, Thomson R, Juonala M, Patel DA, Viikari JS, Marniemi J, Srinivasan SR, Berenson GS, Dwyer T, Venn A. Utility of currently recommended pediatric dyslipidemia classifications in predicting dyslipidemia in adulthood: evidence from the Childhood Determinants of Adult Health (CDAH) study, Cardiovascular Risk in Young Finns Study, and Bogalusa Heart Study. Circulation. 2008;117:32–42. doi: 10.1161/CIRCULATIONAHA.107.718981. [DOI] [PubMed] [Google Scholar]
- 13.Gliksman MD, Lazarus R, Wilson A. Differences in serum lipids in Australian children: is diet responsible? Int J Epidemiol. 1993;22:247–254. doi: 10.1093/ije/22.2.247. [DOI] [PubMed] [Google Scholar]
- 14.Magnussen CG, Thomson R, Cleland VJ, Ukoumunne OC, Dwyer T, Venn A. Factors affecting the stability of blood lipid and lipoprotein levels from youth to adulthood: evidence from the Childhood Determinants of Adult Health Study. Arch Pediatr Adolesc Med. 2011;165:68–76. doi: 10.1001/archpediatrics.2010.246. [DOI] [PubMed] [Google Scholar]
- 15.Juonala M, Magnussen CG, Venn A, Gall S, Kähönen M, Laitinen T, Taittonen L, Lehtimäki T, Jokinen E, Sun C, Viikari JS, Dwyer T, Raitakari OT. Parental smoking in childhood and brachial artery flow-mediated dilatation in young adults: The Cardiovascular Risk in Young Finns study and the Childhood Determinants of Adult Health study. Arterioscler Thromb Vasc Biol. 2012;32:1024–1031. doi: 10.1161/ATVBAHA.111.243261. [DOI] [PubMed] [Google Scholar]
- 16.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007;120:340–345. doi: 10.1542/peds.2006-1699. [DOI] [PubMed] [Google Scholar]
- 17.Khoury PR, Morrison JA, Laskarzewski P, Kelly K, Mellies MJ, King P, Larsen R, Glueck CJ. Relationships of education and occupation to coronary heart disease risk factors in school children and adults: the Princeton School District Study. Am J Epidemiol. 1981;113:378–395. doi: 10.1093/oxfordjournals.aje.a113106. [DOI] [PubMed] [Google Scholar]
- 18.Neufeld EJ, Mietus-Snyder M, Beiser AS, Baker AL, Newburger JW. Passive cigarette smoking and reduced HDL cholesterol levels in children with high-risk lipid profiles. Circulation. 1997;96:1403–1407. doi: 10.1161/01.cir.96.5.1403. [DOI] [PubMed] [Google Scholar]
- 19.Glantz SA, Parmley WW. Passive smoking and heart disease. Mechanisms and risk. JAMA. 1995;273:1047–1053. [PubMed] [Google Scholar]
- 20.Kallio K, Jokinen E, Saarinen M, Hämäläinen M, Volanen I, Kaitosaari T, Rönnemaa T, Viikari J, Raitakari OT, Simell O. Arterial intima-media thickness, endothelial function, and apolipoproteins in adolescents frequently exposed to tobacco smoke. Circ Cardiovasc Qual Outcomes. 2010;3:196–203. doi: 10.1161/CIRCOUTCOMES.109.857771. [DOI] [PubMed] [Google Scholar]
- 21.Poulton R, Caspi A, Milne BJ, Thomson WM, Taylor A, Sears MR, Moffitt TE. Association between children’s experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002;360:1640–1645. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleland VJ, Ball K, Magnussen C, Dwyer T, Venn A. Socioeconomic position and the tracking of physical activity and cardiorespiratory fitness from childhood to adulthood. Am J Epidemiol. 2009;170:1069–1077. doi: 10.1093/aje/kwp271. [DOI] [PubMed] [Google Scholar]
- 23.Gall SL, Abbott-Chapman J, Patton GC, Dwyer T, Venn A. Intergenerational educational mobility is associated with cardiovascular disease risk behaviours in a cohort of young Australian adults: The Childhood Determinants of Adult Health (CDAH) Study. BMC Public Health. 2010;10:55. doi: 10.1186/1471-2458-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen LB, Wedderkopp N, Hansen HS, Cooper AR, Froberg K. Biological cardiovascular risk factors cluster in Danish children and adolescents: the European Youth Heart Study. Prev Med. 2003;37:363–367. doi: 10.1016/s0091-7435(03)00145-2. [DOI] [PubMed] [Google Scholar]
- 25.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 26.Raitakari OT, Porkka KV, Viikari JS, Rönnemaa T, Åkerblom HK. Clustering of risk factors for coronary heart disease in children and adolescents. The Cardiovascular Risk in Young Finns Study. Acta Paediatr. 1994;83:935–940. doi: 10.1111/j.1651-2227.1994.tb13176.x. [DOI] [PubMed] [Google Scholar]
- 27.Pearson TA, Palaniappan LP, Artinian NT, Carnethon MR, Criqui MH, Daniels SR, Fonarow GC, Fortmann SP, Franklin BA, Galloway JM, Goff DC, Jr, Heath GW, Frank AT, Kris-Etherton PM, Labarthe DR, Murabito JM, Sacco RL, Sasson C, Turner MB on behalf of the American Heart Association Council on Epidemiology and Prevention. American Heart Association Guide for Improving Cardiovascular Health at the Community Level, 2013 Update: A Scientific Statement for Public Health Practitioners, Healthcare Providers, and Health Policy Makers. Circulation. 2013 doi: 10.1161/CIR.0b013e31828f8a94. [DOI] [PubMed] [Google Scholar]
- 28.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 29.Metsios GS, Flouris AD, Angioi M, Koutedakis Y. Passive smoking and the development of cardiovascular disease in children: a systematic review. Cardiol Res Pract. 2010;2011 doi: 10.4061/2011/587650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leino M, Porkka KV, Raitakari OT, Laitinen S, Taimela S, Viikari JS. Influence of parental occupation on coronary heart disease risk factors in children. The Cardiovascular Risk in Young Finns Study. Int J Epidemiol. 1996;25:1189–1195. doi: 10.1093/ije/25.6.1189. [DOI] [PubMed] [Google Scholar]
- 31.Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA. 1996;275:1233–1240. [PubMed] [Google Scholar]
- 32.Juonala M, Juhola J, Magnussen CG, Wurtz P, Viikari JS, Thomson R, Seppälä I, Hernesniemi J, Kähönen M, Lehtimäki T, Hurme M, Telama R, Mikkilä V, Eklund C, Räsänen L, Hintsanen M, Keltikangas-Järvinen L, Kivimäki M, Raitakari OT. Childhood environmental and genetic predictors of adulthood obesity: the cardiovascular risk in young Finns study. J Clin Endocrinol Metab. 2011;96:E1542–9. doi: 10.1210/jc.2011-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]