Abstract
It is now well accepted that Vibrio cholerae, the causative agent of the water-borne disease cholera, is acquired from environmental sources where it persists between outbreaks of the disease. Recent advances in molecular technology have demonstrated that this bacterium can be detected in areas where it has not previously been isolated, indicating a much broader, global distribution of this bacterium outside of endemic regions. The environmental persistence of V. cholerae in the aquatic environment can be attributed to multiple intra- and interspecific strategies such as responsive gene regulation and biofilm formation on biotic and abiotic surfaces, as well as interactions with a multitude of other organisms. This review will discuss some of the mechanisms that enable the persistence of this bacterium in the environment. In particular, we will discuss how V. cholerae can survive stressors such as starvation, temperature, and salinity fluctuations as well as how the organism persists under constant predation by heterotrophic protists.
Keywords: starvation adaptation, biofilms, chitin, zooplankton, protozoa, predation, stress, viable but non-culturable
INTRODUCTION
While it is likely to have been responsible for human infections and mortality throughout human history, cholera outbreaks have only been formally known to science since 1817 (Pollitzer, 1954). Sir John Snow was credited in 1849 as being the first person to connect contaminated water with cholera outbreaks and to use that information as an infection control strategy (Snow, 1855). In addition to being the genesis of modern epidemiology, his observation may also be the first study on the ecology of Vibrio cholerae. However, it took another 120 years for V. cholerae to be recognized as an autochthonous aquatic bacterium rather than a human pathogen that is a transient resident of the aquatic environment (Colwell et al., 1977). V. cholerae has over 200 serogroups, with O1 and O139 being the causative agents of cholera, due to their carriage of the genes encoding cholera toxin (CT) and the toxin co-regulated pilus (TCP; Chatterjee et al., 2007). Surveys performed in non-endemic areas have shown that the majority of V. cholerae strains isolated are non-toxigenic (Faruque et al., 2004; Haley et al., 2012; Islam et al., 2013), which suggests that associations with the human host is only one small aspect of the V. cholerae life cycle and is not necessary for environmental persistence.
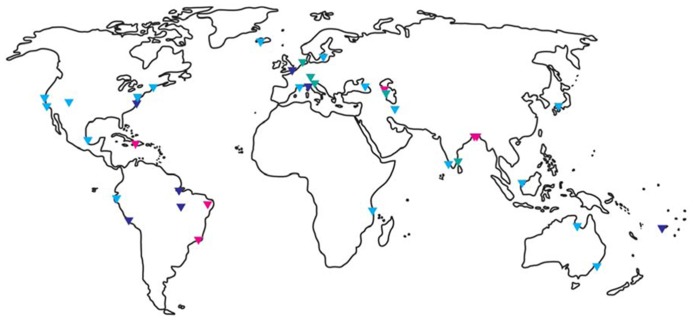
Vibrio cholerae inhabits a vast geographical range from the tropics (e.g., the Bay of Bengal where pandemics still occur, e.g., Albert et al., 1993; Huq et al., 2005; de Magny et al., 2011) to temperate waters world-wide (e.g., USA, South America, Australia, Sweden, and Italy, e.g., Vezzulli et al., 2009; Collin and Rehnstam-Holm, 2011; Schuster et al., 2011; Islam et al., 2013; Tall et al., 2013; Figure 1). An increasing understanding of the ecology of V. cholerae, along with advances in molecular detection has demonstrated that this bacterium is a cosmopolitan aquatic species that is capable of causing illness in humans (Sack et al., 2004).
FIGURE 1.
Global distribution of Vibrio cholerae. Triangles indicate where V. cholerae was detected by molecular and/or culture-based methods. Red indicates O1/O139 detection, light blue non-O1/non-O139 detection, and dark blue did not specify. Referenced studies here are only a small fraction of the studies published for certain areas and should guide as an example. North – and Middle America: (Colwell et al., 1981; Ogg et al., 1989; Blackwell and Oliver, 2008; Lizárraga-Partida et al., 2009; Hill et al., 2011; Dickinson et al., 2013), South America: (Franco et al., 1997; Lipp et al., 2003; Leal et al., 2008; Martinelli Filho et al., 2010; Sá et al., 2012); Africa: (Taviani et al., 2008); Europe: (Andersson and Ekdahl, 2006; Covazzi Harriague et al., 2008; Kirschner et al., 2008; Vezzulli et al., 2009, Vezzulli et al., 2011; Böer et al., 2013; Cantet et al., 2013; Tall et al., 2013); Middle East: (Bakhshi et al., 2009; Grim et al., 2010; Gurbanov et al., 2011; Rashid et al., 2013); Asia Pacific: (Islam et al., 1994, 2013; Desmarchelier et al., 1995; Miyagi et al., 2003; Alam et al., 2006; Vimala et al., 2010; de Magny et al., 2011; Singh et al., 2012).
The capability to survive in many different environmental niches is largely due to the evolution of a range of adaptive responses that allow V. cholerae to survive stressors such as nutrient deprivation, fluctuations in salinity and temperature and to resist predation by heterotrophic protists and bacteriophage. One such strategy is the conversion into a viable but non-culturable (VBNC) state during unfavorable conditions (Colwell, 2000; Thomas et al., 2006). Additionally, V. cholerae attaches to abiotic and biotic surfaces (chitinous as well as gelatinous zoo- and phytoplankton) as biofilms (e.g., Huq et al., 1996; Akselman et al., 2010; Shikuma and Hadfield, 2010). Biofilm formation is associated with increased stress resistance, increased access to nutrients and as a means of dispersal when attached to living, mobile hosts (Costerton et al., 1995; Hall-Stoodley et al., 2004). Here, the current understanding of how V. cholerae is able to adapt to, and persist in the aquatic environment is summarized.
SURFACE COLONIZATION AND BIOFILM FORMATION ENHANCE V. cholerae PERSISTENCE
For aquatic bacteria, surface attachment provides a selective advantage through access to nutrients that accumulate at the liquid–surface interface (Dawson et al., 1981). Therefore, surface adhesion may be a survival strategy that allows bacteria to persist in nutrient-limited natural environments (Dawson et al., 1981; Figure 2). Additionally, some biotic surfaces may provide nutrients for attached bacteria (e.g., chitin; Nalin et al., 1979). Thus, it is not surprising that V. cholerae has been detected on many abiotic and biotic surfaces, including ship hulls (Shikuma and Hadfield, 2010), zooplankton (Tamplin et al., 1990; Epstein, 1993; Huq et al., 2005; Turner et al., 2009), macroalgae (Hood and Winter, 1997), and as floating aggregates (Alam et al., 2006).
FIGURE 2.
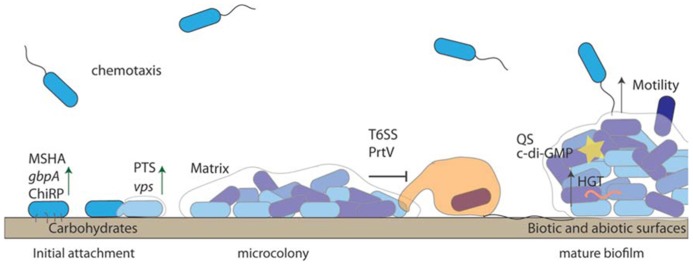
Biofilm formation enhances Vibrio cholerae persistence. V. cholerae uses chemotaxis to detect suitable surfaces for attachment. Substrate components, such as sugar concentrations of the conditioning film, play a role in the reversibly attached cells “decision” to become permanently attached. Permanent attachment is mediated by pili (ChiRP and MSHA) and outer membrane proteins such as GbpA. Biofilm formation is re-enforced through the production of VPS, which is controlled by QS (yellow star) and c-di-GMP. Anti-protozoal mechanisms such as T6SS protect surface attached bacteria. V. cholerae within biofilms undergo horizontal gene transfer (HGT), which may aid in survival.
Vibrio cholerae attachment is mediated by pili, which are surface expressed proteins, comprised of pilin subunits that promote surface attachment and subsequent biofilm formation. The ability of V. cholerae to attach to a range of surfaces is reflected in the variation in pilin subunits, and hence variation in pili, expressed by V. cholerae (Boyd and Waldor, 2002; Aagesen and Häse, 2012). One ecologically important substratum is chitin, and V. cholerae, as are most Vibrionaceae, is chitinolytic and possesses multiple conserved genes to attach to and degrade chitin (Meibom et al., 2004; Hunt et al., 2008). This organic polymer of N-acetylglucosamine (GlcNAc/NAG) is the second most abundant organic polymer in nature and is an excellent carbon source for bacteria (Rinaudo, 2006; Martínez et al., 2009). The binding of V. cholerae to chitin involves the GlcNAc binding protein, GbpA (Kirn et al., 2005; Stauder et al., 2010), as well as the mannose sensitive hemagglutinin (MSHA), which is a type IV pilus (Chiavelli et al., 2001; Figure 2). Furthermore, the TCP, which is a colonization factor of human intestinal epithelia, has a duel role in association with chitin. TCP is required for differentiation of attached biofilms, and undifferentiated biofilms lacking TCP have reduced ecological fitness, as they are less efficient at degrading chitin (Reguera and Kolter, 2005).
After initial surface attachment, V. cholerae forms “matrix-enclosed, surface-associated communities” or biofilms (Yildiz and Visick, 2009). V. cholerae biofilm formation is enhanced through the actions of type IV pili, flagella and production of the biofilm matrix, Vibrio polysaccharide (VPS; Watnick and Kolter, 1999). VPS is involved in cell immobilization, microcolony formation, and biofilm maturation (Watnick and Kolter, 1999; Watnick et al., 2001). High and low VPS producing V. cholerae colony types are referred to as “rugose” and “smooth,” respectively, with the rugose having a higher protective effect toward a variety of stresses, including chlorine (Rice et al., 1992; Morris et al., 1996; Yildiz and Schoolnik, 1999), low pH (Zhu and Mekalanos, 2003), osmotic and oxidative stress (Wai et al., 1998), anti-bacterial serum (Morris et al., 1996), SDS (Fong et al., 2006), phage (Nesper et al., 2001), and heterotrophic protists (Sun et al., 2013). The importance of VPS for protection in the environment is still unknown as there are few published reports on the occurrence of rugose V. cholerae in the environment (Ali et al., 2002; Jubair et al., 2012).
The structural genes for VPS production are encoded on two carbohydrate biosynthesis operons located on the large chromosome, which encodes many essential housekeeping genes (Yildiz and Schoolnik, 1999; Fong et al., 2010). The vpsI operon contains the genes vpsA to vpsK and the vpsII operon contains the genes vpsL to vpsQ. The six genes located between the two vps operons (rbmA–F) are also involved in biofilm formation (Fong and Yildiz, 2007; Absalon et al., 2011; Berk et al., 2012). The requirement for sugars in the synthesis of VPS is an important determinant for the control of biofilm formation (discussed in Section “V. cholerae Responses to Environmental Stresses – Bottom-up Control of V. cholerae”). In addition to sugars, multiple regulators control the expression of VPS. For example VPS biosynthesis is positively regulated by VpsR (Yildiz et al., 2001) and VpsT (Casper-Lindley and Yildiz, 2004) in a c-di-GMP-dependent manner (Krasteva et al., 2010; Srivastava et al., 2011). C-di-GMP is an intracellular secondary messenger that controls the surface association of bacteria in response to environmental conditions (Yildiz, 2008).
Bacterial cell–cell communication, or quorum sensing (QS), is critical for biofilm maturation and subsequent dispersal (Liu et al., 2007; Muller et al., 2007). At high V. cholerae cell densities, the QS response regulator, HapR, positively regulates the transcription of hapA encoding the hemagglutinin protease (HAP; Jobling and Holmes, 1997; Zhu et al., 2002), cytR, a repressor of biofilm development, flagellum biosynthesis genes (Yildiz et al., 2004), and represses VPS production and toxR, the regulator of virulence (Jobling and Holmes, 1997; Zhu et al., 2002; Hammer and Bassler, 2003; Zhu and Mekalanos, 2003; Yildiz et al., 2004). It is proposed that the coordination of QS and c-di-GMP controlled traits allows for survival through rapid adaptation to environmental conditions. For example, the switch from a free-swimming to an attached lifestyle (Yildiz and Visick, 2009; Srivastava and Waters, 2012) enables natural competency and horizontal gene transfer (HGT; Lo Scrudato and Blokesch, 2012) as well as provides enhanced predation resistance (Matz et al., 2005). Mechanisms such as biofilm formation enable the persistence of V. cholerae and are not limited to interactions with nutritive biotic factors. Indeed, as described in the following sections, many abiotic factors including temperature, salinity, and pH influence the expression of adaptive traits.
“VIABLE BUT NON-CULTURABLE” V. cholerae IN PLANKTON
In contrast to starved cells, VBNC cells fail to grow on culture media normally used to grow them, and are often reduced in size but remain metabolically active (Nilsson et al., 1991; McDougald et al., 1998; Oliver, 2010). Since the discovery that V. cholerae can enter the VBNC state (Xu et al., 1982), many bacteria, pathogens as well as non-pathogens, have been shown to enter the VBNC state under unfavorable conditions (McDougald et al., 1998, 1999; Oliver, 2005, 2010). Factors known to induce VBNC formation in V. cholerae include extremes in temperature and salinity as well as nutrient deprivation (Colwell et al., 1985; Ravel et al., 1995; Carroll et al., 2001; González-Escalona et al., 2006; Thomas et al., 2006; Mishra et al., 2012). VBNC cells of V. cholerae have been detected on the surface of higher organisms, such as crustaceans and algae in the plankton and benthos, attached to chironomid egg masses, as well as suspended in bacterioplankton (e.g., Louis et al., 2003; Binsztein et al., 2004; Alam et al., 2007; Halpern et al., 2007). Interestingly, V. cholerae appears predominately as VBNC cells within the bacterioplankton and as culturable cells in biofilm consortia, either as aggregates or attached to biotic and abiotic surfaces (Alam et al., 2006). The importance of the VBNC state in cholera epidemiology was demonstrated by Mishra et al. (2012), where virulence and colonization traits were actively expressed in VBNC V. cholerae incubated in freshwater microcosms.
For the VBNC response to impart protection allowing for persistence during unfavorable conditions, the cells must be able to resuscitate and divide when conditions become favorable (McDougald et al., 1998). For example, Vibrio vulnificus enters the VBNC state and can be resuscitated when incubated in environmental diffusion chambers in the marine environment (Oliver et al., 1995). Just as there are numerous conditions that induce VBNC formation in different species, there are numerous factors that induce resuscitation, including temperature upshift (Nilsson et al., 1991; Mishra et al., 2012) or an increase in nutrients (Binsztein et al., 2004; Senoh et al., 2010).
VBNC V. cholerae cells have also been shown to regain culturability by passage through animal digestive tracts (Colwell et al., 1985; Alam et al., 2007; Asakura et al., 2007). Furthermore, the ingestion by human volunteers of V. cholerae cells that had been VBNC for as long as 7 weeks resulted in colonization of the intestine and excretion of culturable cells (Colwell et al., 1996). Thus, VBNC cells represent an important environmental reservoir of V. cholerae as an agent of disease. However, VBNC cells remain capable of resuscitation for a limited time, and eventually, these cells lose the ability to resuscitate (Weichart et al., 1997). For example, VBNC cells can be resuscitated after co-incubation with eukaryotic cell lines, but resuscitation does not occur for cells that have been VBNC for a prolonged time (more than 91 days; Senoh et al., 2010).
Recently, QS has been implicated in the regulation of the VBNC state. For example, transition of culturable V. cholerae to the VBNC state involves biofilm formation and was shown to be dependent on QS (Kamruzzaman et al., 2010). In accordance with these results, VBNC cells from surface waters in Bangladesh have been resuscitated by natural or chemically synthesized QS autoinducers, as high colony forming unit (CFU) counts were detected after 4–5 h of exposure to two different autoinducers (Bari et al., 2013).
One hypothesis for the non-culturability of viable cells on common agar plates is that accumulation of reactive oxygen species (ROS) in the non-growing VBNC cells is detrimental when growth is initiated after nutrient addition. Thus, increased nutrient could lead to an imbalance in metabolism resulting in the production of ROS and cell death (Bloomfield et al., 1998). In fact, treatment of VBNC Escherichia coli with catalase or peroxide-degrading compounds can restore culturability (Mizunoe et al., 1999) and elimination of hydrogen peroxide from starved cultures of E. coli can prevent VBNC formation (Arana et al., 1992). Furthermore, loss of culturability of V. vulnificus under low temperature incubation has been correlated with loss of catalase activity, making the cells ROS sensitive (Kong et al., 2004).
It was recently hypothesized that VBNC cells resuscitate in a stochastic manner rather than in response to environmental parameters (Epstein, 2009). The authors argue that some cells of a dormant community will randomly revive from dormancy and if conditions are favorable, they will grow. Thus these revived cells can be likened to “scouts” inspecting environmental conditions (Buerger et al., 2012a,b). If conditions are not permissive for growth, the scouts will die, resulting in the loss of only a small fraction of the population. However, if conditions are favorable, then the genetic pool is amplified and maintained. The authors demonstrated that sampled marine and soil bacteria randomly became culturable during long term incubation in the wells of microtiter plates containing single cells. Furthermore, strains that were slow growing initially, with a cultivation time of 3–4 weeks could be sub-cultured within 48–72 h (Buerger et al., 2012b). In this way, the VBNC state represents a low cost population-based strategy that allows bacteria to remain dormant in the environment for extended periods, and to potentially either revive when an appropriate cue is present, e.g., an inducing signal, or to randomly test their environment to subsequently grow when conditions are favorable. Although stochastic VBNC resuscitation was not tested with V. cholerae, it has implications for identifying resuscitation cues and for understanding triggers of V. cholerae growth and cholera outbreaks.
Vibrio cholerae RESPONSES TO ENVIRONMENTAL STRESSES – BOTTOM-UP CONTROL OF V. cholerae
The occurrence of Vibrio spp. in the environment is correlated with temperature, salinity, and phyto- as well as zooplankton (Turner et al., 2009, 2013; Johnson et al., 2010; Asplund et al., 2011). High water temperature is a strong predictor for the presence of Vibrio spp. (Blackwell and Oliver, 2008; Lama et al., 2011; Johnson et al., 2012), as they are mainly detected in warmer waters (above 15°C). Many studies have demonstrated that the abundance of Vibrio spp. follows a seasonal pattern that is dictated to a large extent by temperature (e.g., Louis et al., 2003; Binsztein et al., 2004). Increased temperature can influence the attachment of V. cholerae to chitinous zooplankton. At temperatures above 15°C, attachment to chitin increases significantly due to an increase in the expression of the MSHA pilus and the colonization factor, GbpA (Castro-Rosas and Escartìn, 2005; Turner et al., 2009; Stauder et al., 2010). In contrast, despite the water temperatures in the Chesapeake Bay being above 19°C, V. cholerae was found more often in the water column, as planktonic cells, than attached to plankton (Louis et al., 2003). Thus, in addition to elevated temperature, other factors must influence biofilm formation or dispersal, demonstrating the importance of environmental surveying, collecting, and interpreting metadata to determine those factors that influence cholera epidemics.
Temperature fluctuations due to seasonal changes, as well as freshwater influx can strongly influence the salinity of marine water bodies. Open ocean waters have an average salinity of 35 ppt. However, near coastal and estuarine areas the salinity can drop due to freshwater input from rivers or rain run-off (Jutla et al., 2011), and can increase in areas with higher solar evaporation, especially in the tropics. Vibrio spp. grow preferably at salinities <25 ppt (e.g., Jiang, 2001; Thomas et al., 2006; Baker-Austin et al., 2010). In high salinity environments V. cholerae increases the production of the protective pigment, melanin (Coyne and al-Harthi, 1992), which provides UV resistance (Valeru et al., 2009). The relationship between V. cholerae occurrence and salinity appears to be variable, with some studies reporting a significant correlation (Singleton et al., 1982; Johnson et al., 2010), while others demonstrate a lack of correlation between the occurrence of the organism and salinity (Johnson et al., 2012). For example, Stauder et al. (2010) showed that different salinities had no effect on attachment to surfaces, which is important for environmental persistence (as discussed in Section “ Association with Other Organisms”).
Seasonal fluctuations are often correlated with changing nutrient concentrations, as rain run-off is generally higher in spring/autumn and in coastal and estuarine areas. This can lead to higher phytoplankton abundance, followed by zooplankton blooms (e.g., Lobitz et al., 2000; Huq et al., 2005), which provide the chitinous surfaces that harbor bacteria such as V. cholerae. This may enable overall numbers of the organism to increase in the environment even though bacterivorous predators are also more abundant.
Nutrient sources in the environment are not uniformly distributed but occur as microscale patches, influenced by localized events such as cell lysis and waste excretion (Blackburn et al., 1998). Planktonic bacteria use motility and chemotaxis to take advantage of such nutrient patches (for a review of see, Stocker and Seymour, 2012). V. cholerae possesses a single sheathed polar flagella (Hranitzky et al., 1980) powered by sodium motive force (Kojima et al., 1999). The number of duplicated chemotaxis-related genes possessed by V. cholerae indicates the importance of this response for environmental survival (Heidelberg et al., 2000). V. cholerae have multiple chemotaxis genes, however not all are required for chemotaxis under standard laboratory conditions, suggesting that the other genes act as accessory chemotactic genes or have as yet undiscovered functions in the environment (Gosink et al., 2002). V. cholerae has been shown to be chemotactic toward all amino acids (Freter and O’Brien, 1981), suggesting that proteins, peptides, or amino acids may be important nutrient sources in the aquatic environment. In addition, V. cholerae upregulates chemotaxis genes in response to chitin oligosaccharides, facilitating detection and attachment to chitinous organisms (Meibom et al., 2004).
The ability of V. cholerae to persist in the environment is intrinsically linked to biofilm formation and VPS synthesis, both of which allow for the exploitation of nutrients available at the surface. Concentrations of sugars, phosphorus, and nitrogen influence attachment and biofilm formation V. cholerae cells. The presence of glucose and mannose induce VPS synthesis during biofilm development (Kierek and Watnick, 2003; Moorthy and Watnick, 2004). The phosphoenolpyruvate phosphotransferase system (PTS) is one of the major sugar transport systems in V. cholerae and activation of PTS results in derepression of VPS gene transcription and thus increased biofilm formation (Houot and Watnick, 2008; Houot et al., 2010). In addition, a V. cholerae PTS that responds to intracellular nitrogen concentrations, is believed to repress VPS production, however the receptor molecule and signaling pathway are still unknown (Houot et al., 2010).
Phosphorous also affects surface colonization. In phosphorus depleted environments, V. cholerae adopts a free-swimming planktonic lifestyle that is mediated by a two-component system, PhoBR. The histidine kinase, PhoR, phosphorylates the response regulator, PhoB, resulting in further repression of VPS production (Pratt et al., 2009; Sultan et al., 2010).
Planktonic V. cholerae cells have been shown to settle in response to extracellular DNA (eDNA), which is a component of the pre-established biofilm matrix (Haugo and Watnick, 2002). This occurs by repression of CytR, which in turn represses VPS and biofilm formation (Haugo and Watnick, 2002). Since V. cholerae is rich in DNases (Focareta and Manning, 1991), the eDNA maybe utilized as a nutrient source (Seper et al., 2011).
Since nutrient availability fluctuates in the aquatic environment, the ability to store essential nutrients is an important trait for bacteria that live a “feast-to-famine lifestyle.” In bacteria, glycogen is stored in granules and can serve as a carbon source during periods of starvation (Preiss and Romeo, 1994). Under nutrient rich conditions V. cholerae increases glycogen storage precursors (Kan et al., 2004). In addition, glycogen granules are present in nutrient poor rice water stools shed by patients with cholera (Bourassa and Camilli, 2009), indicating that glycogen storage may provide nutrients to V. cholerae as it passages from the human host into the aquatic environment. In addition to glycogen storage, the ability to store inorganic phosphorus (Pi) facilitates protection against environmental stresses such as acidity, salinity fluctuations, and the damaging effects of hydrogen peroxide, as it is required for activity of the general stress response regulator, RpoS (Jahid et al., 2006). V. cholerae is also able to store Pi within membrane bound granules at 100 times the concentrations achieved by E. coli (Ogawa et al., 2000). V. cholerae mutants deficient in Pi storage displayed reduced attachment to abiotic surfaces, decreased motility and a delayed adaptation to high calcium media (200 mM) (Ogawa et al., 2000).
In addition to carbon and phosphorous, iron is also a growth limiting factor required for cellular metabolism as it is a component of many cofactors (Wackett et al., 1989) and has low solubility in aquatic environments (Martin, 1992). Iron concentrations in the aquatic environment are highly variable and are generally correlated with water depth (Martin and Michael Gordon, 1988). V. cholerae has evolved several iron transport systems and receptors that enable persistence in low iron environments (Heidelberg et al., 2000; Wyckoff et al., 2006, 2007). These iron acquisition systems include a catechol siderophore, vibriobactin (Griffiths et al., 1984), and a transport system, Feo, that can take up ferrous iron (Wyckoff et al., 2006). Most iron acquisition genes, such as irgA (Goldberg et al., 1991), are repressed by the ferric uptake regulator (Fur) in environments with sufficient iron, as Fe(II)-Fur binds to the promoter of iron-regulated genes, preventing their expression (Bagg and Neilands, 1987). V. cholerae can also make use of siderophores secreted by other microorganisms, such as fluvibactin synthesized by Vibrio fluvialis, as it possesses the required receptors and uptake systems (Yamamoto et al., 1993).
In nutrient limited environments, V. cholerae can enter a starvation state, in which the cells are non-growing but culturable. In a recent laboratory study, Jubair et al. (2012) described the long-term starvation survival of V. cholerae (700 days). The authors suggest the term “persister phenotype” to differentiate starved cells from the VBNC state. The growth of persister cells was enhanced in the presence of phosphate and chitin, both important nutrients, which further highlights their importance for V. cholerae survival. An earlier study on the behavior of V. cholerae starved for 40 days showed that chitin attachment ligands were maintained (Pruzzo et al., 2003). These findings demonstrate the importance of association with chitinous organisms with details of specific interactions discussed in the Section “Association with Other Organisms.”
TOP-DOWN CONTROL BY PREDATORY MICROGRAZERS
While availability of nutrients exerts “bottom-up” control of V. cholerae, predation by heterotrophic protists is one of the major mortality factors faced by bacteria in the environment (Hahn and Höfle, 2001; Matz and Kjelleberg, 2005). As part of the bacterioplankton, V. cholerae is under constant predation pressure by phagotrophic protists and other bacterivorous members of the zooplankton community. The long-term persistence and seasonal accumulation of V. cholerae is dependent on how it responds to this stress. Microcosm studies of natural bacterioplankton communities from the Gulf of Mexico showed that ciliates and heterotrophic nanoflagellates (HNFs) efficiently eliminate V. cholerae from environmental water samples (Martínez Pérez et al., 2004). In addition, ciliates as well as flagellates can feed on V. cholerae, with grazing rates of up to 600–2,000 bacteria cell-1 h-1 (Macek et al., 1997). Control of V. cholerae numbers by heterotrophic protists was also demonstrated by Worden et al. (2006), where V. cholerae growth rates of up to 2.5 doublings per day were countered by heavy grazing mortality by HNFs. During intense phytoplankton blooms, these growth rates increased to more than four doublings per day, allowing V. cholerae to overcome grazing pressure, potentially attaining sufficient numbers in the environment to reach an infectious dose.
Vibrio cholerae cells encased in a biofilm matrix are protected from predation by HNFs, while planktonic cells are rapidly eliminated (Matz et al., 2005). Predation induces biofilm formation and a smooth to rugose morphological shift, due to an increase in VPS production (Matz et al., 2005). VPS has subsequently been confirmed to be partly responsible for biofilm grazing resistance, where the V. cholerae cells encased in VPS were protected from predators (Sun et al., 2013). In addition to physical protection provided by biofilms, the high cell density in V. cholerae biofilms provides a sufficient quorum to promote expression of several QS-regulated anti-protozoal factors that cannot accumulate in the planktonic phase.
The importance of QS for protection from protozoal predation is supported by field tests demonstrating that QS-deficient V. cholerae was more susceptible to grazing than the wild type. However, the QS mutant strain did not lose all grazing resistance, suggesting that V. cholerae regulates anti-protozoal activities by a combination of QS and other regulatory systems (Erken et al., 2011). VPS mutants were less resistant than the wild type strain to surface grazing by the amoeba, Acanthamoeba castellanii and the HNF, Rhynchomonas nasuta, but were more resistant than the hapR mutant strain, indicating that QS is more protective than VPS against predators (Sun et al., 2013). QS has been shown to regulate secreted compounds that provide resistance from functionally different predators such as Tetrahymena pyriformis, Cafeteria roenbergensis, and Caenorhabditis elegans, e.g., an uncharacterized anti-protozoal factor (Matz et al., 2005) and the PrtV protease (Vaitkevicius et al., 2006).
The type VI secretion system (T6SS) also functions as an anti-predation mechanism that is inhibitory against Dictyostelium discoideum, mammalian macrophages, and E. coli (Pukatzki et al., 2006; MacIntyre et al., 2010). Three proteins (VgrG-1, -2, and -3) secreted by the T6SS form syringe-like structures, puncturing the cell membrane and delivering a virulence factor, VasX, into D. discoideum (Pukatzki et al., 2007; Miyata et al., 2011). The expression of another major component of T6SS, Hcp, is positively regulated by QS in V. cholerae (Ishikawa et al., 2009). Although all V. cholerae strains have this system, expression differs between them (Unterweger et al., 2012). For example, pandemic El Tor strains do not express T6SS under laboratory conditions while in some non-O1/non-O139 strains T6SS is constitutively expressed (Miyata et al., 2010).
Another mechanism for surviving protozoan predation is the ability of the bacterium to survive digestion. Both clinical and environmental strains of V. cholerae can survive intracellularly in a range of amoeba (Abd et al., 2004, 2005; Jain et al., 2006). Several studies have demonstrated that V. cholerae growth is enhanced when associated with free-living amoeba (Thom et al., 1992; Sandström et al., 2010; Valeru et al., 2012), further demonstrating the role amoeba play as environmental reservoirs of V. cholerae. In addition to surviving within amoebic trophozoites, V. cholerae cells have been found in the stress resistant cysts formed by amoeba, providing protection from environmental stresses (Thom et al., 1992; Abd et al., 2004), as well as a vehicle for dispersal throughout the aquatic environment (Thom et al., 1992; Brown and Barker, 1999). Thus, amoeba cysts could potentially facilitate the spread of cholera (Winiecka-Krusnell and Linder, 2001). Although many reports have characterized the relationship between V. cholerae and amoeba (Thom et al., 1992; Abd et al., 2005, 2007; Sandström et al., 2010), very little is known about the mechanisms that facilitate intracellular survival, although survival of the acidic conditions encountered within the digestive vacuoles has been attributed to an inducible acid tolerance response (Merrell and Camilli, 1999). In addition, ToxR has been shown to be important for survival in amoeba (Valeru et al., 2012). The authors suggest that it may be the ToxR-regulated outer membrane proteins, OmpU and OmpT that are responsible for enhanced survival. Experimentally, attraction and attachment to a protozoan host cell has yet to be shown (Abd et al., 2009, 2011). However, adhesins such as MSHA or capsule/LPS O side chain are not involved (Lock et al., 1987; Abd et al., 2009).
There is a lack of knowledge regarding the type and function of other virulence factors in facilitating intracellular survival in protozoa, especially when compared with other bacteria such as Legionella spp. and Salmonella spp. (Rowbotham, 1980; Bozue and Johnson, 1996; Brandl et al., 2005). There are many important questions that need to be addressed regarding V. cholerae–protozoa interactions, including how prevalent these interactions are in the environment and whether they facilitate resuscitation from the VBNC state. In addition, it is important to explore the prevalence of survival and passage through predatory protists and whether the bacterium remains viable in fecal pellets. A further understanding of the roles higher organisms play in the enhancement of V. cholerae fitness traits is required to understand the persistence and spread of the pathogen in the environment.
In addition to aforementioned predation pressure by phagotrophic protists, phage, and predatory bacteria also affect the abundance and serogroup prevalence of V. cholerae in the environment. For example, the CTXφ phage carries the genes encoding CT and is required for conversion of non-toxigenic to toxigenic strains (Miller and Mekalanos, 1988; Pearson et al., 1993; Waldor and Mekalanos, 1996). Phage predation has shaped cholera epidemics in Bangladesh, where high concentrations of phage are detected after an initial peak in cholera cases and numbers of V. cholerae cells in the aquatic environment (Faruque et al., 2005). Following the increase in phage numbers, the number of cholera cases decreases. An increase in phage numbers in the environment was also correlated with an increase in V. cholerae lytic bacteriophage in patient stool samples, with one of the predominant bacteriophage species belonging to the Myoviridae family (Seed et al., 2011). Environmental surveys have detected Myoviridae in regions where cholera outbreaks have occurred, such as Peru (Talledo et al., 2003), Kolkata (Sen and Ghosh, 2005), and Kenya (Maina et al., 2013). Control of V. cholerae by phage is supported by a continuous culture experiment, which suggests that V. cholerae populations may be influenced by phage to a larger extent than by nutrient limitation (Wei et al., 2011). Predatory bacteria such as Bdellovibrio sp. also prey on V. cholerae (Chen et al., 2012) and might also shape V. cholerae occurrence in the environment. However, there is limited information on the interactions between predatory bacteria and V. cholerae.
ASSOCIATION WITH OTHER ORGANISMS
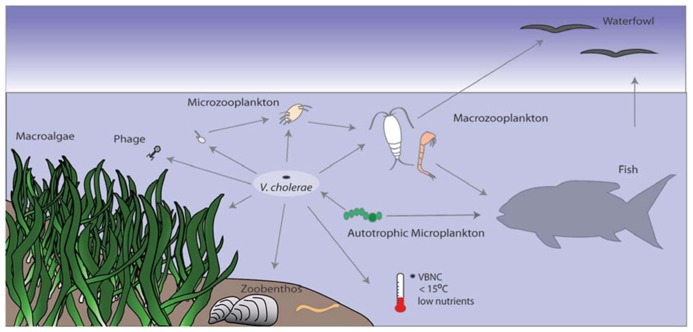
Vibrio cholerae is an integral part of the aquatic environment and in addition to heterotrophic protists, interacts with a wide range of organisms. It has been demonstrated to interact with water fowl (Halpern et al., 2008), fish (Kiiyukia et al., 1992; Senderovich et al., 2010), chironomids (Broza and Halpern, 2001; Halpern et al., 2006), mussels (Deriu et al., 2002; Collin et al., 2012), cyanobacteria (Islam et al., 1999), diatoms (Binsztein et al., 2004; Seeligmann et al., 2008), and dinoflagellates (Binsztein et al., 2004; Akselman et al., 2010; Figure 3). The association of V. cholerae with zooplankton has been a topic of study since the discovery of cells attached to the surface of copepods in the early 1980s (Huq et al., 1983; Tamplin et al., 1990). Zooplankton are an important part of the aquatic food web, grazing on autotrophic and heterotrophic bacterio-, nano-, and micro-plankton and are in turn preyed upon by larger plankton, such as insect and crustacean larvae and fish. One well-studied interaction is that between V. cholerae and chitinous zooplankton, e.g., copepods and cladocerans (Nalin et al., 1979; Huq et al., 1983; Rawlings et al., 2007). For example, pivotal experiments link the transmission of cholera with zooplankton (Huq et al., 1996, 2005; Colwell et al., 2003). In a now classic experiment, the filtration of water through readily available sari cloth reduced V. cholerae numbers by 99% (Huq et al., 1996). This method proved to be effective in field trials in reducing the incidence of cholera cases and was continued by villagers as a treatment for drinking water (Colwell et al., 2003; Huq et al., 2010). de Magny et al. (2011) suggested the use of different zooplankter to predict cholera epidemics as they demonstrated that the cladocerans, Monia spp. and Diphanosoma spp. as well as the rotifer Brachionus angularis, were significantly correlated with the presence of V. cholerae and with cholera outbreaks. V. cholerae has repeatedly been found to be associated with the copepod Acartia tonsa, which appears to harbor higher numbers of V. cholerae than co-occurring copepods (e.g., Huq et al., 1983; Binsztein et al., 2004; Rawlings et al., 2007; Lizárraga-Partida et al., 2009, for further information, see Pruzzo et al., 2008).
FIGURE 3.
Vibrio cholerae interactions with other organisms and the environment. V. cholerae is part of the bacterioplankton in aquatic environments. It is under predation pressure by protozoa and bacteriophage and is thus incorporated into the microbial loop. Low temperature and nutrient conditions can trigger the VBNC state, from which it resuscitates under more favorable conditions. V. cholerae can also attach to autotrophic organisms such as phytoplankton or macroalgae, which can provide a carbon source. Attachment to chitinous zooplankton and gelatinous egg masses (e.g., chironomids) provide nutrients and also facilitate HGT. Fish and birds feed on plankton or mussels that might harbor V. cholerae and can potentially spread the bacterium across long distances.
The predominantly attached lifestyle of V. cholerae enables it to use many different biotic surfaces as nutrient sources. In addition to degrading chitin, V. cholerae has the ability to degrade the egg masses of chironomids (Broza and Halpern, 2001; Halpern et al., 2004). The production of the QS-regulated HAP is necessary for the degradation of the gelatinous matrix of the egg masses (Halpern et al., 2003). Although high numbers of V. cholerae were found attached to the egg masses (3.9 × 104 per egg mass; Halpern et al., 2007) 99.5% of the attached cells were other species, e.g., Acinetobacter, Aeromonas, Klebsiella, Shewanella, and Pseudomonas. These species may benefit from the nutrients that are released by V. cholerae as it degrades the egg mass. Alternatively, these species may have a negative impact on V. cholerae by expressing bacteriocins or competing for nutrients and space, which may in part explain why the majority of V. cholerae on the egg masses, 99.7%, were VBNC (Halpern, 2011). V. cholerae has been shown to be associated with chironomids in all four stages of development, from egg to adult (Broza and Halpern, 2001; Halpern et al., 2003; Broza et al., 2005), suggesting these insect eggs and larvae can serve as vectors for the transmission of cholera. Indeed, chironomids that were collected in air 3 km away from a water source were found to harbor V. cholerae and thus, these midges can carry the pathogen from one body of water to another (Broza et al., 2005). Although no toxigenic serogroups of V. cholerae have been detected in association with chironomids to date, it remains possible that these could also be associated with chironomids (Halpern, 2011).
The association between V. cholerae and phytoplankton has been well studied (e.g., Tamplin et al., 1990; Lobitz et al., 2000; Turner et al., 2009). Autotrophic protists, such as diatoms and dinoflagellates (Binsztein et al., 2004; Eiler et al., 2006), cyanobacteria (Islam et al., 1999; Eiler et al., 2007) as well as macroalgae (Vugia et al., 1997; Haley et al., 2012) support V. cholerae growth (e.g., Vezzulli et al., 2010). Various laboratory and environmental studies have shown that V. cholerae cells attach to microalgae cells. In a study off the coast of Argentina, Seeligmann et al. (2008) detected 1–10 VBNC V. cholerae cells attached to a single algal cell. It was suggested that attachment to phytoplankton might enable V. cholerae to survive prolonged exposure in freshwater environments due to the nutrients and salts excreted by the phytoplankton cells (Islam et al., 1989; Tamplin et al., 1990; Binsztein et al., 2004). Nutrients supplied by phytoplankton, e.g., due to a massive bloom, can also support explosive growth of V. cholerae (Mouriño-Pérez et al., 2003). In fact, remote sensing of chlorophyll a has been proposed as a method to predict cholera outbreaks (Lobitz et al., 2000).
Attachment of V. cholerae to macroalgae is induced by the plant-derived polyamine, norspermidine (Hamana and Matsuzaki, 1982). Norspermidine is bound by NspS, a periplasmic spermidine-binding protein that interacts with the periplasmic portion of the membrane protein, MbaA, resulting in induction of biofilm formation (Karatan et al., 2005). Mannitol, which is a product of photosynthesis by brown algae and secreted at high concentrations (Yamguchi et al., 1969; Ymele-Leki et al., 2013), induces V. cholerae settlement and VPS-dependent biofilm formation, resulting in transcription of mtlA, encoding a mannitol specific transport protein (Ymele-Leki et al., 2013). Once mannitol is transported into the V. cholerae cell it is possibly used as a carbon source or an osmoprotectant (Ymele-Leki et al., 2013).
Vibrio cholerae has also been detected in the gut of various species of fish that these feed on phyto- as well as zooplankton (Senderovich et al., 2010). V. cholerae numbers as high as 5 × 103 cells per gram of intestine content were detected in fish sampled from different marine and freshwater environments in Israel (Senderovich et al., 2010). Non-O1 V. cholerae has also been detected in the kidneys, livers, and spleens of diseased, or homogenates of healthy ayu fish sampled from different rivers in Japan (Kiiyukia et al., 1992). Although no isolate carried CT genes, the supernatant of the cultures produced fluid accumulation in suckling mice. In addition to fish, waterfowl have been connected to the spread of V. cholerae (Ogg et al., 1989). Sea birds feed on zooplankton as well as phytoplankton and come in contact with these organisms by swimming on the water. Here, these planktonic organisms can then attach to bird feathers and thus V. cholerae can potentially be spread by air (Halpern et al., 2008).
Most research on environmental V. cholerae has focused on the occurrence of the bacterium within the planktonic community or on the interactions of V. cholerae with planktonic organisms. However, recent research has shown that benthic communities also harbor high numbers of Vibrios, including V. cholerae (e.g., Covazzi Harriague et al., 2008; Vezzulli et al., 2009; Collin and Rehnstam-Holm, 2011). For example, bivalves are benthic filter feeders connecting the plankton and the benthos by feeding on the plankton. Bivalves such as mussels and oysters can harbor high numbers of pathogenic Vibrio spp. in their tissue and are an important niche for these bacteria (e.g., Olafsen et al., 1993; Maugeri et al., 2001; Kirs et al., 2011). Food poisoning resulting from the ingestion of contaminated raw or undercooked seafood is a major threat to human health. While infection by V. vulnificus and Vibrio parahaemolyticus from ingestion of seafood are the most common (Wright and Harwood, 2013), mussels can also harbor high numbers of V. cholerae and thus are a potential health threat (e.g., Murphree and Tamplin, 1995; Bauer et al., 2006; Haley et al., 2012).
The bivalve immune system consists of hemocytes (phagocytic cells) and the hemolymph (i.e., lysosomal enzymes and antimicrobial peptides; Mitta et al., 2000; Pruzzo et al., 2005). In order to reside in bivalve tissues, bacteria need to survive the antimicrobial activity of the hemolymph and engulfment by the hemocytes. Vibrios are resistant to depuration treatments (Murphree and Tamplin, 1995) and show resistance toward the hemocytes of the blue mussel Mytilus edulis (Hernroth et al., 2010). Some Vibrios were able to inhibit filtration in adult M. edulis, which was not correlated with the binding of the bacteria to the gills of the mussels (Birkbeck et al., 1987), suggesting another mechanism is involved. Interestingly V. cholerae strains of different origin have different retention times in mussels (Collin et al., 2012). An environmental V. cholerae strain isolated from the blue mussel was both taken up and eliminated much faster than a clinical non-O1/O139 strain. The clinical strain had a much longer retention time, implying that pathogenic strains have better fitness in the mussel than environmental strains. This has implications for control measures such as depuration, as they will be less effective at removing clinical strains than environmental strains. In addition, Collin et al. (2012) identified a highly virulent El Tor strain that was not ingested at all, indicating that bivalves did not eliminate this pathogenic strain from the water column. These results highlight the importance of interaction of V. cholerae with other organisms in its environment and the evolution and selection for virulent strains.
In addition to being incorporated into the benthos by filter feeders, V. cholerae can be isolated from sediments in numbers that are much higher than in the planktonic phase (Covazzi Harriague et al., 2008; Vezzulli et al., 2009). Sediments may therefore also act as a reservoir for cholera, especially in colder months, seeding the water column when temperatures rise (Vezzulli et al., 2009). Interestingly, in this study nematodes accounted for the highest abundance of the meiofauna, and bacterivorous nematodes accounted for 50% of the total. This suggests that Vibrio spp. are under high grazing pressure and top-down control by these nematodes (Vezzulli et al., 2009). In a laboratory study with C. elegans, Vaitkevicius et al. (2006) showed that V. cholerae kills the nematode after ingestion by secreting the extracellular protease PrtV. Neither CT nor TCP were required for the killing. Interestingly, PrtV was also required to prevent grazing by the flagellate C. roenbergensis and the ciliate T. pyriformis. In a ΔhapR strain, the ability to kill the nematode was strongly diminished. This is in accordance with the role of the QS response regulator, hapR, which is important for grazing resistance in the laboratory (Matz et al., 2005) as well as in the environment (Erken et al., 2011). Thus, V. cholerae has evolved or acquired a number of genetic systems that facilitate its ability to resist top-down control exerted by predatory eukaryotes.
CONCLUSIONS
Vibrio cholerae is a significant pathogen that has played an important role in human history. Its role in the spread of disease and in epidemics has been reported for more than 150 years and the organism has even played an important role in establishment of modern epidemiology. While the mechanisms leading to infection and epidemics have been well studied, the ecology and mechanisms that underpin environmental persistence have been less well documented. Interestingly, environmental V. cholerae strains are largely represented by non-toxigenic strains and indeed, environmental strains display a high degree of genetic variability which has been suggested to aid in V. cholerae environmental stress resistance and subsequent persistence. The bacterium has an array of genetic systems involved in stress resistance, when faced with nutrient starvation, iron limitation, or changes in salinity and temperature. One such adaptation is the ability to grow as a biofilm on a range of abiotic and biotic surfaces. This not only increases resistance to stress, but may also directly provide access to nutrients, such as when attached to chitinous surfaces. Biofilm formation has also been directly linked to avoidance of predation by microeukaryotes. Predation resistance can be provided either by physical protection offered by the biofilm, the production of anti-predator compounds or defensive molecules or both. Perhaps not surprisingly, some of the gene systems involved in anti-predator defenses are the same as those associated with virulence during human infection. This may support the co-incidental virulence hypothesis that suggests that virulence factors evolve, at least in part, from the competition between predator and prey rather than against a human host. V. cholerae is a common inhabitant of many marine and freshwater habitats and this is most likely because it has evolved a range of strategies to enable its persistence in the natural environment. The identification and elucidation of these mechanisms, from ecological, evolutionary and molecular perspectives are likely to deliver exciting discoveries for the next 150 years.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Scott Rice and Dr. Sharon Longford as well as the anonymous reviewers for useful comments and suggestions provided for earlier versions of this article. Work in the authors laboratory was supported by the Centre for Marine Bio-Innovation, University of New South Wales, the Advanced Environmental Biotechnology Centre, the Nanyang Environment and Water Research Institute, and the Singapore Centre on Environmental Life Sciences Engineering, Nanyang Technological University, and grants from the Australian Research Council.
REFERENCES
- Aagesen A, Häse C. (2012). Sequence analyses of type IV pili from Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus. Microb. Ecol. 64 509–524 10.1007/s00248-012-0021-2 [DOI] [PubMed] [Google Scholar]
- Abd H., Saeed A., Weintraub A., Nair G. B, Sandström G. (2007). Vibrio cholerae O1 strains are facultative intracellular bacteria, able to survive and multiply symbiotically inside the aquatic free-living amoeba Acanthamoeba castellanii. FEMS Microbiol. Ecol. 60 33–39 10.1111/j.1574-6941.2006.00254.x [DOI] [PubMed] [Google Scholar]
- Abd H., Saeed A., Weintraub A, Sandström G. (2009). Vibrio cholerae O139 requires neither capsule nor LPS O side chain to grow inside Acanthamoeba castellanii. J. Med. Microbiol. 58 125–131 10.1099/jmm.0.004721-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd H., Shanan S., Saeed A, Sandström G. (2011). Survival of Vibrio cholerae inside Acanthamoeba and detection of both microorganisms from natural water samples may point out the amoeba as a protozoal host for V. cholerae. J. Bacteriol. Parasitol. 4 109 [Google Scholar]
- Abd H., Weintraub A, Sandström G. (2004). Interaction between Vibrio cholerae and Acanthamoeba castellanii. Microb. Ecol. Health Dis. 16 51–57 10.1080/08910600410029190 [DOI] [Google Scholar]
- Abd H., Weintraub A, Sandström G. (2005). Intracellular survival and replication of Vibrio cholerae O139 in aquatic free-living amoebae. Environ. Microbiol. 7 1003–1008 10.1111/j.1462-2920.2005.00771.x [DOI] [PubMed] [Google Scholar]
- Absalon C., Van Dellen K., Watnick P. I. (2011). A communal bacterial adhesin anchors biofilm and bystander cells to surfaces. PLoS Pathog. 7:e1002210 10.1371/journal.ppat.1002210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akselman R., Jurquiza V., Costagliola M. C., Fraga S. G., Pichel M., Hozbor C., et al. (2010). Vibrio cholerae O1 found attached to the dinoflagellate Noctiluca scintillans in Argentine shelf waters. Mar. Biodivers. Rec. 3 e120 10.1017/S1755267210001077 [DOI] [Google Scholar]
- Alam M., Sultana M., Nair G. B., Sack R. B., Sack D. A., Siddique A. K., et al. (2006). Toxigenic Vibrio cholerae in the aquatic environment of Mathbaria, Bangladesh. Appl. Environ. Microbiol. 72 2849–2855 10.1128/AEM.72.4.2849-2855.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M., Sultana M., Nair G. B., Siddique A. K., Hasan N. A., Sack R. B., et al. (2007). Viable but nonculturable Vibrio cholerae O1 in biofilms in the aquatic environment and their role in cholera transmission. Proc. Natl. Acad. Sci. U.S.A. 104 17801–17806 10.1073/pnas.0705599104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M., Ansaruzzaman M., Bardhan P., Faruque A., Faruque S., Islam M., et al. (1993). Large epidemic of cholera-like disease in Bangladesh caused by Vibrio cholerae 0139 synonym Bengal. Lancet 342 387–390 10.1016/0140-6736(93)92811-7 [DOI] [PubMed] [Google Scholar]
- Ali A., Rashid M. H., Karaolis D. K. (2002). High-frequency rugose exopolysaccharide production by Vibrio cholerae. Appl. Environ. Microbiol. 68 5773–5778 10.1128/AEM.68.11.5773-5778.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson Y., Ekdahl K. (2006). Wound infections due to Vibrio cholerae in Sweden after swimming in the Baltic Sea, summer 2006. Euro. Surveill. 11 E060803.2 [DOI] [PubMed] [Google Scholar]
- Arana I., Muela A., Iriberri J., Egea L., Barcina I. (1992). Role of hydrogen peroxide in loss of culturability mediated by visible light in Escherichia coli in a freshwater ecosystem. Appl. Environ. Microbiol. 58 3903–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura H., Ishiwa A., Arakawa E., Makino S. I., Okada Y., Yamamoto S., et al. (2007). Gene expression profile of Vibrio cholerae in the cold stress-induced viable but non-culturable state. Environ. Microbiol. 9 869–879 10.1111/j.1462-2920.2006.01206.x [DOI] [PubMed] [Google Scholar]
- Asplund M. E., Rehnstam-Holm A. S., Atnur V., Raghunath P., Saravanan V., Härnström K., et al. (2011). Water column dynamics of Vibrio in relation to phytoplankton community composition and environmental conditions in a tropical coastal area. Environ. Microbiol. 13 2738–2751 10.1111/j.1462-2920.2011.02545.x [DOI] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. (1987). Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry 26 5471–5477 10.1021/bi00391a039 [DOI] [PubMed] [Google Scholar]
- Baker-Austin C., Stockley L., Rangdale R., Martinez-Urtaza J. (2010). Environmental occurrence and clinical impact of Vibrio vulnificus and Vibrio parahaemolyticus: a European perspective. Environ. Microbiol. Rep. 2 7–18 10.1111/j.1758-2229.2009.00096.x [DOI] [PubMed] [Google Scholar]
- Bakhshi B., Barzelighi H. M., Adabi M., Lari A. R., Pourshafie M. (2009). A molecular survey on virulence associated genotypes of non-O1 non-O139 Vibrio cholerae in aquatic environment of Tehran, Iran. Water Res. 43 1441–1447 10.1016/j.watres.2008.12.025 [DOI] [PubMed] [Google Scholar]
- Bari S. M. N., Roky M. K., Mohiuddin M., Kamruzzaman M., Mekalanos J. J., Faruque S. M. (2013). Quorum-sensing autoinducers resuscitate dormant Vibrio cholerae in environmental water samples. Proc. Natl. Acad. Sci. U.S.A. 110 9926–9931 10.1073/pnas.1307697110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A., Ostensvik O., Florvag M., Ormen O., Rorvik L. M. (2006). Occurrence of Vibrio parahaemolyticus, V. cholerae, and V. vulnificus in Norwegian blue mussels(Mytilus edulis). Appl. Environ. Microbiol. 72 3058–3061 10.1128/AEM.72.4.3058-3061.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk V., Fong J. C. N., Dempsey G. T., Develioglu O. N., Zhuang X., Liphardt J., et al. (2012). Molecular architecture and assembly principles of Vibrio cholerae biofilms. Science 337 236–239 10.1126/science.1222981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binsztein N., Costagliola M. C., Pichel M., Jurquiza V., Ramiez F. C., Akselman R., et al. (2004). Viable but nonculturable Vibrio cholerae O1 in the aquatic environment of Argentina. Appl. Environ. Microbiol. 70 7481–7486 10.1128/AEM.70.12.7481-7486.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkbeck T., McHenery J., Nottage A. (1987). Inhibition of filtration in bivalves by marine Vibrios. Aquaculture 67 247–248 10.1016/0044-8486(87)90045-7 [DOI] [Google Scholar]
- Blackburn N., Fenchel T., Mitchell J. (1998). Microscale nutrient patches in planktonic habitats shown by chemotactic bacteria. Science 282 2254–2256 10.1126/science.282.5397.2254 [DOI] [PubMed] [Google Scholar]
- Blackwell K. D., Oliver J. D. (2008). The ecology of Vibrio vulnificus, Vibrio cholerae, and Vibrio parahaemolyticus in North Carolina estuaries. J. Microbiol. 46 146–153 10.1007/s12275-007-0216-2 [DOI] [PubMed] [Google Scholar]
- Bloomfield S. F., Stewart G. S. A. B., Dodd C. E. R., Booth I. R, Power E. G. M. (1998). The viable but non-culturable phenomenon explained? Microbiology 144 1–3 10.1099/00221287-144-1-1 [DOI] [PubMed] [Google Scholar]
- Bourassa L., Camilli A. (2009). Glycogen contributes to the environmental persistence and transmission of Vibrio cholerae. Mol. Microbiol. 72 124–138 10.1111/j.1365-2958.2009.06629.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böer S., Heinemeyer E. A., Luden K., Erler R., Gerdts G., Janssen F., et al. (2013). Temporal and spatial distribution patterns of potentially pathogenic Vibrio spp. at recreational beaches of the German North Sea. Microb. Ecol. 65 1052–1067 10.1007/s00248-013-0221-4 [DOI] [PubMed] [Google Scholar]
- Boyd E. F., Waldor M. K. (2002). Evolutionary and functional analyses of variants of the toxin-coregulated pilus protein TcpA from toxigenic Vibrio cholerae non-O1/non-O139 serogroup isolates. Microbiology 148 1655–1666 [DOI] [PubMed] [Google Scholar]
- Bozue J. A., Johnson W. (1996). Interaction of Legionella pneumophila with Acanthamoeba castellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect. Immun. 64 668–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl M. T., Rosenthal B. M., Haxo A. F., Berk S. G. (2005). Enhanced survival of Salmonella enterica in vesicles released by a soilborne Tetrahymena species. Appl. Environ. Microbiol. 71 1562–1569 10.1128/AEM.71.3.1562-1569.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. R. W., Barker J. (1999). Unexplored reservoirs of pathogenic bacteria: protozoa and biofilms. Trends Microbiol. 7 46–50 10.1016/S0966-842X(98)01425-5 [DOI] [PubMed] [Google Scholar]
- Broza M., Gancz H., Halpern M., Kashi Y. (2005). Adult non-biting midges: possible windborne carriers of Vibrio cholerae non-O1 non-O139. Environ. Microbiol. 7 576–585 10.1111/j.1462-2920.2005.00745.x [DOI] [PubMed] [Google Scholar]
- Broza M., Halpern M. (2001). Pathogen reservoirs. Chironomid egg masses and Vibrio cholerae. Nature 412 40 10.1038/35083691 [DOI] [PubMed] [Google Scholar]
- Buerger S., Spoering A., Gavrish E., Leslin C., Ling L., Epstein S. (2012a). Microbial scout hypothesis and microbial discovery. Appl. Environ. Microbiol. 78 3229–3233 10.1128/AEM.07308-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger S., Spoering A., Gavrish E., Leslin C., Ling L., Epstein S. (2012b). Microbial scout hypothesis, stochastic exit from dormancy, and the nature of slow growers. Appl. Environ. Microbiol. 78 3221–3228 10.1128/AEM.07307-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantet F., Hervio-Heath D., Caro A., Le Mennec C., Monteil C., Quéméré C., et al. (2013). Quantification of Vibrio parahaemolyticus, Vibrio vulnificus and Vibrio cholerae in French Mediterranean coastal lagoons. Res. Microbiol. 164 867–874 10.1016/j.resmic.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J., Mateescu M., Chava K., Colwell R. R., Bej A. (2001). Response and tolerance of toxigenic Vibro cholerae O1 to cold temperatures. Antonie Van Leeuwenhoek 79 377–384 10.1023/A:1012004725373 [DOI] [PubMed] [Google Scholar]
- Casper-Lindley C., Yildiz F. H. (2004). VpsT is a transcriptional regulator required for expression of vps biosynthesis genes and the development of rugose colonial morphology in Vibrio cholerae O1 El Tor. J. Bacteriol. 186 1574–1578 10.1128/JB.186.5.1574-1578.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Rosas J, Escartìn E. F. (2005). Increased tolerance of Vibrio cholerae O1 to temperature, pH, or drying associated with colonization of shrimp carapaces. Int. J. Food Microbiol. 102 195–201 10.1016/j.ijfoodmicro.2004.12.015 [DOI] [PubMed] [Google Scholar]
- Chatterjee A., Dutta P. K., Chowdhury R. (2007). Effect of fatty acids and cholesterol present in bile on expression of virulence factors and motility of Vibrio cholerae. Infect. Immun. 75 1946–1953 10.1128/IAI.01435-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Young S., Berhane T. K., Williams H. N. (2012). Predatory Bacteriovorax communities ordered by various prey species. PLoS ONE 7:e34174 10.1371/journal.pone.0034174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavelli D. A., Marsh J. W., Taylor R. K. (2001). The mannose-sensitive hemagglutinin of Vibrio cholerae promotes adherence to zooplankton. Appl. Environ. Microbiol. 67 3220–3225 10.1128/AEM.67.7.3220-3225.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin B., Rehnstam-Holm A. S. (2011). Occurrence and potential pathogenesis of Vibrio cholerae, Vibrio parahaemolyticus and Vibrio vulnificus on the South Coast of Sweden. FEMS Microbiol. Ecol. 78 306–313 10.1111/j.1574-6941.2011.01157.x [DOI] [PubMed] [Google Scholar]
- Collin B., Rehnstam-Holm A. S., Lindmark B., Pal A., Wai S. N., Hernroth B. (2012). The origin of Vibrio cholerae influences uptake and persistence in the blue mussel Mytilus edulis. J. Shellfish Res. 31 87–92 10.2983/035.031.0111 [DOI] [Google Scholar]
- Colwell R. R. (2000). Viable but nonculturable bacteria: a survival strategy. J. Infect. Chemother. 6 121–125 10.1007/PL00012151 [DOI] [PubMed] [Google Scholar]
- Colwell R. R., Brayton P., Herrington D., Tall B., Huq A., Levine M. M. (1996). Viable but non-culturable Vibrio cholerae O1 revert to a cultivable state in the human intestine. World J. Microbiol. Biotechnol. 12 28–31 10.1007/BF00327795 [DOI] [PubMed] [Google Scholar]
- Colwell R. R., Brayton P. R., Grimes D. J., Roszak D. B., Huq S. A., Palmer L. M. (1985). Viable but non-culturable Vibrio cholerae and related pathogens in the environment: implications for release of genetically engineered microorganisms. Biotechnology 3 817–820 10.1038/nbt0985-817 [DOI] [Google Scholar]
- Colwell R. R., Huq A., Islam M. S., Aziz K. M., Yunus M., Khan N. H., et al. (2003). Reduction of cholera in Bangladeshi villages by simple filtration. Proc. Natl. Acad. Sci. U.S.A. 100 1051–1055 10.1073/pnas.0237386100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell R. R., Kaper J., Joseph S. (1977). Vibrio cholerae, Vibrio parahaemolyticus, and other vibrios: occurrence and distribution in Chesapeake Bay. Science 198 394–396 [PubMed] [Google Scholar]
- Colwell R. R., Seidler R. J., Kaper J., Joseph S. W., Garges S., Lockman H., et al. (1981). Occurrence of Vibrio cholerae serotype O1 in Maryland and Louisiana estuaries. Appl. Environ. Microbiol. 41 555–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Lewandowski Z., Caldwell D. E., Korber D. R., Lappin-Scott H. M. (1995). Microbial biofilms. Annu. Rev. Microbiol. 49 711–745 10.1146/annurev.mi.49.100195.003431 [DOI] [PubMed] [Google Scholar]
- Covazzi Harriague A., Brino M. D., Zampini M., Albertelli G., Pruzzo C., Misic C. (2008). Vibrios in association with sedimentary crustaceans in three beaches of the northern Adriatic Sea (Italy). Mar. Pollut. Bull. 56 574–579 10.1016/j.marpolbul.2007.12.011 [DOI] [PubMed] [Google Scholar]
- Coyne V. E., al-Harthi L. (1992). Induction of melanin biosynthesis in Vibrio cholerae. Appl. Environ. Microbiol. 58 2861–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. P., Humphrey B. A., Marshall K. C. (1981). Adhesion: a tactic in the survival strategy of a marine Vibrio during starvation. Curr. Microbiol. 6 195–199 10.1007/BF01566971 [DOI] [Google Scholar]
- de Magny G. C., Mozumder P. K., Grim C. J., Hasan N. A., Naser M. N., Alam M., et al. (2011). Role of zooplankton diversity in Vibrio cholerae population dynamics and in the incidence of cholera in the Bangladesh Sundarbans. Appl. Environ. Microbiol. 77 6125–6132 10.1128/AEM.01472-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deriu A., Sechi L. A., Molicotti P., Spanu M. L., Zanetti S. (2002). Virulence genes in halophilic Vibrio spp. isolated in common mussels. New Microbiol. 25 93–96 [PubMed] [Google Scholar]
- Desmarchelier P., Wong F., Mallard K. (1995). An epidemiological study of Vibrio cholerae O1 in the Australian environment based on rRNA gene polymorphisms. Epidemiol. Infect. 115 435–446 10.1017/S0950268800058593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson G., Lim K. Y., Jiang S. C. (2013). Quantitative microbial risk assessment of pathogenic Vibrios in marine recreational waters of Southern California. Appl. Environ. Microbiol. 79 294–302 10.1128/AEM.02674-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiler A., Gonzalez-Rey C., Allen S., Bertilsson S. (2007). Growth response of Vibrio cholerae and other Vibrio spp. to cyanobacterial dissolved organic matter and temperature in brackish water FEMS Microbiol. Ecol. 60 411–418 10.1111/j.1574-6941.2007.00303.x [DOI] [PubMed] [Google Scholar]
- Eiler A., Johansson M., Bertilsson S. (2006). Environmental influences on Vibrio populations in northern temperate and boreal coastal waters (Baltic and Skagerrak Seas). Appl. Environ. Microbiol. 72 6004–6011 10.1128/AEM.00917-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein P. R. (1993). Algal blooms in the spread and persistence of cholera. Biosystems 31 209–221 10.1016/0303-2647(93)90050-M [DOI] [PubMed] [Google Scholar]
- Epstein S. (2009). “General model of microbial uncultivability,” in Uncultivated Microorganisms ed. Epstein S. S. (Berlin: Springer; ) 131–159 [Google Scholar]
- Erken M., Weitere M., Kjelleberg S., McDougald D. (2011). In situ grazing resistance of Vibrio cholerae in the marine environment. FEMS Microbiol. Ecol. 76 504–512 10.1111/j.1574-6941.2011.01067.x [DOI] [PubMed] [Google Scholar]
- Faruque S. M., Chowdhury N., Kamruzzaman M., Dziejman M., Rahman M. H., Sack D. A., et al. (2004). Genetic diversity and virulence potential of environmental Vibrio cholerae population in a cholera-endemic area. Proc. Natl. Acad. Sci. U.S.A. 101 2123–2128 10.1073/pnas.0308485100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruque S. M., Islam M. J., Ahmad Q. S., Faruque A. S. G., Sack D. A., Nair G. B., et al. (2005). Self-limiting nature of seasonal cholera epidemics: role of host-mediated amplification of phage. Proc. Natl. Acad. Sci. U.S.A. 102 6119–6124 10.1073/pnas.0502069102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focareta T., Manning P. A. (1991). Distinguishing between the extracellular DNases of Vibrio cholerae and development of a transformation system. Mol. Microbiol. 5 2547–2555 10.1111/j.1365-2958.1991.tb02101.x [DOI] [PubMed] [Google Scholar]
- Fong J. C., Karplus K., Schoolnik G. K., Yildiz F. H. (2006). Identification and characterization of RbmA, a novel protein required for the development of rugose colony morphology and biofilm structure in Vibrio cholerae. J. Bacteriol. 188 1049–1059 10.1128/JB.188.3.1049-1059.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong J. C., Syed K. A., Klose K. E., Yildiz F. H. (2010). Role of Vibrio polysaccharide (vps) genes in VPS production, biofilm formation and Vibrio cholerae pathogenesis. Microbiology 156 2757–2769 10.1099/mic.0.040196-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong J. C., Yildiz F. H. (2007). The rbmBCDEF gene cluster modulates development of rugose colony morphology and biofilm formation in Vibrio cholerae. J. Bacteriol. 189 2319–2330 10.1128/JB.01569-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A. A., Fix A. D., Prada A., Paredes E., Palomino J. C., Wright A. C., et al. (1997). Cholera in Lima, Peru, correlates with prior isolation of Vibrio cholerae from the environment. Am. J. Epidemiol. 146 1067–1075 10.1093/oxfordjournals.aje.a009235 [DOI] [PubMed] [Google Scholar]
- Freter R, O’Brien P. C. (1981). Role of chemotaxis in the association of motile bacteria with intestinal mucosa: chemotactic responses of Vibrio cholerae and description of motile nonchemotactic mutants. Infect. Immun. 34 215–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. B., Boyko S. A., Calderwood S. B. (1991). Positive transcriptional regulation of an iron-regulated virulence gene in Vibrio cholerae. Proc. Natl. Acad. Sci. U.S.A. 88 1125–1129 10.1073/pnas.88.4.1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Escalona N., Fey A., Höfle M. G., Espejo R. T., A. Guzmán C. (2006). Quantitative reverse transcription polymerase chain reaction analysis of Vibrio cholerae cells entering the viable but non-culturable state and starvation in response to cold shock. Environ. Microbiol. 8 658–666 10.1111/j.1462-2920.2005.00943.x [DOI] [PubMed] [Google Scholar]
- Gosink K. K., Kobayashi R., Kawagishi I., Hase C. C. (2002). Analyses of the roles of the three cheA homologs in chemotaxis of Vibrio cholerae. J. Bacteriol. 184 1767–1771 10.1128/JB.184.6.1767-1771.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G. L., Sigel S. P., Payne S. M., Neilands J. B. (1984). Vibriobactin, a siderophore from Vibrio cholerae. J. Biol. Chem. 259 383–385 [PubMed] [Google Scholar]
- Grim C. J., Jaiani E., Whitehouse C. A., Janelidze N., Kokashvili T., Tediashvili M., et al. (2010). Detection of toxigenic Vibrio cholerae O1 in freshwater lakes of the former Soviet Republic of Georgia. Environ. Microbiol. Rep. 2 2–6 10.1111/j.1758-2229.2009.00073.x [DOI] [PubMed] [Google Scholar]
- Gurbanov S., Akhmadov R., Shamkhalova G., Akhmadova S., Haley B., Colwell R. R., et al. (2011). Occurrence of Vibrio cholerae in municipal and natural waters and incidence of cholera in Azerbaijan. Ecohealth 8 468–477 10.1007/s10393-012-0756-8 [DOI] [PubMed] [Google Scholar]
- Hahn M. W, Höfle M. G. (2001). Grazing of protozoa and its effect on populations of aquatic bacteria. FEMS Microbiol. Ecol. 35 113–121 10.1111/j.1574-6941.2001.tb00794.x [DOI] [PubMed] [Google Scholar]
- Haley B. J., Chen A., Grim C. J., Clark P., Diaz C. M., Taviani E., et al. (2012). Vibrio cholerae in a historically cholera-free country. Environ. Microbiol. Rep. 4 381–389 10.1111/j.1758-2229.2012.00332.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L., Costerton J. W., Stoodley P. (2004). Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2 95–108 10.1038/nrmicro821 [DOI] [PubMed] [Google Scholar]
- Halpern M. (2011). “Chironomids and Vibrio cholerae,” in Beneficial Microorganisms in Multicellular Life Forms eds Rosenberg E., Gophna U. (Berlin: Springer; ) 43–56 [Google Scholar]
- Halpern M., Broza Y. B., Mittler S., Arakawa E., Broza M. (2004). Chironomid egg masses as a natural reservoir of Vibrio cholerae non-O1 and non-O139 in freshwater habitats. Microb. Ecol. 47 341–349 10.1007/s00248-003-2007-6 [DOI] [PubMed] [Google Scholar]
- Halpern M., Gancz H., Broza M., Kashi Y. (2003). Vibrio cholerae hemagglutinin/protease degrades chironomid egg masses. Appl. Environ. Microbiol. 69 4200–4204 10.1128/AEM.69.7.4200-4204.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M., Landsberg O., Raats D., Rosenberg E. (2007). Culturable and VBNC Vibrio cholerae: interactions with chironomid egg masses and their bacterial population. Microb. Ecol. 53 285–293 10.1007/s00248-006-9094-0 [DOI] [PubMed] [Google Scholar]
- Halpern M., Raats D., Lavion R., Mittler S. (2006). Dependent population dynamics between chironomids (nonbiting midges) and Vibrio cholerae. FEMS Microbiol. Ecol. 55 98–104 10.1111/j.1574-6941.2005.00020.x [DOI] [PubMed] [Google Scholar]
- Halpern M., Senderovich Y., Izhaki I. (2008). Waterfowl: the missing link in epidemic and pandemic cholera dissemination? PLoS Pathog. 4:e1000173 10.1371/journal.ppat.1000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamana K., Matsuzaki S. (1982). Widespread occurrence of norspermidine and norspermine in eukaryotic algae. J. Biochem. 91 1321–1328 [DOI] [PubMed] [Google Scholar]
- Hammer B. K., Bassler B. L. (2003). Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 50 101–104 10.1046/j.1365-2958.2003.03688.x [DOI] [PubMed] [Google Scholar]
- Haugo A. J., Watnick P. I. (2002). Vibrio cholerae CytR is a repressor of biofilm development. Mol. Microbiol. 45 471–483 10.1046/j.1365-2958.2002.03023.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberg J. F., Eisen J. A., Nelson W. C., Clayton R. A., Gwinn M. L., Dodson R. J., et al. (2000). DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406 477–483 10.1038/35020000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernroth B., Lothigius Å., Bölin I. (2010). Factors influencing survival of enterotoxigenic Escherichia coli, Salmonella enterica (serovar Typhimurium) and Vibrio parahaemolyticus in marine environments. FEMS Microbiol. Ecol. 71 272–280 10.1111/j.1574-6941.2009.00803.x [DOI] [PubMed] [Google Scholar]
- Hill V. R., Cohen N., Kahler A. M., Jones J. L., Bopp C. A., Marano N., et al. (2011). Toxigenic Vibrio cholerae O1 in water and seafood, Haiti. Emerg. Infect. Dis. 17 2147 10.3201/eid1711.110748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood M. A., Winter P. A. (1997). Attachment of Vibrio cholerae under various environmental conditions and to selected substrates. FEMS Microbiol. Ecol. 22 215–223 10.1111/j.1574-6941.1997.tb00373.x [DOI] [Google Scholar]
- Houot L., Chang S., Pickering B. S., Absalon C., Watnick P. I. (2010). The phosphoenolpyruvate phosphotransferase system regulates Vibrio cholerae biofilm formation through multiple independent pathways. J. Bacteriol. 192 3055–3067 10.1128/JB.00213-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houot L., Watnick P. I. (2008). A novel role for enzyme I of the Vibrio cholerae phosphoenolpyruvate phosphotransferase system in regulation of growth in a biofilm. J. Bacteriol. 190 311–320 10.1128/JB.01410-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hranitzky K. W., Mulholland A., Larson A. D., Eubanks E. R., Hart L. T. (1980). Characterization of a flagellar sheath protein of Vibrio cholerae. Infect. Immun. 27 597–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. E., Gevers D., Vahora N. M., Polz M. F. (2008). Conservation of the chitin utilization pathway in the Vibrionaceae. Appl. Environ. Microbiol. 74 44–51 10.1128/AEM.01412-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq A., Sack R. B., Nizam A., Longini I. M., Nair G. B., Ali A., et al. (2005). Critical factors influencing the occurrence of Vibrio cholerae in the environment of Bangladesh. Appl. Environ. Microbiol. 71 4645–4654 10.1128/AEM.71.8.4645-4654.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq A., Small E., West P., Huq M., Rahman R., Colwell R. R. (1983). Ecological relationship between Vibrio cholerae and planktonic copepods. Appl. Environ. Microbiol. 45 275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq A., Xu B., Chowdhury M. A., Islam M. S., Montilla R., Colwell R. R. (1996). A simple filtration method to remove plankton-associated Vibrio cholerae in raw water supplies in developing countries. Appl. Environ. Microbiol. 62 2508–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq A., Yunus M., Sohel S. S., Bhuiya A., Emch M., Luby S. P., et al. (2010). Simple sari cloth filtration of water is sustainable and continues to protect villagers from cholera in Matlab, Bangladesh. MBio 1e0003410 10.1128/mBio.00034-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Rompikuntal P. K., Lindmark B., Milton D. L., Wai S. N. (2009). Quorum sensing regulation of the two hcp alleles in Vibrio cholerae O1 strains. PLoS ONE 4:e6734 10.1371/journal.pone.0006734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam A., Labbate M., Djordjevic S. P., Alam M., Darling A., Melvold J., et al. (2013). Indigenous Vibrio cholerae strains from a non-endemic region are pathogenic. Open Biol. 3 120181 10.1098/rsob.120181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M., Hasan M., Miah M., Yunus M., Zaman K., Albert M. (1994). Isolation of Vibrio cholerae O139 synonym Bengal from the aquatic environment in Bangladesh: implications for disease transmission. Appl. Environ. Microbiol. 60 1684–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. S., Drasar B. S., Bradley D. J. (1989). Attachment of toxigenic Vibrio cholerae 01 to various freshwater plants and survival with a filamentous green alga, Rhizoclonium fontanum. J. Trop. Med. Hyg. 92 396–401 [PubMed] [Google Scholar]
- Islam M. S., Rahim Z., Alam M. J., Begum S., Moniruzzaman S. M., Umeda A., et al. (1999). Association of Vibrio cholerae O1 with the cyanobacterium, Anabaena sp.,elucidated by polymerase chain reaction and transmission electron microscopy. Trans. R. Soc. Trop. Med. Hyg. 93 36–40 10.1016/S0035-9203(99)90171-2 [DOI] [PubMed] [Google Scholar]
- Jahid I. K., Silva A. J., Benitez J. A. (2006). Polyphosphate stores enhance the ability of Vibrio cholerae to overcome environmental stresses in a low-phosphate environment. Appl. Environ. Microbiol. 72 7043–7049 10.1128/AEM.00924-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain V., Dongre M., Raychaudhuri S. (2006). Interaction of Vibrio cholerae O139 with an intestinal parasite, Entamoeba histolytica. J. Med. Microbiol. 55 1755–1756 10.1099/jmm.0.46812-0 [DOI] [PubMed] [Google Scholar]
- Jiang S. C. (2001). Vibrio cholerae in recreational beach waters and tributaries of Southern California. Hydrobiologia 460 157–164 10.1023/A:1013152407425 [DOI] [Google Scholar]
- Jobling M. G., Holmes R. K. (1997). Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol. Microbiol. 26 1023–1034 10.1046/j.1365-2958.1997.6402011.x [DOI] [PubMed] [Google Scholar]
- Johnson C., Flowers A., Noriea N., Zimmerman A., Bowers J., Depaola A., et al. (2010). Relationships between environmental factors and pathogenic Vibrios in the Northern Gulf of Mexico. Appl. Environ. Microbiol. 76 7076–7084 10.1128/AEM.00697-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. N., Bowers J. C., Griffitt K. J., Molina V., Clostio R. W., Pei S., et al. (2012). Ecology of Vibrio parahaemolyticus and Vibrio vulnificus in the coastal and estuarine waters of Louisiana, Maryland, Mississippi, and Washington (United States). Appl. Environ. Microbiol. 78 7249–7257 10.1128/AEM.01296-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubair M., Morris J. G., Jr., Ali A. (2012). Survival of Vibrio cholerae in nutrient-poor environments is associated with a novel “persister” phenotype. PLoS ONE 7:e45187 10.1371/journal.pone.0045187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutla A. S., Akanda A. S., Griffiths J. K., Colwell R. R., Islam S. (2011). Warming oceans, phytoplankton, and river discharge: implications for cholera outbreaks. Am. J. Trop. Med. Hyg. 85 303–308 10.4269/ajtmh.2011.11-0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamruzzaman M., Udden S. N., Cameron D. E., Calderwood S. B., Nair G. B., Mekalanos J. J., et al. (2010). Quorum-regulated biofilms enhance the development of conditionally viable, environmental Vibrio cholerae. Proc. Natl. Acad. Sci. U.S.A. 107 1588–1593 10.1073/pnas.0913404107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan B., Habibi H., Schmid M., Liang W., Wang R., Wang D., et al. (2004). Proteome comparison of Vibrio cholerae cultured in aerobic and anaerobic conditions. Proteomics 4 3061–3067 10.1002/pmic.200400944 [DOI] [PubMed] [Google Scholar]
- Karatan E., Duncan T. R., Watnick P. I. (2005). NspS, a predicted polyamine sensor, mediates activation of Vibrio cholerae biofilm formation by norspermidine. J. Bacteriol. 187 7434–7443 10.1128/JB.187.21.7434-7443.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierek K., Watnick P. I. (2003). Environmental determinants of Vibrio cholerae biofilm development. Appl. Environ. Microbiol. 69 5079–5088 10.1128/AEM.69.9.5079-5088.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiiyukia C., Nakajima A., Nakai T., Muroga K., Kawakami H., Hashimoto H. (1992). Vibrio cholerae non-O1 isolated from ayu fish (Plecoglossus altivelis) in Japan. Appl. Environ. Microbiol. 58 3078–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn T. J., Jude B. A., Taylor R. K. (2005). A colonization factor links Vibrio cholerae environmental survival and human infection. Nature 438 863–866 10.1038/nature04249 [DOI] [PubMed] [Google Scholar]
- Kirs M., Depaola A., Fyfe R., Jones J., Krantz J., Van Laanen A., et al. (2011). A survey of oysters Crassostrea gigas in New Zealand for Vibrio parahaemolyticus and Vibrio vulnificus. Int. J. Food Microbiol. 147 149–153 10.1016/j.ijfoodmicro.2011.03.012 [DOI] [PubMed] [Google Scholar]
- Kirschner A. K. T., Schlesinger J., Farnleitner A. H., Hornek R., Süß B., Golda B., et al. (2008). Rapid growth of planktonic Vibrio cholerae non-O1/non-O139 strains in a large alkaline lake in Austria: dependence on temperature and dissolved organic carbon quality. Appl. Environ. Microbiol. 74 2004–2015 10.1128/AEM.01739-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S., Yamamoto K., Kawagishi I., Homma M. (1999). The polar flagellar motor of Vibrio cholerae is driven by an Na+ motive force. J. Bacteriol. 181 1927–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong I. S., Bates T. C., Hülsmann A., Hassan H., Smith B. E., Oliver J. D. (2004). Role of catalase and oxyR in the viable but nonculturable state of Vibrio vulnificus. FEMS Microbiol. Ecol. 50 133–142 10.1016/j.femsec.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Krasteva P. V., Fong J. C., Shikuma N. J., Beyhan S., Navarro M. V., Yildiz F. H., et al. (2010). Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science 327 866–868 10.1126/science.1181185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lama J. R., Seas C. R., León-Barúa R., Gotuzzo E., Sack R. B. (2011). Environmental temperature, cholera, and acute diarrhoea in adults in Lima, Peru. J. Health Popul. Nutr. 22 399–403 [PubMed] [Google Scholar]
- Leal N. C., De Araújo Figueiroa Â. C. T., Cavalcanti V. O., Da Silva S. C., Leal-Balbino T. C., De Almeida A. M. P., et al. (2008). Characterization of Vibrio cholerae isolated from the aquatic basins of the State of Pernambuco, Brazil. Trans. R. Soc. Trop. Med. Hyg. 102 272–276 10.1016/j.trstmh.2007.12.008 [DOI] [PubMed] [Google Scholar]
- Lipp E. K., Rivera I. N., Gil A. I., Espeland E. M., Choopun N., Louis V. R., et al. (2003). Direct detection of Vibrio cholerae and ctxA in Peruvian coastal water and plankton by PCR. Appl. Environ. Microbiol. 69 3676–3680 10.1128/AEM.69.6.3676-3680.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Stirling F. R., Zhu J. (2007). Temporal quorum-sensing induction regulates Vibrio cholerae biofilm architecture. Infect. Immun. 75 122–126 10.1128/IAI.01190-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizárraga-Partida M. L., Mendez-Gomez E., Rivas-Montano A. M., Vargas-Hernandez E., Portillo-Lopez A., Gonzalez-Ramirez A. R., et al. (2009). Association of Vibrio cholerae with plankton in coastal areas of Mexico. Environ. Microbiol. 11 201–208 10.1111/j.1462-2920.2008.01753.x [DOI] [PubMed] [Google Scholar]
- Lo Scrudato M., Blokesch M. (2012). The regulatory network of natural competence and transformation of Vibrio cholerae. PLoS Genet. 8:e1002778 10.1371/journal.pgen.1002778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobitz B., Beck L., Huq A., Wood B., Fuchs G., Faruque A. S., et al. (2000). Climate and infectious disease: use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc. Natl. Acad. Sci. U.S.A. 97 1438–1443 10.1073/pnas.97.4.1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock R., Öhman L., Dahlgren C. (1987). Phagocytic recognition mechanisms in human granulocytes and Acanthamoeba castellanii using type 1 fimbriated Escherichia coli as phagocytic prey. FEMS Microbiol. Lett. 44 135–140 [Google Scholar]
- Louis V. R., Russek-Cohen E., Choopun N., Rivera I. N. G., Gangle B., Jiang S. C., et al. (2003). Predictability of Vibrio cholerae in Chesapeake Bay. Appl. Environ. Microbiol. 69 2773–2785 10.1128/AEM.69.5.2773-2785.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macek M., Carlos G., Memije P, Ramãrez P. (1997). Ciliate–Vibrio cholerae interactions within a microbial loop: an experimental study. Aquat. Microb. Ecol. 13 257–266 10.3354/ame013257 [DOI] [Google Scholar]
- MacIntyre D. L., Miyata S. T., Kitaoka M., Pukatzki S. (2010). The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc. Natl. Acad. Sci. U.S.A. 107 19520–19524 10.1073/pnas.1012931107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina A. N., Mwaura F. B., Oyugi J., Goulding D., Toribio A. L., Kariuki S. (2013). Characterization of Vibrio cholerae bacteriophages isolated from the environmental waters of the Lake Victoria region of Kenya. Curr. Microbiol. 1–7 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. H. (1992). “Iron as a limiting factor in oceanic productivity,” in Primary Productivity and Biogeochemical Cycles in the Sea eds Falkowski P., Woodhead A. (New York: Plenum Press; ) 123–137 [Google Scholar]
- Martin J. H, Michael Gordon R. (1988). Northeast Pacific iron distributions in relation to phytoplankton productivity. Deep-Sea Res. 35 177–196 10.1016/0198-0149(88)90035-0 [DOI] [Google Scholar]
- Martinelli Filho J. E., Lopes R. M., Rivera I. N. G., Colwell R. R. (2010). Vibrio cholerae O1 detection in estuarine and coastal zooplankton. J. Plankton Res. 33 51–62 10.1093/plankt/fbq093 [DOI] [Google Scholar]
- Martïnez J. P., Falomir M. P., Gozalbo D. (2009). “Chitin: a structural biopolysaccharide,” in eLS. John Wiley & Sons, Ltd. [Google Scholar]
- Martïnez Pérez M. E., Macek M., Castro Galván M. T. (2004). Do protozoa control the elimination of Vibrio cholerae in brackish water? Int. Rev. Hydrobiol. 89 215–227 10.1002/iroh.200310644 [DOI] [Google Scholar]
- Matz C., Kjelleberg S. (2005). Off the hook – how bacteria survive protozoan grazing. Trends Microbiol. 13 302–307 10.1016/j.tim.2005.05.009 [DOI] [PubMed] [Google Scholar]
- Matz C., McDougald D., Moreno A. M., Yung P. Y., Yildiz F. H., Kjelleberg S. (2005). Biofilm formation and phenotypic variation enhance predation-driven persistence of Vibrio cholerae. Proc. Natl. Acad. Sci. U.S.A. 102 16819–16824 10.1073/pnas.0505350102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maugeri T., Caccamo D., Gugliandolo C. (2001). Potentially pathogenic Vibrios in brackish waters and mussels. J. Appl. Microbiol. 89 261–266 10.1046/j.1365-2672.2000.01096.x [DOI] [PubMed] [Google Scholar]
- McDougald D., Rice S. A., Kjelleberg S. (1999). New perspectives on the viable but nonculturable response. Biologia-bratislava 54 617–624 [Google Scholar]
- McDougald D., Rice S. A., Weichart D., Kjelleberg S. (1998). Nonculturability: adaptation or debilitation? FEMS Microbiol. Ecol. 25 1–9 10.1111/j.1574-6941.1998.tb00455.x [DOI] [Google Scholar]
- Meibom K. L., Li X. B., Nielsen A. T., Wu C. Y., Roseman S., Schoolnik G. K. (2004). The Vibrio cholerae chitin utilization program. Proc. Natl. Acad. Sci. U.S.A. 101 2524–2529 10.1073/pnas.0308707101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell D. S., Camilli A. (1999). The cadA gene of Vibrio cholerae is induced during infection and plays a role in acid tolerance. Mol. Microbiol. 34 836–849 10.1046/j.1365-2958.1999.01650.x [DOI] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. (1988). A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170 2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A., Taneja N., Sharma M. (2012). Viability kinetics, induction, resuscitation and quantitative real-time polymerase chain reaction analyses of viable but nonculturable Vibrio cholerae O1 in freshwater microcosm. J. Appl. Microbiol. 112 945–953 10.1111/j.1365-2672.2012.05255.x [DOI] [PubMed] [Google Scholar]
- Mitta G., Vandenbulcke F., Roch P. (2000). Original involvement of antimicrobial peptides in mussel innate immunity. FEBS Lett. 486 185–190 10.1016/S0014-5793(00)02192-X [DOI] [PubMed] [Google Scholar]
- Miyagi K., Nakano T., Yagi T., Hanafusa M., Imura S., Honda T., et al. (2003). Survey of Vibrio cholerae O1 and its survival over the winter in marine water of Port of Osaka. Epidemiol. Infect. 131 613–619 10.1017/S0950268803008756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S. T., Kitaoka M., Brooks T. M., McAuley S. B., Pukatzki S. (2011). Vibrio cholerae requires the type VI secretion system virulence factor VasX to kill Dictyostelium discoideum. Infect. Immun. 79 2941–2949 10.1128/IAI.01266-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S. T., Kitaoka M., Wieteska L., Frech C., Chen N., Pukatzki S. (2010). The Vibrio cholerae type VI secretion system: evaluating its role in the human disease cholera. Front. Microbiol. 1:117 10.3389/fmicb.2010.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizunoe Y., Wai S. N., Takade A., Yoshida S. I. (1999). Restoration of culturability of starvation-stressed and low-temperature-stressed Escherichia coli O157 cells by using H2O2-degrading compounds. Arch. Microbiol. 172 63–67 10.1007/s002030050741 [DOI] [PubMed] [Google Scholar]
- Moorthy S., Watnick P. I. (2004). Genetic evidence that the Vibrio cholerae monolayer is a distinct stage in biofilm development. Mol. Microbiol. 52 573–587 10.1111/j.1365-2958.2004.04000.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. G., Jr., Sztein M. B., Rice E. W., Nataro J. P., Losonsky G. A., Panigrahi P., et al. (1996). Vibrio cholerae O1 can assume a chlorine-resistant rugose survival form that is virulent for humans. J. Infect. Dis. 174 1364–1368 10.1093/infdis/174.6.1364 [DOI] [PubMed] [Google Scholar]
- Mouriño-Pérez R. R., Worden A. Z., Azam F. (2003). Growth of Vibrio cholerae O1 in red tide waters off California. Appl. Environ. Microbiol. 69 6923–6931 10.1128/AEM.69.11.6923-6931.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J., Miller M. C., Nielsen A. T., Schoolnik G. K., Spormann A. M. (2007). vpsA- and luxO-independent biofilms of Vibrio cholerae. FEMS Microbiol. Lett. 275 199–206 10.1111/j.1574-6968.2007.00884.x [DOI] [PubMed] [Google Scholar]
- Murphree R. L., Tamplin M. L. (1995). Uptake and retention of Vibrio cholerae O1 in the Eastern oyster, Crassostrea virginica. Appl. Environ. Microbiol. 61 3656–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalin D., Daya V., Reid A., Levine M., Cisneros L. (1979). Adsorption and growth of Vibrio cholerae on chitin. Infect. Immun. 25 768–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesper J., Lauriano C. M., Klose K. E., Kapfhammer D., Kraiss A., Reidl J. (2001). Characterization of Vibrio cholerae O1 El Tor galU and galE mutants: influence on lipopolysaccharide structure, colonization, and biofilm formation. Infect. Immun. 69 435–445 10.1128/IAI.69.1.435-445.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L., Oliver J., Kjelleberg S. (1991). Resuscitation of Vibrio vulnificus from the viable but nonculturable state. J. Bacteriol. 173 5054–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa N., Tzeng C. M., Fraley C. D., Kornberg A. (2000). Inorganic polyphosphate in Vibrio cholerae: genetic, biochemical, and physiologic features. J. Bacteriol. 182 6687–6693 10.1128/JB.182.23.6687-6693.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg J. E., Ryder R. A., Smith H. L., Jr (1989). Isolation of Vibrio cholerae from aquatic birds in Colorado and Utah. Appl. Environ. Microbiol. 55 95–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafsen J. A., Mikkelsen H. V., Giæver H. M, Høvik Hansen G. (1993). Indigenous bacteria in hemolymph and tissues of marine bivalves at low temperatures. Appl. Environ. Microbiol. 59 1848–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. D. (2005). The viable but nonculturable state in bacteria. J. Microbiol. 43 93–100 [PubMed] [Google Scholar]
- Oliver J. D. (2010). Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol. Rev. 34 415–425 [DOI] [PubMed] [Google Scholar]
- Oliver J. D., Hite F., McDougald D., Andon N. L., Simpson L. M. (1995). Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in an estuarine environment. Appl. Environ. Microbiol. 61 2624–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. D., Woods A., Chiang S. L., Mekalanos J. J. (1993). CTX genetic element encodes a site-specific recombination system and an intestinal colonization factor. Proc. Natl. Acad. Sci. U.S.A. 90 3750–3754 10.1073/pnas.90.8.3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitzer R. (1954). History of the disease. Cholera. Bull. World Health Organ. 10 421 [PMC free article] [PubMed] [Google Scholar]
- Pratt J. T., McDonough E., Camilli A. (2009). PhoB regulates motility, biofilms, and cyclic di-GMP in Vibrio cholerae. J. Bacteriol. 191 6632–6642 10.1128/JB.00708-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J., Romeo T. (1994). Molecular biology and regulatory aspects of glycogen biosynthesis in bacteria. Prog. Nucleic Acid Res. Mol. Biol. 47 299–329 10.1016/S0079-6603(08)60255-X [DOI] [PubMed] [Google Scholar]
- Pruzzo C., Gallo G., Canesi L. (2005). Persistence of Vibrios in marine bivalves: the role of interactions with haemolymph components. Environ. Microbiol. 7 761–772 10.1111/j.1462-2920.2005.00792.x [DOI] [PubMed] [Google Scholar]
- Pruzzo C., Tarsi R., Del Mar Lleò M., Signoretto C., Zampini M., Pane L., et al. (2003). Persistence of adhesive properties in Vibrio cholerae after long-term exposure to sea water. Environ. Microbiol. 5 850–858 10.1046/j.1462-2920.2003.00498.x [DOI] [PubMed] [Google Scholar]
- Pruzzo C., Vezzulli L., Colwell R. R. (2008). Global impact of Vibrio cholerae interactions with chitin. Environ. Microbiol. 10 1400–1410 10.1111/j.1462-2920.2007.01559.x [DOI] [PubMed] [Google Scholar]
- Pukatzki S., Ma A. T., Revel A. T., Sturtevant D., Mekalanos J. J. (2007). Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc. Natl. Acad. Sci. U.S.A. 104 15508–15513 10.1073/pnas.0706532104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukatzki S., Ma A. T., Sturtevant D., Krastins B., Sarracino D., Nelson W. C., et al. (2006). Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. U.S.A. 103 1528–1533 10.1073/pnas.0510322103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid A., Haley B. J., Rajabov M., Ahmadova S., Gurbanov S., Colwell R. R., et al. (2013). Detection of Vibrio cholerae in environmental waters including drinking water reservoirs of Azerbaijan. Environ. Microbiol. Rep. 5 30–38 10.1111/j.1758-2229.2012.00369.x [DOI] [PubMed] [Google Scholar]
- Ravel J., Knight I. T., Monahan C. E., Hill R. T., Colwell R. R. (1995). Temperature-induced recovery of Vibrio cholerae from the viable but nonculturable state: growth or resuscitation? Microbiology 141 377–383 10.1099/13500872-141-2-377 [DOI] [PubMed] [Google Scholar]
- Rawlings T. K., Ruiz G. M., Colwell R. R. (2007). Association of Vibrio cholerae O1 El Tor and O139 Bengal with the Copepods Acartia tonsa and Eurytemora affinis. Appl. Environ. Microbiol. 73 7926–7933 10.1128/AEM.01238-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera G., Kolter R. (2005). Virulence and the environment: a novel role for Vibrio cholerae toxin-coregulated pili in biofilm formation on chitin. J. Bacteriol. 187 3551–3555 10.1128/JB.187.10.3551-3555.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice E. W., Johnson C. J., Clark R. M., Fox K. R., Reasoner D. J., Dunnigan M. E., et al. (1992). Chlorine and survival of “rugose” Vibrio cholerae. Lancet 340 740 10.1016/0140-6736(92)92289-R [DOI] [PubMed] [Google Scholar]
- Rinaudo M. (2006). Chitin and chitosan: properties and applications. Prog. Polym. Sci. 31 603–632 10.1016/j.progpolymsci.2006.06.001 [DOI] [Google Scholar]
- Rowbotham T. J. (1980). Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33 1179–1183 10.1136/jcp.33.12.1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sá L. L. C., Vale E. R. V., Garza D. R, Vicente A. C. P. (2012). Vibrio cholerae O1 from superficial water of the Tucunduba Stream, Brazilian Amazon. Braz. J. Microbiol. 43 635–638 10.1590/S1517-83822012000200027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack D. A., Sack R. B., Nair G. B., Siddique A. K. (2004). Cholera. Lancet 363 223–233 10.1016/S0140-6736(03)15328-7 [DOI] [PubMed] [Google Scholar]
- Sandström G., Saeed A., Abd H. (2010). Acanthamoeba polyphaga is a possible host for Vibrio cholerae in aquatic environments. Exp. Parasitol. 126 65–68 10.1016/j.exppara.2009.09.021 [DOI] [PubMed] [Google Scholar]
- Schuster B. M., Tyzik A. L., Donner R. A., Striplin M. J., Almagro-Moreno S., Jones S. H., et al. (2011). Ecology and genetic structure of a northern temperate Vibrio cholerae population related to toxigenic isolates. Appl. Environ. Microbiol. 77 7568–7575 10.1128/AEM.00378-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed K. D., Bodi K. L., Kropinski A. M., Ackermann H. W., Calderwood S. B., Qadri F., et al. (2011). Evidence of a dominant lineage of Vibrio cholerae-specific lytic bacteriophages shed by cholera patients over a 10-year period in Dhaka, Bangladesh. MBio 2 10.1128/mBio.00334-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeligmann C. T., Mirande V., Tracanna B. C., Silva C., Aulet O., Cecilia M., et al. (2008). Phytoplankton-linked viable non-culturable Vibrio cholerae O1 (VNC) from rivers in Tucumán, Argentina. J. Plankton Res. 30 367–377 10.1093/plankt/fbn008 [DOI] [Google Scholar]
- Sen A., Ghosh A. N. (2005). New Vibrio cholerae O1 biotype El Tor bacteriophages. Virol. J. 2 28 10.1186/1743-422X-2-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senderovich Y., Izhaki I., Halpern M. (2010). Fish as reservoirs and vectors of Vibrio cholerae. PLoS ONE 5:e8607 10.1371/journal.pone.0008607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoh M., Ghosh-Banerjee J., Ramamurthy T., Hamabata T., Kurakawa T., Takeda M., et al. (2010). Conversion of viable but nonculturable Vibrio cholerae to the culturable state by co-culture with eukaryotic cells. Microbiol. Immunol. 54 502–507 10.1111/j.1348-0421.2010.00245.x [DOI] [PubMed] [Google Scholar]
- Seper A., Fengler V. H. I., Roier S., Wolinski H., Kohlwein S. D., Bishop A. L., et al. (2011). Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation. Mol. Microbiol. 82 1015–1037 10.1111/j.1365-2958.2011.07867.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikuma N. J., Hadfield M. G. (2010). Marine biofilms on submerged surfaces are a reservoir for Escherichia coli and Vibrio cholerae. Biofouling 26 39–46 10.1080/08927010903282814 [DOI] [PubMed] [Google Scholar]
- Singh R., Narayan V., McLenachan P., Winkworth R. C., Mitra S., Lockhart P. J., et al. (2012). Detection and diversity of pathogenic Vibrio from Fiji. Environ. Microbiol. Rep. 4 403–411 10.1111/j.1758-2229.2012.00344.x [DOI] [PubMed] [Google Scholar]
- Singleton F. L., Attwell R. W., Jangi M. S., Colwell R. R. (1982). Influence of salinity and organic nutrient concentration on survival and growth of Vibrio cholerae in aquatic microcosms. Appl. Environ. Microbiol. 43 1080–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow J. (1855). On the Mode of Communication of Cholera. New Burlington Street, London: John Churchill [Google Scholar]
- Srivastava D., Harris R. C., Waters C. M. (2011). Integration of cyclic di-GMP and quorum sensing in the control of vpsT and aphA in Vibrio cholerae. J. Bacteriol. 193 6331–6341 10.1128/JB.05167-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D., Waters C. M. (2012). A tangled web: regulatory connections between quorum sensing and cyclic di-GMP. J. Bacteriol. 194 4485–4493 10.1128/JB.00379-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauder M., Vezzulli L., Pezzati E., Repetto B., Pruzzo C. (2010). Temperature affects Vibrio cholerae O1 El Tor persistence in the aquatic environment via an enhanced expression of GbpA and MSHA adhesins. Environ. Microbiol. Rep. 2 140–144 10.1111/j.1758-2229.2009.00121.x [DOI] [PubMed] [Google Scholar]
- Stocker R., Seymour J. R. (2012). Ecology and physics of bacterial chemotaxis in the ocean. Microbiol. Mol. Biol. Rev. 76 792–812 10.1128/MMBR.00029-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan S. Z., Silva A. J., Benitez J. A. (2010). The PhoB regulatory system modulates biofilm formation and stress response in El Tor biotype Vibrio cholerae. FEMS Microbiol. Lett. 302 22–31 10.1111/j.1574-6968.2009.01837.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Kjelleberg S., McDougald D. (2013). Relative contributions of Vibrio polysaccharide and quorum sensing to the resistance of Vibrio cholerae to predation by heterotrophic protists. PLoS ONE 8:e56338 10.1371/journal.pone.0056338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tall A., Hervio-Heath D., Teillon A., Boisset-Helbert C., Delesmont R., Bodilis J., et al. (2013). Diversity of Vibrio spp. isolated at ambient environmental temperature in the eastern English Channel as determined by pyrH sequencing. J. Appl. Microbiol. 114 1713–1724 10.1111/jam.12181 [DOI] [PubMed] [Google Scholar]
- Talledo M., Rivera I. N., Lipp E. K., Neale A., Karaolis D., Huq A., et al. (2003). Characterization of a Vibrio cholerae phage isolated from the coastal water of Peru. Environ. Microbiol. 5 350–354 10.1046/j.1462-2920.2003.00411.x [DOI] [PubMed] [Google Scholar]
- Tamplin M., Gauzens A., Huq A., Sack D., Colwell R. R. (1990). Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl. Environ. Microbiol. 56 1977–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taviani E., Ceccarelli D., Lazaro N., Bani S., Cappuccinelli P., Colwell R. R., et al. (2008). Environmental Vibrio spp., isolated in Mozambique, contain a polymorphic group of integrative conjugative elements and class 1 integrons. FEMS Microbiol. Ecol. 64 45–54 10.1111/j.1574-6941.2008.00455.x [DOI] [PubMed] [Google Scholar]
- Thom S., Warhurst D., Drasar B. S. (1992). Association of Vibrio cholerae with fresh water amoebae. J. Med. Microbiol. 36 303–306 10.1099/00222615-36-5-303 [DOI] [PubMed] [Google Scholar]
- Thomas K. U., Joseph N., Raveendran O., Nair S. (2006). Salinity-induced survival strategy of Vibrio cholerae associated with copepods in Cochin backwaters. Mar. Pollut. Bull. 52 1425–1430 10.1016/j.marpolbul.2006.04.011 [DOI] [PubMed] [Google Scholar]
- Turner J. W., Good B., Cole D., Lipp E. K. (2009). Plankton composition and environmental factors contribute to Vibrio seasonality. ISME J. 3 1082–1092 10.1038/ismej.2009.50 [DOI] [PubMed] [Google Scholar]
- Turner J. W., Malayil L., Guadagnoli D., Cole D., Lipp E. K. (2013). Detection of Vibrio parahaemolyticus, Vibrio vulnificus and Vibrio cholerae with respect to seasonal fluctuations in temperature and plankton abundance. Environ. Microbiol. 10.1111/1462-2920.12246 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Unterweger D., Kitaoka M., Miyata S. T., Bachmann V., Brooks T. M., Moloney J., et al. (2012). Constitutive type VI secretion system expression gives Vibrio cholerae intra- and interspecific competitive advantages. PLoS ONE 7:e48320 10.1371/journal.pone.0048320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitkevicius K., Lindmark B., Ou G., Song T., Toma C., Iwanaga M., et al. (2006). A Vibrio cholerae protease needed for killing of Caenorhabditis elegans has a role in protection from natural predator grazing. Proc. Natl. Acad. Sci. U.S.A. 103 9280–9285 10.1073/pnas.0601754103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeru S., Wai S., Saeed A., Sandström G., Abd H. (2012). ToxR of Vibrio cholerae affects biofilm, rugosity and survival with Acanthamoeba castellanii. BMC Res. Notes 5:33 10.1186/1756-0500-5-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeru S. P., Rompikuntal P. K., Ishikawa T., Vaitkevicius K., Sjoling A., Dolganov N., et al. (2009). Role of melanin pigment in expression of Vibrio cholerae virulence factors. Infect. Immun. 77 935–942 10.1128/IAI.00929-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzulli L., Brettar I., Pezzati E., Reid P. C., Colwell R. R., Höfle M. G., et al. (2011). Long-term effects of ocean warming on the prokaryotic community: evidence from the vibrios. ISME J. 6 21–30 10.1038/ismej.2011.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzulli L., Pezzati E., Moreno M., Fabiano M., Pane L., Pruzzo C., et al. (2009). Benthic ecology of Vibrio spp. and pathogenic Vibrio species in a coastal Mediterranean environment (La Spezia Gulf, Italy). Microb. Ecol. 58 808–818 10.1007/s00248-009-9542-8 [DOI] [PubMed] [Google Scholar]
- Vezzulli L., Pruzzo C., Huq A., Colwell R. R. (2010). Environmental reservoirs of Vibrio cholerae and their role in cholera. Environ. Microbiol. Rep. 2 27–33 10.1111/j.1758-2229.2009.00128.x [DOI] [PubMed] [Google Scholar]
- Vimala B., Thong K. L., Chong V. (2010). Isolation, detection and genomic differentiation of Vibrio cholerae and Vibrio parahaemolyticus in Bachok, Kelantan. Malays. J. Sci. 29 1–10 [Google Scholar]
- Vugia D. J., Shefer A. M., Douglas J., Greene K. D., Bryant R. G., Werner S. B. (1997). Cholera from raw seaweed transported from the Philippines to California. J. Clin. Microbiol. 35 284–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L., Orme-Johnson W., Walsh C. (1989). “Transition metal enzymes in bacterial metabolism,” in Metal Ions and Bacteria eds Beveridge T. J., Doyle R. J. (New York, NY: Wiley-Interscience; ) 165–206 [Google Scholar]
- Wai S. N., Mizunoe Y., Takade A., Kawabata S. I., Yoshida S. I. (1998). Vibrio cholerae O1 Strain TSI-4 produces the exopolysaccharide materials that determine colony morphology, stress resistance, and biofilm formation. Appl. Environ. Microbiol. 64 3648–3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldor M. K., Mekalanos J. J. (1996). Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272 1910–1914 10.1126/science.272.5270.1910 [DOI] [PubMed] [Google Scholar]
- Watnick P. I., Kolter R. (1999). Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 34 586–595 10.1046/j.1365-2958.1999.01624.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick P. I., Lauriano C. M., Klose K. E., Croal L., Kolter R. (2001). The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Mol. Microbiol. 39 223–235 10.1046/j.1365-2958.2001.02195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Kirby A., Levin B. R. (2011). The population and evolutionary dynamics of Vibrio cholerae and its bacteriophage: conditions for maintaining phage-limited communities. Am. Nat. 178 715–725 10.1086/662677 [DOI] [PubMed] [Google Scholar]
- Weichart D., McDougald D., Jacobs D., Kjelleberg S. (1997). In situ analysis of nucleic acids in cold-induced nonculturable Vibrio vulnificus. Appl. Environ. Microbiol. 63 2754–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winiecka-Krusnell J., Linder E. (2001). Bacterial infections of free-living amoebae. Res. Microbiol. 152 613–619 10.1016/S0923-2508(01)01240-2 [DOI] [PubMed] [Google Scholar]
- Worden A. Z., Seidel M., Smriga S., Wick A., Malfatti F., Bartlett D., et al. (2006). Trophic regulation of Vibrio cholerae in coastal marine waters. Environ. Microbiol. 8 21–29 10.1111/j.1462-2920.2005.00863.x [DOI] [PubMed] [Google Scholar]
- Wright A. C., Harwood V. J. (2013). “Vibrios,” in Foodborne Infections and Intoxications 4th Edn eds Glenn J. M., Morris P. (San Diego: Academic Press; ) 113–128 [Google Scholar]
- Wyckoff E. E., Mey A. R., Leimbach A., Fisher C. F., Payne S. M. (2006). Characterization of ferric and ferrous iron transport systems in Vibrio cholerae. J. Bacteriol. 188 6515–6523 10.1128/JB.00626-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff E. E., Mey A. R., Payne S. M. (2007). Iron acquisition in Vibrio cholerae. Biometals 20 405–416 10.1007/s10534-006-9073-4 [DOI] [PubMed] [Google Scholar]
- Xu H. S., Roberts N., Singleton F. L., Attwell R. W., Grimes D. J., Colwell R. R. (1982). Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb. Ecol. 8 313–323 10.1007/BF02010671 [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Okujo N., Fujita Y., Saito M., Yoshida T., Shinoda S. (1993). Structures of two polyamine-containing catecholate siderophores from Vibrio fluvialis. J. Biochem. 113 538–544 [DOI] [PubMed] [Google Scholar]
- Yamguchi T., Ikawa T., Nisizawa K. (1969). Pathway of mannitol formation during photosynthesis in brown algae. Plant Cell Physiol. 10 425–440 [Google Scholar]
- Yildiz F. H. (2008). Cyclic dimeric GMP signaling and regulation of surface-associated developmental programs. J. Bacteriol. 190 781–783 10.1128/JB.01852-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz F. H., Dolganov N. A., Schoolnik G. K. (2001). VpsR, a member of the response regulators of the two-component regulatory systems, is required for expression of vps biosynthesis genes and EPS(ETr)-associated phenotypes in Vibrio cholerae O1 El Tor. J. Bacteriol. 183 1716–1726 10.1128/JB.183.5.1716-1726.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz F. H., Liu X. S., Heydorn A., Schoolnik G. K. (2004). Molecular analysis of rugosity in a Vibrio cholerae O1 El Tor phase variant. Mol. Microbiol. 53 497–515 10.1111/j.1365-2958.2004.04154.x [DOI] [PubMed] [Google Scholar]
- Yildiz F. H., Schoolnik G. K. (1999). Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl. Acad. Sci. U.S.A. 96 4028–4033 10.1073/pnas.96.7.4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz F. H., Visick K. L. (2009). Vibrio biofilms: so much the same yet so different. Trends Microbiol. 17 109–118 10.1016/j.tim.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ymele-Leki P., Houot L., Watnick P. I. (2013). Mannitol and the mannitol-specific enzyme IIB subunit activate Vibrio cholerae biofilm formation. Appl. Environ. Microbiol. 79 4675–4683 10.1128/AEM.01184-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Mekalanos J. J. (2003). Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell 5 647–656 10.1016/S1534-5807(03)00295-8 [DOI] [PubMed] [Google Scholar]
- Zhu J., Miller M. B., Vance R. E., Dziejman M., Bassler B. L., Mekalanos J. J. (2002). Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. U.S.A. 99 3129–3134 10.1073/pnas.052694299 [DOI] [PMC free article] [PubMed] [Google Scholar]