Abstract
Rationale: The current management of lymphoma requires accurate diagnosis and subtyping of de novo lymphoma and of relapsed or refractory lymphoma in known cases. The role of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) in the clinical management of lymphomas is unclear.
Objectives: To investigate the use of EBUS-TBNA in the diagnosis of de novo and relapsed mediastinal lymphomas.
Methods: A total of 2,256 consecutive patients who underwent EBUS-TBNA in a tertiary center between February 2008 and April 2013 were prospectively evaluated. The diagnostic accuracy and clinical use of EBUS-TBNA in 100 cases of de novo or suspected relapsed mediastinal lymphoma was investigated by comparing EBUS-TBNA diagnosis with the final diagnosis.
Measurements and Main Results: De novo mediastinal lymphoma was correctly diagnosed by EBUS-TBNA in 45 (88%) of 51 and relapsed lymphoma in 15 (100%) of 15 lymphoma cases. EBUS-TBNA accurately established a diagnosis other than lymphoma in 32 (97%) of 33 patients with suspected lymphoma relapse. Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of EBUS-TBNA in the diagnosis of mediastinal lymphoma were 89%, 97%, 98%, 83%, and 91%, respectively. Sensitivity of EBUS-TBNA in subtyping lymphomas into high-grade non-Hodgkin lymphoma, low-grade non-Hodgkin lymphoma, and Hodgkin lymphoma was 90%, 100%, and 79%, respectively. EBUS-TBNA diagnosis was adequate for clinical management in 84 (84%) of 100 cases.
Conclusions: Multimodality evaluation of EBUS-TBNA can be successful in the diagnosis of de novo mediastinal lymphomas and is ideally suited in distinguishing lymphoma relapse from alternative pathologies; it is least sensitive in subtyping Hodgkin lymphoma.
Keywords: lymphoma, fine-needle aspiration, cytology, endobronchial ultrasound
At a Glance Commentary
Scientific Knowledge on the Subject
The role of endobronchial ultrasound transbronchial needle aspiration in lymphoma diagnosis and management has not been established.
What This Study Adds to the Field
This study demonstrates that this minimally invasive technique can be used in the diagnosis and management of mediastinal lymphoma, thereby obviating the need for more invasive surgical biopsies.
Lymphomas account for 20% of primary mediastinal tumors in adults and involvement of mediastinum by systemic lymphoma is common (1). The diagnosis and classification of lymphoma no longer relies on pure morphologic characteristics of tissue specimens. The World Health Organization recommends the use of a mixture of diagnostic modalities (cytomorphology, immunophenotype, cytogenetics, and molecular features) for accurate subclassification (2). As a consequence, fine-needle aspiration cytology (FNAC) has received attention as an alternative to histology on excisional or core biopsies, which represent the gold standard for lymphoma diagnosis (3–8). The development of endobronchial ultrasound (EBUS), which allows ultrasound-guided aspiration of mediastinal and pulmonary lymph nodes and masses (EBUS transbronchial needle aspiration [TBNA]), offers the opportunity to incorporate FNAC to the diagnosis and management of mediastinal lymphoid neoplasms, which had hitherto required sampling by more invasive techniques, such as mediastinoscopy, mediastinotomy, or surgical thoracoscopy. Although there is support for the use of EBUS-TBNA in lung cancer (9–12) and in the evaluation of isolated mediastinal lymphadenopathy (13), there is limited information of its value in the diagnosis and management of lymphoma (14–18). The ability of FNAC in general (5) and EBUS-TBNA specifically (14, 18) to correctly diagnose and subtype lymphoma has been questioned. In this study we investigated the accuracy of diagnosis and subtyping of mediastinal lymphoma by multimodality evaluation of EBUS-TBNA and assessed its role in the investigation of suspected lymphoma relapse.
Methods
All patients who underwent EBUS-TBNA in a tertiary center between February 2008 and April 2013 were prospectively evaluated to assess the diagnostic accuracy and clinical use of EBUS-TBNA in cases of de novo and suspected relapsed mediastinal lymphoma.
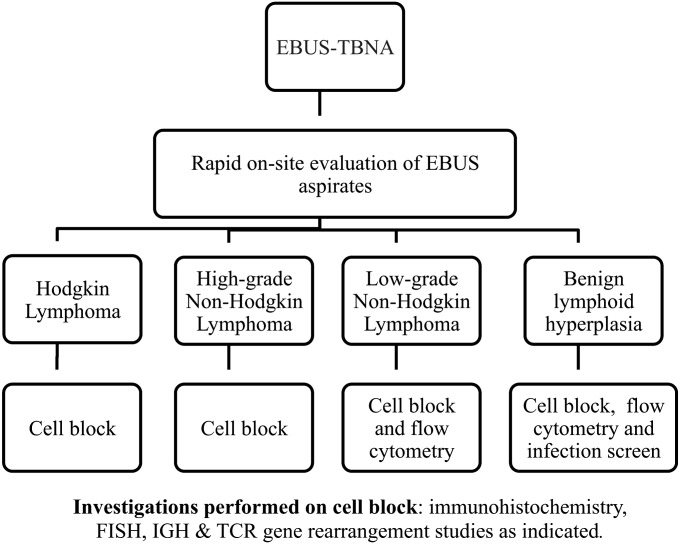
EBUS-TBNA was performed by two consultant pulmonologists as previously described (19–21). The size, nodal station, and number of aspirates for each node sampled were recorded in real-time. A biomedical scientist prepared EBUS-derived aspirates for rapid on-site morphologic evaluation of air-dried smears by a consultant cytopathologist, who provided real-time assessment of the aspirates and allowed triage of cell suspensions for diagnostic tests (Figure 1) (19–21). The same cytopathologist reviewed all multimodality data and issued a diagnosis (referred to as EBUS-TBNA diagnosis from here on). The hemato-oncology multidisciplinary team (MDT) at our institution independently reviewed the EBUS-TBNA diagnosis, assessed its adequacy for diagnosis and management, and determined the need for tissue biopsy. Tissue biopsy was obtained in cases with EBUS diagnosis of probable lymphoma, or unequivocal lymphoma that could not be subtyped; as part of staging investigations; and at the discretion of the MDT if confirmation of EBUS diagnosis was deemed appropriate. The hemato-oncology MDT then issued a diagnosis based on all available data (referred to as final diagnosis from here on). The EBUS-TBNA and final diagnoses were recorded, once issued, on the study electronic database. Lymphoma cases diagnosed at our institution during the same period were also assessed to identify any additional cases that had been previously investigated by EBUS-TBNA.
Figure 1.
Flow chart illustrating pathway for diagnostic tests performed on EBUS-derived aspirates following rapid on site evaluation by consultant cytopathologist. EBUS = endobronchial ultrasound; FISH = fluorescent in situ hybridization; IGH = immunoglobulin heavy chain; TBNA = transbronchial needle aspiration; TCR = T-cell receptor.
Immunocytochemistry on sections cut from the cell blocks was performed with an automated immunostainer (Leica Bondmax, Leica Microsystems [UK] Ltd, Milton Keynes, UK) using commercially available antibodies. Flow cytometry was performed on a Navios flow cytometer (Beckman Coulter, High Wycombe, UK) using a four-color protocol according to manufacturer’s instructions and using routine protocols. Fluorescent in situ hybridization was performed on sections cut from cell blocks using commercially available fusion and break-apart probes.
High-grade B-cell non-Hodgkin lymphoma (NHL) was diagnosed on morphology and appropriate immunohistochemistry on EBUS-derived cell block. Diffuse large B-cell lymphoma (DLBCL) was further subclassified in accordance with the Hans algorithm (22) into (1) germinal center B-cell type (GCB), (2) activated B-cell type (ABC), and (3) unclassified subtype. T-cell NHL was diagnosed on morphologic criteria; an appropriate, often entity-specific immunophenotype; and evidence of clonality (CD4/CD8 restriction or polymerase chain reaction for T-cell receptor gene rearrangements).
The diagnosis of low-grade B-cell NHL was based on morphology and by identifying a light-chain restricted B-cell population either by flow cytometry or cell block immunohistochemistry. Further subclassification into chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL-SLL), follicular lymphoma, mantle cell lymphoma, and marginal zone lymphoma was made on the basis of morphologic criteria and the demonstration of a specific immunophenotype. Cases that did not meet these criteria but had unequivocal morphologic and flow cytometry evidence of clonal B-cell expansion were classified as low-grade B-cell lymphoma.
Classical Hodgkin lymphoma (CHL) was diagnosed on EBUS-TBNA aspirates when there was unequivocal and/or immunophenotypic evidence of Hodgkin lymphoma. Probable Hodgkin lymphoma was defined when these criteria could not be satisfied or there were aberrant morphologic or immunophenotypic features.
Benign reactive lymphadenopathy was diagnosed on EBUS-TBNA by the presence of mixed population of mature lymphocytes and tingible body macrophages, an appropriate lymphocyte subpopulation, and lack of clonal expansion on flow cytometry.
Additional detail on EBUS-TBNA sample preparation, ancillary testing, and criteria for lymphoma subtyping is provided in the online supplement.
Results
Patient Clinical Characteristics
Patient clinical characteristics are shown in Table 1. We accrued 100 cases from 2,256 (4.4%) consecutive patients who had been evaluated by EBUS-TBNA during the study period. Fifty-one were male and 49 female. Their median age was 61 years (range, 24–88 yr). Six (6%) of 100 cases had prior diagnosis of carcinoma and two (2%) cases had received treatment for HIV infection. Ten patients presented with a mediastinal mass and 90 with mediastinal lymphadenopathy.
TABLE 1.
PATIENT CLINICAL CHARACTERISTICS
| Final Diagnosis |
|||||
|---|---|---|---|---|---|
| Hodgkin Lymphoma | High-Grade NHL | Low-Grade NHL | Nonlymphoma Diagnosis | Total | |
| Total number |
24 |
12 |
30 |
34 |
100 |
| De novo lymphoma |
22 |
11 |
18 |
1* |
52 |
| Lymphoma follow-up |
2 |
1 |
12 |
33 |
48 |
| Median (range) age, yr |
54 (24–84) |
61 (25–85) |
63 (59–85) |
66 (25–88) |
61 (24–88) |
| Male/female |
12/12 |
5/7 |
18/12 |
16/18 |
51/49 |
| Indication |
|
|
|
|
|
| Mediastinal mass |
8 |
1 |
1 |
0 |
10 |
| Mediastinal lymphadenopathy |
16 |
11 |
29 |
34 |
90 |
| Isolated |
11 |
8 |
14 |
1 |
34 |
| HIV positive |
2 |
0 |
0 |
0 |
2 |
| Evaluation in known carcinoma |
0 |
2 |
4 |
0 |
6 |
| Evaluation in known lymphoma |
3 |
1 |
11 |
33 |
48 |
| Lymph node stations† |
|
|
|
|
|
| Total |
44 |
15 |
59 |
67 |
185 |
| 2R/L |
4 |
0 |
3 |
4 |
11 |
| 4R/L |
20 |
8 |
22 |
20 |
70 |
| 7 |
12 |
4 |
19 |
15 |
50 |
| 10-12R/L |
8 |
3 |
15 |
28 |
54 |
| Mean (range) lymph node size, cm |
1.8 (0.5–4) |
1.9 (0.9–4) |
1.5 (0.5–3) |
1.2 (0.5–2) |
1.6 (0.5–4) |
| Mean (range) number of passes per lymph node | 6.7 (2–13) | 5.3 (3–9) | 4.7 (2–11) | 4.1 (2–10) | 5.2 (2–12) |
Definition of abbreviation: NHL = non-Hodgkin lymphoma.
False-positive diagnosis of probable non-Hodgkin lymphoma.
Refers to delineation of lymph node stations by endobronchial ultrasound based on the new International Association of Study of Lung Cancer lymph node map.
A total of 185 lymph nodes and 10 masses were sampled by EBUS-TBNA. Mean lymph node size was 1.61 cm (range, 0.5–4 cm). Lower paratracheal lymph nodes (stations 4R and 4L) were the commonest lymph node stations sampled (38%). Fifty-four (29%) lymph nodes at stations 10–12R/L would have been accessible only by thoracotomy or surgical thoracoscopy. Two or more lymph node stations were sampled by EBUS-TBNA in 31 (61%) of 51 de novo lymphoma cases and 11 (73%) of 15 lymphoma follow-up cases. The mean number of passes per lymph node was 5.1 (range, 2–13). There were no major complications.
Final Diagnosis
EBUS-TBNA established a definite diagnosis of lymphoma in 59 (89%) of 66 cases; of probable lymphoma in six (9%) cases (including one false positive); and was nondiagnostic in one (2%) case (Table 2). It established a diagnosis other than lymphoma in 32 (97%) of 33 cases. There were no additional cases of de novo or relapsed lymphoma diagnosed at our institution, which serves as a tertiary center for hemato-oncology and thoracic surgery, during the study period.
TABLE 2.
COMPARISON BETWEEN EBUS-TBNA AND FINAL DIAGNOSES
| EBUS-TBNA diagnosis (n = 93) | Final Diagnosis (n = 100) |
|||
|---|---|---|---|---|
| High-grade NHL | Low-grade NHL | Hodgkin Lymphoma | Nonlymphoma Diagnosis | |
| High-grade B/T NHL (n = 10) |
10 |
0 |
0 |
0 |
| Probable high-grade NHL (n = 1) |
0 |
0 |
0 |
1 |
| Low-grade B-NHL (n = 30) |
0 |
30 |
0 |
0 |
| Hodgkin lymphoma (n = 19) |
0 |
0 |
19 |
0 |
| Probable Hodgkin lymphoma (n = 6) |
1 |
0 |
5 |
0 |
| Nonlymphoma diagnosis in suspected lymphoma relapse cases (n = 32) |
0 |
0 |
0 |
32 |
| Inadequate (n = 2) | 1 | 0 | 0 | 1 |
Definition of abbreviations: EBUS-TBNA = endobronchial ultrasound transbronchial needle aspiration; NHL = non-Hodgkin lymphoma.
Paired tissue was available in (1) 16 cases with unequivocal EBUS-TBNA diagnosis of lymphoma (six cases of Hodgkin lymphoma, four cases of high-grade NHL, and six cases of low-grade lymphoma); (2) seven cases with EBUS-TBNA diagnosis of probable lymphoma (six cases of probable Hodgkin and one case of probable high-grade NHL); and (3) five cases of suspected relapsed low-grade B-cell lymphoma with a diagnosis other than lymphoma. Tissue was obtained by mediastinoscopy in 20 of these cases and from other sites, as part of subsequent staging investigations, in eight cases (bone marrow biopsy in four; excision lung biopsy and biopsies from liver, buttock, and paraspinal masses, respectively, in four). There was 100% concordance between EBUS-TBNA and tissue diagnoses in Groups 1 and 3 and in five (71%) of seven in Group 2. Tissue biopsy in a case of de novo T-cell NHL yielded insufficient material for full immunophenotyping. This was achieved after EBUS-TBNA. Paired tissue comparison was obtained in 8 of the first 10 cases of lymphoma diagnosed by EBUS-TBNA and in the first 30 months of the study in 68% of cases. The overall sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of EBUS-TBNA in diagnosing lymphoma was 89%, 97%, 98%, 83%, and 91%, respectively. Cases with an EBUS diagnosis of probable lymphoma and those that were inadequate were classified as false negative. Sensitivity of EBUS-TBNA in subtyping lymphomas into high-grade NHL, low-grade NHL, and Hodgkin lymphoma was 90%, 100%, and 79%, respectively. EBUS-TBNA diagnosis was deemed sufficient for clinical management by independent review of all data by the hemato-oncology MDT in 52 (79%) of 66 lymphoma cases; 32 (97%) of 33 lymphoma follow-up cases with nonlymphoma diagnosis; and in one false-positive case of probable lymphoma. Overall, EBUS-TBNA diagnosis was adequate for clinical management in 84 (84%) of 100 cases.
Lymphoma Subtyping
High-grade B- and T-cell NHL.
EBUS-TBNA diagnosis of high-grade NHL was established in 10 (83%) of 12 cases (Tables 2 and 3). DLBCL was diagnosed by EBUS-TBNA in six cases. Immunohistochemistry subclassified these into GCB (one of six); ABC (four of six); or unclassified subtype (one of six). Fluorescent in situ hybridization was successfully performed in all cases; one showed a MYC rearrangement indicating poor prognosis. No B-cell lymphoma 2 (BCL-2) or BCL-6 to immunoglobulin heavy chain locus (IGH) rearrangements (IGH-BCL2 or IGH-BCL-6) were identified in any cases. Tissue biopsy was not considered necessary in these six cases.
TABLE 3.
IMMUNOPHENOTYPE OF HIGH-GRADE B- AND T-CELL NHL*
| |
Immunohistochemistry on Cell Block |
FISH: IGH/MYC | PCR: T-Cell Gene Rearrangement | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | CD20 | CD79a | BCL-6 | CD10 | CD3 | CD5 | CD4 | CD25 | MUM-1 | BCL-2 | CD30 | EBER | MIB-1 (%) | ||
| Immunoprofile and cytogenetic characterization of DLBCL on EBUS-TBNA | |||||||||||||||
| 1 |
+ |
+ |
+ |
− |
|
|
|
|
+ |
+ |
− |
nd |
90 |
+ |
|
| 2 |
+ |
+ |
− |
− |
|
|
|
|
+ |
|
+ |
− |
80 |
− |
|
| 3 |
+ |
+ |
+ |
− |
|
|
|
|
+ |
+ |
− |
− |
90 |
− |
|
| 4 |
+ |
+ |
+ |
+ |
|
|
|
|
+ |
− |
+ |
− |
60 |
− |
|
| 5 |
+ |
+ |
+ |
+ |
|
|
|
|
− |
− |
+ |
− |
80 |
− |
|
| 6 |
+ |
+ |
− |
− |
|
|
|
|
+ |
+ |
+ |
nd |
90 |
nd as necrotic |
|
| 7 |
+ |
+ |
+ |
− |
|
|
|
|
+ |
+ |
− |
− |
80 |
+ |
|
| Immunoprofile and molecular characterization of T-cell lymphoma on EBUS-TBNA | |||||||||||||||
| 8 |
|
|
|
|
+ |
− |
− |
− |
+ |
nd |
+ |
+ |
70 |
|
Clonal |
| 9 |
|
|
|
|
+ |
− |
+ |
+ |
+ |
+ |
+ |
− |
65 |
|
Clonal |
| 10 |
|
|
|
|
+ |
− |
+ |
+ |
+ |
+ |
− |
− |
100 |
|
nd |
| 11 | + | − | + | + | + | + | + | − | nd | nd | |||||
Definition of abbreviations: BCL = B-cell lymphoma oncogene; DLBCL = diffuse large B-cell lymphoma; EBER = Epstein-Barr virus–encoded RNA; EBUS-TBNA = endobronchial ultrasound transbronchial needle aspiration; FISH = fluorescence in situ hybridization; IGH = immunoglobulin heavy chain; MIB-1 = proliferation index assessed by MIB-1 staining; MUM = multiple myeloma oncogene-1; MYC = myelocytomatosis oncogene; nd = not done; NHL = non-Hodgkin lymphoma; PCR = polymerase chain reaction.
One case of inadequate EBUS-TBNA not shown.
There was insufficient diagnostic material after EBUS-TBNA in one case; this was diagnosed as DLBCL after tissue biopsy. There was one false-positive case, diagnosed as necrotic lymphoma suspicious of DLBCL (Table 3, Case 6). A surgical biopsy was recommended but declined by the patient; subsequent imaging showed resolution of lymphadenopathy. We speculate that infectious mononucleosis that could not be confirmed because of the necrotic nature of the aspirate simulated a high-grade lymphoma in this case.
EBUS-TBNA diagnosis of de novo CD30+ ALK-1 negative peripheral T-cell lymphoma in two cases and de novo and relapsed adult T-cell lymphoma-leukemia was established in two cases, respectively (Table 3). Cell block immunohistochemistry showed that the lymphoid cells expressed CD45, MUM-1, T-cell markers, and CD30, but were negative for CD20, CD79a, BCL-6, CD10, and anaplastic lymphoma kinase -1 (ALK-1). The adult T-cell leukemia/lymphoma (ATLL) cases expressed CD25 strongly and human T lymphotropic virus serology was positive.
Low-grade B-cell NHL.
EBUS-TBNA diagnosis of low-grade B-cell NHL was established in all 30 cases. These were subtyped as CLL-SLL (seven cases); follicular lymphoma (seven cases); mantle cell lymphoma (two cases); and marginal zone lymphoma (four cases). Ten cases were labeled as low-grade B-cell lymphoma. Four of these cases were de novo lymphomas that could not be further subtyped and six were in patients with known low-grade NHL. There was no evidence of high-grade transformation on morphologic grounds in any of these cases.
Hodgkin lymphoma.
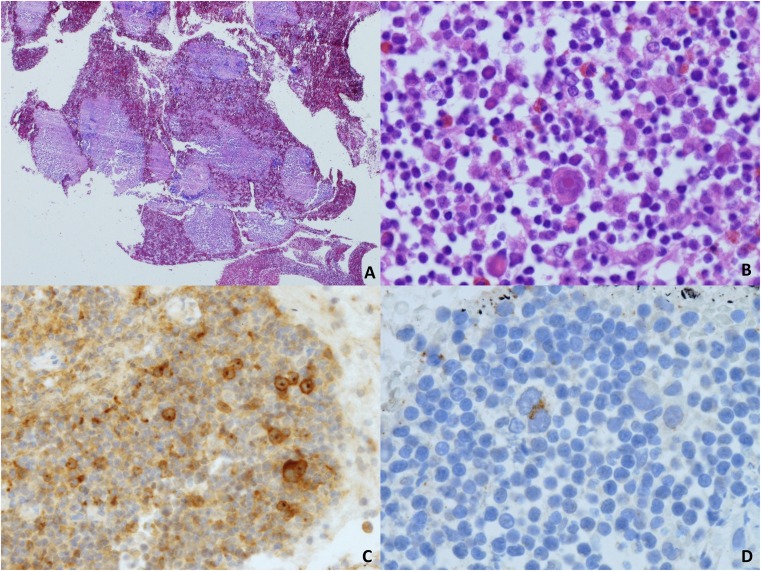
EBUS-TBNA diagnosis of Hodgkin lymphoma was made in 19 (79%) of 24 cases (Table 2). Of these, 22 represented de novo diagnosis. Relapsed CHL was diagnosed post treatment in two cases. Thirteen cases (54%) were confirmed as CHL by a complete immunophenotype on EBUS-TBNA (Figure 2, Table 4). No tissue biopsy was deemed necessary in these 13 cases. In the remaining 12 cases, tissue biopsy was obtained. In 6 of 12 there was unequivocal morphologic evidence of Hodgkin lymphoma but insufficient material for complete immunophenotyping. Tissue biopsy established diagnosis of CHL in all cases. In six further cases with an EBUS-TBNA diagnosis of probable Hodgkin lymphoma, paired tissue biopsy confirmed CHL in five cases. One case, with an EBUS-TBNA diagnosis of probable CHL with aberrant CD20 expression, was diagnosed as DLBCL on tissue biopsy.
Figure 2.
(A) Scanning magnification view to show large numbers of microbiopsy fragments obtained using a 21-gauge needle (hematoxylin and eosin, ×2). (B) Higher magnification of one of these fragments showing a typical Hodgkin cell in an inflammatory milieu with eosinophils (hematoxylin and eosin, ×20). (C) Immunohistochemistry for CD30 (×10). (D) Reed-Sternberg cell expressing CD15 (×40).
TABLE 4.
EBUS-TBNA DIAGNOSIS AND SUBTYPING OF HODGKIN LYMPHOMA
| Case | EBUS-TBNA Site* | Immunophenotype by Immunohistochemistry on EBUS-TBNA Cell Block |
||||||
|---|---|---|---|---|---|---|---|---|
| CD45 | CD30 | CD15 | MUM-1 | EBER | CD20 | CD3 | ||
| 1 |
4R/7/10R |
− |
+ |
+ |
+ |
+ |
− |
− |
| 2 |
4R |
− |
+ |
+ |
+ |
+ |
− |
− |
| 3 |
4R/7 |
− |
+ |
− |
+ |
− |
− |
− |
| 4 |
4R |
− |
+ |
+ |
− |
− |
− |
− |
| 5 |
4R |
− |
+ |
− |
nd |
nd |
− |
− |
| 6 |
4R/7 |
− |
+ |
+ |
+ |
+ |
− |
− |
| 7 |
4L/10L |
− |
+ |
− |
+ |
+ |
− |
− |
| 8 |
4R/4L |
− |
+ |
+ |
− |
− |
− |
− |
| 9 |
2R/4R |
− |
+ |
+ |
+ |
− |
− |
− |
| 10 |
4R |
− |
+ |
− |
+ |
− |
− |
− |
| 11 |
4R/2R/4L |
− |
+ |
+ |
+ |
+ |
− |
− |
| 12 |
7/12L |
− |
+ |
− |
+ |
+ |
− |
− |
| 13 | 2R/7 | − | + | + | + | − | − | − |
Definition of abbreviations: EBER = Epstein-Barr virus\x{2013}encoded RNA; EBUS-TBNA = endobronchial ultrasound transbronchial needle aspiration; MUM = multiple myeloma oncogene; nd = not done.
Refers to delineation of lymph node stations by EBUS based on the new International Association of Study of Lung Cancer lymph node map.
The accuracy of EBUS-TBNA diagnosis and subtyping of Hodgkin lymphoma improved with experience; only 3 (27%) of 11 patients were fully immunophenotyped on EBUS-TBNA in the first 2 years in contrast to 10 (77%) of 13 in the last 3 years.
Nonlymphoma EBUS Diagnosis
Among the suspected lymphoma relapse cases, an alternative diagnosis was revealed in 33 (68%) of 48 cases and EBUS-TBNA established this in 32 (97%) of 33 cases (Table 5). Nonlymphoma diagnoses include lung cancer, granulomatous lymphadenitis, mycobacterium tuberculosis, bronchogenic cyst, and benign reactive lymphadenitis. In all cases of benign reactive lymphadenitis, follow-up period was at least 6 months; in 8 of 12 cases follow-up was for 12 months or longer. Flow cytometry was negative for clonal expansion in all cases of benign reactive lymphadenitis. A nondiagnostic aspirate was obtained in one case of Hodgkin lymphoma, which was subsequently shown to have granulomatous lymphadenitis after excision biopsy of new cervical lymph node.
TABLE 5.
EBUS-TBNA DIAGNOSIS IN SUSPECTED LYMPHOMA RELAPSE CASES
| EBUS-TBNA diagnosis | Primary Diagnosis |
|||||
|---|---|---|---|---|---|---|
| Hodgkin Lymphoma | High-Grade NHL | Low-Grade NHL | Cutaneous Lymphoma | Other* | Total | |
| Lymphoma relapse |
2 |
1 |
12 |
0 |
0 |
15 |
| Lung cancer† |
1 |
2 |
3 |
1 |
1 |
8 |
| Granulomatous inflammation |
2 |
2 |
3 |
2 |
0 |
9 |
| Mycobacterial lymphadenitis |
0 |
0 |
2 |
0 |
0 |
2 |
| Benign reactive lymphadenitis |
4 |
2 |
4 |
1 |
1 |
12‡ |
| Bronchogenic cyst |
0 |
0 |
0 |
1 |
0 |
1 |
| Nondiagnostic |
1§ |
0 |
0 |
0 |
0 |
1 |
| Total | 10 | 7 | 24 | 5 | 2 | 48 |
Definition of abbreviations: EBUS-TBNA = endobronchial ultrasound transbronchial needle aspiration; NHL = non-Hodgkin lymphoma.
Gastric Burkitt lymphoma and Waldenström macroglobulinemia.
Adenocarcinoma (n = 4); squamous cell carcinoma (n = 1); and small-cell lung cancer (n = 3).
Tissue confirmation of benign reactive lymphadenitis was obtained in five of these cases by mediastinoscopy. In the remainder, EBUS-TBNA diagnosis was confirmed on follow-up.
Granulomatous lymphadenitis diagnosed after subsequent excision biopsy of new cervical lymph node.
Discussion
Histology on excisional or core biopsies is the gold standard for lymphoma diagnosis. The fact that EBUS-TBNA diagnosis relies on FNAC could therefore limit its use as a diagnostic modality, particularly in cases of de novo mediastinal lymphoma. However, the ability to generate cell blocks from EBUS-TBNA allows cytologic material to be treated as a biopsy and for histologic sections to be cut, while cell suspensions for flow cytometry and molecular testing could also be retained. Although most of the cells within the cell block are disaggregated, small fragments of tissue or slender cores are often identified; this can allow immunocytochemistry and ancillary tests to be interpreted in the context of the architecture, which is important in lymphoma diagnosis. Previous studies that evaluated the role of EBUS-TBNA in lymphoma diagnosis (14–18) reported mostly on cases with suspected disease relapse; they included only a very small number of high-grade NHL or Hodgkin lymphomas and provided limited data on further lymphoma subclassification. In cases of de novo lymphoma, the diagnostic accuracy of EBUS-TBNA was found to be very low, despite underrepresentation of Hodgkin and high-grade NHL cases in these patient cohorts (25–51%), which questions its clinical role in this context (14, 18). Moreover, the ability of FNAC in general and EBUS-TBNA specifically to accurately subtype lymphoma has been questioned (5, 14, 18). The use of EBUS-TBNA in the diagnosis of mediastinal lymphomas is therefore uncertain and of questionable value in cases of de novo lymphoma.
In this study, we report on the largest and most comprehensively evaluated cohort of patients diagnosed with mediastinal lymphoma by multimodality evaluation of EBUS-TBNA thus far. The inclusion of a comparatively large number of de novo lymphoma, in particular Hodgkin and high-grade NHL, is a novel feature of this study and distinguishes it from recent studies that evaluated the role of EBUS-TBNA and EUS-FNA in lymphoma diagnosis. We acknowledge that randomized studies are necessary to fully establish the role of EBUS-TBNA, compared with other techniques, in the diagnosis and management of mediastinal lymphomas; however, this pragmatic prospective study, similar in design to that of Navani and colleagues (11) that evaluated the role of EBUS-TBNA in lung cancer subtyping, allows us to draw meaningful conclusions on the potential role of this minimally invasive and low-morbidity technique.
The sensitivity and specificity of EBUS-TBNA diagnosis of lymphoma and more specifically Hodgkin and high-grade NHL in our series is superior to the reported sensitivities of EBUS-TBNA (14–18) and EUS-FNAC (23–26), and compare well with the reported sensitivities of mediastinoscopy (27, 28) and CT-guided FNAC (3) in diagnosing lymphoma. The accuracy of EBUS-TBNA in establishing the diagnosis of lymphoma in our study would have been influenced by the exclusion of cases subsequently diagnosed with lymphoma. We believe this is highly unlikely, because there were no additional cases of lymphoma diagnosed at our institution during the study period that had been previously investigated by EBUS-TBNA. In support of this we highlight the fact that the number of lymphoma cases in our study (n = 66 of 2,256 cases) compares favorably with that for cervical mediastinoscopy (n = 51 of 2,145 cases) (27).
In our study, the need for comparison between histology and EBUS-TBNA diagnoses was independently determined by the hemato-oncology MDT. Such comparison was available in 28 cases and was found to be concordant in 26 (93%). There was discordance between EBUS-TBNA and tissue diagnoses only in cases where EBUS-TBNA was suspicious but not definite of the diagnosis of lymphoma. In view of these findings and because of the strength and completeness of EBUS-TBNA diagnosis, paired tissue confirmation of EBUS-TBNA diagnosis was deemed unnecessary in the remainder of the cases. Moreover, in 30% of these cases, paired tissue confirmation would have required thoracotomy or surgical thoracoscopy, which could not be justified on clinical grounds.
We perform EBUS in our institution with integrated real-time evaluation of aspirates by a consultant pathologist. In the case of lymphoma, this has enabled us to individualize sampling to the most cellular target; to make decisions on the number of passes that are performed to maximize yield; and to triage aspirates for ancillary testing (immunohistochemistry, flow cytometry, cytogenetics, or molecular tests). We speculate that this approach to EBUS-TBNA and sample evaluation might explain the superior diagnostic accuracy of EBUS-TBNA reported in this compared with previous studies. Randomized studies are necessary to address the true value of EBUS-TBNA with or without rapid on-site morphologic evaluation in lymphoma diagnosis and management; however, the rarity of lymphoma cases among the total evaluated by EBUS-TBNA (4.4% in our series over 5 yr) poses significant challenges in performing such prospective studies.
We found that low-grade NHL was most amenable to diagnosis by EBUS-TBNA. We had no false-positive or -negative cases in this category. High-grade NHL was equally amenable to diagnosis. Insufficient diagnostic material was obtained by EBUS-TBNA in one case, and there was one false-positive case, caused by interpretative error and not by lack of diagnostic material. Diagnostic accuracy was lowest for Hodgkin lymphoma, which can be difficult to diagnose by FNAC because of the paucity of Reed-Sternberg cells and their admixture with benign cells. Differentiating lymphoma from rare causes of mediastinal lymphadenopathy can also be achieved using EBUS-TBNA. For example, we have previously shown that multimodality evaluation of EBUS-TBNA can be successfully used to diagnose and subclassify thymoma (19) and the rare mid-line carcinoma (29).
EBUS, unlike alternative techniques that are available to sample the mediastinum, can systematically evaluate the superior mediastinum to intrapulmonary nodal stations bilaterally in one setting; it also allows the safe sampling of lymph nodes as small as 5 mm in short axis. This capability would be especially useful in the evaluation of suspected relapsed lymphoma, in particular low-grade B-cell NHL, which has an indolent course that is characterized by frequent relapses. The clinical use of this was demonstrated in this study, because in 61% of de novo lymphoma and 73% of lymphoma follow-up cases two or more lymph node stations were sampled for diagnostic purposes and 29% of lymph nodes sampled would have been inaccessible by any approach other than thoracotomy or surgical thoracoscopy. Interestingly, relapsed lymphoma was confirmed in only 15 (31%) of 48 cases in which it was suspected. In the remainder, alternative causes, which included lung cancer, granulomatous inflammation, benign reactive lymphadenitis, and mycobacterial infection, were found. EBUS-TBNA is therefore ideally suited to answer whether there is residual or relapsed lymphoma in the mediastinum. Moreover, the ability to sample multiple lymph nodes by EBUS-TBNA in one setting could reduce the likelihood of missing transformation in cases of known lymphoma. Because of its minimally invasive nature, it is also potentially suitable for sequential sampling of target lesions for clinical or research purposes. Interestingly, we have successfully performed global gene expression profiling on EBUS-TBNA from benign and tumor-infiltrated lymph nodes in patients with known lung cancer and lymphoma in routine clinical practice (30).
When compared with histology, FNAC has been shown to be of limited value as a diagnostic technique in the clinical management of lymphoma, because of low accuracy in lymphoma subtyping (5). We therefore explored this question in relation to EBUS-TBNA. Overall, EBUS-TBNA diagnosis of lymphoma was deemed sufficient for clinical management by independent review of all the multimodality data by the hemato-oncology MDT in 52 (79%) of 66 cases, which compares favorably with FNAC in general (5). Full immunophenotyping was achieved by EBUS-TBNA in 83% of high-grade NHL. In our view this requires cell blocks for immunohistochemistry, which we believe is superior to immunophenotyping by flow cytometry because it allows evaluation of many nuclear antigens (BCL-6, MUM-1, EBER) that are difficult to assess on flow cytometry. Using multimodality testing, we successfully applied the Hans algorithm in all DLBCL cases, allowing their further subclassification into GCB (good prognosis) and ABC phenotypes (poor prognosis) (22), although outcome data after rituximab-based therapy indicate that this distinction may be of diminished relevance (31, 32). Furthermore, we were able to perform cytogenetic analysis for MYC, IGH–BCL-2, and BCL-6 rearrangement in all our DLBCL cases to identify aggressive subtypes (presence of IGH-MYC, MYC rearrangement, or concomitant presence of MYC and IGH–BCL-2 and/or BCL-6 rearrangements) (33, 34). We also demonstrate subclassification of low-grade NHL, using a combination of immunohistochemistry on cell block and flow cytometry into CLL-SLL, follicular lymphoma, mantle cell lymphoma, and marginal zone lymphoma (35–37). Moreover, adequate material was available after this multimodality testing approach for IGH-TCR gene rearrangement studies by polymerase chain reaction, when this was required. Several cases (9 of 26) were classified as low-grade B-cell lymphomas without being further subtyped. Most were lymphoma follow-up cases and EBUS-TBNA provided sufficient information for clinical management to proceed in this patient group without supplemental biopsy. EBUS-TBNA performed least well in cases of Hodgkin lymphoma, because full immunophenotyping was achieved in only 55% of cases; it improved, however, to 77% in the last 3 years compared with 27% in the first 2 years. Despite the fact that EBUS-TBNA performed least well in cases of Hodgkin lymphoma, it nonetheless prevented mediastinoscopy in more than 50% of these cases.
We conclude that multimodality evaluation of EBUS-TBNA can be successfully used in the diagnosis, subtyping, and clinical management of de novo and relapsed mediastinal lymphomas in routine clinical practice. EBUS-TBNA is ideally suited in distinguishing lymphoma relapse in the mediastinum from alternative pathologies and can therefore prevent surgical biopsies in most of such patients.
Acknowledgments
Acknowledgment
The authors thank their consultant pathology colleagues (Drs. A. Chandra, F. Chang, B. Gill-Barman, U. Mahadeva, E. McLean, and P. Menon at Guy’s and St Thomas’s NHS Foundation Trust; and Dr. G. Dixon at London Bridge Hospital) and Clinical Nurse Specialist A. Quinn for their expert contribution to their endobronchial ultrasound service. They also thank all clinicians that referred patients for endobronchial ultrasound transbronchial needle aspiration since 2008.
Footnotes
Supported in part by the Department of Health via the National Institutes of Health Research comprehensive Biomedical Research Centre award to Guy’s and St Thomas’ National Health Service Foundation Trust in partnership with King’s College London (G.S.).
Author Contributions: Conception and design, G.S. and M.T.M. Analysis and interpretation, M.T.M., R.B., P.A.F., and G.S. Endobronchial ultrasound was performed by G.S. and R.B., M.T.M. reviewed all pathology. Drafting the manuscript, G.S. Contribution to final manuscript, M.T.M., R.B., P.A.F., and G.S.
Originally Published in Press as DOI: 10.1164/rccm.201303-0462OC on September 18, 2013
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
Refrences
- 1.Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest. 2005;128:2893–2909. doi: 10.1378/chest.128.4.2893. [DOI] [PubMed] [Google Scholar]
- 2.Sverdlow SH, Campo E, Harris NL, Jaffe ES, Pilleri SA, Stein H, Theile J. Lyon: IARC; 2008. WHO classification of tumours of haemopoetic and lymphoid tissue. [Google Scholar]
- 3.Assaad MW, Pantanowitz L, Otis CN. Diagnostic accuracy of image-guided percutaneous fine needle aspiration biopsy of the mediastinum. Diagn Cytopathol. 2007;35:705–709. doi: 10.1002/dc.20738. [DOI] [PubMed] [Google Scholar]
- 4.Gong JZ, Williams DC, Jr, Liu K, Jones C. Fine-needle aspiration in non-Hodgkin lymphoma: evaluation of cell size by cytomorphology and flow cytometry. Am J Clin Pathol. 2002;117:880–888. doi: 10.1309/16UL-W4PX-HRL7-7V88. [DOI] [PubMed] [Google Scholar]
- 5.Hehn ST, Grogan TM, Miller TP. Utility of fine-needle aspiration as a diagnostic technique in lymphoma. J Clin Oncol. 2004;22:3046–3052. doi: 10.1200/JCO.2004.02.104. [DOI] [PubMed] [Google Scholar]
- 6.Jennings CD, Foon KA. Recent advances in flow cytometry: application to the diagnosis of hematologic malignancy. Blood. 1997;90:2863–2892. [PubMed] [Google Scholar]
- 7.Meda BA, Buss DH, Woodruff RD, Cappellari JO, Rainer RO, Powell BL, Geisinger KR. Diagnosis and subclassification of primary and recurrent lymphoma. The usefulness and limitations of combined fine-needle aspiration cytomorphology and flow cytometry. Am J Clin Pathol. 2000;113:688–699. doi: 10.1309/0Q7F-QTGM-6DPD-TLGY. [DOI] [PubMed] [Google Scholar]
- 8.Nicol TL, Silberman M, Rosenthal DL, Borowitz MJ. The accuracy of combined cytopathologic and flow cytometric analysis of fine-needle aspirates of lymph nodes. Am J Clin Pathol. 2000;114:18–28. doi: 10.1309/MN6J-4NJY-C5CG-1PLH. [DOI] [PubMed] [Google Scholar]
- 9.Annema JT, van Meerbeeck JP, Rintoul RC, Dooms C, Deschepper E, Dekkers OM, De Leyn P, Braun J, Carroll NR, Praet M, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA. 2010;304:2245–2252. doi: 10.1001/jama.2010.1705. [DOI] [PubMed] [Google Scholar]
- 10.Herth FJ, Annema JT, Eberhardt R, Yasufuku K, Ernst A, Krasnik M, Rintoul RC. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol. 2008;26:3346–3350. doi: 10.1200/JCO.2007.14.9229. [DOI] [PubMed] [Google Scholar]
- 11.Navani N, Brown JM, Nankivell M, Woolhouse I, Harrison RN, Jeebun V, Munavvar M, Ng BJ, Rassl DM, Falzon M, et al. Suitability of endobronchial ultrasound-guided transbronchial needle aspiration specimens for subtyping and genotyping of non-small cell lung cancer: a multicenter study of 774 patients. Am J Respir Crit Care Med. 2012;185:1316–1322. doi: 10.1164/rccm.201202-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasufuku K, Pierre A, Darling G, de Perrot M, Waddell T, Johnston M, da Cunha Santos G, Geddie W, Boerner S, Le LW, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer J Thorac Cardiovasc Surg 20111421393–1400.e1391 [DOI] [PubMed] [Google Scholar]
- 13.Navani N, Lawrence DR, Kolvekar S, Hayward M, McAsey D, Kocjan G, Falzon M, Capitanio A, Shaw P, Morris S, et al. REMEDY Trial Investigators. Endobronchial ultrasound-guided transbronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: a prospective trial. Am J Respir Crit Care Med. 2012;186:255–260. doi: 10.1164/rccm.201203-0393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iqbal S, DePew ZS, Kurtin PJ, Sykes AM, Johnson GB, Edell ES, Habermann TM, Maldonado F. Endobronchial ultrasound and lymphoproliferative disorders: a retrospective study. Ann Thorac Surg. 2012;94:1830–1834. doi: 10.1016/j.athoracsur.2012.08.051. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy MP, Jimenez CA, Bruzzi JF, Mhatre AD, Lei X, Giles FJ, Fanning T, Morice RC, Eapen GA. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thorax. 2008;63:360–365. doi: 10.1136/thx.2007.084079. [DOI] [PubMed] [Google Scholar]
- 16.Ko HM, da Cunha Santos G, Darling G, Pierre A, Yasufuku K, Boerner SL, Geddie WR.Diagnosis and subclassification of lymphomas and non-neoplastic lesions involving mediastinal lymph nodes using endobronchial ultrasound-guided transbronchial needle aspiration Diagn Cytopathol[online ahead of print] 31 May 2011; DOI: 10.1002/dc.21741 [DOI] [PubMed] [Google Scholar]
- 17.Marshall CB, Jacob B, Patel S, Sneige N, Jimenez CA, Morice RC, Caraway N. The utility of endobronchial ultrasound-guided transbronchial needle aspiration biopsy in the diagnosis of mediastinal lymphoproliferative disorders. Cancer Cytopathol. 2011;119:118–126. doi: 10.1002/cncy.20134. [DOI] [PubMed] [Google Scholar]
- 18.Steinfort DP, Conron M, Tsui A, Pasricha SR, Renwick WE, Antippa P, Irving LB. Endobronchial ultrasound-guided transbronchial needle aspiration for the evaluation of suspected lymphoma. J Thoracic Oncol. 2010;5:804–809. doi: 10.1097/jto.0b013e3181d873be. [DOI] [PubMed] [Google Scholar]
- 19.Moonim MT, Breen R, Gill-Barman B, Santis G. Diagnosis and subclassification of thymoma by minimally invasive fine needle aspiration directed by endobronchial ultrasound: a review and discussion of four cases. Cytopathology. 2012;23:220–228. doi: 10.1111/j.1365-2303.2012.01007.x. [DOI] [PubMed] [Google Scholar]
- 20.Neat MJ, Foot NJ, Hicks A, Breen R, Wilkins B, McLean E, Santis G.ALK rearrangements in EBUS-derived transbronchial needle aspiration cytology in lung cancer Cytopathologyonline ahead of print]. 1 Apr2013. DOI: 10.1111/cyt.12060 [DOI] [PubMed] [Google Scholar]
- 21.Santis G, Angell R, Nickless G, Quinn A, Herbert A, Cane P, Spicer J, Breen R, McLean E, Tobal K. Screening for EGFR and KRAS mutations in endobronchial ultrasound derived transbronchial needle aspirates in non-small cell lung cancer using COLD-PCR. PLoS ONE. 2011;6:e25191. doi: 10.1371/journal.pone.0025191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 23.Catalano MF, Nayar R, Gress F, Scheiman J, Wassef W, Rosenblatt ML, Kochman M. EUS-guided fine needle aspiration in mediastinal lymphadenopathy of unknown etiology. Gastrointest Endosc. 2002;55:863–869. doi: 10.1067/mge.2002.124637. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro A, Pereira D, Escalón MP, Goodman M, Byrne GE., Jr EUS-guided biopsy for the diagnosis and classification of lymphoma. Gastrointest Endosc. 2010;71:851–855. doi: 10.1016/j.gie.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, Wang KK, Clain JE, Wiersema MJ. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485–491. doi: 10.1067/mge.2001.112841. [DOI] [PubMed] [Google Scholar]
- 26.Yasuda I, Tsurumi H, Omar S, Iwashita T, Kojima Y, Yamada T, Sawada M, Takami T, Moriwaki H, Soehendra N. Endoscopic ultrasound-guided fine-needle aspiration biopsy for lymphadenopathy of unknown origin. Endoscopy. 2006;38:919–924. doi: 10.1055/s-2006-944665. [DOI] [PubMed] [Google Scholar]
- 27.Lemaire A, Nikolic I, Petersen T, Haney JC, Toloza EM, Harpole DH, Jr, D’Amico TA, Burfeind WR.Nine-year single center experience with cervical mediastinoscopy: complications and false negative rate Ann Thorac Surg 2006821185–1189.discussion 1189–1190 [DOI] [PubMed] [Google Scholar]
- 28.Porte H, Roumilhac D, Eraldi L, Cordonnier C, Puech P, Wurtz A. The role of mediastinoscopy in the diagnosis of mediastinal lymphadenopathy. Eur J Cardiothorac Surg. 1998;13:196–199. doi: 10.1016/s1010-7940(97)00324-2. [DOI] [PubMed] [Google Scholar]
- 29.Santis G, Landau D, Harrison-Phipps K, Neat M, Moonim MT. Successful radical treatment of midline carcinoma with t(15;19) diagnosed by endobronchial ultrasound-derived transbronchial needle aspiration. J Clin Oncol. 2011;29:e327–329. doi: 10.1200/JCO.2010.32.7973. [DOI] [PubMed] [Google Scholar]
- 30.Lee R, Cousins DJ, Ortiz-Zapater E, Breen R, McLean E, Santis G.Gene expression profiling of endobronchial ultrasound (EBUS)-derived cytological fine needle aspirates from hilar and mediastinal lymph nodes in non-small cell lung cancer Cytopathologyonline ahead of print] 10 Dec 2012. DOI: 10.1111/cyt.12034 [DOI] [PubMed] [Google Scholar]
- 31.Castillo JJ, Beltran BE, Song MK, Ilic I, Leppa S, Nurmi H, Seki R, Uccella S, Li JM, Treaba DO, et al. The Hans algorithm is not prognostic in patients with diffuse large B-cell lymphoma treated with R-CHOP. Leuk Res. 2012;36:413–417. doi: 10.1016/j.leukres.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Meyer PN, Fu K, Greiner TC, Smith LM, Delabie J, Gascoyne RD, Ott G, Rosenwald A, Braziel RM, Campo E, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large b-cell lymphoma treated with rituximab. J Clin Oncol. 2011;29:200–207. doi: 10.1200/JCO.2010.30.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akyurek N, Uner A, Benekli M, Barista I. Prognostic significance of MYC, BCL2, and BCL6 rearrangements in patients with diffuse large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Cancer. 2012;118:4173–4183. doi: 10.1002/cncr.27396. [DOI] [PubMed] [Google Scholar]
- 34.Foot NJ, Dunn RG, Geoghegan H, Wilkins BS, Neat MJ. Fluorescence in situ hybridisation analysis of formalin-fixed paraffin-embedded tissue sections in the diagnostic work-up of non-Burkitt high grade B-cell non-Hodgkin’s lymphoma: a single centre’s experience. J Clin Pathol. 2011;64:802–808. doi: 10.1136/jclinpath-2011-200015. [DOI] [PubMed] [Google Scholar]
- 35.Ensani F, Mehravaran S, Irvanlou G, Aghaipoor M, Vaeli S, Hajati E, Khorgami Z, Nasiri S.Fine-needle aspiration cytology and flow cytometric immunophenotyping in diagnosis and classification of non-Hodgkin lymphoma in comparison to histopathology Diagn Cytopathol 201240305–310.10.1002/dc.21561 [DOI] [PubMed] [Google Scholar]
- 36.Jorgensen JL. State of the Art Symposium: flow cytometry in the diagnosis of lymphoproliferative disorders by fine-needle aspiration. Cancer. 2005;105:443–451. doi: 10.1002/cncr.21455. [DOI] [PubMed] [Google Scholar]
- 37.Zeppa P, Vigliar E, Cozzolino I, Troncone G, Picardi M, De Renzo A, Grimaldi F, Pane F, Vetrani A, Palombini L. Fine needle aspiration cytology and flow cytometry immunophenotyping of non-Hodgkin lymphoma: Can we do better? Cytopathology. 2010;21:300–310. doi: 10.1111/j.1365-2303.2009.00725.x. [DOI] [PubMed] [Google Scholar]