Abstract
Rationale: A hallmark of pulmonary tuberculosis (TB) is the formation of granulomas. However, the immune factors that drive the formation of a protective granuloma during latent TB, and the factors that drive the formation of inflammatory granulomas during active TB, are not well defined.
Objectives: The objective of this study was to identify the underlying immune mechanisms involved in formation of inflammatory granulomas seen during active TB.
Methods: The immune mediators involved in inflammatory granuloma formation during TB were assessed using human samples and experimental models of Mycobacterium tuberculosis infection, using molecular and immunologic techniques.
Measurements and Main Results: We demonstrate that in human patients with active TB and in nonhuman primate models of M. tuberculosis infection, neutrophils producing S100 proteins are dominant within the inflammatory lung granulomas seen during active TB. Using the mouse model of TB, we demonstrate that the exacerbated lung inflammation seen as a result of neutrophilic accumulation is dependent on S100A8/A9 proteins. S100A8/A9 proteins promote neutrophil accumulation by inducing production of proinflammatory chemokines and cytokines, and influencing leukocyte trafficking. Importantly, serum levels of S100A8/A9 proteins along with neutrophil-associated chemokines, such as keratinocyte chemoattractant, can be used as potential surrogate biomarkers to assess lung inflammation and disease severity in human TB.
Conclusions: Our results thus show a major pathologic role for S100A8/A9 proteins in mediating neutrophil accumulation and inflammation associated with TB. Thus, targeting specific molecules, such as S100A8/A9 proteins, has the potential to decrease lung tissue damage without impacting protective immunity against TB.
Keywords: inflammation, tuberculosis, neutrophil, S100A8/A9 proteins, granuloma
At a Glance Commentary
Scientific Knowledge on the Subject
A hallmark of pulmonary tuberculosis (TB) in humans and experimentally infected animals is the formation of granulomas. However, the immune factors that drive the formation of the protective granuloma during latent TB, and the factors that drive the inflammatory granulomas formed during active TB, are not well defined.
What This Study Adds to the Field
This study demonstrates the dominant presence of neutrophils producing S100 proteins within the inflammatory lung granulomas of patients with active TB. This study also describes a link between S100A8/A9 protein induction, neutrophil accumulation, and pathology associated with the inflammatory granuloma formed during TB, because S100A8/A9 deficiency in mice reverses exacerbated inflammation during TB. In addition, this study demonstrates the potential use of S100A8/A9 proteins along with neutrophil-attracting chemokines in serum as surrogate biomarkers to assess inflammation and disease severity in TB in humans.
Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB), infects one-third of the world’s population. Although most infected individuals develop latent TB (LTBI), 5–10% of infected individuals develop active TB (ATB). In addition, although most patients infected with LTBI remain asymptomatic, they have approximately 10% lifetime risk of developing ATB. A hallmark of pathology during TB in both humans and experimentally infected animals is the formation of granulomas in the lung (1). Recently, we described that protective granulomas found during LTBI are associated with formation of ectopic lymphoid structures, which harbor T cells near Mtb-infected macrophages to mediate Mtb control (2). In contrast, although large granulomas are found in the lungs during ATB, they lack ectopic lymphoid structures, and thus do not sufficiently mediate Mtb control (2). Incidentally, extensive infiltrative patterns mediating immune pathology in the lung are associated with respiratory failure and increased mortality rates in patients with TB (3). Thus, identifying the factors that mediate formation of the inflammatory granulomas seen in ATB will allow for generation of new therapies that can treat the inflammation associated with TB.
In mouse models of Mtb infection, neutrophil accumulation is associated with increased pathology in genetically susceptible mice strains (4, 5), and under conditions of exacerbated inflammation (6). In addition, a neutrophil-driven human blood transcriptional signature was seen in patients with ATB (7), and neutrophils are the predominant cells infected with replicating Mtb in patients with ATB (8). Despite the association of neutrophil accumulation with increased susceptibility to TB, the molecular signals and cellular components orchestrating neutrophil recruitment and accumulation, and the mechanisms mediating inflammation during TB remain poorly defined. In the current study, we demonstrate that in human patients with ATB and in nonhuman primate (NHP) models of Mtb infection, neutrophils producing proinflammatory S100 proteins are dominant within the inflammatory lung granulomas seen during ATB. S100A8/A9 proteins promote recruitment of neutrophils and monocytes (9) by inducing production of proinflammatory cytokines and chemokines (10), and by influencing leukocyte trafficking (11). Using the mouse model of TB, we demonstrate that exacerbated lung inflammation seen as a result of neutrophilic accumulation is dependent on the proinflammatory cytokine IL-17, and induction of S100A8/A9 proteins. Importantly, our data show that measurement of S100A8/A9 levels in the serum is directly associated with the extent of inflammatory lung damage and disease severity observed in human patients with ATB. Together, our study demonstrates that neutrophil accumulation is predominant within the inflammatory lesions associated with ATB, and that IL-17–dependent induction of S100 proteins is a pathway by which the pathology is mediated during TB.
Methods
Human Tissue Samples and Patient Diagnosis
Human samples were collected on approval from the Ethics Committee of the National Institute for Respiratory Diseases, KwaZulu-Natal Research Institute for TB and HIV, Durban, South Africa; National Institute for Research in Tuberculosis, Chennai, India; and The American British Cowdray Medical Center, Mexico City, Mexico. Full details are available in the online supplement.
Mtb Infection in NHP
Groups of NHP (Indian rhesus macaques) were used in this study at the Tulane National Primate Research Center, Covington, Louisiana, as previously described (2). For LTBI, NHPs were infected with low dose of Mtb CDC1551 (200–500 CFU) by the aerosol route (2). For ATB, NHPs were infected with high dose of Mtb CDC1551 by aerosol infection (∼5,000 CFU) as previously described (2). Full details in are in the online supplement.
Mtb Infection in Mice
C57BL/6 (B6), Diversity Outbred (DO) (12), and Ifng−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). S100a9−/− mice (13) were obtained on the B6 background and bred at the Children’s Hospital of Pittsburgh, Pittsburgh, PA. All mice were maintained and used in accordance with approved University of Pittsburgh IACUC guidelines. Mtb strain H37Rv was cultured as described previously (2), and mice were aerosol infected with approximately 100 CFU of bacteria as described previously (14). Full details are described in the online supplement.
Lung Cell Preparation and Flow Cytometry
Lung cell suspensions were stained with fluorochrome-labeled antibodies specific for CD11b(M1/70) and Gr1 (RB6–8C5) or isotype control antibodies (2). Full details are in the online supplement.
Immunohistochemistry
Lung lobes were instilled with 10% neutral buffered formalin and embedded in paraffin. Lung sections were stained with hematoxylin and eosin and inflammatory features were evaluated by light microscopy (Research Histology Core, University of Pittsburgh). For immunofluorescent staining, formalin-fixed lung sections were stained as previously described (2). Full details are in the online supplement.
In Situ Hybridization
Paraffin-embedded tissue sections were deparaffinized and in situ hybridization was performed as previously described (2). Full details are in the online supplement.
Generation of Lung Cell Types
Lung alveolar macrophages were obtained by bronchoalveolar lavage (2), and primary HBE cells were provided by the Tissue Core Laboratory at the University of Pittsburgh or purchased from Cambrex (Lonza, Allendale, NJ). Full details are in the online supplement. Lung neutrophils from Mtb-infected mice were isolated using a commercially kit and resulted in purity of greater than 88% (Miltenyi Biotech, Auburn, CA).
Protein Estimation by ELISA
Protein levels for cytokines and chemokines in culture supernatants, serum, or lung homogenates were measured using a mouse or human Luminex assay (Linco/Millipore, Billerica, MA). S100A8/A9 protein levels were measured in mouse lung homogenates and culture supernatants (15) and in human serum (16) by ELISA as described before.
Results
S100A8/A9 Proteins Are Surrogate Biomarkers for Lung Inflammation in ATB
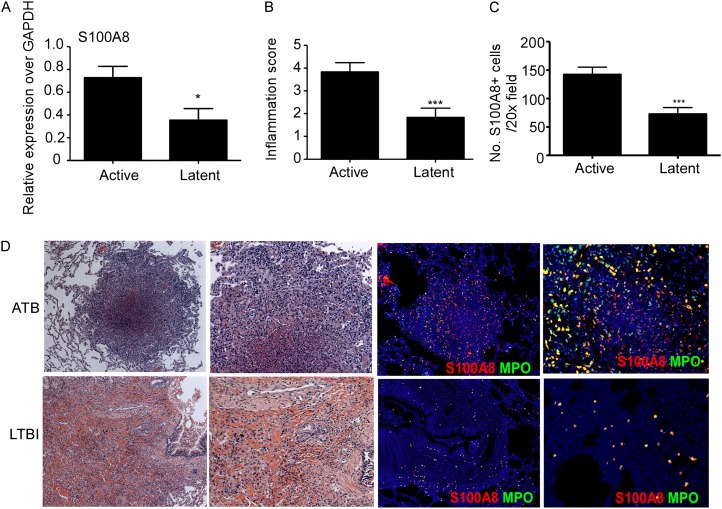
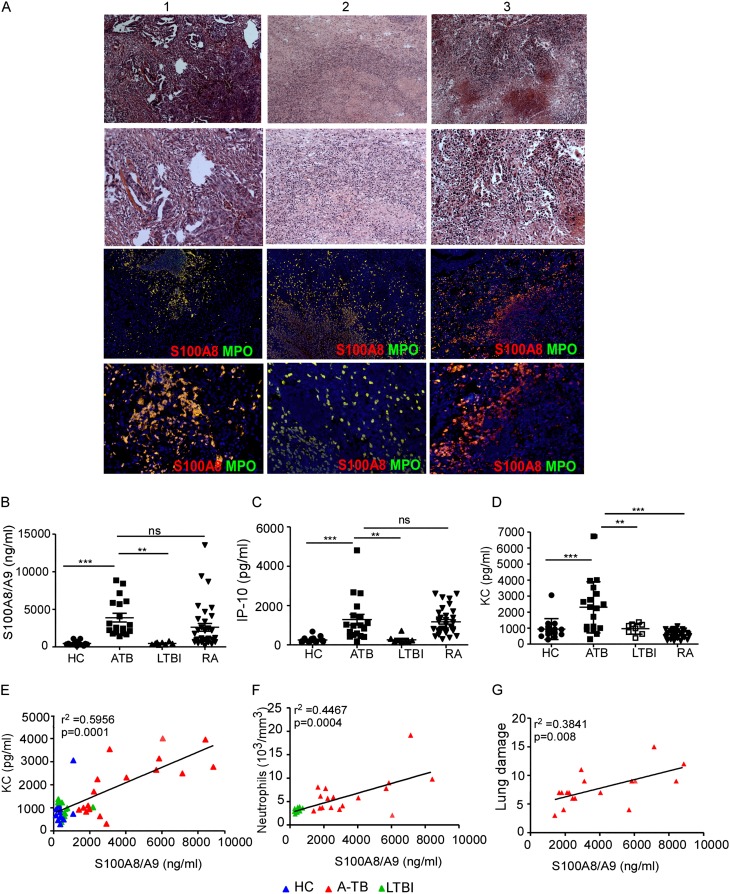
Granulomas harboring lymphoid structures are protective and mediate Mtb control during LTBI (2). However, the immune mechanisms that mediate the formation of inflammatory granulomas seen during ATB are not well defined. Because neutrophil-associated transcriptional blood signature is seen in patients with ATB (7), and S100 A8 and A9 proteins comprise approximately 45% of the cytosol of neutrophils (17), we first determined if S100-producing neutrophils accumulated within lung granulomas in TB. Therefore, we characterized the presence of S100-producing neutrophils in NHPs that were experimentally infected with aerosolized Mtb in which similar to human infection, immune control results in LTBI, and the absence of immune control results in ATB (18). We found increased expression of total lung S100A8 mRNA in NHP with ATB (Figure 1A), and increased accumulation of S100A8-producing neutrophils within the large inflammatory pulmonary lesions of NHPs with ATB (Figures 1B–1D; see Figure E1A in the online supplement). In contrast, fewer S100A8-producing neutrophils were observed within smaller inflammatory lung lesions of NHPs with LTBI (Figures 1B–1D; see Figure E1B), and granulomas predominantly contained lymphoid follicles (2). Increased levels of S100A8/A9 proteins have been previously associated with other inflammatory diseases, such as rheumatoid arthritis (RA) (19). We found that human lung inflammatory lesions from patients with ATB also harbored large numbers of S100-producing neutrophils (Figure 2A). Importantly, significantly higher levels of S100A8/A9 proteins were detected in serum of patients with ATB, when compared with serum obtained from either patients with LTBI or healthy control subjects (HC) from a cohort in Mexico (Figure 2B). Consistent with previous studies (7), increased induced protein-10 (IP-10) protein levels were also detected in serum from patients with ATB (Figure 2C). Importantly, we show that keratinocyte chemoattractant (KC), a neutrophil-attracting chemokine, was also increased in serum from patients with ATB when compared with LTBI and HC serum (Figure 2D). These results were further validated in two additional cohorts of human samples obtained from South Africa (see Figures E2A–E2C) and India (see Figures E2D–E2F), where increased levels of S100 A8/A9, KC, and IP-10 were observed in serum from patients with ATB. In addition, although high levels of S100A8/A9 and IP-10 proteins were detected in RA patient serum (Figures 2B and 2C), elevated expression of KC was only detected in serum from patients with ATB and not RA, LTBI, and HC serum (Figure 2D). Serum S100A8/A9 protein levels in patients with ATB showed positive linear correlation with expression of serum KC levels (Figure 2E), numbers of neutrophils in peripheral blood (Figure 2F), and most importantly with the extent of lung inflammatory damage assessed by chest radiographs in patients with ATB (Figure 2G). These data together provide new evidence that measurement of serum S100A8/A9 proteins along with chemokines, such as KC, can potentially serve as surrogate biomarkers for assessing inflammation and disease severity during ATB.
Figure 1.
S100A8/A9 expression coincides with severity of inflammation in nonhuman primates (NHP) with active tuberculosis (ATB). NHPs aerosol infected with Mycobacterium tuberculosis CDC1551 exhibited either latent TB infection (LTBI) or ATB as described under Methods. (A) mRNA expression levels of S100A8 from lung granulomatous lesions obtained from ATB and LTBI NHPs was determined by reverse transcriptase polymerase chain reaction and expression relative to glyceraldehyde phosphate dehydrogenase (GAPDH) expression shown. (B) The inflammation score was determined by a pathologist as mild, moderate, or severe and degree of inflammation annotated as inflammatory score was scored from 1 to 4 (with 4 reflecting maximum pathology) for each NHP sample described. (C) Formalin-fixed paraffin embedded lungs from ATB and LTBI were stained with hematoxylin and eosin or with antibodies specific for S100A8 (red) and MPO (green) and the number of S100A8+ neutrophils counted. (D) A representative image from ATB and LTBI sample is shown (left, ×100 original magnification; right, ×200 original magnification). Staining from an individual NHP shown. The data points represent the mean (±SD) of values from 4–7 NHPs (A–C). *P ≤ 0.05, ***P ≤ 0.0005.
Figure 2.
S100A8/A9 expression correlates with active disease and lung inflammation in humans with active tuberculosis (ATB). Formalin-fixed paraffin embedded lung sections from patients with ATB (A) were stained with hematoxylin and eosin or with antibodies specific for S100A8 (red) and MPO (green). Three representative human ATB samples (A) are shown (top, hematoxylin and eosin, original magnification ×100; bottom, ×200). Immunohistochemistry, 4 × 4 mosaic (top, ×200 original magnification; bottom, ×200) (A). S100A8/A9 (B), induced protein-10 (IP-10) (C), and keratinocyte chemoattractant (KC) (D) protein levels were measured in serum collected from healthy control subjects (HC), ATB, latent TB infection (LTBI), and patients with rheumatoid arthritis (RA) using ELISA or Luminex assays. Linear correlation analysis of S100A8/9 protein levels and KC (E), S100A8/A9 protein levels and neutrophils in peripheral blood (F), and S100A8/A9 levels and lung damage score (G) in HCs (blue triangles), patients with ATB (red triangles), and patients with LTBI (green triangles) was performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). The data points represent the mean (±SD) of values from 15 HCs, 17 ATB, 8 LTBI, and 33 RA patients (B–G). **P ≤ 0.005, ***P ≤ 0.0005. ns = not significant.
Inflammation in a Genetically Diverse Mtb-infected Mouse Population Is Associated with Increased IL-17 and S100A8/A9 Protein Production
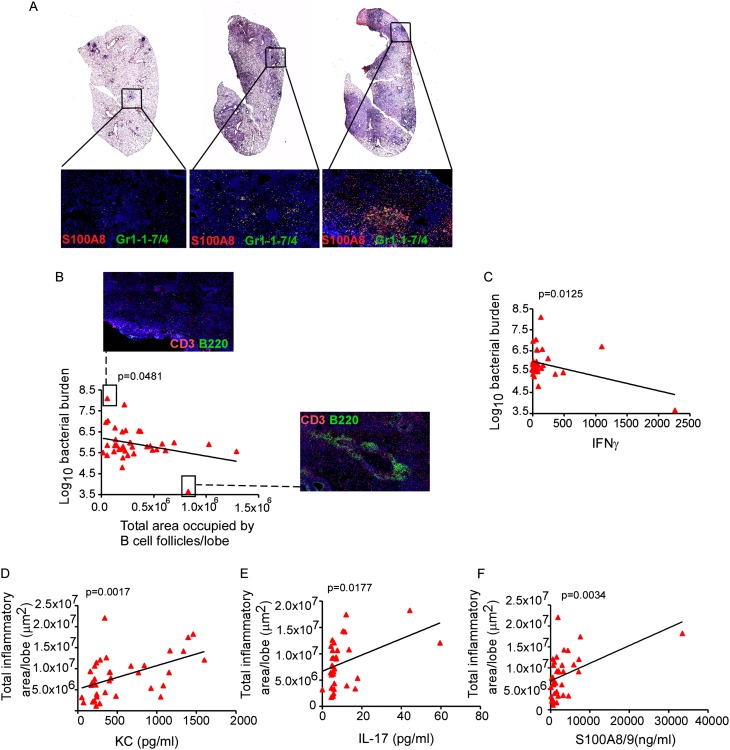
Recent studies suggest a correlation between neutrophil infiltration, pathology, and increased susceptibility to TB (4, 20). Thus, using the newly available DO mouse population, which is derived from partially inbred collaborative cross strains and maintained by randomized outcrossing (12), we next addressed if genetic diversity in a mouse population would result in variable inflammatory responses after Mtb infection, and whether inflammation severity was associated with expression of IL-17 and S100A8/A9 proteins. After Mtb infection, the DO mouse population displayed considerable variability in lung inflammatory responses (Figure 3A), and susceptibility to Mtb infection (Figure 3B). In support of our recent findings (2), mice that harbored lower lung Mtb burdens contained well-organized B-cell lymphoid follicles (Figure 3B), and elevated levels of the cytokine IFN-γ (Figure 3C). In contrast, mice that exhibited increased pulmonary inflammation harbored more S100A8-expressing Gr1+ cells (Figure 3A) and correlated with increased expression of lung KC protein (Figure 3D). IL-17 is a proinflammatory cytokine (21), and we found that increased pulmonary inflammation also correlated with increased lung IL-17 protein levels in DO Mtb-infected mice (Figure 3E). In addition, we found that lung S100A8/A9 levels in DO mice correlated with increased inflammation observed within granulomas (Figure 3F). These data show that in a genetically diverse mouse population, although protection against Mtb infection correlates with formation of lymphoid structures within granulomas, increased production of IL-17 and S100A8/A9 protein levels correlates with exacerbated inflammatory granulomas.
Figure 3.
Inflammation in Mycobacterium tuberculosis (Mtb) infected genetically diverse Diversity Outbred (DO) mouse population is associated with increased IL-17 production and S100A8/A9 proteins. (A) Genetically diverse mice from DO strain were aerosol infected with approximately 100 CFU of Mtb H37Rv and on Day 60 postinfection, formalin-fixed paraffin embedded (FFPE) lung sections were stained with hematoxylin and eosin or analyzed by immunofluorescence using antibodies specific for S100A8 (red) and Gr1-1-7/4 (green). FFPE lung sections were also analyzed by immunofluorescence using antibodies specific for B220 (green) and CD3 (red), and the total area occupied by B-cell follicles per lobe quantified using the morphometric tool of the Zeiss Axioplan (Carl Zeiss, Oberkochen, Germany) (4 × 4 mosaic, ×200 original magnification). Lung CFU was determined by plating. Linear correlation analysis between total area occupied by B-cell follicles per lung lobe and bacterial burden (B) and lung IFN-γ levels and bacterial burden (C) was performed using GraphPad Prism. A representative image demonstrating absence of B-cell follicles in a mouse exhibiting the highest Mtb bacterial burden and a representative image showing well-formed B-cell follicles in a mouse exhibiting the lowest Mtb burden is also shown (×100 original magnification). Total area occupied by inflammatory lesions per lobe was quantified in the Mtb-infected hematoxylin and eosin–stained FFPE lungs using the morphometric tool of the Zeiss Axioplan microscope. Lung protein levels of keratinocyte chemoattractant (KC) (D), IL-17 (E), and S100A8/A9 proteins (F) were measured and linear regression analysis was determined using GraphPad Prism. The data points represent values from 37 mice (A–F).
IL-17 Overexpression Induces S100A8/A9 Protein and Lung Inflammation during TB
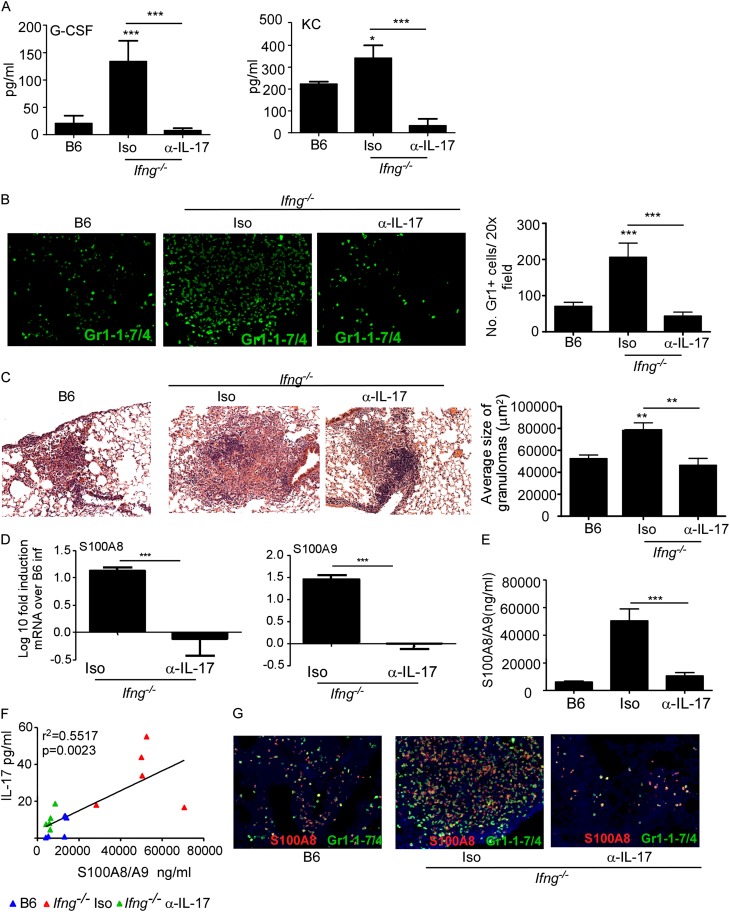
Our data show that increased IL-17 and S100A8/A9 protein production in genetically diverse Mtb-infected mice correlates with increased pulmonary inflammation. Thus, we next addressed if IL-17 was the primary inducer of S100A8/A9 in mediating neutrophilic accumulation and inflammation during TB. IFN-γ is a negative regulator of IL-17 during mycobacterial infections (22). Thus, mice deficient in the cytokine IFN-γ (Ifng−/−) produce increased IL-17 levels that coincide with increased neutrophil accumulation and exacerbated lung inflammation (6). Accordingly, we found Mtb-infected Ifng−/− mice expressed increased levels of lung IL-17 (see Figure E3A), granulocyte colony–stimulating factor (G-CSF), and KC (see Figures E3B and E3C). In contrast, expression of IFN-γ–inducible chemokines, such as IFN-γ, IP-10, and regulated upon activation normal, was decreased in Ifng−/− Mtb-infected lungs (see Figures E3D and E3E). Furthermore, lungs of Ifng−/− Mtb-infected mice formed large inflammatory lesions (see Figure E3F) and harbored higher bacterial burden (see Figure E3G). To formally prove that the exacerbated inflammation in Ifng−/− mice was IL-17 dependent, we neutralized IL-17 in Ifng−/− Mtb-infected mice and found decreased lung G-CSF and KC levels (Figure 4A), impaired neutrophil accumulation (Figure 4B), and reduction in size of inflammatory lung lesions (Figure 4C). These data together suggest that IL-17 overexpression drives neutrophilic accumulation and mediates exacerbated inflammation during TB.
Figure 4.
IL-17–dependent induction of S100A8/A9 proteins in Ifng−/− Mycobacterium tuberculosis (Mtb)-infected mice mediates lung inflammation. B6, Ifng−/− mice were aerosol infected with approximately 100 CFU Mtb H37Rv. Starting at Day 9 postinfection, groups of Ifng−/− infected mice received either isotype control antibody (Iso) or IL-17 neutralizing antibody (α−IL-17) (300 μg/mouse every 48 h) and samples for the below described analyses were harvested on Day 30 postinfection. Induction of granulocyte colony–stimulating factor (G-CSF) and keratinocyte chemoattractant (KC) (A) levels were measured in lung homogenates from infected mice. Formalin-fixed paraffin embedded lung sections were stained with rat antimouse Ly6G/Ly6C and rat antimouse Ly6-B.2 antigen to detect pulmonary neutrophils in mice (Gr1-1-7/4, green) (B) or underwent hematoxylin and eosin staining (C). The number of Gr1+ cells per ×20 field were counted (B) or average size of inflammatory lesions were quantified using the morphometric tool of the Zeiss Axioplan microscope (C) (×100 original magnification for hematoxylin and eosin images; ×200 original magnification for fluorescent images). Log10 fold induction of S100A8 or S100A9 mRNA in B6 and different groups of Ifng−/− Mtb-infected lungs was measured by reverse transcriptase polymerase chain reaction and fold induction in Ifng−/− infected lungs over B6 Mtb-infected lungs is shown (D). Protein levels of S100A8/A9 (E) or IL-17 (F) were measured from lung homogenates by ELISA and linear regression analysis was determined using GraphPad Prism. Red triangles, Ifn−/− Mtb infected isotype control treated mice; green triangles, Ifng−/− Mtb-infected mice treated with IL-17 neutralizing antibody; blue triangles, B6 Mtb-infected mice (F). Formalin-fixed paraffin embedded lung sections from Mtb-infected mice were assayed by immunofluorescence staining for Gr1-1-7/4 (green) and S100A8 (red) (G). The data points represent the mean (±SD) of values from 4–6 mice (A–G). *P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.0005. One experiment representative of two is shown.
S100 proteins exhibit potent proinflammatory properties (15, 23, 24), and S100 mRNA is induced by IL-17 treatment in keratinocytes (25). Furthermore, IL-17 treatment also induced S100A8 and A9 mRNA in primary human bronchial epithelial cells (see Figure E4A). Thus, we hypothesized that during TB, the IL-17–dependent mechanism mediating exacerbated inflammation was through the induction of S100A8/A9 proteins. Although S100A8 and S100A9 mRNA were expressed in low levels within inflammatory lesions of B6 Mtb-infected mice, Ifng−/− Mtb-infected lungs expressed higher levels of S100A8 and A9 mRNA, which localized within inflammatory lesions (see Figure E4B). Gr1+ cells localized within the inflammatory lesions were the predominant cellular source of S100A8 (see Figure E4C), and significantly increased S100A8/A9 protein levels were detected in Ifng−/− Mtb-infected lungs (see Figures E4C and E4D). Importantly, increased induction of S100A8 and S100A9 mRNA (Figure 4D), their increased localization in the inflammatory lesions (see Figure E5A), and increased S100A8/A9 protein levels (Figure 4E) in Ifng−/− Mtb-infected lungs were all reversed on IL-17 neutralization. In addition, IL-17 overproduction in Ifng−/− Mtb-infected lungs correlated positively with expression of S100A8/A9 protein (Figure 4F). Likewise, IL-17 neutralization decreased the accumulation of S100A8-producing Gr1+ neutrophils in the inflammatory pulmonary lesions of Ifng−/− Mtb-infected lungs (Figure 4G). Interestingly, there was no difference in lung bacterial burden between Ifng−/− Mtb-infected mice and Ifng−/− Mtb-infected mice that received IL-17 neutralizing antibody (see Figure E5B).
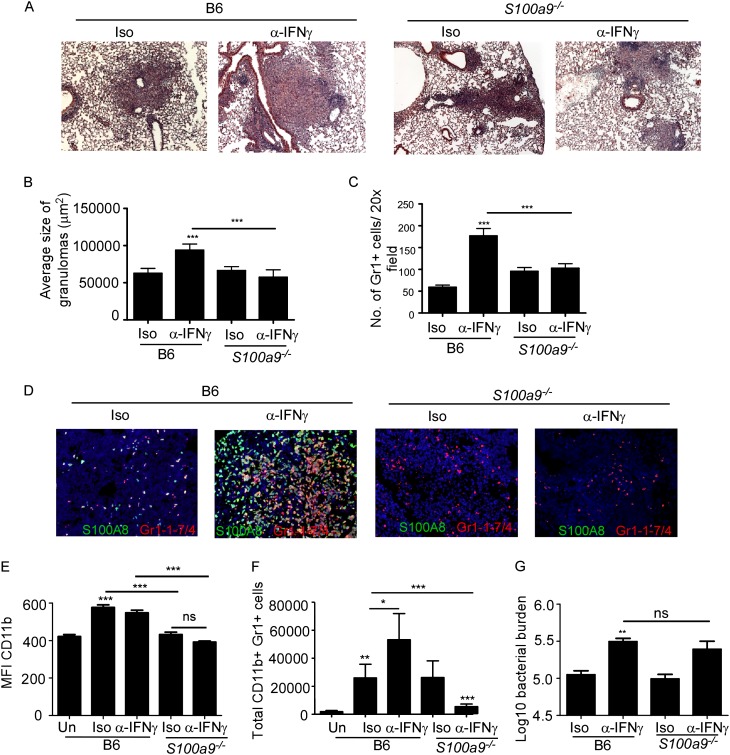
To prove the role of S100A8/A9 in mediating the exacerbated inflammation under conditions of IL-17 overexpression, we treated Mtb-infected B6 mice and S100a9−/− mice with IFN-γ neutralizing antibodies and monitored disease progression. S100a9−/− mice do not express S100A8 protein (13). IFN-γ neutralization in B6 Mtb-infected mice resulted in exacerbated inflammation (Figures 5A and 5B) and increased accumulation of S100A8-producing Gr1+ neutrophils (Figures 5C and 5D). In contrast, IFN-γ neutralization in Mtb-infected S100a9−/− mice resulted in loss of lung inflammation (Figures 5A and 5B) and reversed neutrophil accumulation (Figures 5C and 5D). We obtained similar reversal in lung inflammation (see Figures E6A and E6B) and neutrophil accumulation (see Figures E6C and E6D) when S100A9 neutralizing antibodies (26) were delivered to Ifng−/− Mtb-infected mice. S100A8/A9 proteins can promote recruitment and infiltration of neutrophils and monocytes (9) by directly inducing proinflammatory chemokine production (10). Accordingly, alveolar macrophages isolated from S100a9−/− mice on exposure to Mtb in vitro produced significantly reduced levels of KC and G-CSF (see Figures E7A and E7B). In addition, neutrophils isolated from lungs of Mtb-infected Ifng−/− mice produced high levels of S100A8/A9 proteins (1344.44 ± 782.77 ng/ml) and on exposure to IL-17, induced KC and G-CSF production (see Figures E7C and E7D). S100A8/A9 proteins also promote neutrophil chemotaxis and adhesion by enhancing CD11b expression (13, 27, 28), a molecule critical for lung neutrophil infiltration (29). CD11b expression on lung neutrophils increased in B6 Mtb-infected mice but did not increase in S100a9−/− Mtb-mice mice (Figure 5E), and coincided with reduced neutrophil accumulation in S100a9−/− Mtb-infected lungs (Figure 5F). Interestingly, although IFN-γ deficiency increased bacterial burden, absence of S100A8/A9 by itself and under conditions of IFN-γ deficiency did not impact lung Mtb bacterial burdens (Figure 5G; see Figure E6E). In addition, alveolar macrophages from B6 and S100a9−/− mice similarly controlled Mtb infection in vitro (see Figure E7E). These data together demonstrate that IL-17 overexpression, through an S100A8/A9-dependent pathway, mediates exacerbated neutrophil recruitment and lung inflammation during TB. However, S100A8/A9 proteins do not participate in protective immunity against Mtb infection.
Figure 5.
S100A8/A9 proteins mediate exacerbated inflammation during Mycobacterium tuberculosis (Mtb) infection. B6 and S100a9−/− mice were infected with approximately 100 CFU Mtb H37Rv. From Day 9 postinfection, B6 and S100a9−/− Mtb-infected mice received either isotype (Iso) or IFN-γ neutralizing antibody (α−IFN-γ) (300 μg/mouse every 48 h) and samples for the analyses below were collected on Day 30 postinfection. Formalin-fixed paraffin embedded lung sections were stained with hematoxylin and eosin (A and B) or analyzed by immunofluorescence using antibodies specific for Gr1-1-7/4 (red) and S100A8 (green) (C and D). Average size of inflammatory lesions (B) was quantified in the Mtb-infected lungs using the morphometric tool of the Zeiss Axioplan microscope or number of Gr1+ cells per ×20 field counted (C) (×100 original magnification for hematoxylin and eosin images; ×200 original magnification for fluorescent images). Single cell suspensions from lungs of Mtb-infected mice were stained with antibodies specific for CD11b and Gr1 and the mean fluorescent intensity (MFI) of CD11b expression (E) and number of lung neutrophils (Gr1+ CD11b+) determined by flow cytometry (F). Lung bacterial burden in Mtb-infected mice was determined by plating (G). The data points represent the mean (±SD) of values from 4–6 mice (A–G). *P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.0005. ns = not significant. One experiment representative of two is shown.
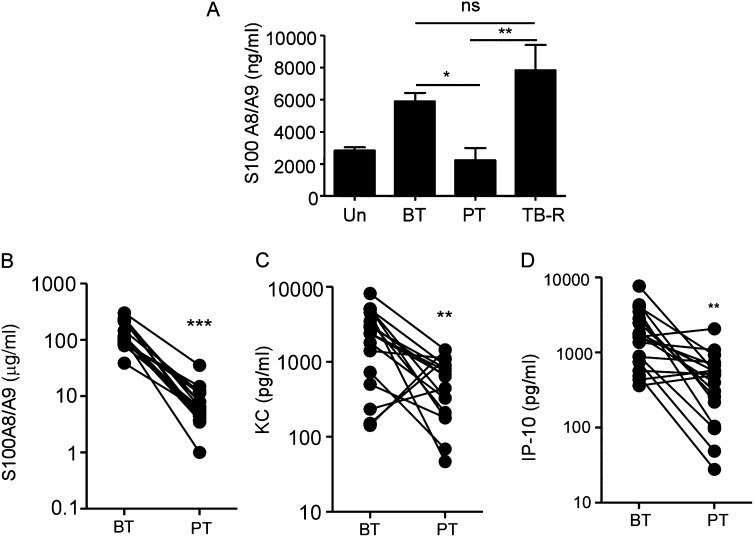
We also found that lung S100A8/A9 protein levels in chronically Mtb-infected B6 mice decreased on antibiotic treatment (Figure 6A). On completion of antibiotic therapy in B6 Mtb-infected mice, Mtb lung burdens increased (30), and this also coincided with increased lung S100A8/A9 protein levels (Figure 6A). Importantly, we found that the high levels of serum S100A8/A9, KC, and IP-10 proteins detected in the patients with ATB also decreased postantibiotic treatment (Figures 6B–6D). These data together suggest that monitoring S100A8/A9 levels in serum could be a potential surrogate biomarker for TB reactivation.
Figure 6.
S100A8/A9 protein levels decrease with Mycobacterium tuberculosis clearance and are surrogate indicators of tuberculosis (TB) reactivation. (A) S100A8/A9 lung protein levels in mice left untreated (Un), M. tuberculosis infected before antibiotic therapy (BT) or treated with antibiotic therapy (PT), or allowed to reactivate (TB-R) was determined by ELISA (n = 4–5). Serum S100A8/A9 proteins (B), keratinocyte chemoattractant (KC) (C), and induced protein-10 (IP-10) (D) levels in patients with ATB before treatment (BT) with antibiotics, or post-treatment (PT) was determined in the samples from India. The data points represent the mean (±SD) of values from 20 patients with ATB (B–D). *P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.0005. ns = not significant.
Discussion
Although granulomas are found in latent and active forms of TB (31), the immunologic differences between a “functional” protective granuloma that limits infection during LTBI, and an inflammatory granuloma not capable of controlling infection during ATB, are just beginning to be addressed (2, 31). In the current study, we describe that a dominant feature of the inflammatory granulomas found in lungs of NHPs with ATB and human patients with ATB is the presence of S100-producing neutrophils. In addition, using mouse models of TB, we demonstrate that a pathway by which the neutrophilic inflammation mediates pathology during TB is through the induction of S100A8/A9 proteins. One of the major challenges in current TB control measures is the unavailability of reliable biomarkers that can distinguish between ATB and LTBI in humans. Thus, our work also describes how S100A8/A9 protein expression in serum along with chemokines, such as KC, can be potentially used as surrogate biomarkers to assess lung inflammation and disease severity during TB. Thus, our new “working hypothesis” proposes that a protective TB granuloma formed during LTBI is predominantly organized with lymphoid follicles, where T cells can effectively activate macrophages to control Mtb infection (2). In contrast, during ATB, we propose that lung granulomas contain fewer protective lymphoid follicles (2), but instead, accumulate S100A8/A9-producing neutrophils, which likely mediate inflammation, pathology, and clinical disease without contributing to protection.
Despite the recent associations of neutrophils with increased susceptibility (4) and inflammation (6) to TB, the molecular mechanisms mediating neutrophil accumulation, inflammation, and resulting pathology have not been well studied. Transmigration of leukocytes to inflammatory sites is dependent on the process of rolling of leukocytes on activated endothelial cell surfaces and is mediated by binding of endothelial selectins to respective ligands on leukocytes. Accordingly, S100A8/A9 proteins have a well-described role in leukocyte migration (32). For example, S100A8/A9 proteins can promote recruitment and infiltration of neutrophils and monocytes (9) by inducing production of both proinflammatory cytokines and chemokines (10), and mediating neutrophil chemotaxis and adhesion by enhancing CD11b expression (13, 27, 28). Our study demonstrates that lung pathology mediated as a result of neutrophilic inflammation during TB is S100A8/A9 dependent. Accordingly, in our study, S100a9−/− macrophages produced lower levels of KC and G-CSF in vitro on Mtb exposure. Under condition of exacerbated inflammation, S100A8/A9 deficiency also coincided with reduced accumulation of neutrophils in the Mtb-infected lung. Despite this inflammatory role for S100A8/A9 proteins in TB, our data show that S100A8/A9 proteins have no role in bacterial control, because S100 deficiency both under normal conditions and under conditions of IFN-γ deficiency show no defects in bacterial control.
The role for IL-17 in neutrophil recruitment, particularly to mediate host defense and protective immunity against extracellular pathogens, is now well described (21). In these models, IL-17 drives the induction of G-CSF and neutrophil-attracting chemokines, such as KC, MIP2 to mediate granulopoeisis, and neutrophil recruitment to mucosal sites (21). However, IL-17 production is dispensable in protective immunity against Mtb (33). However, an inflammatory role for IL-17 during mycobacterial infections has been suggested, where IL-17 overexpression either caused by immune dysregulation (6, 34, 35) or repeated antigenic exposure (36) results in increased neutrophil recruitment and exacerbated inflammation. The exact molecular mechanism by which IL-17 directly, or through the induction of effector molecules, drives this inflammatory phenotype during TB is not known. In this study, we show that the IL-17–dependent induction of S100A8/A9 proteins is one contributing pathway that mediates neutrophil recruitment and associated inflammation. Accordingly, increased expression of IL-17 under conditions of cytokine dysregulation and in models of genetic diversity correlates with increased production of S100A8/A9 proteins and increased disease severity as reflected by increased lung inflammatory granulomas harboring S100-producing neutrophils. The use of newly available genetic resources, such as the DO mice to study the inflammatory and protective immune correlates of TB, allows genetic variability similar to human population to be studied in animal models; however, a limitation of this model is the enormous genetic diversity, which requires larger cohorts to be studied to arrive at conclusions. IL-17 depletion in Ifng−/− Mtb-infected mice reverses exacerbated inflammation in the lung and provides formal proof that overexpression of IL-17 mediates exacerbated inflammation during TB. In addition, we demonstrate that IL-17 can induce both S100A8 and S100A9 mRNA in vitro in bronchial epithelial cells and in vivo in the lungs of Mtb-infected mice. Consistent with our findings, IL-17 independently and synergistically with IL-22 can also induce S100 A7, A8, A9 mRNA in human keratinocytes (25). In addition, during TB, in models of cytokine dysregulation and genetic diversity in mice, our data show an association between the levels of S100A8/A9 proteins detected and the extent of inflammation within the lung during TB. Importantly, S100A8/A9 levels in serum correlate with the number of neutrophils, levels of neutrophils-attracting chemokines, such as KC, and the extent of lung inflammation in human patients with ATB. Our current data also support human studies where IL-17 expression is elevated in patients with ATB, and thought to be associated with higher antigen load (37, 38). In contrast, other studies have shown increased IL-17 production in patients with LTBI (39, 40) and HC (40, 41), or no increases in IL-17 levels, even at disease sites (42). These discrepancies may well be associated with inherent differences in the type of antigen used, and the duration of the in vitro assays, and future studies will no doubt need to further address this. It is also plausible that the S100A8/A9 pathway described in this study is also involved in other inflammatory diseases, such as inflammatory bowel disease (43), RA (16), psoriasis (44), and cystic fibrosis (45), all diseases where both S100A8/A9 proteins and IL-17 have been implicated in disease progression and pathology (21).
In summary, we demonstrate a dominant inflammatory role for S100A8/A9 proteins in mediating neutrophil accumulation and associated pathology during ATB. In addition, our data suggest that levels of S100A8/A9 proteins, along with chemokines, such as KC, can be potentially used as surrogate markers of lung inflammation during TB, and support the idea of a neutrophil-driven blood transcriptional signature recently identified in patients with ATB (7). We also propose that S100A8/A9 levels in serum can be monitored to track early reactivation of active disease in patients with LTBI within TB-endemic areas, and in potentially high-risk populations. Because the immune pathology caused by inflammation during TB is associated with higher mortality (3), targeting specific molecules, such as S100A8/A9, has the potential to decrease lung tissue damage without impacting protective immunity against TB.
Acknowledgments
Acknowledgment
The authors thank Hillary Cleveland for mice breeding, and Dr. James Kreindler for help with the human bronchial epithelial cell cultures.
Footnotes
Supported by the Children’s Hospital of Pittsburgh; NIH grants HL105427 to S.A.K., AI060422 to T.A.R., and T32 AI065380-08 to S.S.; grants of the Interdisciplinary Center of Clinical Research of the University of Muenster (Vo2/014/09) and the German Research Foundation (DFG) CRC Transregio 128-A2 and CRC 1009 B8 to T.V.; NIH grants RR026006, AI091457, RR020159, and Tulane Primate Center base grant RR000164 to D.K.; the Department of Medicine, University of Rochester, and NIH grant AI91036 to J.R.-M.; grant 115497 from the National Council of Science and Technology of Mexico (Conacyt) to J.Z.; and NIH grants AI058131 and AI076389 to A.J.C.S. A.J.C.S. is a Burroughs Welcome Investigator in the Pathogenesis of Infectious Disease.
Author Contributions: R.G., J.R.-M., D.K., J.Z., and S.A.K. designed the experiments. R.G., L.M., S.S., D.T., S.M., K.C.M., J.R.-M., B.A.F.J., R.B.-S., A.C.-L., J.R., T.V., B.C., N.P.K., and P.T. did the experiments. T.S.R.-R., M.S., E.B.-V., J.B.-H., J.Z., V.O.K., A.J.C.S., and S.B. provided human samples. R.G. and S.A.K. wrote the paper. J.K., D.K., M.S., T.A.R., J.R., T.V., and S.A.K. edited the paper. A.J.C.S., D.K., T.A.R., T.V., J.R.-M., J.Z., and S.A.K. provided the funding.
Originally Published in Press as DOI: 10.1164/rccm.201304-0803OC on September 18, 2013
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Saunders BM, Cooper AM. Restraining mycobacteria: role of granulomas in mycobacterial infections. Immunol Cell Biol. 2000;78:334–341. doi: 10.1046/j.1440-1711.2000.00933.x. [DOI] [PubMed] [Google Scholar]
- 2.Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, Selman M, Becerril-Villanueva E, Baquera-Heredia J, Pavon L, et al. CXCR5+ T helper cells mediate protective immunity against tuberculosis. J Clin Invest. 2013;123:712–726. doi: 10.1172/JCI65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacks LV, Pendle S. Factors related to in-hospital deaths in patients with tuberculosis. Arch Intern Med. 1998;158:1916–1922. doi: 10.1001/archinte.158.17.1916. [DOI] [PubMed] [Google Scholar]
- 4.Eruslanov EB, Lyadova IV, Kondratieva TK, Majorov KB, Scheglov IV, Orlova MO, Apt AS. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect Immun. 2005;73:1744–1753. doi: 10.1128/IAI.73.3.1744-1753.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pichugin AV, Yan BS, Sloutsky A, Kobzik L, Kramnik I. Dominant role of the sst1 locus in pathogenesis of necrotizing lung granulomas during chronic tuberculosis infection and reactivation in genetically resistant hosts. Am J Pathol. 2009;174:2190–2201. doi: 10.2353/ajpath.2009.081075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nandi B, Behar SM. Regulation of neutrophils by interferon-{gamma} limits lung inflammation during tuberculosis infection. J Exp Med. 2011;208:2251–2262. doi: 10.1084/jem.20110919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eum SY, Kong JH, Hong MS, Lee YJ, Kim JH, Hwang SH, Cho SN, Via LE, Barry CE., III Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137:122–128. doi: 10.1378/chest.09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lackmann M, Rajasekariah P, Iismaa SE, Jones G, Cornish CJ, Hu S, Simpson RJ, Moritz RL, Geczy CL. Identification of a chemotactic domain of the pro-inflammatory S100 protein CP-10. J Immunol. 1993;150:2981–2991. [PubMed] [Google Scholar]
- 10.Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M, Yamamoto H, Chazin WJ, Nakatani Y, Yui S, Makino H. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res Ther. 2006;8:R69. doi: 10.1186/ar1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryckman C, McColl SR, Vandal K, de Medicis R, Lussier A, Poubelle PE, Tessier PA. Role of S100A8 and S100A9 in neutrophil recruitment in response to monosodium urate monohydrate crystals in the air-pouch model of acute gouty arthritis. Arthritis Rheum. 2003;48:2310–2320. doi: 10.1002/art.11079. [DOI] [PubMed] [Google Scholar]
- 12.Svenson KL, Gatti DM, Valdar W, Welsh CE, Cheng R, Chesler EJ, Palmer AA, McMillan L, Churchill GA. High-resolution genetic mapping using the Mouse Diversity outbred population. Genetics. 2012;190:437–447. doi: 10.1534/genetics.111.132597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manitz MP, Horst B, Seeliger S, Strey A, Skryabin BV, Gunzer M, Frings W, Schonlau F, Roth J, Sorg C, et al. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol Cell Biol. 2003;23:1034–1043. doi: 10.1128/MCB.23.3.1034-1043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 15.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 16.Frosch M, Strey A, Vogl T, Wulffraat NM, Kuis W, Sunderkotter C, Harms E, Sorg C, Roth J. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2000;43:628–637. doi: 10.1002/1529-0131(200003)43:3<628::AID-ANR20>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 17.Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem. 1991;266:7706–7713. [PubMed] [Google Scholar]
- 18.Mehra S, Golden NM, Dutta NK, Midkiff CC, Alvarez X, Doyle LA, Asher M, Russell-Lodrigue K, Monjure C, Roy CJ, et al. Reactivation of latent tuberculosis in rhesus macaques by co-infection with simian virus I. J Med Primatol. 2011;40:485–494. doi: 10.1111/j.1600-0684.2011.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen YS, Yan W, Geczy CL, Brown MA, Thomas R. Serum levels of soluble receptor for advanced glycation end products and of S100 proteins are associated with inflammatory, autoantibody, and classical risk markers of joint and vascular damage in rheumatoid arthritis. Arthritis Res Ther. 2009;11:R39. doi: 10.1186/ar2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowe DM, Redford PS, Wilkinson RJ, O'Garra A, Martineau AR. Neutrophils in tuberculosis: friend or foe? Trends Immunol. 2012;33:14–25. doi: 10.1016/j.it.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Kolls JK, Khader SA. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010;21:443–448. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruz A, Khader S, Torrado E, Fraga A, Pearl J, Pedrosa J, Cooper A, Castro ACE. IFN-γ regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection1. J Immunol. 2006;177:1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 23.van Lent PL, Grevers L, Blom AB, Sloetjes A, Mort JS, Vogl T, Nacken W, van den Berg WB, Roth J. Myeloid-related proteins S100A8/S100A9 regulate joint inflammation and cartilage destruction during antigen-induced arthritis. Ann Rheum Dis. 2008;67:1750–1758. doi: 10.1136/ard.2007.077800. [DOI] [PubMed] [Google Scholar]
- 24.Loser K, Vogl T, Voskort M, Lueken A, Kupas V, Nacken W, Klenner L, Kuhn A, Foell D, Sorokin L, et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat Med. 2010;16:713–717. doi: 10.1038/nm.2150. [DOI] [PubMed] [Google Scholar]
- 25.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cesaro A, Anceriz N, Plante A, Page N, Tardif MR, Tessier PA. An inflammation loop orchestrated by S100A9 and calprotectin is critical for development of arthritis. PLoS ONE. 2012;7:e45478. doi: 10.1371/journal.pone.0045478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newton RA, Hogg N. The human S100 protein MRP-14 is a novel activator of the beta 2 integrin Mac-1 on neutrophils. J Immunol. 1998;160:1427–1435. [PubMed] [Google Scholar]
- 28.Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003;170:3233–3242. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- 29.Gao XP, Liu Q, Broman M, Predescu D, Frey RS, Malik AB. Inactivation of CD11b in a mouse transgenic model protects against sepsis-induced lung PMN infiltration and vascular injury. Physiol Genomics. 2005;21:230–242. doi: 10.1152/physiolgenomics.00291.2004. [DOI] [PubMed] [Google Scholar]
- 30.Scanga C, Mohan V, Joseph H, Yu K, Chan J, Flynn J. Reactivation of latent tuberculosis: variations on the Cornell murine model. Infect Immun. 1999;67:4531–4538. doi: 10.1128/iai.67.9.4531-4538.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flynn JL, Chan J, Lin PL. Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol. 2011;4:271–278. doi: 10.1038/mi.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goyette J, Geczy CL. Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids. 2011;41:821–842. doi: 10.1007/s00726-010-0528-0. [DOI] [PubMed] [Google Scholar]
- 33.Khader SA, Guglani L, Rangel-Moreno J, Gopal R, Fallert Junecko BA, Fountain JJ, Martino C, Pearl JE, Tighe M, Lin YY, et al. IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J Immunol. 2011;187:5402–5407. doi: 10.4049/jimmunol.1101377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desvignes L, Ernst JD. Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity. 2009;31:974–985. doi: 10.1016/j.immuni.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozakiewicz L, Chen Y, Xu J, Wang Y, Dunussi-Joannopoulos K, Ou Q, Flynn JL, Porcelli SA, Jacobs WR, Jr, Chan J. B cells regulate neutrophilia during Mycobacterium tuberculosis infection and BCG vaccination by modulating the interleukin-17 response. PLoS Pathog. 2013;9:e1003472. doi: 10.1371/journal.ppat.1003472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cruz A, Fraga AG, Fountain JJ, Rangel-Moreno J, Torrado E, Saraiva M, Pereira DR, Randall TD, Pedrosa J, Cooper AM, et al. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J Exp Med. 2010;207:1609–1616. doi: 10.1084/jem.20100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jurado JO, Pasquinelli V, Alvarez IB, Pena D, Rovetta AI, Tateosian NL, Romeo HE, Musella RM, Palmero D, Chuluyan HE, et al. IL-17 and IFN-gamma expression in lymphocytes from patients with active tuberculosis correlates with the severity of the disease. J Leukoc Biol. 2012;91:991–1002. doi: 10.1189/jlb.1211619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng MY, Wang ZH, Yao CY, Jiang LN, Jin QL, Wang J, Li BQ. Interleukin 17-producing gamma delta T cells increased in patients with active pulmonary tuberculosis. Cell Mol Immunol. 2008;5:203–208. doi: 10.1038/cmi.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stern JN, Keskin DB, Romero V, Zuniga J, Encinales L, Li C, Awad C, Yunis EJ. Molecular signatures distinguishing active from latent tuberculosis in peripheral blood mononuclear cells, after in vitro antigenic stimulation with purified protein derivative of tuberculin (PPD) or Candida: a preliminary report. Immunol Res. 2009;45:1–12. doi: 10.1007/s12026-008-8024-2. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Zhang M, Liao M, Graner MW, Wu C, Yang Q, Liu H, Zhou B. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am J Respir Crit Care Med. 2010;181:734–742. doi: 10.1164/rccm.200909-1463OC. [DOI] [PubMed] [Google Scholar]
- 41.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matthews K, Wilkinson KA, Kalsdorf B, Roberts T, Diacon A, Walzl G, Wolske J, Ntsekhe M, Syed F, Russell J, et al. Predominance of interleukin-22 over interleukin-17 at the site of disease in human tuberculosis. Tuberculosis (Edinb) 2011;91:587–593. doi: 10.1016/j.tube.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foell D, Wittkowski H, Ren Z, Turton J, Pang G, Daebritz J, Ehrchen J, Heidemann J, Borody T, Roth J, et al. Phagocyte-specific S100 proteins are released from affected mucosa and promote immune responses during inflammatory bowel disease. J Pathol. 2008;216:183–192. doi: 10.1002/path.2394. [DOI] [PubMed] [Google Scholar]
- 44.Benoit S, Toksoy A, Ahlmann M, Schmidt M, Sunderkotter C, Foell D, Pasparakis M, Roth J, Goebeler M. Elevated serum levels of calcium-binding S100 proteins A8 and A9 reflect disease activity and abnormal differentiation of keratinocytes in psoriasis. Br J Dermatol. 2006;155:62–66. doi: 10.1111/j.1365-2133.2006.07198.x. [DOI] [PubMed] [Google Scholar]
- 45.McMorran BJ, Patat SA, Carlin JB, Grimwood K, Jones A, Armstrong DS, Galati JC, Cooper PJ, Byrnes CA, Francis PW, et al. Novel neutrophil-derived proteins in bronchoalveolar lavage fluid indicate an exaggerated inflammatory response in pediatric cystic fibrosis patients. Clin Chem. 2007;53:1782–1791. doi: 10.1373/clinchem.2007.087650. [DOI] [PubMed] [Google Scholar]