Abstract
Rationale: After lung transplantation, insults to the allograft generally result in one of four histopathologic patterns of injury: (1) acute rejection, (2) lymphocytic bronchiolitis, (3) organizing pneumonia, and (4) diffuse alveolar damage (DAD). We hypothesized that DAD, the most severe form of acute lung injury, would lead to the highest risk of chronic lung allograft dysfunction (CLAD) and that a type I immune response would mediate this process.
Objectives: Determine whether DAD is associated with CLAD and explore the potential role of CXCR3/ligand biology.
Methods: Transbronchial biopsies from all lung transplant recipients were reviewed. The association between the four injury patterns and subsequent outcomes were evaluated using proportional hazards models with time-dependent covariates. Bronchoalveolar lavage (BAL) concentrations of the CXCR3 ligands (CXCL9/MIG, CXCL10/IP10, and CXCL11/ITAC) were compared between allograft injury patterns and “healthy” biopsies using linear mixed-effects models. The effect of these chemokine alterations on CLAD risk was assessed using Cox models with serial BAL measurements as time-dependent covariates.
Measurements and Main Results: There were 1,585 biopsies from 441 recipients with 62 episodes of DAD. An episode of DAD was associated with increased risk of CLAD (hazard ratio, 3.0; 95% confidence interval, 1.9–4.7) and death (hazard ratio, 2.3; 95% confidence interval, 1.7–3.0). There were marked elevations in BAL CXCR3 ligand concentrations during DAD. Furthermore, prolonged elevation of these chemokines in serial BAL fluid measurements predicted the development of CLAD.
Conclusions: DAD is associated with marked increases in the risk of CLAD and death after lung transplantation. This association may be mediated in part by an aberrant type I immune response involving CXCR3/ligands.
Keywords: lung transplantation, chronic lung allograft dysfunction, bronchiolitis obliterans syndrome, diffuse alveolar damage, CXC chemokines
At a Glance Commentary
Scientific Knowledge on the Subject
Chronic lung allograft dysfunction (CLAD) is a major factor limiting long-term survival after lung transplantation. The pathogenesis of CLAD remains unknown with no effective treatments. Thus, the identification and avoidance of CLAD risk factors is critical.
What This Study Adds to the Field
This study identifies diffuse alveolar damage as a major risk factor for CLAD development. Furthermore, it provides insight into a possible mechanism for CLAD pathogenesis: the role of aberrant CXCR3/ligand biology causing persistent allograft injury. The CXCR3/ligand axis may represent a potential therapeutic target to decrease allograft injury and CLAD development among lung transplant recipients.
Lung transplantation has one of the highest mortality rates among solid organ transplants: 48% at 5 years and 71% at 10 years (1). Chronic lung allograft dysfunction (CLAD) is the major factor limiting long-term survival (2). There is accumulating evidence that CLAD has two distinct phenotypes: bronchiolitis obliterans syndrome (BOS) and restrictive allograft syndrome (RAS). These subtypes of CLAD seem to differ in their clinical characteristics and prognosis (3–5). CLAD has traditionally been recognized as BOS with progressive irreversible obstruction on pulmonary function testing (PFT) caused by fibroobliteration of the small airways. Among double lung transplant recipients (LTRs), RAS has recently been identified as another phenotype of CLAD with restriction on PFT caused by fibrosis of the lung parenchyma (4). RAS seems to have significantly higher mortality compared with BOS (3–5). Because there is no known effective treatment for CLAD or its subtypes (BOS and RAS), the identification and avoidance of risk factors are critical.
Prior studies have shown an association between various immune and nonimmune mediated “insults” to the lung allograft and the development of CLAD. Examples of these allograft “insults” include acute rejection (AR) (6–8), primary graft dysfunction (PGD) (9–12), autoimmune reactivity (13, 14), antibody-mediated rejection (15–17), air pollution (18, 19), gastroesophageal reflux (20, 21), and several respiratory infections and colonizations (22–28). Despite these numerous insults, there are only a limited number of histopathologic patterns of acute allograft injury. Foremost is the prototypical pattern of AR, consisting of perivascular mononuclear infiltrates that may extend to the interstitium and alveolar spaces (29). Lymphocytic bronchiolitis (LB) is an airway-centered pattern characterized by a peribronchiolar mononuclear cell infiltration (29). Organizing pneumonia (OP) is characterized by excessive proliferation of granulation tissue within alveolar ducts and alveoli (29).
Diffuse alveolar damage (DAD) has an initial exudative phase with epithelial and endothelial breakdown, alveolar protein leak, edema formation, macrophage activation, and neutrophil recruitment. The proliferative phase is characterized by type II pneumocyte and fibroblast proliferation, extracellular matrix deposition, and marked interstitial thickening. DAD is considered the most severe form of acute lung allograft injury and can be caused by a number of insults including PGD, infection, sepsis, drugs or toxins, and severe AR. DAD is commonly observed in the allograft, particularly in the immediate postoperative period because of PGD (6, 30). Although several studies have evaluated DAD caused by PGD, there is a paucity of information on the pathogenesis and clinical consequences of DAD occurring after the perioperative period.
Histopathologically, the four allograft injury patterns have a common theme: they all involve the extravasation and infiltration of leukocytes into the area of injury. CXCL9/MIG, CXCL10/IP10, and CXCL11/ITAC are ELR-CXC chemokines (CXCR3 ligands) that signal through a shared G protein–coupled receptor, CXCR3 (31, 32). These chemokines, induced by interferon-γ, act as potent chemoattractants for mononuclear cells (e.g., activated T cells and natural killer cells). Our group has previously demonstrated the role of CXCR3/ligand biology in the pathogenesis of allograft rejection and dysfunction in animal studies (33, 34).
The allograft injury patterns (AR, LB, OP, DAD) are determined by interactions between the host (recipient), allograft (donor), and “insult.” Thus, we hypothesized that the pattern and severity of allograft injury would be a stronger determinant of CLAD than the various insults causing them. Specifically, the most severe form of lung injury, DAD, should be associated with the highest risk of CLAD. We further hypothesized that this association is mediated in part by an aberrant type I immune response involving the CXCR3/ligand biologic axis.
Methods
With IRB approval, we performed a retrospective review of all LTRs at University of California at Los Angeles between January 1, 2000 and December 31, 2010. Patients received surveillance bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial biopsy (TBBX) at 1, 3, 6, and 12 months post-transplant, and during episodes of clinical deterioration. Approximately 6–14 TBBXs were obtained at each bronchoscopy. Biopsies were interpreted by one of three pulmonary pathologists according to International Multidisciplinary Consensus Statement on Idiopathic Interstitial Pneumonias (DAD and OP) (35) and International Society for Heart and Lung Transplantation criteria (AR and LB) (29, 36). LB was graded as present or absent until March 1, 2009, and thereafter graded according to the revised 2007 International Society for Heart and Lung Transplantation criteria (0, B1R, B2R, or ungradable) (29). To exclude events from the immediate postoperative period (e.g., PGD), TBBXs collected less than 15 days from transplantation were excluded. Any episodes of DAD occurring after 15 days that were attributed to persistent PGD were also excluded.
Immunosuppression and antimicrobial prophylaxis were administered in accordance with the University of California at Los Angeles lung transplantation protocols as previously described (27). In general, episodes of AR were treated with three doses of solumedrol, 500–1,000 mg intravenously, followed by prednisone, 0.5 mg/kg daily for 1 week and tapered by 5 mg per week down to the previous dose. Management of DAD was by discretion of the transplant pulmonologist. Treatment regimens included high-dose solumedrol, intravenous immunoglobulin, plasmapheresis, basiliximab, or thymoglobulin. Spirometry was performed every 1–2 weeks during the first 3 months post-transplant and every 4–12 weeks thereafter. CLAD was defined as a sustained drop of at least 20% in the FEV1 from the average of the two best post-transplant FEV1 measurements (7, 37). CLAD was further classified as RAS or BOS based on spirometry and chest computed tomography (CT) per the 2013 definition by Sato and coworkers (38). RAS was defined as ΔFVC%/ΔFEV1% greater than 0.5 and chest CT showing ground-glass opacification, interstitial reticulation, or interlobular septal thickening. Recipients with CLAD who did not fulfill the criteria for RAS were considered to have the BOS phenotype. Allograft mortality was defined as death or retransplant.
Biopsy data were collected from surgical pathology reports and coded for the presence or absence of DAD, AR (grade A2 or greater), LB, and OP. A biopsy described as ungradable was considered to be a missing value. A subset of patients were consented for the collection of BAL fluid (BALF) for research purposes at the time of their bronchoscopies. Three 60-ml aliquots of isotonic saline were instilled into the subsegmental bronchus in the lingula, right middle lobe, or area of interest and pooled. After centrifugation, the supernatant was collected and stored at −80°C. Chemokine concentrations were measured using Luminex technologies or standard ELISA: CXCL9 bead assay (Bio-Rad Life Science Research, Hercules, CA), CXCL10 bead assay (Millipore, Billerica, MA), and CXCL11 DuoSet kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The lower limit of detection for CXCL9, CXCL10, and CXCL11 were 19.2, 14.0, and 1.7 pg/ml, respectively. All BALF samples were unconcentrated. Immunohistochemistry (IHC) was performed on formalin-fixed, paraffin-embedded tissue for the localization of CXCR3 and its chemokine ligands as previously described (28, 39). Details are provided in the online supplement.
Cox proportional hazards models with time-dependent cumulative counts for DAD, AR, LB, and OP were constructed to take into effect the multiple samples per patient and the frequency (recurrence) of the injury patterns. For each of the injury patterns, the cumulative count increased from zero to one at the first episode of injury, from one to two at the second episode, and so forth. In addition to the four injury patterns, the univariable models included other plausible demographic variables associated with poor clinical outcomes: age greater than 70, male sex, single lung transplant, pretransplant diagnosis, and time of transplant. The time of transplant was divided into tertiles: before 9/1/05, 9/1/05 to 2/13/08, and after 2/13/08 (reference group). The final model was determined using a backward selection method (P < 0.05) using all covariates from the univariable models. Subgroup analyses among bilateral LTRs were performed to evaluate the association between the injury patterns and phenotypes of CLAD (RAS and BOS) using cause-specific proportional hazards models with time-dependent cumulative counts for DAD, AR, LB, and OP.
Log-transformed chemokine concentrations were compared between the “healthy” and “pathologic” biopsies using a linear mixed-effects model to account for repeated measurements. Time from transplant (<90 d vs. ≥90 d) was included as a covariate. To explore whether the prolonged alterations of these chemokines are predictive of CLAD, serial CXCR3 ligand concentrations were included as time-dependent variables in Cox models for CLAD. Analyses were performed with SAS v9.3 (SAS Institute Inc., Cary, NC).
Results
Histopathologic Findings
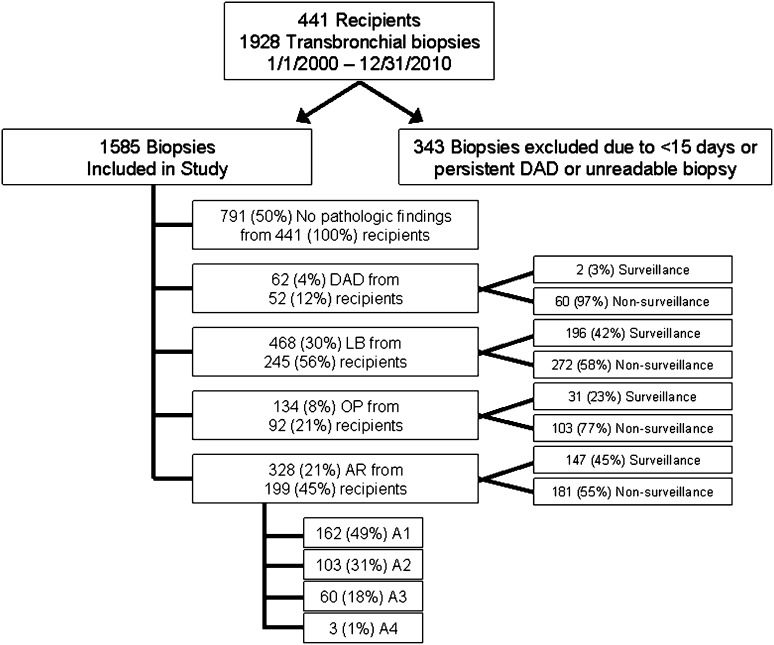
In total, there were 1,928 bronchoscopies with TBBXs from 441 transplant recipients during the period of study. Three hundred forty-three TBBXs were excluded from the analysis because of collection during the first 15 days post-transplant (n = 322), persistent PGD (n = 3), and ungradable samples (n = 18). There remained 1,585 biopsies from 441 recipients available for analysis (Figure 1).
Figure 1.
Study profile. A1 = grade A1; A2 = grade A2; A3 = grade A3; A4 = grade A4; AR = acute rejection; DAD = diffuse alveolar damage; LB = lymphocytic bronchiolitis; OP = organizing pneumonia.
There were 328 (21%) biopsies with a perivascular AR pattern in 199 (45%) recipients. These were graded as follows: 162 (49%) grade A1, 103 (31%) grade A2, 60 (18%) grade A3, and 3 (1%) grade A4. There were 468 (30%) biopsies with LB from 245 (56%) recipients, 134 (8%) biopsies with OP from 92 (21%) recipients, and 62 (4%) biopsies with DAD from 52 (12%) recipients. One hundred seventy-seven TBBXs had concurrent injury patterns. The most common cooccurrence was between AR and LB (n = 87) and LB and OP (n = 67). DAD occurred with LB (n = 35), OP (n = 19), and AR (n = 8). Seven hundred and ninety-one (50%) biopsies from 441 (100%) recipients had no evidence of histopathology and were classified as “healthy biopsies.”
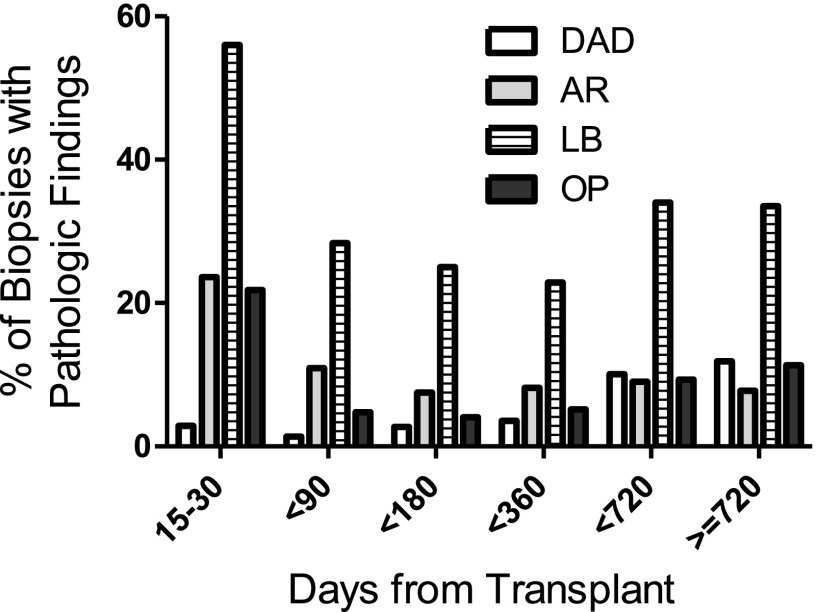
The prevalence of the histopathologic injury patterns varied by the time from transplant (Figure 2). In general, the frequency per biopsy of AR, LB, and OP were greatest during the first 15–30 days post-transplant with observed frequencies of 23%, 55%, and 21%, respectively. In contrast, the frequency of DAD (after excluding cases caused by PGD) started near 2% during the first 15–30 days and increased to over 9% after the first year. Table 1 lists the patient characteristics by whether or not they ever had an episode of DAD.
Figure 2.
Prevalence of pathologic findings by time from transplant. AR = acute rejection (grade A2 or higher); DAD = diffuse alveolar damage; LB = lymphocytic bronchiolitis, OP = organizing pneumonia.
TABLE 1.
BASELINE PATIENT CHARACTERISTICS BY DAD STATUS (EVER HAD DAD ON TRANSBRONCHIAL BIOPSY)
| |
Never DAD |
Ever DAD |
||
|---|---|---|---|---|
| n | % | n | % | |
| Number of subjects |
389 |
88.2 |
52 |
11.8 |
| Median age |
61 |
|
58 |
|
| Male sex |
227 |
58.4 |
32 |
61.5 |
| Single lung transplant |
175 |
45.0 |
13 |
25.0 |
| Diagnosis |
|
|
|
|
| IPF |
179 |
46.0 |
16 |
30.8 |
| COPD |
111 |
28.5 |
12 |
23.1 |
| Scleroderma |
19 |
4.9 |
4 |
7.7 |
| Other ILD |
16 |
4.1 |
4 |
7.7 |
| Cystic fibrosis |
14 |
3.6 |
2 |
3.8 |
| Pulmonary HTN |
12 |
3.1 |
7 |
13.5 |
| Non-CF bronchiectasis |
11 |
2.8 |
— |
0.0 |
| AAT deficiency |
9 |
2.3 |
1 |
1.9 |
| Sarcoidosis |
8 |
2.1 |
3 |
5.8 |
| BOS |
5 |
1.3 |
2 |
3.8 |
| LAM |
3 |
0.8 |
— |
0.0 |
| PLCH |
1 |
0.3 |
— |
0.0 |
| Other |
1 |
0.3 |
1 |
1.9 |
| Induction |
|
|
|
|
| ATG |
215 |
55.3 |
32 |
61.5 |
| Basiliximab |
173 |
44.5 |
19 |
36.5 |
| None | 1 | 0.3 | 1 | 1.9 |
Definition of abbreviations: AAT = α1-antitrypsin; ATG = thymoglobulin; BOS = bronchiolitis obliterans syndrome; CF = cystic fibrosis; COPD = chronic obstructive pulmonary disease; DAD = diffuse alveolar damage; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; HTN = hypertension; LAM = lymphangioleiomyomatosis; PLCH = pulmonary Langerhans cell histiocytosis.
Freedom from CLAD after Histopathologic Lung Allograft Injury Patterns
Proportional hazards models for CLAD were constructed with time-dependent variables for the four injury patterns and plausible demographic variables associated with poor clinical outcomes (Table 2). In the univariable models, an episode of DAD was associated with the highest risk of CLAD (hazard ratio [HR], 3.1; 95% confidence interval [CI], 2.0–4.9), followed by AR (HR, 1.5; 95% CI, 1.2–1.9), OP (HR, 1.3; 95% CI, 1.1–1.7), and LB (HR, 1.3; 95% CI, 1.2–1.5). None of the demographic variables were associated with CLAD. In the multivariable model, DAD remained the strongest predictor of CLAD (HR, 3.0; 95% CI, 1.9–4.7), followed by single lung transplant (HR, 1.4; 95% CI, 1.01–1.91), AR (HR, 1.3; 95% CI, 1.03–1.69), and LB (HR, 1.2; 95% CI, 1.03–1.37) (Table 2). For the injury patterns, the HRs represent the increase in risk for a single episode of the injury.
TABLE 2.
COX PROPORTIONAL HAZARDS MODEL FOR CLAD
| |
Univariable |
Multivariable* |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| DAD |
3.09† |
1.95 |
4.88 |
2.96† |
1.86 |
4.72 |
| AR (≥grade A2) |
1.52† |
1.22 |
1.89 |
1.32‡ |
1.03 |
1.69 |
| LB |
1.33† |
1.17 |
1.51 |
1.19‡ |
1.03 |
1.37 |
| OP |
1.34§ |
1.09 |
1.65 |
|
|
|
| Age >70 |
1.69 |
0.86 |
3.32 |
|
|
|
| Male sex |
1.24 |
0.90 |
1.71 |
|
|
|
| Single lung transplant |
1.35 |
0.99 |
1.85 |
1.39‡ |
1.01 |
1.91 |
| Dx: CF |
0.50 |
0.19 |
1.36 |
|
|
|
| Dx: IPF |
0.85 |
0.62 |
1.17 |
|
|
|
| Dx: PH |
1.51 |
0.77 |
2.97 |
|
|
|
| Dx: COPD |
1.07 |
0.77 |
1.50 |
|
|
|
| Time of Tx: first tertile |
0.68 |
0.43 |
1.07 |
|
|
|
| Time of Tx: second tertile | 1.00 | 0.66 | 1.52 | |||
Definition of abbreviations: AR = acute rejection; CF = cystic fibrosis; CI = confidience interval; CLAD = chronic lung allograft dysfunction; COPD = chronic obstructive pulmonary disease; DAD = diffuse alveolar damage; HR = hazard ratio; IPF = idiopathic pulmonary fibrosis; LB = lymphocytic bronchiolitis; OP = organizing pneumonia; PH = pulmonary hypertension; Tx = transplantation.
Time of Tx: first tertile = before 9/1/05; second tertile = 9/1/05 to 2/13/08. Reference group = after 2/13/08.
Multivariable model includes only the variables listed: DAD, AR, LB, single lung transplant.
P < 0.001.
P < 0.05.
P < 0.01.
We further evaluated the association between the injury patterns and the phenotypes of CLAD (BOS and RAS) in subset analyses among double lung transplants. Of the 47 DAD episodes among double LTRs, 31 (66%) developed CLAD. Twenty-six of these 31 LTRs with DAD and CLAD had CT scans available for review: 21 (81%) met criteria for RAS based on the combined spirometric and CT definition, whereas the remaining 5 (19%) were considered BOS. In the multivariable model, DAD (HR, 6.5; 95% CI, 3.3–12.5) and AR (HR, 1.8; 95% CI, 1.1–2.8) were both associated with development of RAS (Table 3). AR was the only injury pattern associated with the development of BOS (HR, 1.9; 95% CI, 1.2–3.1). We were unable to estimate the risk of BOS after DAD because of limited sample size.
TABLE 3.
CAUSE-SPECIFIC PROPORTIONAL HAZARDS MODEL FOR CLAD PHENOTYPES AMONG DOUBLE LUNG TRANSPLANT RECIPIENTS
| |
Univariable |
Multivariable* |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| RAS |
|
|
|
|
|
|
| DAD |
6.42† |
3.31 |
12.45 |
6.46† |
3.33 |
12.54 |
| AR (≥grade A2) |
1.74‡ |
1.10 |
2.73 |
1.77‡ |
1.12 |
2.81 |
| LB |
1.36 |
0.99 |
1.86 |
|
|
|
| OP |
1.47‡ |
1.04 |
2.07 |
|
|
|
| BOS |
|
|
|
|
|
|
| DAD |
— |
— |
— |
— |
— |
— |
| AR (≥grade A2) |
1.92§ |
1.20 |
3.07 |
1.92§ |
1.20 |
3.07 |
| LB |
1.50‡ |
1.08 |
2.08 |
|
|
|
| OP | 1.14 | 0.66 | 1.95 | |||
Definition of abbreviations: AR = acute rejection; BOS = bronchiolitis obliterans syndrome; CI = confidence interval; CLAD = chronic lung allograft dysfunction; DAD = diffuse alveolar damage; HR = hazard ratio; LB = lymphocytic bronchiolitis; OP = organizing pneumonia; RAS = restrictive allograft syndrome.
Multivariable model includes only the injury patterns listed. Includes double lung transplants only.
P < 0.001.
P < 0.05.
P < 0.01.
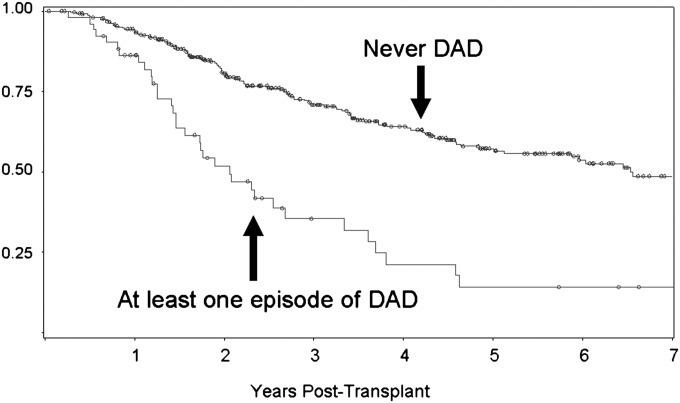
Given the large HRs observed for DAD, Kaplan-Meier curves for freedom from CLAD were constructed and stratified by whether or not the recipient ever had an episode of DAD. The 5-year incidence of CLAD was 86% in recipients with at least one episode of DAD versus 43% in recipients with no episodes of DAD (P = 0.0001) (Figure 3). For the recipients who developed CLAD after DAD, the median time from DAD to CLAD onset was 149 days.
Figure 3.
Kaplan-Meier plot for freedom from chronic lung allograft dysfunction in lung transplant recipients with and without ever having an episode of diffuse alveolar damage (DAD).
Allograft Mortality after Histopathologic Lung Allograft Injury Patterns
Allograft mortality was defined as death (n = 189) or retransplant (n = 8). In the univariable models, an episode of DAD (HR, 3.4; 95% CI, 2.6–4.4), AR (HR, 1.3; 95% CI, 1.1–1.6), LB (HR, 1.2; 95% CI, 1.1–1.3), OP (HR, 1.5; 95% CI, 1.3–1.7), CLAD (HR, 4.1; 95% CI, 3.0–5.7), and cystic fibrosis diagnosis (HR, 0.2; 95% CI, 0.05–0.9) were all significant predictors of allograft mortality (Table 4). In the multivariable model, only DAD (HR, 2.3; 95% CI, 1.7–3.0), CLAD (HR, 3.3; 95% CI, 2.3–4.6), OP (HR, 1.3; 95% CI, 1.1–1.5), and age greater than 70 (HR, 2.1; 95% CI, 1.1–4.0) remained significant (Table 4).
TABLE 4.
COX PROPORTIONAL HAZARDS MODEL FOR ALLOGRAFT MORTALITY
| |
Univariable |
Multivariable* |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| DAD |
3.37† |
2.57 |
4.42 |
2.25† |
1.67 |
3.03 |
| AR (≥grade A2) |
1.32‡ |
1.10 |
1.59 |
|
|
|
| LB |
1.23† |
1.13 |
1.33 |
|
|
|
| OP |
1.47† |
1.25 |
1.72 |
1.25§ |
1.05 |
1.48 |
| CLAD |
4.14† |
3.01 |
5.69 |
3.27† |
2.32 |
4.60 |
| Age >70 |
1.76 |
0.98 |
3.17 |
2.08§ |
1.09 |
3.98 |
| Male sex |
1.24 |
0.93 |
1.66 |
|
|
|
| Single lung transplant |
1.19 |
0.90 |
1.58 |
|
|
|
| Dx: CF |
0.21§* |
0.05 |
0.85 |
|
|
|
| Dx: IPF |
1.14 |
0.86 |
1.51 |
|
|
|
| Dx: PH |
1.33 |
0.70 |
2.51 |
|
|
|
| Dx: COPD |
1.04 |
0.77 |
1.40 |
|
|
|
| Time of Tx: first tertile |
1.35 |
0.87 |
2.09 |
|
|
|
| Time of Tx: second tertile | 1.46 | 0.95 | 2.24 | |||
Definition of abbreviations: Allograft Mortality = death or retransplant; AR = acute rejection; CF = cystic fibrosis; CI = confidence interval; CLAD = chronic lung allograft dysfunction; COPD = chronic obstructive pulmonary disease; DAD = diffuse alveolar damage; HR = hazard ratio; IPF = idiopathic pulmonary fibrosis; LB = lymphocytic bronchiolitis; OP = organizing pneumonia; PH = pulmonary hypertension; Tx = transplantation.
Time of Tx: first tertile = before 9/1/05; second tertile = 9/1/05 to 2/13/08. Reference group = after 2/13/08.
Multivariable model includes only the variables listed: DAD, OP, CLAD, age greater than 70.
P < 0.001.
P < 0.01.
P < 0.05.
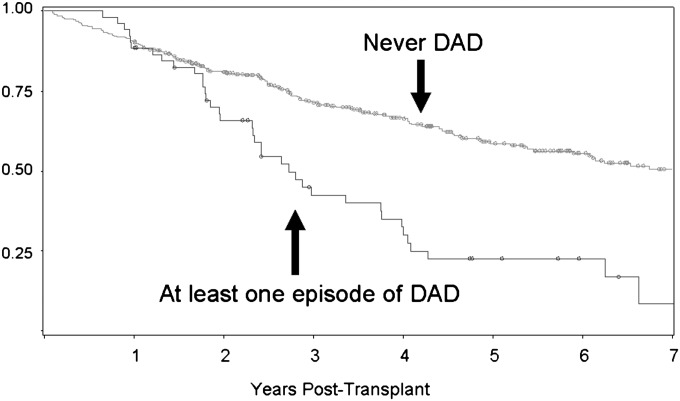
Kaplan-Meier curves for allograft mortality were constructed, stratified by whether or not the recipient ever had an episode of DAD. The 5-year incidence of allograft mortality was 78% in recipients with at least one episode of DAD versus 41% in recipients with no episodes of DAD (P = 0.0001) (Figure 4). For the recipients with allograft mortality after DAD, the median time from DAD to allograft mortality was 278 days.
Figure 4.
Kaplan-Meier plot for allograft survival in lung transplant recipients with and without ever having an episode of diffuse alveolar damage (DAD). Allograft survival = freedom from death or retransplant.
Etiology of DAD
We attempted to determine the specific cause of DAD using microbiology cultures, TBBX cultures and stains for organisms, radiographic studies, and clinical notes by the treating physician. In most cases (n = 39) there was no discernable cause for the DAD. When the etiology for DAD could be identified, the most common was viral infection: cytomegalovirus (n = 3), parainfluenza (n = 4), bocavirus (n = 1), and one case of mixed human metapneumovirus-coronavirus (n = 1). Other etiologies for DAD included nonviral pulmonary infections (n = 6), aspiration (n = 4), severe AR (n = 3), and recurrent sarcoid (n = 1).
BALF Concentrations of Chemokines during DAD, AR, and LB
DAD, AR, and LB all predicted CLAD development in the multivariable Cox model. We tested the hypothesis that a type I immune response involving CXCR3/ligand biology is involved in the association between DAD, AR, LB, and CLAD development. BALF CXCR3 ligand concentrations were compared between the biopsies without pathologic findings (“healthy biopsies”) and those with DAD, AR, and LB. In total, we assayed 496 BALF samples from 224 recipients. There were 325 “healthy” samples from 194 recipients, 20 DAD samples from 18 recipients, 70 AR samples from 56 recipients, and 197 LB samples from 129 recipients. Median BALF CXCL9 concentrations were 19-fold higher during DAD, fourfold higher during AR, and threefold higher during LB as compared with “healthy” biopsies (Table 5). Similarly, BALF CXCL10 concentrations were increased during DAD, AR, and LB, but the increases were less pronounced. BALF CXCL11 concentrations were mildly elevated during DAD, but not during AR or LB.
TABLE 5.
MEDIAN BALF CHEMOKINE CONCENTRATIONS DURING DAD, AR, AND LB
| |
Healthy (pg/ml) | DAD |
AR |
LB |
|||
|---|---|---|---|---|---|---|---|
| pg/ml | P Value* | pg/ml | P Value* | pg/ml | P Value* | ||
| Sample size |
n = 325 |
n = 20 |
|
n = 70 |
|
n = 197 |
|
| No. of recipients |
n = 194 |
n = 18 |
|
n = 56 |
|
n = 129 |
|
| CXCL9 |
270.0 |
5234.6 |
0.0002 |
998.0 |
<0.0001 |
865.1 |
<0.0001 |
| CXCL10 |
115.3 |
206.8 |
0.0211 |
221.2 |
0.0112 |
212.7 |
0.0007 |
| CXCL11 | 81.1 | 95.5 | 0.0221 | 83.8 | 0.5370 | 83.7 | 0.0990 |
Definition of abbreviations: AR = acute rejection; BALF = bronchoalveolar lavage fluid; DAD = diffuse alveolar damage; LB = lymphocytic bronchiolitis.
Time from transplant (<3 mo vs. ≥3 mo) included as covariate.
Mixed-effects model with log-transformed chemokine levels.
BALF Chemokines and the Risk of CLAD
Given the increased BALF concentrations of the CXCR3 ligands during DAD, AR, and LB, we explored whether prolonged elevation of these chemokines would be predictive of CLAD. Cox models for CLAD were constructed with serial BAL measurements of the CXCR3 ligands as time-dependent covariates. All three CXCR3 ligand concentrations were associated with an increased risk of CLAD. Ten-fold increases in CXCL9, CXCL10, and CXCL11 were associated with HRs of 1.52 (95% CI, 1.1–1.9), 1.45 (95% CI, 1.1–1.9), and 2.25 (95% CI, 1.1–4.6), respectively (Table 6).
TABLE 6.
UNIVARIABLE COX PROPORTIONAL HAZARDS MODEL FOR CLAD USING BALF CHEMOKINE CONCENTRATIONS
| Chemokine | HR* | 95% CI | P Value | |
|---|---|---|---|---|
| CXCL9 |
1.52 |
1.12 |
1.90 |
0.0004 |
| CXCL10 |
1.45 |
1.12 |
1.88 |
0.0044 |
| CXCL11 | 2.25 | 1.11 | 4.57 | 0.0245 |
Definition of abbreviations: BALF = bronchoalveolar lavage fluid; CI = confidence interval; CLAD = chronic lung allograft dysfunction; HR = hazard ratio.
HRs are for a 10-fold increase in chemokine concentrations.
Cellular Sources of the Chemokines and Their Shared Receptor CXCR3
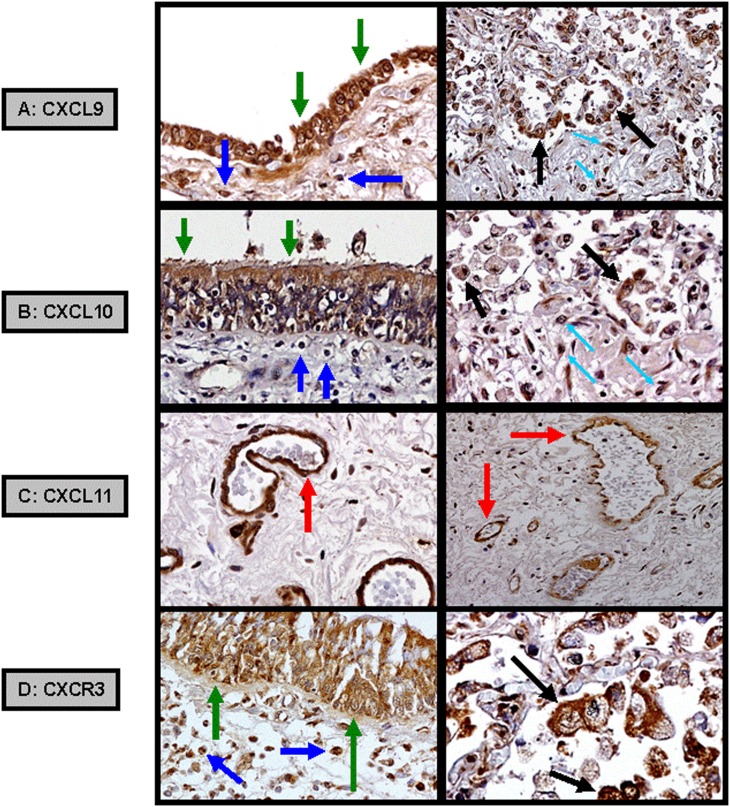
We used IHC on lung biopsy tissue to determine the cellular sources of these chemokines and their shared receptor, CXCR3, during DAD (n = 5). Both CXCL9 and CXCL10 were expressed by airway bronchial epithelial cells, subepithelial and interstitial infiltrating mononuclear cells, and alveolar macrophages. In contrast, CXCL11 was predominately expressed by vascular endothelial cells. CXCR3 was expressed by bronchial epithelial cells, infiltrating mononuclear cells, and alveolar macrophages (Figure 5).
Figure 5.
Immunohistochemistry demonstrating (A) CXCL9 and (B) CXCL10 expressed from allograft airway bronchial epithelial cells (green arrows), subepithelial (dark blue arrows) and interstitial (light blue arrows) infiltrating mononuclear cells, and alveolar macrophages (black arrows). (C) CXCL11 expressed from allograft pulmonary vascular endothelial cells (red arrows). (D) CXCR3 expressed from airway bronchial epithelial cells, infiltrating mononuclear cells, and alveolar macrophages.
Discussion
In contrast to prior studies of CLAD that focused on specific insults to the allograft (e.g., PGD, gastroesophageal reflux, infections), this study focused on the common pathways of allograft injury (AR, LB, OP, DAD) that are determined by interactions between the host, allograft, and insult. We evaluated 1,585 TBBXs from 441 LTRs and found that DAD, the most severe form of lung injury, was associated with the highest risk of subsequent CLAD (HR, 3.0; 95% CI, 1.9–4.7) and death (HR, 2.3; 95% CI, 1.7–3.0). We further hypothesized that the association between DAD and CLAD would be mediated in part by an aberrant type I immune response involving CXCR3/ligand biology. Using BALF analysis, we found marked elevations in BALF CXCR3 ligand concentrations during DAD. Furthermore, the chronic elevation of these chemokines as determined by serial BALF measurements predicted the development of CLAD. Taken together, these findings support the potential role of the CXCR3/ligand biologic axis in the continuum of DAD to CLAD.
Previous studies have repeatedly implicated AR as the major risk factor for the development of CLAD (6–8, 40). Several studies have also reported an association between LB and CLAD (40–42). Consistent with these studies, we found that both AR and LB were associated with CLAD in univariable and multivariable models. Of the acute lung allograft injury patterns, DAD was associated with the highest risk of CLAD in both univariable and multivariable analyses. Remarkably, those with an episode of DAD had a 5-year incidence of CLAD of 86% as compared with only 43% for those without, suggesting that DAD is an ominous sign of future graft dysfunction.
A recent study by Sato and coworkers (43) evaluated the development of CLAD, BOS, and RAS after DAD among bilateral LTRs using PFTs with lung volume measurements. They found that “early DAD” (<3 mo post-transplant) increased the risk of BOS (HR, 1.24; 95% CI, 1.04–1.47), whereas “late DAD” (>3 mo post-transplant) increased the risk of RAS (HR, 36.8; 95% CI, 18.3–14.1). Because we do not routinely obtain lung volume measurements at our center, we used the surrogate spirometric and CT definition of RAS in bilateral LTRs, as advocated by Sato and coworkers (38). In concordance with the results of Sato and coworkers, we found that non-PGD–associated episodes of DAD (>15 d post-transplant) were strongly associated with RAS (HR, 6.5; 95% CI, 3.3–12.5). AR was associated with both RAS (HR, 1.8; 95% CI, 1.1–2.8) and BOS (HR, 1.9; 95% CI, 1.2–3.1). These subset analysis were limited by small sample size, but support the strong association between DAD and the development of CLAD, particularly the RAS phenotype.
We found that DAD was associated with a profound increase in the risk of death, even after controlling for CLAD. Recipients who had a single episode of DAD had a 5-year allograft mortality of 78% compared with only 41% for those without DAD. These results conflict with the study by Sato and coworkers (43) that found no association between “late-onset” DAD and overall mortality. Further studies are required to reconcile this discrepancy.
Similar to previous studies (6, 43, 44), most cases of DAD did not have a discernible cause. In many cases, recipients were treated with antimicrobials before their bronchoscopy, lowering the yield for an infectious diagnosis. Additionally, the more sensitive polymerase chain reaction–based respiratory virus panel became routine at our institution only in 2009. When an etiology could be determined, infection (viral and nonviral) was still the most common cause of DAD, followed by aspiration and severe AR.
We hypothesized, based on our group’s prior animal data, that the association between DAD and CLAD would be mediated in part by an aberrant type I immune response involving CXCR3/ligand biology. In rodent models of AR and CLAD, we demonstrated that these chemokines recruit mononuclear cells through their shared receptor, CXCR3, leading to allograft fibroplasia (33). Inhibition of CXCR3 or its ligands resulted in a profound attenuation of allograft rejection and dysfunction (33, 34, 45). Smaller clinical studies by our group and others have demonstrated increased BALF concentrations of the CXCR3 ligands during both AR (33, 46) and CLAD (33, 47). The current study evaluated 496 BALF samples from 224 recipients and found elevations of CXCL9 and CXCL10 during AR and LB. As expected, there was even greater elevation of these chemokines during DAD, paralleling the risk for subsequent CLAD development. To further evaluate the involvement of CXCR3/ligand interactions in the pathogenesis of CLAD, we explored whether chronically elevated BALF CXCR3 ligand concentrations increased the risk of CLAD. Using Cox models with serial measurement of the CXCR3 ligands as time-dependent covariates, we found that all three ligands were predictive of CLAD development.
To further characterize these chemokine expression patterns, IHC studies were conducted on biopsies during DAD. Both CXCL9 and CXCL10 were expressed primarily by bronchial epithelial cells, infiltrating mononuclear cells, and alveolar macrophages. In contrast, CXCL11 was predominately expressed by vascular endothelial cells. CXCR3 was expressed by bronchial epithelial cells, infiltrating mononuclear cells, and alveolar macrophages. Collectively, these findings support a coordinated effort by chemokines from various compartments within the lung. CXCL11 acts to recruit CXCR3 expressing mononuclear cells from the blood stream. Once in the lung, the CXCR3 expressing mononuclear cells move to the sites of lung allograft injury along a CXCL9 and/or CXCL10 chemokine gradient. This compartmentalization of chemokines may explain the apparent contradiction of CXCL11: less pronounced increases in the BALF but higher effect estimates for CLAD. Although CXCL11 is less detectable in the alveolar compartment (BALF), its augmentation in the vasculature may have an important effect on mononuclear cell trafficking and CLAD development.
The major limitation of this study is the potential for confounding inherent to retrospective studies. For example, patients experiencing clinical deterioration may have had more frequent biopsies leading to a higher incidence of histopathologic findings. Furthermore, patients who were found to have allograft injury may have received treatment that was not taken into account. At our institution, patients routinely received augmented immunosuppression for AR but not for DAD. Additionally, we do not have data for LB grading, a factor that may have weakened the association between LB, CLAD, and its subtypes (BOS and RAS). Lastly, our study design accounted only for lung allograft injuries that were captured by transbronchial biopsies. Undoubtedly, there were episodes of allograft injury that were missed because of the infrequency of TBBX sampling.
We believe that a major strength of this study is the identification of a potential mechanism to explain the association between DAD, AR, LB, and CLAD development: specifically the role of CXCR3/ligand biology in the propagation of a damaging “inflammatory milieu.” The allograft injury patterns likely represent a deleterious cycle of cell damage, type I immune response mediated in part by CXCR3/ligands, recruitment of injurious mononuclear cells into the allograft causing further cell damage and eventual fibroproliferation. This study evaluated serial BALF and histopathology in 224 LTRs and to our knowledge is the largest study to evaluate chemokine expression patterns during lung allograft injury.
In conclusion, our results suggest that the histopathologic finding of DAD is an ominous harbinger for the development of CLAD and death. The findings in this study represent a first step in elucidating a mechanism linking DAD to CLAD. Future studies should investigate the involvement of this chemokine pathway and other cytokine pathways (e.g., Th2, Th17) after allograft injury and the pharmacologic disruption of these interactions as a strategy to decrease CLAD development among LTRs.
Footnotes
Supported by NIH/National Center for Advancing Translational Science University of California at Los Angeles CTSI Grant Number UL1TR000124 (M.Y.S.), K23 HL094746 (S.S.W.), and R01 HL112990-01 (J.A.B.).
Author Contributions: All authors have made a substantial contribution to the acquisition, analysis, or interpretation of data, and revision and final approval of the manuscript to be published. Conception and design, M.Y.S., S.S.W., A.D., D.M.S., A.L.G., J.P.L., and J.A.B. Acquisition of data, analysis, and interpretation, M.Y.S., S.S.W., N.L., V.P., M.C.F., R.M.E., and J.A.B. Drafting and editing of the manuscript, M.Y.S., S.S.W., R.S., A.A., D.J.R., J.P.L., and J.A.B.
Originally Published in Press as DOI: 10.1164/rccm.201305-0861OC on September 24, 2013
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI. The registry of the International Society for Heart and Lung Transplantation: twenty-eighth adult lung and heart-lung transplant report–2011. J Heart Lung Transplant. 2011;30:1104–1122. doi: 10.1016/j.healun.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Weigt SS, DerHovanessian A, Wallace WD, Lynch JP, III, Belperio JA. Bronchiolitis obliterans syndrome: the Achilles’ heel of lung transplantation. Semin Respir Crit Care Med. 2013;34:336–351. doi: 10.1055/s-0033-1348467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verleden GM, Vos R, Verleden SE, De Wever W, De Vleeschauwer SI, Willems-Widyastuti A, Scheers H, Dupont LJ, Van Raemdonck DE, Vanaudenaerde BM. Survival determinants in lung transplant patients with chronic allograft dysfunction. Transplantation. 2011;92:703–708. doi: 10.1097/TP.0b013e31822bf790. [DOI] [PubMed] [Google Scholar]
- 4.Sato M, Waddell TK, Wagnetz U, Roberts HC, Hwang DM, Haroon A, Wagnetz D, Chaparro C, Singer LG, Hutcheon MA, et al. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant. 2011;30:735–742. doi: 10.1016/j.healun.2011.01.712. [DOI] [PubMed] [Google Scholar]
- 5.Sato M, Hwang DM, Waddell TK, Singer LG, Keshavjee S. Progression pattern of restrictive allograft syndrome after lung transplantation. J Heart Lung Transplant. 2013;32:23–30. doi: 10.1016/j.healun.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 6.Burton CM, Iversen M, Carlsen J, Andersen CB. Interstitial inflammatory lesions of the pulmonary allograft: a retrospective analysis of 2697 transbronchial biopsies. Transplantation. 2008;86:811–819. doi: 10.1097/TP.0b013e3181852f02. [DOI] [PubMed] [Google Scholar]
- 7.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 8.Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant. 2002;21:271–281. doi: 10.1016/s1053-2498(01)00360-6. [DOI] [PubMed] [Google Scholar]
- 9.Christie JD, Kotloff RM, Ahya VN, Tino G, Pochettino A, Gaughan C, DeMissie E, Kimmel SE. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171:1312–1316. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, Patterson GA, Trulock EP, Hachem RR. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175:507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 11.Huang HJ, Yusen RD, Meyers BF, Walter MJ, Mohanakumar T, Patterson GA, Trulock EP, Hachem RR. Late primary graft dysfunction after lung transplantation and bronchiolitis obliterans syndrome. Am J Transplant. 2008;8:2454–2462. doi: 10.1111/j.1600-6143.2008.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitson BA, Prekker ME, Herrington CS, Whelan TP, Radosevich DM, Hertz MI, Dahlberg PS. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant. 2007;26:1004–1011. doi: 10.1016/j.healun.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Sumpter TL, Wilkes DS. Role of autoimmunity in organ allograft rejection: a focus on immunity to type V collagen in the pathogenesis of lung transplant rejection. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1129–L1139. doi: 10.1152/ajplung.00330.2003. [DOI] [PubMed] [Google Scholar]
- 14.Wilkes DS, Heidler KM, Yasufuku K, Devito-Haynes L, Jankowska-Gan E, Meyer KC, Love RB, Burlingham WJ. Cell-mediated immunity to collagen V in lung transplant recipients: correlation with collagen V release into BAL fluid. J Heart Lung Transplant. 2001;20:167. doi: 10.1016/s1053-2498(00)00308-9. [DOI] [PubMed] [Google Scholar]
- 15.Snyder LD, Wang Z, Chen DF, Reinsmoen NL, Finlen-Copeland CA, Davis WA, Zaas DW, Palmer SM. Implications for human leukocyte antigen antibodies after lung transplantation: a 10-year experience in 441 patients. Chest. 2013;144:226–233. doi: 10.1378/chest.12-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girnita AL, Duquesnoy R, Yousem SA, Iacono AT, Corcoran TE, Buzoianu M, Johnson B, Spichty KJ, Dauber JH, Burckart G, et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. 2005;5:131–138. doi: 10.1111/j.1600-6143.2004.00650.x. [DOI] [PubMed] [Google Scholar]
- 17.Palmer SM, Davis RD, Hadjiliadis D, Hertz MI, Howell DN, Ward FE, Savik K, Reinsmoen NL. Development of an antibody specific to major histocompatibility antigens detectable by flow cytometry after lung transplant is associated with bronchiolitis obliterans syndrome. Transplantation. 2002;74:799–804. doi: 10.1097/00007890-200209270-00011. [DOI] [PubMed] [Google Scholar]
- 18.Nawrot TS, Vos R, Jacobs L, Verleden SE, Wauters S, Mertens V, Dooms C, Hoet PH, Van Raemdonck DE, Faes C, et al. The impact of traffic air pollution on bronchiolitis obliterans syndrome and mortality after lung transplantation. Thorax. 2011;66:748–754. doi: 10.1136/thx.2010.155192. [DOI] [PubMed] [Google Scholar]
- 19.Verleden SE, Scheers H, Nawrot TS, Vos R, Fierens F, Geenens R, Yserbyt J, Wauters S, Verbeken EK, Nemery B, et al. Lymphocytic bronchiolitis after lung transplantation is associated with daily changes in air pollution. Am J Transplant. 2012;12:1831–1838. doi: 10.1111/j.1600-6143.2012.04134.x. [DOI] [PubMed] [Google Scholar]
- 20.Blondeau K, Mertens V, Vanaudenaerde BA, Verleden GM, Van Raemdonck DE, Sifrim D, Dupont LJ. Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J. 2008;31:707–713. doi: 10.1183/09031936.00064807. [DOI] [PubMed] [Google Scholar]
- 21.D’Ovidio F, Mura M, Tsang M, Waddell TK, Hutcheon MA, Singer LG, Hadjiliadis D, Chaparro C, Gutierrez C, Pierre A, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005;129:1144–1152. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 22.Billings JL, Hertz MI, Savik K, Wendt CH. Respiratory viruses and chronic rejection in lung transplant recipients. J Heart Lung Transplant. 2002;21:559–566. doi: 10.1016/s1053-2498(01)00405-3. [DOI] [PubMed] [Google Scholar]
- 23.Botha P, Archer L, Anderson RL, Lordan J, Dark JH, Corris PA, Gould K, Fisher AJ. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation. 2008;85:771–774. doi: 10.1097/TP.0b013e31816651de. [DOI] [PubMed] [Google Scholar]
- 24.Khalifah AP, Hachem RR, Chakinala MM, Schechtman KB, Patterson GA, Schuster DP, Mohanakumar T, Trulock EP, Walter MJ. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004;170:181–187. doi: 10.1164/rccm.200310-1359OC. [DOI] [PubMed] [Google Scholar]
- 25.Ruttmann E, Geltner C, Bucher B, Ulmer H, Höfer D, Hangler HB, Semsroth S, Margreiter R, Laufer G, Müller LC. Combined CMV prophylaxis improves outcome and reduces the risk for bronchiolitis obliterans syndrome (BOS) after lung transplantation. Transplantation. 2006;81:1415–1420. doi: 10.1097/01.tp.0000209439.27719.ed. [DOI] [PubMed] [Google Scholar]
- 26.Weigt SS, Derhovanessian A, Liao E, Hu S, Gregson AL, Kubak BM, Saggar R, Plachevskiy V, Fishbein MC, Lynch JP, III, et al. CXCR3 chemokine ligands during respiratory viral infections predict lung allograft dysfunction. Am J Transplant. 2012;12:477–484. doi: 10.1111/j.1600-6143.2011.03859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weigt SS, Elashoff RM, Huang C, Ardehali A, Gregson AL, Kubak B, Fishbein MC, Saggar R, Keane MP, Lynch JP, III, et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am J Transplant. 2009;9:1903–1911. doi: 10.1111/j.1600-6143.2009.02635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weigt SS, Elashoff RM, Keane MP, Strieter RM, Gomperts BN, Xue YY, Ardehali A, Gregson AL, Kubak B, Fishbein MC, et al. Altered levels of CC chemokines during pulmonary CMN predict BOS and mortality post-lung transplantation. Am J Transplant. 2008;8:1512–1522. doi: 10.1111/j.1600-6143.2008.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, Glanville A, Gould FK, Magro C, Marboe CC, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Fisher AJ, Wardle J, Dark JH, Corris PA. Non-immune acute graft injury after lung transplantation and the risk of subsequent bronchiolitis obliterans syndrome (BOS) J Heart Lung Transplant. 2002;21:1206–1212. doi: 10.1016/s1053-2498(02)00450-3. [DOI] [PubMed] [Google Scholar]
- 31.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and MIG: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belperio JA, Keane MP, Burdick MD, Lynch JP, III, Xue YY, Li K, Ross DJ, Strieter RM. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002;169:1037–1049. doi: 10.4049/jimmunol.169.2.1037. [DOI] [PubMed] [Google Scholar]
- 34.Belperio JA, Keane MP, Burdick MD, Lynch JP, III, Zisman DA, Xue YY, Li K, Ardehali A, Ross DJ, Strieter RM. Role of CXCL9/CXCR3 chemokine biology during pathogenesis of acute lung allograft rejection. J Immunol. 2003;171:4844–4852. doi: 10.4049/jimmunol.171.9.4844. [DOI] [PubMed] [Google Scholar]
- 35.American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS executive committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 36.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, Marchevsky A, Ohori NP, Ritter J, Stewart S, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15:1–15. [PubMed] [Google Scholar]
- 37.Cooper JD, Billingham M, Egan T, Hertz MI, Higenbottam T, Lynch J, Mauer J, Paradis I, Patterson GA, Smith C, et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 1993;12:713–716. [PubMed] [Google Scholar]
- 38.Sato M, Saito T, Waddell TK, Singer LG, Keshavjee S.Diagnosis of restrictive allograft syndrome (RAS) without using total lung capacity. Presented at the ISHLT 33rd Annual Meeting and Scientific Sessions. April 24–27, 2013, Montreal, Quebec, Canada. Abstract #262. [Google Scholar]
- 39.Busuttil A, Weigt SS, Keane MP, Xue YY, Palchevskiy V, Burdick MD, Huang C, Zisman DA, Fishbein M, Lynch JP, III, et al. CXCR3 ligands are augmented during the pathogenesis of pulmonary sarcoidosis. Eur Respir J. 2009;34:676–686. doi: 10.1183/09031936.00157508. [DOI] [PubMed] [Google Scholar]
- 40.Glanville AR, Aboyoun CL, Havryk A, Plit M, Rainer S, Malouf MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med. 2008;177:1033–1040. doi: 10.1164/rccm.200706-951OC. [DOI] [PubMed] [Google Scholar]
- 41.Girgis RE, Tu I, Berry GJ, Reichenspurner H, Valentine VG, Conte JV, Ting A, Johnstone I, Miller J, Robbins RC, et al. Risk factors for the development of obliterative bronchiolitis after lung transplantation. J Heart Lung Transplant. 1996;15:1200–1208. [PubMed] [Google Scholar]
- 42.Yousem SA. Lymphocytic bronchitis/bronchiolitis in lung allograft recipients. Am J Surg Pathol. 1993;17:491–496. doi: 10.1097/00000478-199305000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Sato M, Hwang DM, Ohmori-Matsuda K, Chaparro C, Waddell TK, Singer LG, Hutcheon MA, Keshavjee S. Revisiting the pathologic finding of diffuse alveolar damage after lung transplantation. J Heart Lung Transplant. 2012;31:354–363. doi: 10.1016/j.healun.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 44.Chaparro C, Chamberlain D, Maurer J, De Hoyos A, Winton T, Kesten S. Acute lung injury in lung allografts. J Heart Lung Transplant. 1995;14:267–273. [PubMed] [Google Scholar]
- 45.Medoff BD, Wain JC, Seung E, Jackobek R, Means TK, Ginns LC, Farber JM, Luster AD. CXCR3 and its ligands in a murine model of obliterative bronchiolitis: regulation and function. J Immunol. 2006;176:7087–7095. doi: 10.4049/jimmunol.176.11.7087. [DOI] [PubMed] [Google Scholar]
- 46.Agostini C, Calabrese F, Rea F, Facco M, Tosoni A, Loy M, Binotto G, Valente M, Trentin L, Semenzato G. CXCR3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol. 2001;158:1703–1711. doi: 10.1016/S0002-9440(10)64126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neujahr DC, Perez SD, Mohammed A, Ulukpo O, Lawrence EC, Fernandez F, Pickens A, Force SD, Song M, Larsen CP, et al. Cumulative exposure to gamma interferon-dependent chemokines CXCL9 and CXCL10 correlates with worse outcome after lung transplant. Am J Transplant. 2012;12:438–446. doi: 10.1111/j.1600-6143.2011.03857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]