Abstract
Rationale: Statins, or HMG-CoA reductase inhibitors, may aid in the treatment of asthma through their pleiotropic antiinflammatory effects.
Objectives: To examine the effect of statin therapy on asthma-related exacerbations using a large population-based cohort.
Methods: Statin users aged 31 years or greater with asthma were identified from the Population-Based Effectiveness in Asthma and Lung population, which includes data from five health plans. Statin exposure and asthma exacerbations were assessed over a 24-month observation period. Statin users with a statin medication possession ratio greater than or equal to 80% were matched to non–statin users by age, baseline asthma therapy, site of enrollment, season at baseline, and propensity score, which was calculated based on patient demographics and Deyo-Charlson conditions. Asthma exacerbations were defined as two or more oral corticosteroid dispensings, asthma-related emergency department visits, or asthma-related hospitalizations. The association between statin exposure and each of the three outcome measures was assessed using conditional logistic regression.
Measurements and Main Results: Of the 14,566 statin users, 8,349 statin users were matched to a nonuser. After adjusting for Deyo-Charlson conditions that remained unbalanced after matching, among statin users, statin exposure was associated with decreased odds of having asthma-related emergency department visits (odds ratio [OR], 0.64; 95% confidence interval [CI], 0.53–0.77; P < 0.0001) and two or more oral corticosteroid dispensings (OR, 0.90; 95% CI, 0.81–0.99; P = 0.04). There were no differences in asthma-related hospitalizations (OR, 0.91; 95% CI, 0.66–1.24; P = 0.52).
Conclusions: Among statin users with asthma, statin exposure was associated with decreased odds of asthma-related emergency department visits and oral corticosteroid dispensings.
Keywords: HMG-CoA reductase inhibitors, asthma therapy, exacerbations
At a Glance Commentary
Scientific Knowledge on the Subject
HMG-CoA reductase inhibitors have pleiotropic immunomodulatory effects and have been postulated to play a role in the treatment of asthma, a chronic inflammatory condition. Randomized controlled trials have demonstrated conflicting results.
What This Study Adds to the Field
Using a large population-based cohort, this study found that statin exposure was associated with decreased odds of asthma-related emergency department visits and oral corticosteroid dispensing.
HMG-CoA reductase inhibitors, or statins, are widely used for hyperlipidemia and cardiovascular disease. Independent of their cholesterol-lowering properties, statins also demonstrate pleiotropic antiinflammatory and immunomodulatory effects (1, 2). It has therefore been postulated that statins may have a role in the treatment of inflammatory lung diseases, such as asthma (3, 4), a condition affecting more than 300 million people worldwide (5). In murine models of allergic asthma, statins have been shown to attenuate antigen-induced bronchial smooth muscle hyperresponsiveness (6–8), increase lung compliance (7), decrease eosinophilic inflammation in the lung (7), inhibit goblet cell hyperplasia (9), and suppress IL-17 (10) and tumor necrosis factor-α production (7).
A recent population-based study in Taiwan reported that statin use was associated with reduced hospitalization for asthma exacerbations (11). However, as the authors pointed out, inhaled corticosteroids (ICS) are underused to a greater extent in Taiwan compared with other countries, thus the results may not be generalizable to other populations. Randomized controlled trials of statin therapy in subjects with asthma have demonstrated mixed results, and most are limited by small sample sizes and short treatment periods (12–15).
Using a large population-based cohort of subjects with asthma, we examined the association between statin therapy and asthma exacerbations as defined by use of oral corticosteroid (OCS) therapy and asthma-related emergency department (ED) visits or hospitalizations. We hypothesized that statin use would be associated with fewer of these events.
Methods
Study Design
We conducted a propensity score–matched cohort study of statin users and non–statin users in the Population-based Effectiveness in Asthma and Lung Diseases (PEAL) Asthma Network Population, consisting of a subset of patients with asthma.
Subjects
PEAL Network.
The PEAL Network population consists of patients from five sites, including four health maintenance organization plans: (1) Harvard Pilgrim Health Care; (2) Health Partners; (3) Kaiser Permanente Northern California (KPNC); (4) Kaiser Permanente Georgia; and (5) TennCare, the Tennessee Medicaid plan. The PEAL Network population was assembled to study comparative effectiveness in lung diseases including, but not limited to, asthma, cystic fibrosis, bronchopulmonary dysplasia, pulmonary hypertension and pulmonary embolism, chronic bronchitis and emphysema, bronchiectasis, and chronic obstructive pulmonary disease. This study was approved by the Institutional Review Board at each site. Electronic data from subjects from each of the five sites were pooled to form the PEAL Data Warehouse, which includes information on subject demographics, enrollment type, prescribing and dispending of medications, healthcare resource use, copayments, census-based socioeconomic variables, basic anthropometric measures, and death.
PEAL Asthma Population.
Subjects from the PEAL Network population were included in the PEAL Asthma Population if they had a diagnosis of asthma (International Classification of Diseases [ICD]-9 code 493.xx) during an acute inpatient hospital stay, ED visit, ambulatory visit, or nonacute institutional stays during the period of January 1, 2004 to December 31, 2010. This time window varied for each site by up to 1 year, based on data availability. Based on ICD-9 codes, subjects were excluded if they had a diagnosis of cystic fibrosis, immunodeficiency, bronchiectasis, hereditary and degenerative diseases of the central nervous system, psychoses, mental retardation, congestive heart failure, pulmonary hypertension, or pulmonary embolism.
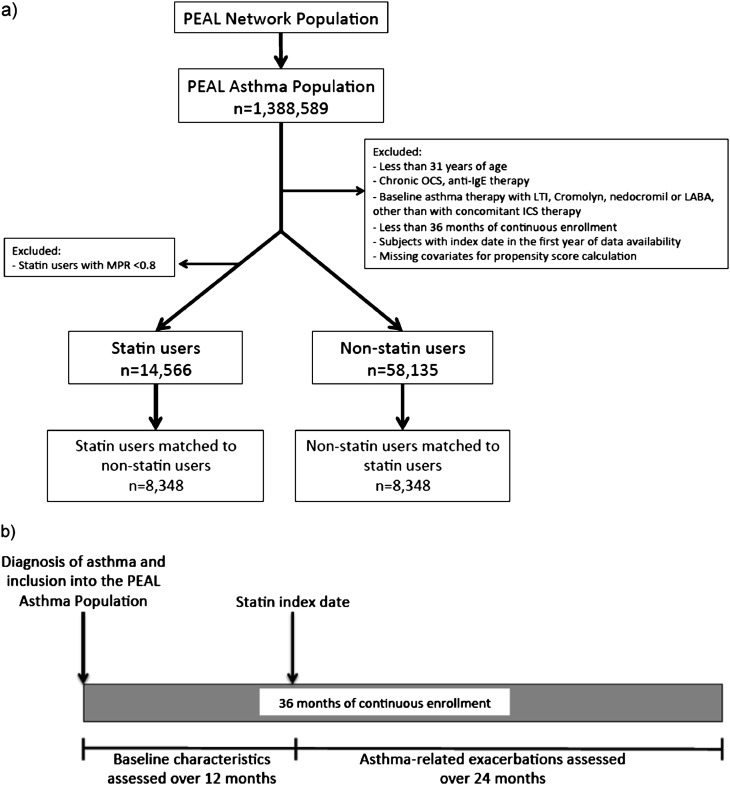
For this analysis, subjects from the PEAL Asthma Population who were on statin therapy were included if they were 31 years or older on their first statin dispensing (index date), because only a minority of statin users were younger than this cutoff, and if they had at least 36 months of continuous enrollment with drug benefits (Figure 1a). Subjects on anti-IgE therapy (omalizumab) or chronic OCS therapy, defined as 180 days or more of continuous OCS supply, were excluded. To ensure that all subjects had at least 12 months of baseline data, subjects with an index date during the first calendar year of data availability were excluded.
Figure 1.
(a) Flow for selection of statin users and nonusers from the Population-based Effectiveness in Asthma and Lung Diseases (PEAL) Asthma Cohort. (b) Timeline for assessment of baseline characteristics and asthma-related outcomes of statin users. Non–statin users were matched to statin users using propensity score and other covariates (see text). ICS = inhaled corticosteroids; LABA = long-acting β-agonist; LTI = leukotriene inhibitors; MPR = medication possession ratio; OCS = oral corticosteroids.
Ascertainment of Statin and Asthma Therapy
Following the above exclusions, subjects on statins and asthma therapy were identified using National Drug Codes for the respective medications. The statin index date was defined as the first dispensing of any statin. Subjects were included if they were continuously enrolled for 12 months before and 24 months after the index date (thus continuously for 36 mo) (Figure 1b). If there is no statin dispensing within the first 36 months of continuous enrollment, the subject was considered a non–statin user and their index date was defined as 12 months into this period. Subjects were classified as statin users if they had a medication possession ratio (MPR) of 80% or greater over the 24 months of follow-up. The MPR was defined as the total number of days of statin supplied during the 24-month follow-up divided by the total number of days over that same period. Subjects with an MPR less than 80% were excluded from this analysis.
In this analysis, baseline asthma therapy was divided into three mutually exclusive categories: (1) any ICS use, including combination therapy; (2) short-acting β-agonist or ipratropium only; and (3) no asthma medication within the timeframe. Given the small sample sizes, unless they were on concomitant ICS therapy, subjects on leukotriene modifiers, cromolyn, nedocromil, or long-acting β-agonists were excluded from this analysis.
Ascertainment of Asthma-related Outcomes
The occurrence of an asthma-related exacerbation, defined as an asthma-related ED visit, hospitalization, or requirement of two or more courses of OCSs (≥3 d each), was recorded over the 24 months post–index date period. Asthma-related ED visits and hospitalizations were defined using ICD-9 codes (primary diagnosis of 493.xx or nonprimary diagnosis of 493.xx in addition to another respiratory diagnosis) (see Table E1 in the online supplement).
Statistical Analysis
Individual propensity scores were calculated using the following covariates: asthma therapy and severity (based on ED visits, hospitalizations, OCS use) over the 12-month preindex period; year of the index date; age category (31–35, 36–40, 41–45, 46–50, 51–55, 56–60, ≥60 yr); sex; race; hypertension; obesity; tobacco use; enrollment in Medicaid; and the Deyo-Charlson conditions of cardiovascular disease, diabetes, diabetes with chronic complications, myocardial infarction, peripheral vascular disease, and renal disease. These variables were included in the propensity score calculation because we judged that they would likely affect whether a subject would be on statin therapy and the likelihood of presenting to the ED or to be hospitalized for asthma. Individuals with missing covariates for the propensity score calculations were excluded. Other Deyo-Charlson conditions, including malignancy, mild and severe liver disease, metastatic solid tumors, hemiplegia or paraplegia, peptic ulcer disease, and rheumatologic disease, were not included in the propensity score model because of their low prevalence. However, they were adjusted for in the conditional logistic regression. The propensity score reflects the probability that an individual was treated with statins conditional on these baseline characteristics. Given that some sites of enrollment had different patterns of use of statins and asthma medications, the modeling and matching of the propensity score was site-specific. Using a 1/1 ratio, in addition to propensity score matching, each statin user was matched to a non–statin user on age category (31–35, 36–65, ≥65 yr), site of enrollment, season of the index date, and baseline asthma medications as defined previously. For propensity score matching, statin users and non–statin users were matched on the logit of the propensity score using calipers of width equal to 0.2 of the standard deviation of the logit of the propensity score without replacement and with a distance tolerance of 1e-8. If several non–statin users were successfully matched using these criteria, then one of them was chosen randomly as the match. Multivariate analyses were conducted using conditional logistic regression to assess the association between statin exposure and each of the three asthma-related outcomes of interest. Covariates included Deyo-Charlson conditions that remained unbalanced after matching. To explore the extent of residual unmeasured confounding, a sensitivity analysis for matched samples was performed (16). P values were two-sided. Multivariate and propensity score matching was conducted using the package “Matching” in R 2.15.1 (www.r-project.org) (17). All other analyses were performed using SAS (version 9.3; SAS Institute Inc., Cary, NC).
Results
Patient Characteristics
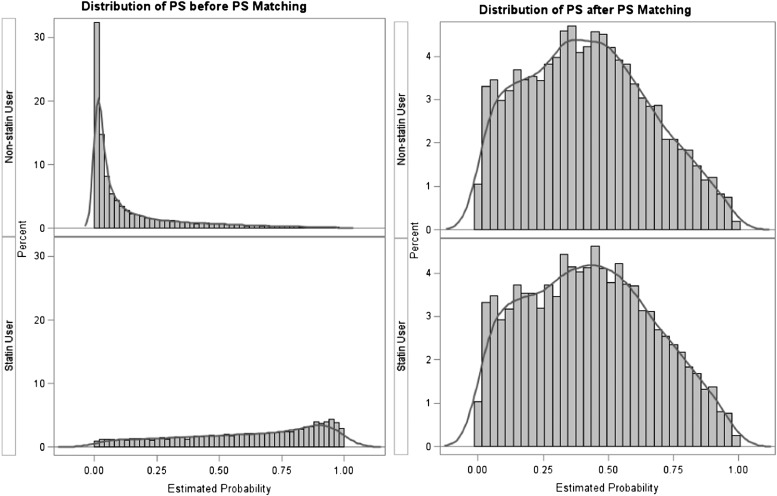
Of the 1,388,589 subjects in the PEAL Asthma cohort, 72,704 subjects met the inclusion and exclusion criteria. Three subjects were excluded because of missing gender values, leaving 14,566 statin users and 58,135 non–statin users (Figure 1a). Baseline characteristics of statin users and non–statin users before multivariate and propensity score matching are presented in Table 1. There was a high degree of imbalance between the baseline characteristics. Specifically, statin users were older (mean, 57.9 [SD, 10.8] yr vs. 43.2 [9.3] for non–statin users) and had a higher prevalence of most comorbidities, particularly obesity (18.7% among statin users vs. 8.3% among nonusers), hypertension (54.2% vs. 14.4%), and diabetes (20.8% vs. 2.0%). The distribution of site-specific propensity scores differed between statin users and non–statin users, with most non–statin users having lower propensity scores. An example of the KPNC enrollment site is shown in Figure 2; other figures can be found in Figure E1.
TABLE 1.
BASELINE CHARACTERISTICS OF STATIN USERS AND NON–STATIN USERS BEFORE PROPENSITY SCORE MATCHING
| Statin Users | Non–Statin Users | P Value | |
|---|---|---|---|
| N |
14,566 |
58,135 |
— |
| Sites, % (n) |
|
|
|
| HPHC |
7.4 (1,086) |
18.8 (10,920) |
— |
| Health Partners |
5.8 (842) |
11.7 (6,838) |
|
| KPNC |
83.5 (12,164) |
60.0 (34,852) |
|
| KPGA |
1.3 (185) |
2.8 (1,643) |
|
| TennCare |
2.0 (289) |
6.7 (3,882) |
|
| Age, yr, mean (SD) |
57.9 (10.8) |
43.2 (9.3) |
<0.0001 |
| Female, % (n) |
62.2 (9,065) |
65.1 (37,834) |
<0.0001 |
| Race/ethnicity, % (n) |
|
|
|
| White |
55.4 (8,072) |
36.7 (21,311) |
<0.0001 |
| African American |
5.6 (819) |
7.0 (4,075) |
|
| Hispanic |
9.4 (1,362) |
8.6 (5,006) |
|
| Asian |
9.1 (1,321) |
5.1 (2,978) |
|
| Other/unknown |
20.5 (2,992) |
42.6 (24,765) |
|
| Asthma therapy in the 12-mo preindex period, % (n) |
|
|
|
| Inhaled corticosteroids |
38.7 (5,641) |
31.5 (18,335) |
<0.0001 |
| Short-acting β-agonists only |
12.8 (1,869) |
16.5 (9,571) |
|
| No asthma medication |
48.5 (7,056) |
52.0 (30,229) |
|
| Asthma severity in the 12-mo preindex period, % (n) |
|
|
|
| Oral corticosteroids dispensing |
|
|
|
| 0 |
86.5 (12,595) |
85.2 (49,551) |
<0.0001 |
| 1 |
9.2 (1,341) |
10.5 (6,097) |
|
| 2 |
4.3 (630) |
4.3 (2,487) |
|
| Subjects with ≥1 asthma-related hospitalization |
0.8 (110) |
0.5 (280) |
<0.0001 |
| Subjects with ≥1 asthma-related ED visit |
1.3 (186) |
2.4 (1,404) |
<0.0001 |
| Tobacco use in the 12-mo preindex period, % (n) |
14.8 (2,151) |
14.6 (8,480) |
0.58 |
| Obesity in the 12-mo preindex period, % (n) |
18.7 (2,721) |
8.3 (4,836) |
<0.0001 |
| Hypertension in the 12-mo preindex period, % (n) |
54.2 (7,891) |
14.4 (8,355) |
<0.0001 |
| Deyo-Charlson conditions in the 12-mo preindex period, % (n) |
|
|
|
| Myocardial infarction |
3.4 (501) |
0.1 (52) |
<0.0001 |
| Peripheral vascular disease |
1.7 (243) |
0.2 (110) |
<0.0001 |
| Cerebrovascular disease |
3.7 (536) |
0.4 (215) |
<0.0001 |
| Rheumatologic disease |
1.2 (180) |
0.8 (491) |
<0.0001 |
| Peptic ulcer disease |
0.6 (80) |
0.3 (189) |
<0.0001 |
| Mild liver disease |
0.2 (23) |
0.1 (74) |
0.37 |
| Diabetes |
20.8 (3,029) |
2.0 (1,172) |
<0.0001 |
| Diabetes with chronic complications |
3.8 (560) |
0.2 (133) |
<0.0001 |
| Hemiplegia or paraplegia |
0.4 (60) |
0.0 (24) |
<0.0001 |
| Renal disease |
1.8 (256) |
0.2 (133) |
<0.0001 |
| Moderate or severe liver disease |
0.0 (5) |
0.1 (28) |
0.48 |
| Any malignancy including leukemia and lymphoma |
4.8 (695) |
1.5 (891) |
<0.0001 |
| Metastatic solid tumor | 0.3 (40) | 0.2 (96) | 0.01 |
Definition of abbreviations: ED = emergency department; HPHC = Harvard Pilgrim Health Care; KPGA = Kaiser Permanente Georgia; KPNC = Kaiser Permanente Northern California.
Figure 2.
Distribution of propensity scores for Kaiser Permanente Northern California before and after propensity score (PS) matching. Refer to Figure E1 for other enrollment sites.
To assess imbalances between statin users and nonusers prematching and post-matching, absolute standardized differences were estimated for each of the baseline characteristics (Figure 3). A standardized difference less than 10% indicates a negligible difference between statin users and nonusers (18, 19). In this case, multivariate and propensity score matching balanced baseline characteristics between the two groups. A total of 8,348 statin users were successfully matched to a non–statin user.
Figure 3.
Love plot of the absolute standardized differences for demographics and baseline characteristics between statin users and non–statin users, before (open triangles) and after (closed circles) multivariate and propensity score matching. ED = emergency department; HPHC = Harvard Pilgrim Health Care; KPGA = Kaiser Permanente Georgia; KPNC = Kaiser Permanente Northern California.
Baseline characteristics of statin users and non–statin users after multivariate and propensity score matching are presented in Table 2. The mean age was 53.9 (SD, 9.3) years, with 62.8% females among statin users and 53.9 (SD, 9.1) years, with 62.2% females among non–statin users. After matching, the differences in prevalence of most comorbidities between the two groups were nonsignificant. Baseline asthma therapy and asthma exacerbations in the preindex period were similar between matched and unmatched statin users (see Table E2). A higher percentage of unmatched statin users came from KPNC, reflecting the poor overlap in propensity scores in that population and possibly prescribing practices. Comorbid Deyo-Charlson conditions were also more prevalent among unmatched statin users, reinforcing the observation that statin users differed systematically from non–statin users before multivariate and propensity score matching.
TABLE 2.
BASELINE CHARACTERISTICS OF STATIN USERS AND THE MATCHED NON–STATIN USERS
| Statin Users | Non–Statin Users | P Value | |
|---|---|---|---|
| N |
8,348 |
8,348 |
— |
| Sites, % (n) |
|
|
|
| HPHC |
11.0 (915) |
11.0 (915) |
|
| Health Partners |
6.6 (551) |
6.6 (551) |
|
| KPNC |
78.5 (6,556) |
78.5 (6,556) |
|
| KPGA |
1.3 (105) |
1.3 (105) |
|
| TennCare |
2.6 (221) |
2.6 (221) |
|
| Age, mean (SD) |
53.9 (9.3) |
53.9 (9.1) |
0.99 |
| Female, % (n) |
62.8 (5,240) |
62.2 (5,194) |
0.46 |
| Race/ethnicity, % (n) |
|
|
|
| White |
50.1 (4,186) |
50.0 (4,169) |
0.83 |
| African American |
5.5 (456) |
5.3 (445) |
|
| Hispanic |
8.5 (711) |
8.1 (680) |
|
| Asian |
7.6 (631) |
7.7 (639) |
|
| Other/unknown |
28.3 (2,364) |
28.9 (2,415) |
|
| Asthma therapy in the 12-mo preindex period, % (n) |
|
|
|
| Inhaled corticosteroids |
36.8 (3,069) |
36.8 (3,069) |
1.00 |
| Short-acting β-agonists only |
13.2 (1,104) |
13.2 (1,104) |
|
| No asthma medication |
50.0 (4,175) |
50.0 (4,175) |
|
| Asthma severity in the 12-mo preindex period, % (n) |
|
|
|
| Oral corticosteroid dispensing |
|
|
|
| 0 |
84.5 (7,219) |
86.6 (7,228) |
0.58 |
| 1 |
9.3 (777) |
9.5 (793) |
|
| 2 |
4.2 (352) |
3.9 (327) |
|
| Subjects with ≥1 asthma-related hospitalization |
0.6 (51) |
0.5 (40) |
0.25 |
| Subjects with ≥1 asthma-related ED visit |
1.4 (118) |
1.4 (114) |
0.79 |
| Tobacco use in the 12-mo preindex period, % (n) |
14.9 (1,242) |
14.3 (1,197) |
0.32 |
| Obesity in the 12-mo preindex period, % (n) |
15.6 (1,306) |
14.8 (1,233) |
0.12 |
| Hypertension in the 12-mo preindex period, % (n) |
41.5 (3,468) |
40.4 (3,374) |
0.14 |
| Deyo-Charlson conditions in the 12-mo preindex period, % (n) |
|
|
|
| Myocardial infarction |
0.6 (48) |
0.3 (21) |
0.001 |
| Peripheral vascular disease |
0.7 (57) |
0.5 (40) |
0.08 |
| Cerebrovascular disease |
1.3 (109) |
1.0 (85) |
0.08 |
| Rheumatologic disease |
1.0 (83) |
1.5 (123) |
0.01 |
| Peptic ulcer disease |
0.4 (29) |
0.5 (42) |
0.12 |
| Mild liver disease |
0.1 (10) |
0.3 (22) |
0.03 |
| Diabetes |
7.6 (637) |
6.8 (568) |
0.04 |
| Diabetes with chronic complications |
1.3 (105) |
0.9 (75) |
0.03 |
| Hemiplegia or paraplegia |
0.2 (13) |
0.1 (6) |
0.11 |
| Renal disease |
0.7 (57) |
0.8 (65) |
0.47 |
| Moderate or severe liver disease |
0.0 (1) |
0.0 (3) |
0.32 |
| Any malignancy including leukemia and lymphoma |
3.1 (261) |
3.1 (257) |
0.86 |
| Metastatic solid tumor | 0.1 (8) | 0.3 (27) | 0.001 |
Definition of abbreviations: ED = emergency department; HPHC = Harvard Pilgrim Health Care; KPGA = Kaiser Permanente Georgia; KPNC = Kaiser Permanente Northern California.
Association between Statin Therapy and Asthma-related Outcomes
After adjusting for Deyo-Charlson conditions that remained unbalanced after matching, among statin users, statin exposure was associated with decreased odds of asthma-related ED visits (odds ratio [OR], 0.64; 95% confidence interval [CI], 0.53–0.77; P < 0.0001) and two or more OCS dispensings (OR, 0.90; 95% CI, 0.81–0.99; P = 0.04) (Table 3). No association was observed between statin use and asthma-related hospitalizations (OR, 0.90; 95% CI, 0.66–1.24; P = 0.52). To assess residual unmeasured confounding, a sensitivity analysis for matched samples was performed (see Table E3) using Rosenbaum's approach (16). The sensitivity parameter Γ, a measure of the degree of departure from a study that is free of unmeasured confounding, is varied between 1.0 and 2.0. Γ equals 1 in the absence of unmeasured confounding. The association between statin-use and the outcome of OCS dispensing would become insignificant (P value ≥ 0.05) with a Γ value between 1.3 and 1.4. The association between statin-use and the outcome of asthma-related ED visits would become insignificant with a Γ less than 1.1. Despite our best effort to account for unmeasured confounding compared with previously published observational studies in this field, this suggests that the findings are somewhat sensitive to unmeasured confounding, for ED visits more so than OCS dispensings.
TABLE 3.
EFFECT OF STATIN USE ON OCS DISPENSING, ASTHMA-RELATED ED VISITS, AND HOSPITALIZATIONS
| Statin Users | Non–Statin Users | OR* (95% CI) | P Value* | |
|---|---|---|---|---|
| Two or more OCS dispensings, % (n) |
9.7 (808) |
10.7 (895) |
0.90 (0.81–0.99) |
0.04 |
| Asthma-related ED visit, % (n) |
2.4 (201) |
3.6 (304) |
0.64 (0.53–0.77) |
<0.0001 |
| Asthma-related hospitalization, % (n) | 1.0 (82) | 1.1 (89) | 0.90 (0.66–1.24) | 0.52 |
Definition of abbreviations: CI = confidence interval; ED = emergency department; OCS = oral corticosteroids; OR = odds ratio.
Adjusted for unbalanced covariates after propensity score matching.
A subgroup analysis of ICS users consisting of 3,069 pairs of matched statin and non–statin users showed similar findings. Among statin users, statin exposure was associated with decreased odds of asthma-related ED visits (OR, 0.74; 95% CI, 0.58–0.96; P = 0.03) (Table 4). This association was not statistically significant for OCS dispensings, although an effect size similar to original analysis was observed (OR, 0.90; 95% CI, 0.78–1.04; P = 0.16).
TABLE 4.
EFFECT OF STATIN USE ON OCS DISPENSING, ASTHMA-RELATED ED VISITS, AND HOSPITALIZATIONS AMONG ICS USERS
| Statin Users | Non–Statin Users | OR* (95% CI) | P Value* | |
|---|---|---|---|---|
| Two or more OCS dispensings, % (n) |
14.4 (442) |
15.7 (483) |
0.90 (0.78–1.04) |
0.16 |
| Asthma-related ED visit, % (n) |
3.7 (112) |
4.7 (145) |
0.74 (0.58–0.96) |
0.03 |
| Asthma-related hospitalization, % (n) | 1.4 (42) | 1.5 (47) | 0.93 (0.60–1.46) | 0.76 |
Definition of abbreviations: CI = confidence interval; ED = emergency department; ICS = inhaled corticosteroids; OCS = oral corticosteroids; OR = odds ratio.
Adjusted for unbalanced covariates after propensity score matching.
Discussion
Our study has two key findings. First, we found that among patients with asthma, statin users were less likely to experience asthma-related ED visits. Second, statin users had decreased odds of filling two or more prescriptions of OCS. Furthermore, among subjects on ICS therapy, statin use was associated with decreased odds of asthma-related ED visits.
The association between statin use and decreased asthma exacerbations in our study is in accordance with the findings of recent observational studies. A recent study using the Mississippi Medicaid data found a protective effect of prior statin exposure on asthma-related ED visits and hospitalization in patients with asthma on ICS (20). Methodologically, this study differed from ours because statin exposure was assessed over the 6 months before the index date and the asthma outcomes assessed over the 12 months post index date. The temporal relationship between statin use and its potential benefits on asthma is unclear and future studies are needed to clarify this relationship. Compared with our subjects, this population was also older (62.6 [SD, 12.1] yr). Using a nationwide insurance database in Taiwan, Huang and coworkers (11) found that statin use was associated with a lower incidence of hospitalization in patients with asthma. It is important to note that their population is not directly comparable with ours and thus their findings may not be generalizable. Statin users in the analysis by Huang and coworkers were older (60.7 [SD, 0.4] vs. 53.9 [9.3] in our study), had a lower prevalence of ICS use (11.5% vs. 36.8%), and a higher OCS use (56.9% vs. 13.5%), the latter suggesting that the Taiwan study subjects had worse asthma control. Thus, by studying a different, more heterogeneous population, our study adds to the existing literature on the effects of statins on adverse asthma events.
Our findings contrast with several recent randomized trials assessing the effects of statins in patients with asthma, which demonstrated limited benefits of statins. Hothersall and coworkers (12) found no difference in mean morning PEF between the atorvastatin and placebo treatment periods in atopic subjects with asthma already on an ICS. A similar study that randomized subjects on ICS to simvastatin or placebo supported an additive action of simvastatin and ICS in the reduction of sputum eosinophils (13), whereas others showed no steroid-sparing effect of simvastatin (14) and no improvement in PEF with atorvastatin compared with placebo, or with atorvastatin and ICS compared with ICS alone (15). Most of these trials had small sample sizes and a short treatment period and the studies did not have the power to examine the effect of statins on asthma exacerbations.
A strength of our study is the use of multivariate and propensity score matching to minimize confounding by indication. Before multivariate and propensity score matching, our results suggested that statin users and non–statin users had similar odds of experiencing ED visits and filling OCS even after adjusting for comorbid illnesses. Nevertheless, because statin users were more likely than non–statin users to have comorbid illnesses that could lead to asthma exacerbations, we conducted multivariate and propensity score matching to adjust for additional confounding by indication. Interestingly, the distribution of propensity scores and other baseline comorbid conditions among statin users differed greatly from non–statin users, reinforcing the notion of confounding by indication in observational studies and the importance of accounting for this confounding to obtain valid results. Matching greatly improved these imbalances, allowing statin users to be more comparable with nonusers (Figure 3). We used a multivariate and propensity score matching approach to adjust for multiple covariates simultaneously by the propensity score, and at the same time match exactly on several preselected important characteristics (e.g., age groups, year and season of index date). Compared with other propensity score-based methods, propensity score matching automatically addresses the issue of poor covariate overlap because subjects that do not have comparable control subjects with similar propensity scores will fail to find matched control subjects and will be excluded from subsequent analyses (21, 22). We further adjusted for unbalanced covariates in the conditional logistic regression after matching. These steps eliminated the observed systematic differences between statin users and non–statin users and allowed for direct comparison of the treated and untreated in the matched sample with respect to outcomes (18).
Several limitations in this study deserve mention. First, in our study, multivariate and propensity score matching was successful in about 60% of the statin users and resulted in 8,000 matched pairs for analysis. Although the analysis of this subgroup of statin users may limit the generalizability of our findings, this technique allowed us to make valid conclusions on the association between statins and asthma exacerbations. Second, the sensitivity analysis suggests that our findings are somewhat sensitive to unmeasured bias. The associations between statins and OCS dispensing, and statins and ED visits became statistically nonsignificant with a sensitivity parameter less than 1.5. This suggests that despite our best efforts to adjust for potential confounders using multivariate and propensity score matching, there may be residual unmeasured confounding, a limitation inherent to observational studies. Thus, further prospective studies are needed to confirm these findings. Third, only statin users with an MPR of 80% or greater were included in this study. Although this may provide information on the efficacy of statins on asthma exacerbations, this subgroup of statin users may represent individuals who are more adherent to therapies and thus limit the generalizability of our results for less frequent statin users. Future studies are also needed to explore the dose effect of statins on asthma outcomes. Finally, asthma is a heterogeneous condition (23). However, we did not analyze subgroups of asthma in this study, because it is difficult to accurately differentiate asthma subtypes, such as atopic or allergic asthma, based on the claims-based data. We do not have biomarker data and several medications used for allergies can be purchased over-the-counter. Patients on anti-IgE therapy, such as omalizumab, were also excluded. Previous studies using murine models that have shown an effect from statins have mainly focused on atopic or allergic asthma. It is possible that statins benefit only a subgroup of patients with asthma. In this cohort, about half of the statin users were on an asthma medication (38.7% on an ICS), reflecting a mild-to-moderate population with asthma. Subjects on chronic OCS therapy were also excluded because OCS may be used for medical conditions other than asthma. This may concomitantly affect asthma control and introduce bias. By doing so, we may have excluded a minority of subjects with severe asthma on chronic OCS. Although further studies are needed to explore whether the statins also benefit subjects with more severe asthma, it is interesting to note that similar results were found in populations using more (20) and less (11) ICS. Furthermore, a subgroup analysis among ICS users in our cohort showed a protective association between statin user and asthma-related ED visits. This suggests that statins may have beneficial effects across different degrees of asthma severity. Finally, given the administrative nature of our database, there is a potential misclassification of asthma-related events using ICD-9 codes and of statin exposure (e.g., we did not have access to medication adherence data).
In conclusion, in a large population of patients with asthma, we found that statin exposure was associated with decreased OCS dispensings and asthma-related ED visits. Our study also demonstrated the importance of adjusting for confounding by indication in observational studies, as evidenced by the differences in baseline characteristics between statin users and non–statin users in our population. The use of detailed multivariate and propensity score matching allowed for a more valid comparison of statin users and non–statin users. Future randomized controlled trials specifically designed to examine the effect of statins on asthma exacerbations are needed to confirm these findings.
Acknowledgments
Acknowledgment
The authors are indebted to the Tennessee Bureau of TennCare of the Department of Finance and Administration, and the Tennessee Department of Health, Office of Policy, Planning & Assessment, for providing the TennCare Medicaid data.
Footnotes
Supported by NHLBI (R01 HS019669; PI: S. B. Soumerai). A.W. was supported by K08 HL088046-01A2 from NHLBI.
Author Contributions: All authors made substantial contributions to conception and design. M.G.B., V.F., E.O.K., E.K.L., W.M.V., D.R., T.L., and A.W. made substantial contributions to the acquisition of data. S.M.T., L.L., I.M., and A.W. made substantial contributions to the analysis and interpretation of data. All authors made substantial contributions to the critical revision of the manuscript for important intellectual content and to the final approval of the version to be published.
Originally Published in Press as DOI: 10.1164/rccm.201306-1017OC on October 4, 2013
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dinarello CA. Anti-inflammatory agents: present and future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vandermeer ML, Thomas AR, Kamimoto L, Reingold A, Gershman K, Meek J, Farley MM, Ryan P, Lynfield R, Baumbach J, et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis. 2012;205:13–19. doi: 10.1093/infdis/jir695. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Le W, Ahuja R, Cho DY, Hwang PH, Upadhyay D. Inhibition of inflammatory mediators: role of statins in airway inflammation. Otolaryngol Head Neck Surg. 2011;144:982–987. doi: 10.1177/0194599811400367. [DOI] [PubMed] [Google Scholar]
- 4.Zeki AA, Kenyon NJ, Goldkorn T. Statin drugs, metabolic pathways, and asthma: a therapeutic opportunity needing further research. Drug Metab Lett. 2011;5:40–44. doi: 10.2174/187231211794455217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masoli M, Fabian D, Holt S, Beasley R Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 6.Chiba Y, Arima J, Sakai H, Misawa M. Lovastatin inhibits bronchial hyperresponsiveness by reducing RhoA signaling in rat allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2008;294:L705–L713. doi: 10.1152/ajplung.00531.2007. [DOI] [PubMed] [Google Scholar]
- 7.Zeki AA, Franzi L, Last J, Kenyon NJ. Simvastatin inhibits airway hyperreactivity: implications for the mevalonate pathway and beyond. Am J Respir Crit Care Med. 2009;180:731–740. doi: 10.1164/rccm.200901-0018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad T, Mabalirajan U, Sharma A, Aich J, Makhija L, Ghosh B, Agrawal A. Simvastatin improves epithelial dysfunction and airway hyperresponsiveness: from asymmetric dimethyl-arginine to asthma. Am J Respir Cell Mol Biol. 2011;44:531–539. doi: 10.1165/rcmb.2010-0041OC. [DOI] [PubMed] [Google Scholar]
- 9.Zeki AA, Bratt JM, Rabowsky M, Last JA, Kenyon NJ. Simvastatin inhibits goblet cell hyperplasia and lung arginase in a mouse model of allergic asthma: a novel treatment for airway remodeling? Transl Res. 2010;156:335–349. doi: 10.1016/j.trsl.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imamura M, Okunishi K, Ohtsu H, Nakagome K, Harada H, Tanaka R, Yamamoto K, Dohi M. Pravastatin attenuates allergic airway inflammation by suppressing antigen sensitisation, interleukin 17 production and antigen presentation in the lung. Thorax. 2009;64:44–49. doi: 10.1136/thx.2007.094540. [DOI] [PubMed] [Google Scholar]
- 11.Huang CC, Chan WL, Chen YC, Chen TJ, Chou KT, Lin SJ, Chen JW, Leu HB. Statin use in patients with asthma: a nationwide population-based study. Eur J Clin Invest. 2011;41:507–512. doi: 10.1111/j.1365-2362.2010.02434.x. [DOI] [PubMed] [Google Scholar]
- 12.Hothersall EJ, Chaudhuri R, McSharry C, Donnelly I, Lafferty J, McMahon AD, Weir CJ, Meiklejohn J, Sattar N, McInnes I, et al. Effects of atorvastatin added to inhaled corticosteroids on lung function and sputum cell counts in atopic asthma. Thorax. 2008;63:1070–1075. doi: 10.1136/thx.2008.100198. [DOI] [PubMed] [Google Scholar]
- 13.Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ.Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2, 3-dioxygenase. J Allergy Clin Immunol2010. 126:754–762, e751. [DOI] [PubMed]
- 14.Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Simvastatin in the treatment of asthma: lack of steroid-sparing effect. Thorax. 2010;65:891–896. doi: 10.1136/thx.2010.138990. [DOI] [PubMed] [Google Scholar]
- 15.Braganza G, Chaudhuri R, McSharry C, Weir CJ, Donnelly I, Jolly L, Lafferty J, Lloyd SM, Spears M, Mair F, et al. Effects of short-term treatment with atorvastatin in smokers with asthma—a randomized controlled trial. BMC Pulm Med. 2011;11:16. doi: 10.1186/1471-2466-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenbaum P.Observational studies. New York: Springer; 2002 [Google Scholar]
- 17.Sekhon J.Multivariate and propensity score matching software with automated balance optimization: the matching package for R J Stat Softw 201142(7)1–52. [Google Scholar]
- 18.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, McNeil BJ. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 20.Lokhandwala T, West-Strum D, Banahan BF, Bentley JP, Yang Y.Do statins improve outcomes in patients with asthma on inhaled corticosteroid therapy? A retrospective cohort analysis. BMJ Open2012. 2:e001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin PC.The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Making2009. 29:661–677. [DOI] [PubMed]
- 22.Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, Robins JM. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163:262–270. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 23.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D’Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]