Abstract
Background
Large multispecialty physician group practices, with a central role for primary care practitioners, have been shown to achieve high-quality, low-cost care for patients with chronic disease. We assessed the extent to which informal multispecialty physician networks in Ontario could be identified by using health administrative data to exploit natural linkages among patients, physicians, and hospitals based on existing patient flow.
Methods
We linked each Ontario resident to his or her usual provider of primary care over the period from fiscal year 2008/2009 to fiscal year 2010/2011. We linked each specialist to the hospital where he or she performed the most inpatient services. We linked each primary care physician to the hospital where most of his or her ambulatory patients were admitted for non-maternal medical care. Each resident was then linked to the same hospital as his or her usual provider of primary care. We computed “loyalty” as the proportion of care to network residents provided by physicians and hospitals within their network. Smaller clusters were aggregated to create networks based on a minimum population size, distance, and loyalty. Networks were not constrained geographically.
Results
We identified 78 multispecialty physician networks, comprising 12 410 primary care physicians, 14 687 specialists, and 175 acute care hospitals serving a total of 12 917 178 people. Median network size was 134 723 residents, 125 primary care physicians, and 143 specialists. Virtually all eligible residents were linked to a usual provider of primary care and to a network. Most specialists (93.5%) and primary care physicians (98.2%) were linked to a hospital. Median network physician loyalty was 68.4% for all physician visits and 81.1% for primary care visits. Median non-maternal admission loyalty was 67.4%. Urban networks had lower loyalties and were less self-contained but had more health care resources.
Interpretation
We demonstrated the feasibility of identifying informal multispecialty physician networks in Ontario on the basis of patterns of health care–seeking behaviour. Networks were reasonably self-contained, in that individual residents received most of their care from providers within their respective networks. Formal constitution of networks could foster accountability for efficient, integrated care through care management tools and quality improvement, the ideas behind “accountable care organizations.”
According to widespread evidence, quality of care for patients with chronic disease is suboptimal,1-3 with large variations in the provision of evidence-based services.3-8 Serious gaps in the quality of chronic disease management have been attributed to poor coordination and fragmentation of care.1,2,9-11 Effective chronic disease management requires coordinated, longitudinal care and the engagement of multidisciplinary teams across different sectors.11-13 Large multispecialty physician group practices have been shown to achieve high-quality, low-cost care. Through better ambulatory management of patients’ care, such practices reduce complications and avoid costly readmissions to the hospital and emergency department (ED).13-19
The development of multidisciplinary accountable care organizations in the United States has illustrated the central role of primary care.20 Strong primary care systems are associated with higher quality of care for chronic disease, including increased preventive care and fewer avoidable ED visits and hospital admissions.21-24 Ontario leads other Canadian provinces in deploying a range of primary care models, such as Family Health Teams, using financial incentives to reward continuity and comprehensiveness of care, after-hours coverage, and electronic medical records.25-28 However, primary care reform has paid little attention to integrating specialists and hospitals into the management of patients with chronic disease.
The current health care structure and payment system is focused on acute care and is poorly aligned with the needs of patients who have chronic disease. To incentivize health care providers to offer integrated, coordinated care and to promote collective accountability will require a major reorganization of traditional health care delivery and payment systems.11-13
Although formal multispecialty physician networks are uncommon in Canada, health care providers tend to form informal networks based on the sharing of patients and information. These “virtual” networks, behaving as informal, organic, “self-organizing” systems that consist of primary care physicians, specialists, and the hospitals where their patients are admitted, have likely developed naturally through patients’ travel patterns, long-standing referral patterns, information-sharing, and admission of patients to local hospitals.
Our objective was to identify naturally occurring multispecialty physician networks in Ontario by using health administrative data to exploit linkages among patients, physicians, and hospitals based on existing patient flow and to characterize these networks in terms of population and physician characteristics.
We postulated that this “natural” model of physician organization would reflect the way in which primary care physicians, specialists, and hospitals actually practise together to care for a defined population and that such a structure would permit more accurate and robust evaluation of quality and costs of care. Our overarching goals are to identify which networks provide high-quality, low-cost care and to examine the factors contributing to their efficiency. Understanding what constitutes a high-performing system provides an opportunity to focus policy reforms on promoting and sustaining the best systems.29
Methods
We identified physician networks with the idea of defining “medical neighbourhoods” for chronic disease care.30 A medical neighbourhood consists of primary care physicians (representing patients’ medical homes) and the specialists and hospitals from which their patients receive most of their care. The reason for including hospitals was to foster accountability for hospital readmissions among the multiple providers who might take responsibility for reducing the rates of such events.31,32 While improving the quality of chronic disease care is important, one of the markers for excellent care is avoidance of costly readmissions. Our algorithm for defining networks was similar to that used to derive networks in the United States33,34 but was adapted to reflect the Ontario health care system.
Data sources
Multiple Ontario health administrative databases containing information on all publicly insured hospital and physician services were linked using unique, anonymized, encrypted identifiers for patients and physicians. These databases were the Discharge Abstract Database (available through the Canadian Institute for Health Information) for hospital admissions, procedures and transfers; the National Ambulatory Care Reporting System for ED visits; the Ontario Health Insurance Plan (OHIP) database of physician billings for type and location of service, diagnosis codes, and procedures; the Ontario Drug Benefits Plan for outpatient drug prescriptions for those over 65 years of age; the provincial Registered Persons Database for patient demographic information and deaths; the provincial Client Agency Program Enrolment registry to identify rostered patients and primary care models; the Ontario Physician Workforce Database35 and the Institute for Clinical Evaluative Sciences Physician Database to identify physician activity status, self-designated and functional specialties, and full-time equivalency. Neighbourhood income was derived from Statistics Canada 2006 census estimates. We used the 2008 Rurality Index of Ontario (RIO) to measure rurality, accounting for population size and travel time.36 These data sets were held securely in a linked, de-identified form and were analyzed at the Institute for Clinical Evaluative Sciences.
Eligible residents and physicians
The eligible population consisted of residents of Ontario who were alive and aged less than 100 years on April 1, 2008, including newborns and immigrants who registered with OHIP during the period from fiscal year 2008/2009 to fiscal year 2010/2011, excluding those who had had no contact with the health care system since 2000/2001. Ontario physicians with a valid physician number were eligible for inclusion if they had been in active practice during the period of interest (2008/2009 to 2010/2011), defined as having an active Client Agency Program Enrolment patient roster or at least one OHIP billing. All acute care inpatient institutions, including general, cardiac, children’s, and psychiatric hospitals, were eligible.
Linkage of residents to usual providers of primary care
Primary care physicians were those whose self-designated or functional specialty was general practice, family practice, or community medicine. Pediatricians were considered primary care providers if they billed more than 30 well-baby or well-child visits between 2008/2009 and 2010/2011.37 In the spirit of defining medical homes, we linked Ontario residents to their usual providers of primary care in the following hierarchical manner. Each resident was linked to the primary care physician to whom he or she had been rostered at the midpoint of the period, because that physician was contractually responsible for the resident’s primary care; any rostered resident who died before the midpoint was linked to his or her last primary care physician. Each nonrostered resident was linked to the primary care physician who provided most of the resident’s core primary care services, on the basis of the billing codes used by the Ontario Ministry of Health and Long-Term Care to determine virtual patient rosters (Appendix A). Each remaining unlinked resident was linked to the primary care (preferentially) or other physician who provided the greatest number of ambulatory services, including visits, laboratory tests, diagnostic tests, and prescriptions, counting each type of service once per patient per day. Any resident who had no ambulatory contact with a physician was not linked to a usual provider of primary care.
Linkage of physicians to hospitals
We linked each specialist to the acute care hospital where he or she had provided the highest volume of inpatient services, including visits, procedures, imaging, and diagnostic tests billed during the admission. We did not include same-day surgery or ED visits. Several specialists could provide services to the same inpatient.
Each primary care physician was linked to the hospital where most of his or her patients (defined according to the linkage between residents and usual providers of primary care, described above) were admitted. We reasoned that this hospital would be responsible for discharge planning for the primary care physician’s patients. We used non-maternal medical admissions for hospital assignment, since the focus for patients with chronic disease is avoidable medical admissions, whereas surgery is often regionalized and maternal admissions are not avoidable. Each admitted patient was counted once, to ensure that those who were admitted more frequently would not bias the assignment; for those admitted to more than one hospital, we weighted each admission proportionally to the total number of admissions for that patient. Each unlinked rostering primary care physician was linked to the hospital where most physicians in his or her primary care model were assigned. Each unlinked specialist was linked to the hospital where most of his or her ambulatory patient panel was admitted.
Linkage of residents to hospitals
Each Ontario resident was linked to the hospital where his or her usual provider of primary care was assigned, as this was likely to be the hospital responsible for the patient’s discharge planning. Each unlinked resident who was admitted to a hospital or seen in the ED during the period 2008/2009 to 2010/2011 was directly linked to that hospital.
Aggregation of provider clusters to form networks
A provider cluster comprised the population and physicians linked to a particular hospital. We combined provider clusters to derive multispecialty physician networks, aggregating small clusters that shared patients and were in close proximity, so that the resulting network had a minimum population size and included at least one medium or large hospital. A network consisted of the combined population, physicians, and hospitals linked to its component provider clusters. A satellite network was a collection of small, rural provider clusters that were geographically distant from the large hospital upon which they depended for complex services. For these satellite networks, the population served and the local services provided were very different from those in the nearest large provider cluster. Depending on the purpose, a satellite network could always be aggregated to the nearest network. See the glossary of terms (Appendix B) for additional detail.
We defined 3 indexes of localization that measured the extent of self-containment of provider clusters and networks. Admission loyalty was defined as the percentage of total admissions within a provider cluster’s (or network’s) population that were to the linked hospital (or to network hospitals). Physician loyalty was defined as the percentage of ambulatory visits by a provider cluster’s (or network’s) population that were to physicians linked to the cluster (or network). Primary care loyalty was defined as the percentage of ambulatory visits by a provider cluster’s (or network’s) population that were to primary care physicians linked to the cluster (or network) (Appendix B). High loyalty implied that network providers delivered most of the care for their assigned population.
To aggregate provider clusters, we reviewed the top 4 admission and physician loyalties of each cluster’s population to itself and other clusters to determine where the population was primarily seeking care. We computed travel times between centroids of hospital postal codes with a geographic information system (GIS) that used posted speed limits on the road network. Using a combination of high admission and physician loyalty, indicating shared patients, close geographic proximity, and GIS mapping, we aggregated small provider clusters to form networks of minimum population size 50 000, where possible, respecting hospital governance structures. The minimum population size was based on having sufficient patients to accurately measure network performance for chronic diseases with prevalence less than 10% and being sufficiently large to provide a range of health care services and to implement system improvements.29
Table 1 shows an example of aggregating provider cluster A, which had 27 473 patients (fewer than the specified minimum), with a larger cluster. Provider cluster A’s admission loyalty to itself was moderate (53.8%), since it was small and had few specialists (n = 4), so that patients were admitted to larger nearby hospitals for serious conditions. Hospital B was a large tertiary care centre and was further away than hospital C but was linked to hospital A by a major highway. Provider cluster A’s residents received more services from cluster B than cluster C, as evidenced by a higher percentage of admissions and ambulatory visits to providers in cluster B. We therefore aggregated provider cluster A with provider cluster B.
Table 1.
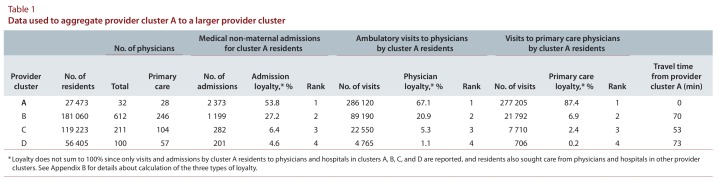
Data used to aggregate provider cluster A to a larger provider cluster
Characteristics of physician networks
We report network population characteristics, in terms of the median and 10th and 90th percentiles, weighted by network population. Characteristics include network size, loyalty, distance between residents and providers, socio-demographic information, prevalence of chronic diseases (including diabetes mellitus, acute myocardial infarction, congestive heart failure, hypertension, chronic obstructive pulmonary disease, and asthma) based on chart-validated algorithms,4,38-42 and primary care model affiliation.26,43
Physician supply was calculated as full-time equivalents (FTEs) per capita network population. FTEs were calculated using total physician payments from all sources, assigning an FTE of 1.00 to physicians who fell between the 40th and 60th percentiles of their specialty.44,45 Primary care continuity was measured as the proportion of ambulatory visits that were with a resident’s usual provider of primary care.5
Ethics approval
The study was approved by the Research Ethics Board of Sunnybrook Health Sciences Centre.
Results
We began with 12 971 629 Ontario residents (including 359 963 immigrants and 435 381 newborns), 27 437 physicians, and 175 acute care hospitals that were eligible to be linked to a network. Overall, 97.5% of primary care physicians (11 888 of 12 193) and 100% of primary care pediatricians (n = 373) were designated as usual providers of primary care. A total of 12 845 793 (99.0%) of the residents were linked to a usual provider of primary care; of these, 70.8% were linked through rostering, 27.1% through use of core primary care services, and 2.1% through other physician services. We were unable to link 125 836 (< 1%) of eligible residents to a usual provider of primary care because they had received no ambulatory physician services over the 3-year period.
The majority of specialists (13 904 of 14 871 [93.5%]) were linked to a hospital through their hospital activity, and the majority of primary care physicians, including primary care pediatricians (12 340 out of 12 566 [98.2%]), were linked to a hospital through admissions of the patients for whom they were the usual providers of primary care. An additional 70 primary care physicians were linked to a hospital through their primary care model, and 783 specialists were linked to a hospital through admissions of their ambulatory patient panel. Most residents (98.8%) were linked to a provider cluster and therefore to a network. For the majority (92.0%) of specialists, inpatient activity was in one hospital. Admissions of patients linked to an individual primary care provider were often to several hospitals, but on average, more than half of admissions for a particular provider (58.0%) were to one hospital. Primary care physicians who could not be linked typically had small, healthy patient panels; since the networks were created to foster chronic disease care, these physicians remained unlinked.
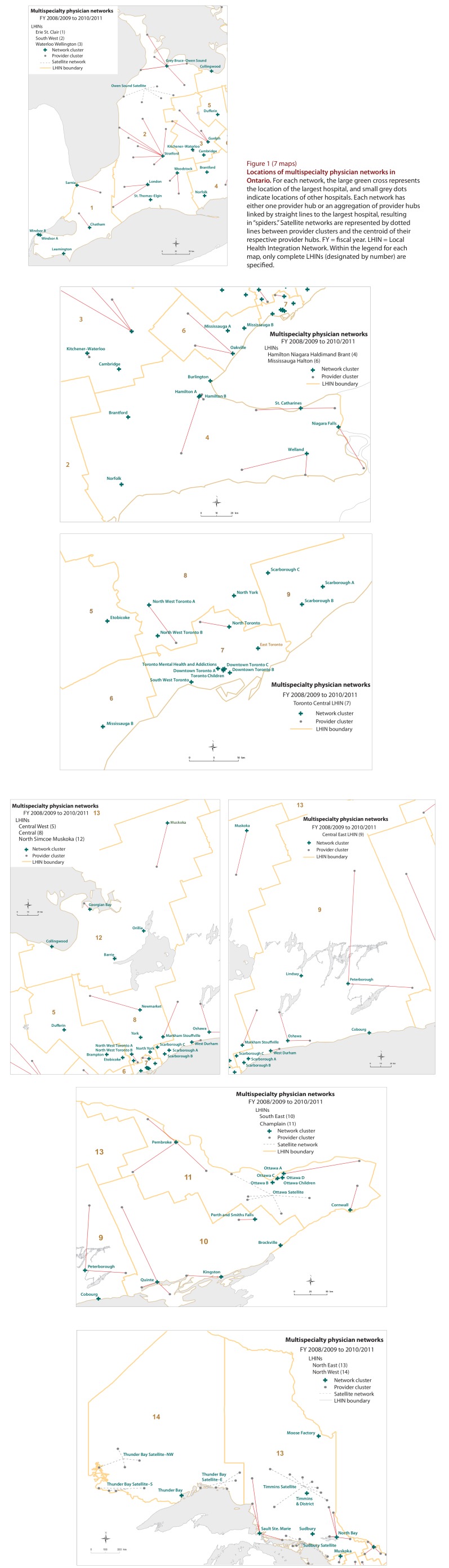
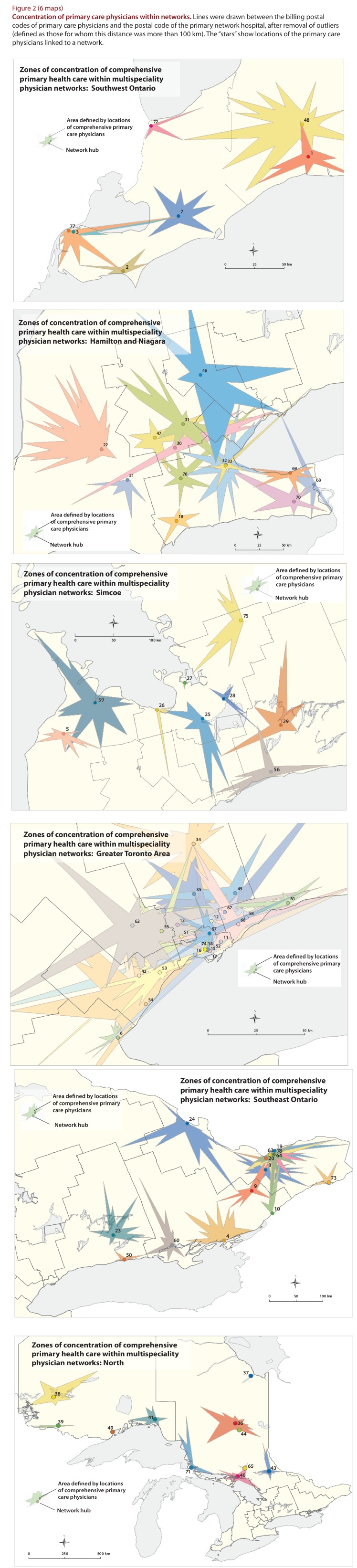
We began the next stage with 175 provider clusters, each containing one acute care hospital, which were aggregated into 78 multispecialty physician networks. The final networks comprised 12 410 primary care physicians, including primary care pediatricians, and 14 687 specialists serving 12 917 178 people. Some networks (n = 38) were large and comprised only one hospital. Other networks (n = 33) comprised more than 1 hospital and appear as “spiders” in Figure 1. There were 7 satellite networks. Although most hospitals in a network were located inside boundaries of the relevant Local Health Integration Network (LHIN), this was not true of the primary care physicians or the population served. Figure 2, which plots the envelope of primary care providers linked to each network on the basis of physicians’ billing postal codes, shows that residents did not seek care within distinct LHIN boundaries and that there was substantial geographic overlap among network catchment areas in urban areas.
Figure 1.
Locations of multispecialty physician networks in Ontario
Figure 2.
Concentration of primary care physicians within networks
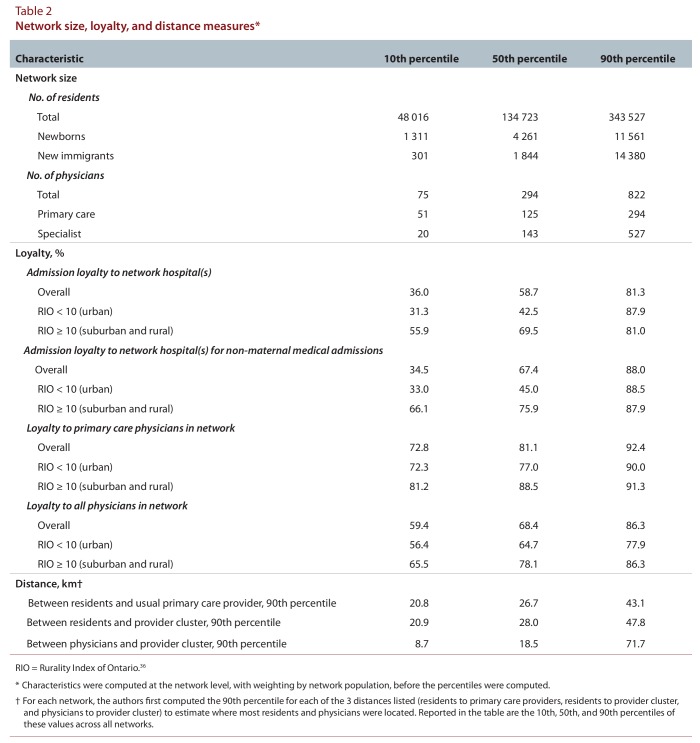
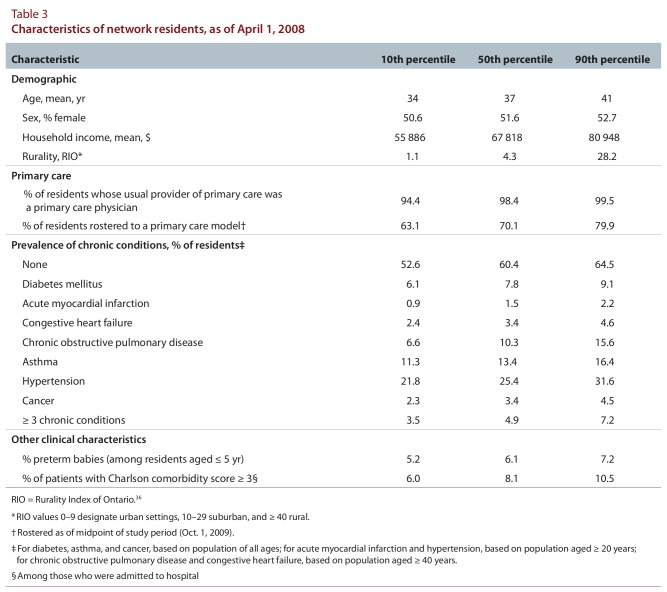
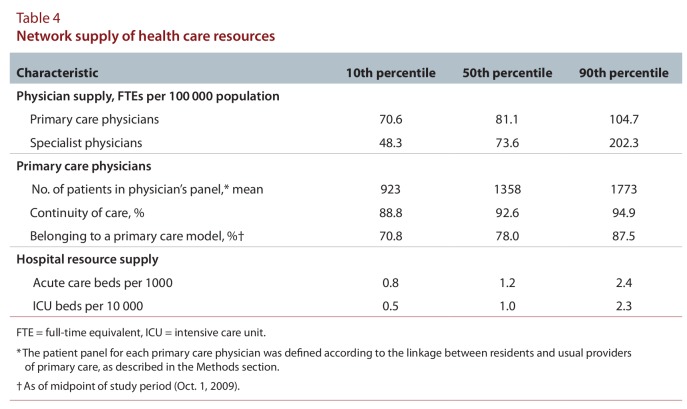
The numbers of residents and physicians linked across networks varied widely (Table 2). In general, admission and physician loyalty were high. Median non-maternal medical admission loyalty was 67.4%. Median network loyalty was 68.4% for all physicians and 81.1% for primary care physicians. Urban networks had large populations, many primary care physicians, and large numbers of specialists, hospital beds, and ICU beds (data not shown), with residents living close to their providers; however, physician and admission loyalties were low. Northern rural networks (RIO ≥ 40) were small, with few specialists, hospital beds, and ICU beds; they had similar loyalties to suburban networks (RIO 10–39). Distances between residents and providers were generally short; 99.1% of primary care physicians practised less than 100 km from their respective network hubs. The prevalence of chronic conditions varied about 2-fold across networks (Table 3). There was modest variability in the proportion of patients rostered to a primary care model. The supply of primary care physicians varied about 1.5-fold, and specialist supply about 4-fold, across networks (Table 4). The supply of acute care and intensive care beds varied 3- to 5-fold.
Table 2.
Network size, loyalty, and distance measures
Table 3.
Characteristics of network residents, as of April 1, 2008
Table 4.
Network supply of health care resources
Interpretation
We identified and characterized informal, multispecialty physician networks in Ontario, which can be viewed as “self-organizing systems of care” that collectively serve their large panels of patients. Physicians in these networks were associated by virtue of sharing care for common patients and admitting patients to the same hospital. Not every physician shared patients or consulted with every other physician in their network, but collectively they shared important resources such as acute care beds, specialists, and inpatient medical technologies that affect patients’ outcomes. Networks were not constrained geographically but were based on where patients sought care, since health care is not delivered according to geographic boundaries. These informal networks provide a snapshot of how care was organized during this period, rather than prescribing how it should be organized.
The main strength of this work lies in its high face validity, based on high loyalty and close proximity among patients and providers. The other main strength is that it permits the identification of a target population (denominator) for reporting quality and performance rates. Most networks had sufficient patients with chronic disease to measure performance and outcomes. Primary care loyalty was high, because such care was provided locally. Physician loyalty was moderately high, since patients could be referred to specialists outside their network; nevertheless, care was generally concentrated among local providers since primary care physicians often refer to the specialists working in the local hospitals where their patients are admitted. Admission loyalty was lower for urban networks, since patients may be admitted to and physicians may have privileges at many nearby hospitals. Other researchers have created physician networks on the basis of shared patients, but they did not aggregate provider clusters to networks, and some did not include hospitals or residents less than 65 years of age.33,46,47
The primary limitation of this study was the fluid nature of health care–seeking behaviour. We linked residents and physicians on the basis of average utilization patterns over 3 years; however, patients may have switched primary care physicians, and both patients and physicians may have moved over this period. Nonetheless, as long as there are no major hospital openings or closures, or changes to workforce policy, the basic network organization should persist. In fact, we applied the same method over the period 2005/2006 to 2007/2008 and obtained similar networks. Another limitation was the possibility that the patients linked to individual primary care physicians were admitted to more than one hospital, so the specificity of assignment to a single hospital was only moderate in urban areas; however, this is the nature of patient flow in large urban areas. We could not link long-term care, Community Care Access Centres, or public health units to the networks, since we did not have the appropriate data to do so. It will be important to add these services in the future, as they play a support role in the community that may help to reduce hospital admissions and readmissions.
This work is aligned with a systems-minded approach to providing chronic disease care and promoting accountability.29,48 The overarching aim is to explore virtual multispecialty physician networks as functional and organizational units for chronic disease care and to better understand systems factors and strategies associated with efficient networks. Large multispecialty physician networks may be the most practical level for targeting reforms for integration of care, since providers are already connected by virtue of caring for the same patients. Such networks comprise large, multidisciplinary groups that work together to manage patients with chronic disease and might be more conducive to evaluation, system interventions, and physician accountability frameworks. These networks could provide the context and information to engage hospitals, physicians, interdisciplinary care providers, and policy-makers in discussions of how to better align medical practice with evidence and how to address resource use and integration across hospital and ambulatory sectors.
It will be no small task to exploit these networks for the purpose of improving care. There is currently no accountability framework that encompasses hospitals, specialists and primary care physicians, and interdisciplinary care providers. The current payment system is a patchwork of global budgets for hospitals, fee for service, capitation, and incentives for primary care, with fee-for-service and alternate funding plans for specialists; none of these modes of payment are aligned with multidisciplinary physician practices. In addition, regional jurisdiction over primary care may be at odds with the locations where patients seek care and where their providers are located. Ontario has recently shown a willingness to embrace new structural models through the creation of a set of pilot Health Links that, in some respects, resemble these networks, to improve care for complex high-needs patients and to reduce avoidable readmissions to hospital.49 The developments in Ontario may provide a nascent organizational structure for vertically integrated networks.
In summary, formal constitution of multispecialty physician networks around existing patterns of patient flow could foster accountability for efficient, integrated care, investment in electronic medical records, care coordination, performance measurement, care management tools, and quality improvement, the concepts behind “accountable care organizations.”29
Acknowledgments
We thank Dr. Julie Bynum for methodological assistance, Alex Kopp for programming assistance, and Ashley Corallo and Nancy MacCallum for administrative and technical support.
Biographies
Therese A. Stukel, PhD, is a Senior Scientist at the Institute for Clinical Evaluative Sciences, Toronto, Ontario; an Adjunct Professor at the Dartmouth Institute for Health Policy and Clinical Practice, Giesel School of Medicine at Dartmouth, Hanover, New Hampshire; and a Professor at the Institute for Health Policy, Management and Evaluation, University of Toronto, Toronto, Ontario.
Richard H. Glazier, MD, MPH, FCFP, is a Senior Scientist at the Institute for Clinical Evaluative Sciences; a Professor in the Department of Family and Community Medicine and the Dalla Lana School of Public Health, University of Toronto; a Scientist at the Centre for Research on Inner City Health in the Keenan Research Centre of the Li Ka Shing Knowledge Institute, St. Michael’s Hospital; and a Clinician Scientist and Family Physician in the Department of Family and Community Medicine, St. Michael’s Hospital, Toronto, Ontario.
Susan E. Schultz, MA, MSc, is an Epidemiologist at the Institute for Clinical Evaluative Sciences, Toronto, Ontario.
Jun Guan, MSc, is a Lead Analyst at the Institute for Clinical Evaluative Sciences, Toronto, Ontario.
Brandon M. Zagorski, MS, is a Data Analyst at the Institute for Clinical Evaluative Sciences, Toronto, Ontario.
Peter Gozdyra, MA, is a Medical Geographer at the Institute for Clinical Evaluative Sciences and the Centre for Research on Inner City Health in the Keenan Research Centre of the Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Toronto, Ontario.
David A. Henry, MBChB, FRCPE(Edin), is the CEO of the Institute for Clinical Evaluative Sciences and a Professor in the Department of Medicine, University of Toronto, Toronto, Ontario.
Appendices
Appendix A.
Comprehensive primary care codes
Appendix B.
Glossary of terms
Footnotes
Competing interests: None declared.
Funding source: Funding was provided by an Emerging Team Grant (ETG92248) in Applied Health Services and Policy Research from the Canadian Institutes of Health Research. The funding agency had no role in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the report for publication. This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
Therese A. Stukel, Richard H. Glazier, Susan E. Schultz, and David A. Henry contributed to the conception and design of the study. Therese Stukel was responsible for the acquisition of data; Susan Schultz, Jun Guan, and Brandon Zagorski performed the analyses; and Peter Gozdyra performed all geographic information system mapping. Statistical expertise was provided by Therese Stukel, Jun Guan, and Brandon M. Zagorski. Therese Stukel, Richard Glazier, Susan Schultz, and David Henry interpreted the results. Therese Stukel drafted the manuscript. All authors critically reviewed the manuscript and approved the final version submitted for publication. Therese Stukel obtained funding for the study and, along with Richard Glazier and Susan Schultz, provided overall supervision. Therese Stukel is the study guarantor.
References
- 1.Institute of Medicine, Committee on Quality of Health Care in America. Crossing the quality chasm: a new health system for the 21st century. Washington (DC): National Academies Press; 2001. [Google Scholar]
- 2.McGlynn Elizabeth A, Asch Steven M, Adams John, Keesey Joan, Hicks Jennifer, DeCristofaro Alison, Kerr Eve A. The quality of health care delivered to adults in the United States. N Engl J Med. 2003 Jun 26;348(26):2635–2645. doi: 10.1056/NEJMsa022615. http://www.nejm.org/doi/abs/10.1056/NEJMsa022615?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov. [DOI] [PubMed] [Google Scholar]
- 3.2008 report on Ontario’s health system. Toronto (ON): Ontario Health Quality Council; 2008. http://www.ohqc.ca/pdfs/ohqc_2008_report_-_english.pdf. [Google Scholar]
- 4.Hux Janet E, Ivis Frank, Flintoft Virginia, Bica Adina. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512–516. doi: 10.2337/diacare.25.3.512. http://www.scholaruniverse.com/ncbi-linkout?id=11874939. [DOI] [PubMed] [Google Scholar]
- 5.Jaakkimainen L, Upshur R E G, Klein-Geltink J E, Leong A, Maaten S, Schultz S E, Wang L, editors. Primary care in Ontario: ICES atlas. Toronto (ON): Institute for Clinical Evaluative Sciences; 2006. http://www.ices.on.ca/file/PC_atlas_prelims_complete.pdf. [Google Scholar]
- 6.Broemeling Anne Marie, Watson Diane E, Prebtani Farrah. Population patterns of chronic health conditions, co-morbidity and healthcare use in Canada: implications for policy and practice. Healthc Q. 2008;11(3):70–76. doi: 10.12927/hcq.2008.19859. http://www.longwoods.com/product.php?productid=19859. [DOI] [PubMed] [Google Scholar]
- 7.Bierman A S, editor. Project for an Ontario women’s health evidence-based report: volume 1. Toronto (ON): St. Michael’s Hospital and Institute for Clinical Evaluative Sciences; 2009/10. http://powerstudy.ca/power-report/volume1/ [Google Scholar]
- 8.Bierman A S, editor. Project for an Ontario women’s health evidence-based report: volume 2. Toronto (ON): Institute for Clinical Evaluative Sciences and St. Michael’s Hospital; 2010. http://powerstudy.ca/power-report/volume2. [Google Scholar]
- 9.Coleman Eric A, Smith Jodi D, Raha Devbani, Min Sung Joon. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005 Sep 12;165(16):1842–1847. doi: 10.1001/archinte.165.16.1842. http://www.scholaruniverse.com/ncbi-linkout?id=16157827. [DOI] [PubMed] [Google Scholar]
- 10.Glazier R H, Moineddin R, Agha M M, Zagorski B, Hall R, Manuel D G, Sibley L, Kopp A. The impact of not having a primary care physician among people with chronic conditions. Toronto (ON): Institute for Clinical Evaluative Sciences; 2008. http://www.ices.on.ca/webpage.cfm?site_id=1&org_id=31&morg_id=0&gsec_id=0&item_id=4903. [Google Scholar]
- 11.Institute of Medicine, Committee on Redesigning Health Insurance Performance Measures, Payment and Performance Improvement Programs. Performance measurement: accelerating improvement. Washington (DC): National Academies Press; 2006. [Google Scholar]
- 12.Crosson F J. The delivery system matters. Health Aff (Millwood) 2005;24(6):1543–1548. doi: 10.1377/hlthaff.24.6.1543. http://content.healthaffairs.org/cgi/doi/10.1377/hlthaff.24.6.1543. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence David M. Chronic disease care: rearranging the deck chairs. Ann Intern Med. 2005 Sep 20;143(6):458–459. doi: 10.7326/0003-4819-143-6-200509200-00010. [DOI] [PubMed] [Google Scholar]
- 14.Rich M W, Beckham V, Wittenberg C, Leven C L, Freedland K E, Carney R M. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995 Nov 2;333(18):1190–1195. doi: 10.1056/NEJM199511023331806. http://www.nejm.org/doi/abs/10.1056/NEJM199511023331806?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov. [DOI] [PubMed] [Google Scholar]
- 15.Wagner E H, Austin B T, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. http://content.healthaffairs.org/cgi/doi/10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 16.Bodenheimer Thomas, Wagner Edward H, Grumbach Kevin. Improving primary care for patients with chronic illness. JAMA. 2002;288(14):1775–1779. doi: 10.1001/jama.288.14.1775. http://jama.ama-assn.org/cgi/doi/10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 17.Bodenheimer Thomas, Wagner Edward H, Grumbach Kevin. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002 Oct 16;288(15):1909–1914. doi: 10.1001/jama.288.15.1909. http://jama.ama-assn.org/cgi/doi/10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 18.Knowler William C, Barrett-Connor Elizabeth, Fowler Sarah E, Hamman Richard F, Lachin John M, Walker Elizabeth A, Nathan David M Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. http://www.nejm.org/doi/abs/10.1056/NEJMoa012512?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chodosh Joshua, Morton Sally C, Mojica Walter, Maglione Margaret, Suttorp Marika J, Hilton Lara, Rhodes Shannon, Shekelle Paul. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med. 2005 Sep 20;143(6):427–438. doi: 10.7326/0003-4819-143-6-200509200-00007. http://www.scholaruniverse.com/ncbi-linkout?id=16172441. [DOI] [PubMed] [Google Scholar]
- 20.Guterman S, Schoenbaum S C, Davis K, Schoen C, Audet A M J, Stremikis K, Zezza M A. High performance accountable care: building on success and learning from experience. New York (NY): The Commonwealth Fund; 2011. http://www.commonwealthfund.org/Publications/Fund-Reports/2011/Apr/High-Performance-Accountable-Care.aspx?page=all. [Google Scholar]
- 21.Macinko James, Starfield Barbara, Shi Leiyu. The contribution of primary care systems to health outcomes within Organization for Economic Cooperation and Development (OECD) countries, 1970-1998. Health Serv Res. 2003;38(3):831–865. doi: 10.1111/1475-6773.00149. http://europepmc.org/abstract/MED/12822915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gill James M, Mainous Arch G, 3rd, Diamond James J, Lenhard M J. Impact of provider continuity on quality of care for persons with diabetes mellitus. Ann Fam Med. 2003;1(3):162–170. doi: 10.1370/afm.22. http://www.annfammed.org/cgi/pmidlookup?view=long&pmid=15043378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starfield Barbara, Shi Leiyu, Macinko James. Contribution of primary care to health systems and health. Milbank Q. 2005;83(3):457–502. doi: 10.1111/j.1468-0009.2005.00409.x. http://europepmc.org/abstract/MED/16202000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ionescu-Ittu Raluca, McCusker Jane, Ciampi Antonio, Vadeboncoeur Alain Michel, Roberge Danièle, Larouche Danielle, Verdon Josée, Pineault Raynald. Continuity of primary care and emergency department utilization among elderly people. CMAJ. 2007 Nov 20;177(11):1362–1368. doi: 10.1503/cmaj.061615. http://www.cmaj.ca/cgi/doi/10.1503/cmaj.061615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamarche P A, Beaulieu M D, Pineault R, Contandriopoulos A P, Denis J L, Haggerty J. Choices for change: the path for restructuring primary healthcare services in Canada. Ottawa (ON): Canadian Health Services Research Foundation; 2003. Nov, http://www.cfhi-fcass.ca/migrated/pdf/researchreports/commissionedresearch/choices_for_change_e.pdf. [Google Scholar]
- 26.Hutchison Brian, Glazier Richard. Ontario’s primary care reforms have transformed the local care landscape, but a plan is needed for ongoing improvement. Health Aff (Millwood) 2013;32(4):695–703. doi: 10.1377/hlthaff.2012.1087. [DOI] [PubMed] [Google Scholar]
- 27.Glazier Richard H, Klein-Geltink Julie, Kopp Alexander, Sibley Lyn M. Capitation and enhanced fee-for-service models for primary care reform: a population-based evaluation. CMAJ. 2009;180(11):E72–E81. doi: 10.1503/cmaj.081316. http://europepmc.org/abstract/MED/19468106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glazier Richard H, Redelmeier Donald A. Building the patient-centered medical home in Ontario. JAMA. 2010 Jun 2;303(21):2186–2187. doi: 10.1001/jama.2010.753. http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2010.753. [DOI] [PubMed] [Google Scholar]
- 29.Shortell Stephen M, Casalino Lawrence P. Health care reform requires accountable care systems. JAMA. 2008 Jul 2;300(1):95–97. doi: 10.1001/jama.300.1.95. http://www.scholaruniverse.com/ncbi-linkout?id=18594045. [DOI] [PubMed] [Google Scholar]
- 30.Fisher Elliott S. Building a medical neighborhood for the medical home. N Engl J Med. 2008 Sep 18;359(12):1202–1205. doi: 10.1056/NEJMp0806233. http://www.nejm.org/doi/abs/10.1056/NEJMp0806233?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epstein A M. Revisiting readmissions—changing the incentives for shared accountability. N Engl J Med. 2009 Apr 2;360(14):1457–1459. doi: 10.1056/NEJMe0901006. http://www.nejm.org/doi/abs/10.1056/NEJMe0901006?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov. [DOI] [PubMed] [Google Scholar]
- 32.Jencks Stephen F. Defragmenting care. Ann Intern Med. 2010 Dec 7;153(11):757–758. doi: 10.1059/0003-4819-153-11-201012070-00010. http://annals.org/article.aspx?doi=10.7326/0003-4819-153-11-201012070-00010. [DOI] [PubMed] [Google Scholar]
- 33.Bynum Julie P W, Bernal-Delgado Enrique, Gottlieb Daniel, Fisher Elliott. Assigning ambulatory patients and their physicians to hospitals: a method for obtaining population-based provider performance measurements. Health Serv Res. 2007;42(1 Pt 1):45–62. doi: 10.1111/j.1475-6773.2006.00633.x. http://europepmc.org/abstract/MED/17355581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher Elliott S, Staiger Douglas O, Bynum Julie P W, Gottlieb Daniel J. Creating accountable care organizations: the extended hospital medical staff. Health Aff (Millwood) 2006 Dec 5;26(1):w44–w57. doi: 10.1377/hlthaff.26.1.w44. http://content.healthaffairs.org/cgi/pmidlookup?view=long&pmid=17148490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.About OPHRDC. Hamilton (ON): Ontario Physician Human Resources Data Centre; [accessed 2013 Jan. 14]. https://www.ophrdc.org/Public/About.aspx. [Google Scholar]
- 36.Kralj B. Measuring rurality – RIO2008 BASIC: methodology and results. Toronto (ON): Ontario Ministry of Health and Long-Term Care; 2008. [accessed 2013 Jan. 15]. http://www.health.gov.on.ca/english/providers/program/uap/docs/up_rio_methodology.pdf. [Google Scholar]
- 37.Guttmann Astrid, Shipman Scott A, Lam Kelvin, Goodman David C, Stukel Therese A. Primary care physician supply and children’s health care use, access, and outcomes: findings from Canada. Pediatrics. 2010;125(6):1119–1126. doi: 10.1542/peds.2009-2821. http://pediatrics.aappublications.org/cgi/pmidlookup?view=long&pmid=20498170. [DOI] [PubMed] [Google Scholar]
- 38.Austin Peter C, Daly Paul A, Tu Jack V. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144(2):290–296. doi: 10.1067/mhj.2002.123839. http://linkinghub.elsevier.com/retrieve/pii/S0002870302000819. [DOI] [PubMed] [Google Scholar]
- 39.Lee Douglas S, Donovan Linda, Austin Peter C, Gong Yanyan, Liu Peter P, Rouleau Jean L, Tu Jack V. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43(2):182–188. doi: 10.1097/00005650-200502000-00012. http://www.scholaruniverse.com/ncbi-linkout?id=15655432. [DOI] [PubMed] [Google Scholar]
- 40.Tu Karen, Campbell Norman R, Chen Zhong-Liang, Cauch-Dudek Karen J, McAlister Finlay A. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007 Apr 14;1(1):18–26. http://www.openmedicine.ca/article/view/17/23. [PMC free article] [PubMed] [Google Scholar]
- 41.Gershon A S, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physician diagnosed COPD in health administrative databases. COPD. 2009;6(5):388–394. doi: 10.1080/15412550903140865. [DOI] [PubMed] [Google Scholar]
- 42.Gershon Andrea S, Wang Chengning, Guan Jun, Vasilevska-Ristovska Jovonka, Cicutto Lisa, To Teresa. Identifying patients with physician-diagnosed asthma in health administrative databases. Can Respir J. 2009;16(6):183–188. doi: 10.1155/2009/963098. http://europepmc.org/abstract/MED/20011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glazier R H, Zagorski B M, Rayner J. Comparison of primary care models in Ontario by demographics, case mix and emergency department use, 2008/09 to 2009/10. Toronto (ON): Institute for Clinical Evaluative Sciences; 2012. http://www.ices.on.ca/file/ICES_Primary%20Care%20Models%20English.pdf. [Google Scholar]
- 44.Full-time equivalent physicians (FTE) report, Canada, 1999-2000 and 2000-2001. Ottawa (ON): Canadian Institute for Health Information; 2003. https://secure.cihi.ca/estore/productSeries.htm?pc=PCC37. [Google Scholar]
- 45.Henry D A, Schultz S E, Glazier R H, Bhatia R S, Dhalla I A, Laupacis A. Payments to Ontario physicians from Ministry of Health and Long-Term Care sources, 1992/93 to 2009/10. Toronto (ON): Institute for Clinical Evaluative Sciences; 2012. http://www.ices.on.ca/file/ICES_PhysiciansReport_2012.pdf. [Google Scholar]
- 46.Manuel Douglas G, Lam Kelvin, Maaten Sarah, Klein-Geltink Julie. Using administrative data to measure the extent to which practitioners work together: “interconnected” care is common in a large cohort of family physicians. Open Med. 2011;5(4):177–182. http://www.openmedicine.ca/article/view/448/437. [PMC free article] [PubMed] [Google Scholar]
- 47.Landon Bruce E, Keating Nancy L, Barnett Michael L, Onnela Jukka-Pekka, Paul Sudeshna, O'Malley A J, Keegan Thomas, Christakis Nicholas A. Variation in patient-sharing networks of physicians across the United States. JAMA. 2012 Jul 18;308(3):265–273. doi: 10.1001/jama.2012.7615. http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2012.7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hackbarth Glenn, Reischauer Robert, Mutti Anne. Collective accountability for medical care—toward bundled Medicare payments. N Engl J Med. 2008 Jul 3;359(1):3–5. doi: 10.1056/NEJMp0803749. http://www.nejm.org/doi/abs/10.1056/NEJMp0803749. [DOI] [PubMed] [Google Scholar]
- 49.Improving care for high-needs patients: McGuinty government linking health providers, offering patients more co-ordinated care [press release] Toronto (ON): Ontario Ministry of Health and Long-Term Care; 2012. Dec 6, [accessed 2012 Dec. 17]. http://news.ontario.ca/mohltc/en/2012/12/improving-care-for-high-needs-patients.html. [Google Scholar]