Abstract
Background
Antibiotic-associated diarrhea (AAD) and Clostridium difficile infection (CDI) are associated with high morbidity, mortality, and health care costs. Probiotics may mitigate the existing disease burden. We performed a systematic review and meta-analysis to evaluate the efficacy of co-administration of probiotics with antibiotics in preventing these adverse outcomes in adult inpatients.
Methods
Systematic searches of MEDLINE (1946 to May 2012), Embase (1980 to May 2012), and the Cochrane Central Register of Controlled Trials were undertaken on May 31, 2012, to identify relevant publications. We searched for randomized controlled trials, published in English, of adult inpatients who were receiving antibiotics and who were randomly assigned to co-administration of probiotics or usual care, with or without the use of placebo. Studies were included if they reported on AAD or CDI (or both) as outcomes. Data for predetermined criteria evaluating study characteristics, methods, and risk of bias were extracted. Trials were given a global rating of good, fair, or poor by at least 2 reviewers. Meta-analyses were performed using a random-effects model, and pooled relative risks (RRs) and 95% confidence intervals (CIs) were calculated.
Results
Sixteen trials met the criteria for inclusion in this review. Four studies were of good quality, 5 were of fair quality, and 7 were of poor quality. Pooled analyses revealed significant reductions in the risks of AAD (RR 0.61, 95% CI 0.47 to 0.79) and CDI (RR 0.37, 95% CI 0.22 to 0.61) among patients randomly assigned to co-administration of probiotics. The number needed to treat for benefit was 11 (95% CI 8 to 20) for AAD and 14 (95% CI 9 to 50) for CDI. With subgroup analysis, significant reductions in rates of both AAD and CDI were retained in the subgroups of good-quality trials, the trials assessing a primarily Lactobacillus-based probiotic formulation, and the trials for which the follow-up period was less than 4 weeks.
Interpretation
Probiotics used concurrently with antibiotics reduce the risk of AAD and CDI
A rise in the use of antibiotics has resulted in a marked increase in antibiotic-associated diarrhea (AAD) and Clostridium difficile infection (CDI).1 A spectrum of adverse sequelae is associated with CDI, including diarrhea, electrolyte abnormalities, sepsis and septic shock, toxic megacolon requiring colectomy, admission to the intensive care unit, and death.2 In response to this devastating infection, a variety of non-antibiotic strategies, such as toxin-binding agents, active immunization, intravenous administration of immune globulin, and fecal transplantation, have been attempted, with variable success.3 Many hospitals are emphasizing infection control measures and antimicrobial stewardship to mitigate disease burden.4 The concurrent administration of probiotics with antibiotics has also been studied as a potential preventive intervention against AAD and CDI.
Randomized controlled trials (RCTs) assessing probiotics for the prevention of AAD and CDI have been limited by low case volumes. Existing systematic reviews and meta-analyses5-9 have grouped disparate populations, such as inpatients with outpatients or adults with children, and have considered clinically distinct entities, such as prevention and treatment of AAD and CDI, as combined outcomes.
Given the high morbidity among inpatients with these adverse outcomes, we conducted a systematic review and meta-analysis to evaluate the efficacy of probiotics administered with antibiotics in reducing these outcomes. We examined AAD and CDI as separate outcomes and limited our review to adult inpatients, because admission to hospital is a potent risk factor for colonization with C. difficile.10
Methods
Data sources and searches
This review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.11 We undertook systematic searches of MEDLINE (1946 to May 2012), Embase (1980 to May 2012), and the Cochrane Central Register of Controlled Trials on May 31, 2012, to identify relevant publications. We employed a sensitive search strategy (Appendix A) using broad keywords to identify both the conditions of interest (“Clostridium difficile,” “antibiotic-associated diarrhea,” and phrase variants) and the intervention of interest (“probiotics” and the names of specific probiotic genera). We also performed a manual search of the reference lists of identified articles to identify and retrieve relevant research studies.
Study selection
One reviewer screened all abstracts for relevance to the topic. Two independent reviewers then screened the abstracts of relevant articles for possible inclusion. We included English-language RCTs of adult inpatients (i.e., patients who had been admitted to medical or surgical wards or to wards devoted to acute care of elderly patients) receiving antibiotics who were randomly assigned to co-administration of probiotics or to usual care, with or without the use of placebo. To be included in the review, a study must have reported the prevention of AAD or CDI (or both) as an outcome. The rate of AAD or CDI was defined as the number of patients who experienced diarrhea or diarrhea with C. difficile positivity by toxin assay or stool culture, respectively, while receiving antibiotics, divided by the number of patients with available end points. If an included publication did not report the necessary data, we contacted the primary authors to obtain original data for our quantitative analysis. If the authors of a study could not provide the necessary data, the study was included in the systematic review but was excluded from the meta-analysis. We excluded studies of probiotics used to prevent CDI recurrence in patients with a previous diagnosis of CDI. We also excluded trials in which antibiotics were used for eradication of Helicobacter pylori, as this represents a distinct clinical end point of treatment augmentation, and H. pylori infection is a condition for which management occurs almost exclusively in the outpatient setting. We excluded studies that were pilot trials of feasibility or tolerability because they did not define AAD or CDI incidence as outcomes of interest. We also excluded studies presented only at conferences, studies of before-and-after comparisons, and non-randomized comparison and cohort studies. Letters, commentaries, reviews, and editorials were excluded if they did not contain original data.
Data extraction and assessment of risk of bias
Two reviewers independently performed a full-text review of each included manuscript. Risk of bias in the included studies was assessed by the same 2 reviewers on the basis of the US Preventive Services Task Force recommendations, which include domains of randomization, blinding, comparability of groups, adequacy of follow-up (>80%), clarity of interventions and outcomes, use of intention-to-treat analysis, and adequacy of study power.12 A data extraction form was used to record the findings for each trial. The reviewers rated the studies as good, fair, or poor on the basis of a predetermined global quality rating scale combining the aforementioned criteria (see Appendix B). Disagreement on quality rating was resolved by a third reviewer.
Data synthesis
Meta-analytic software (RevMan 5.0 from the Cochrane Collaboration) was used to synthesize the results. Relative risk (RR), risk difference (RD), and number needed to treat (NNT) to benefit or to harm, with their respective 95% confidence intervals (CIs), were calculated using the DerSimonian Laird method. The Mantel–Haenszel method was used to weight the studies in the meta-analyses because the events being assessed were rare. We expected clinical and statistical heterogeneity among the studies. Therefore, we used the random-effects model for meta-analyses because it accounts for random variability both within and among studies. Subgroup analyses were planned a priori to assess the effect on results of study quality (good v. fair v. poor), type of probiotic (Lactobacillus-based v. Saccharomyces boulardii–based), and duration of follow-up (< 4 weeks or ≥ 4 weeks). No adjustments were made for multiple analyses. Post hoc meta-regression was performed to identify the independent effects of type of probiotics.
Heterogeneity and assessment of publication bias
Clinical heterogeneity was assessed for population characteristics, type of probiotic supplementation, and quality of studies. Statistical heterogeneity was assessed using the Cochrane Q test and by calculating I-squared (I2) values. A funnel plot was created to assess the possibility of publication bias.
Ethics approval
All of the data are available from completed trials, and thus no ethics approval was necessary.
Results
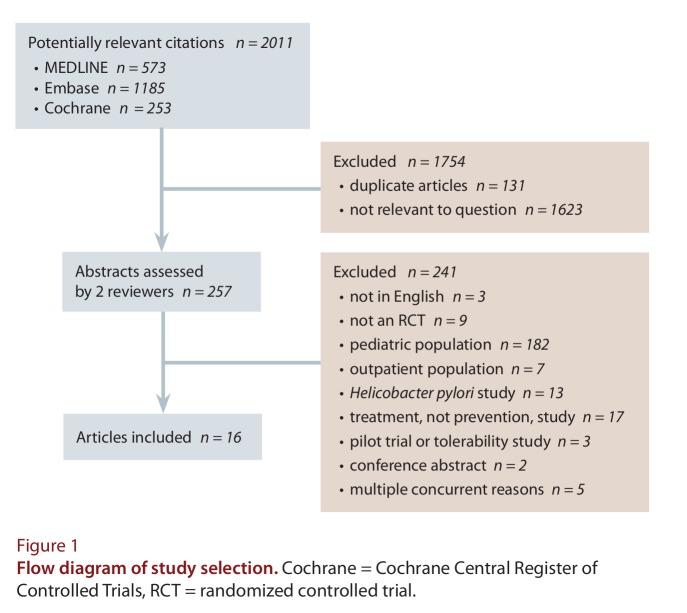
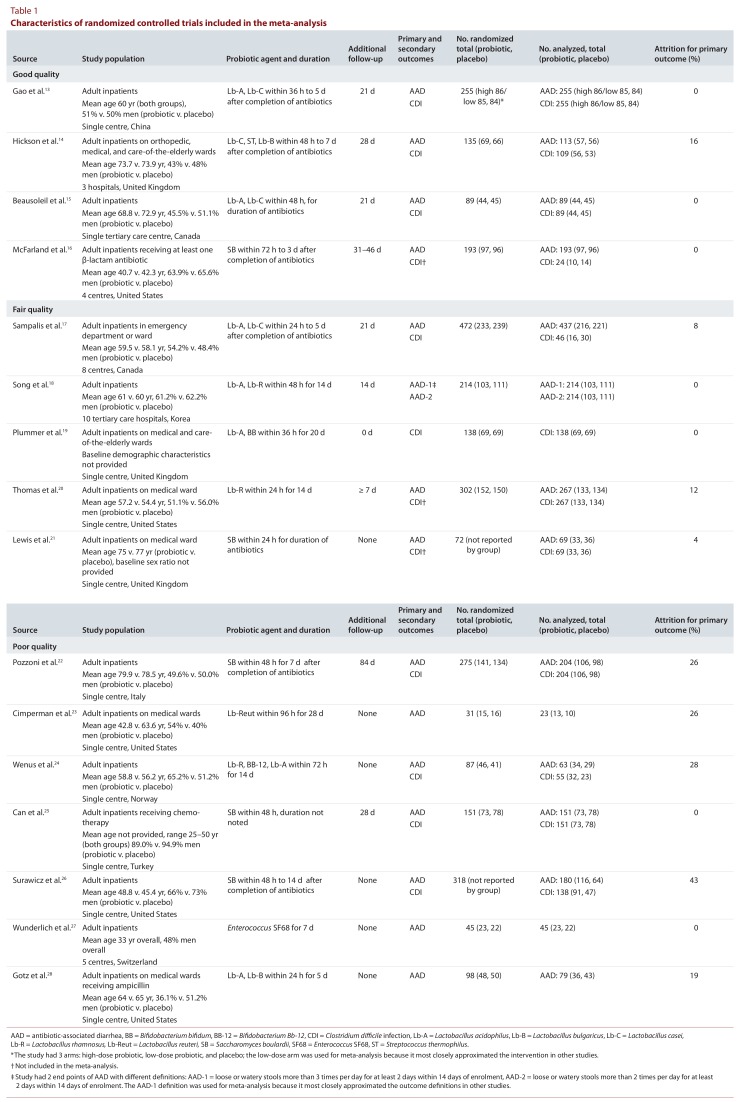
Sixteen studies13-28 were included in our analyses. Details of the selection process are shown in Figure 1, and the baseline characteristics of the studies are reported in Table 1. Only 5 of the studies involved more than one centre,14,16-18,27 and the majority of studies were conducted in the United States or the United Kingdom. Among all of the trials, the range of mean ages for patients randomly assigned to probiotic was 33–79.9 years and to placebo was 33–78.5 years. Men constituted 43%–89% of participants in the probiotic groups and 40%–94.9% in the placebo group. However, the upper limit for proportion of men enrolled was influenced by just one study,25 with the majority of studies including fewer than 75% men.
Figure 1.
Flow diagram of study selection
Table 1.
Characteristics of randomized controlled trials included in the meta-analysis
All of the studies, with one exception,19 examined AAD as a primary outcome. Only 1 trial18 assessed 2 end points of AAD with different definitions, and in that case, the definition that most closely approximated the outcome definitions in other studies was used for meta-analysis. One study13 examined a dose–response relationship using a Lactobacillus acidophilus and Lactobacillus casei co-formulation. In that study, patients were randomly assigned to a high-dose probiotic group, a low-dose probiotic group, or a placebo group. For the purpose of this meta-analysis, we used the data from the low-dose and placebo groups, since that comparison most closely approximated the dosing regimens of the other included RCTs. Ten studies13-15,17-20,23,24,28 used a Lactobacillus-based probiotic, 5 studies16,21,22,25,26 evaluated Saccharomyces boulardii, and 1 study27 assessed Enterococcus species. Twelve studies13-17,19-22,24-26 sought to evaluate CDI as an outcome, with one having CDI as the primary end point.19 Of the 12 studies evaluating CDI, 4 were initially excluded. Two of these studies21,24 did not report CDI event rates because there were insufficient data to detect a difference. An additional 2 studies16,20 reported CDI cases in ways that deviated from the original study protocols, and the outcome definitions were inconsistent with those of the other included studies. We contacted the primary authors of these 4 studies but were able to obtain original data for only one publication24 to generate comparable outcome information. Therefore, all 4 of these studies were included in the systematic review, but only the single study for which original data were acquired24 was ultimately included in the meta-analysis.
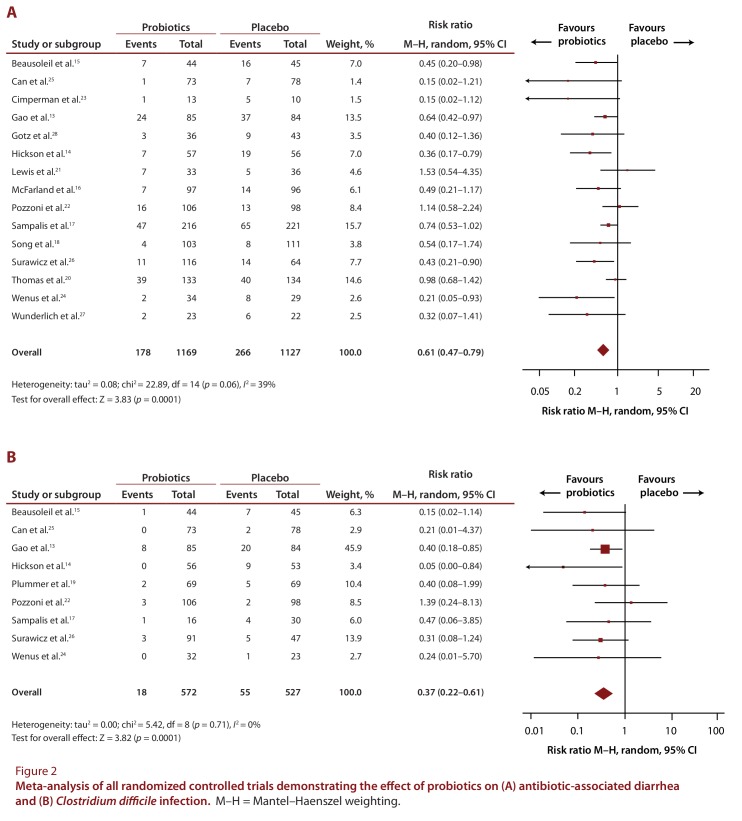
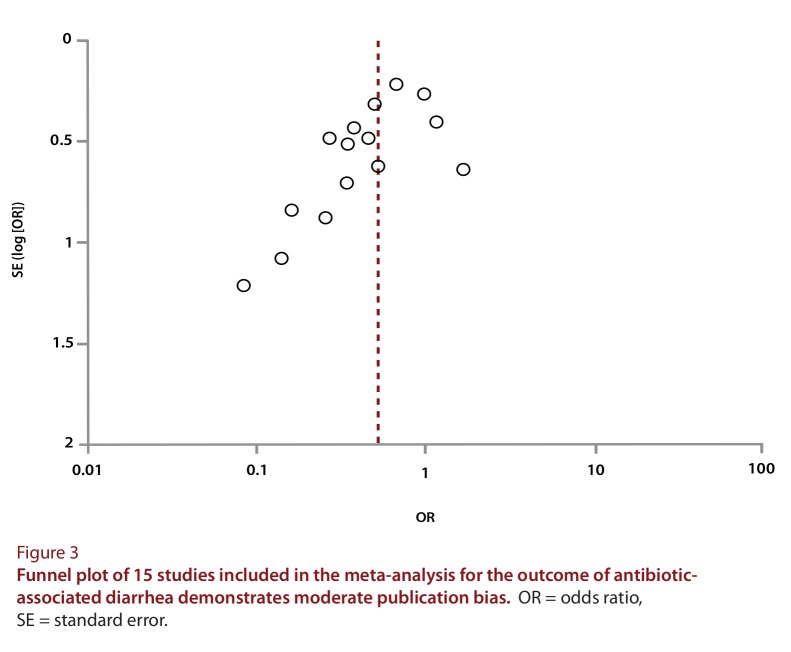
Meta-analysis of included studies demonstrated a statistically significant reduction in the risk of AAD (RR 0.61, 95% CI 0.47 to 0.79; I2= 39%; RD –0.09, 95% CI –0.13 to –0.05; NNT to benefit 11, 95% CI 8 to 20). For CDI, there was a substantially lower number of patients with available end points. The event rates were 18 (3.1%) of 572 patients in the intervention arm and 55 (10.4%) of 527 patients in the placebo arm (RR 0.37, 95% CI 0.22 to 0.61; I2= 0%; RD –0.07, 95% CI –0.11 to –0.02; NNT to benefit 14, 95% CI 9 to 50). The forest plots displaying the effect size by trial, as well as the aggregate effect size, are shown in Figure 2. Because of the small sample sizes and the rarity of outcomes, the CIs for several of the studies cross unity. Studies were heterogeneous in sample size, and the funnel plot (Figure 3) demonstrates a moderate degree of publication bias.
Figure 2.
Meta-analysis of all randomized controlled trials demonstrating the effect of probiotics on (A) antibiotic-associated diarrhea and (B) Clostridium difficile infection.
Figure 3.
Funnel plot of 15 studies included in the meta-analysis for the outcome of antibiotic-associated diarrhea demonstrates moderate publication bias
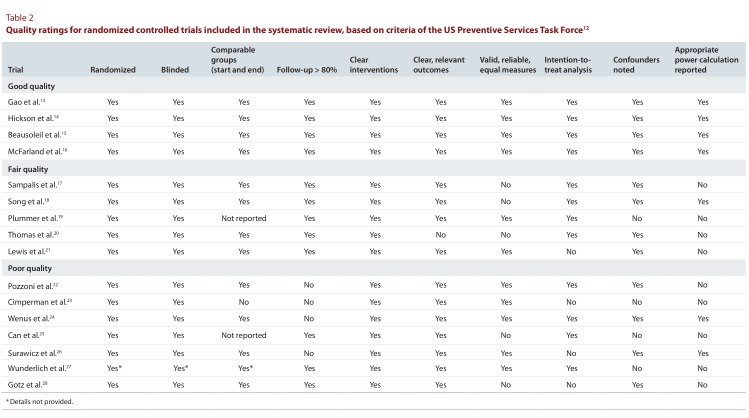
Four of the studies13-16 were rated as having good quality, whereas 5 studies were of fair quality17-21 and 7 were of poor quality22-28 (Table 2).
Table 2.
Quality ratings for randomized controlled trials included in the systematic review, based on criteria of the US Preventative Services Task Force
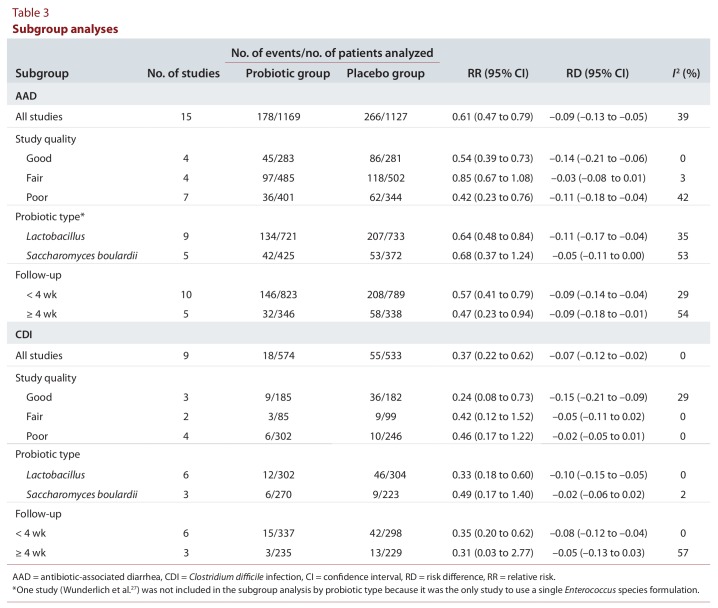
When the results were stratified by study quality (Table 3), the 4 good-quality studies13-16 demonstrated reduction in AAD and CDI with the use of probiotics. These studies shared features that led to their high rating, specifically clear inclusion criteria, interventions, and outcomes. They used validated scales or precise qualitative explanations to define the outcome measures and had reasonably long-term follow-up (between 3 and 7 weeks). The fair-quality studies,17-21 when pooled, demonstrated reduction in AAD and CDI that was not statistically significant. These studies received lower quality ratings because of a lack of clarity or validity in their outcome measures, with the use of liberal, subjective criteria for AAD and CDI that may have resulted in overreporting. Specifically for CDI, 2 of the studies19,21 involved testing for C. difficile toxin on formed stool, which may have led to the inclusion of cases of C. difficile colonization as opposed to the clinically relevant outcome of CDI. All but one22 of the 7 poor-quality studies22-28 showed a significantly lower RR for AAD with the use of probiotics. Four of the poor-quality trials22,24-26 assessed CDI as a secondary outcome, but none of them demonstrated a significant risk reduction. In general, the poor-quality studies were limited by unclear interventions and outcomes (see Table 2). They lacked formal reporting of key study methods, such as the randomization process, blinding methods, and the duration of the intervention or follow-up.
Table 3.
Subgroup analyses
When studies were pooled by type of probiotic, reductions in AAD and CDI were observed regardless of whether a primarily Lactobacillus-based probiotic or an S. boulardii–based formulation was used. However, only the combined analyses of Lactobacillus-based formulations showed reductions that were statistically significant. The similarity in effect size between the 2 groups has some biologic plausibility, given that the benefit of probiotics is thought to derive, at least in part, from recolonization of the gastrointestinal tract with “normal,” non-pathogenic flora, rather than from species-specific effects.29
For short follow-up periods (< 4 weeks), statistically significant reductions in both AAD and CDI were observed. With longer follow-up, only the reduction in AAD, and not that for CDI, remained significant. Statistical heterogeneity (I2) was moderately greater for the subgroup of patients with follow-up of 4 weeks or longer (54% for AAD and 57% for CDI). The literature suggests that AAD and CDI can occur after just one dose of antibiotics and may appear up to several weeks after completion of antibiotic therapy.30 As such, an adequate follow-up period is needed to ensure that most cases are appropriately identified. Our subgroup analysis by follow-up period was dichotomized as less than 4 weeks v. 4 weeks or more, because this time frame reflects a practical and clinically applicable cut-off for ongoing patient surveillance.
Post hoc meta-regression analysis by type of probiotic confirmed the findings of the subgroup analysis. Specifically, the primarily Lactobacillus-based formulation remained significantly effective in reducing AAD. Because of wide variability in the duration of follow-up, we were unable to perform meta-regression of duration of follow-up as a continuous measure.
No life-threatening adverse effects of probiotics were reported in these RCTs. Despite case reports of toxic effects among patients with extenuating circumstances,31-33 probiotics had an excellent safety profile, the most common adverse effect being gastrointestinal upset.
Interpretation
Probiotics can confer health benefits in several ways: by creating nutrient competition, by favourably altering the gut flora, by serving as a barrier against pathogen-receptor binding, by elaborating immunomodulators (such as immunoglobulin A) or trophic factors, and by reducing osmotic diarrhea.34 With recent epidemiologic patterns showing a rise in the occurrence of AAD and CDI among healthier, previously spared populations, as well as among those patients most vulnerable to its complications,35-37 there is an urgent need to find innovative methods for prevention.
Our findings indicate that probiotics given concurrently with antibiotics reduce the risk of AAD and CDI. The results of our meta-analysis are concordant with several prior systematic reviews and meta-analyses,5-9 which varied in terms of the patients assessed and the outcomes defined. In a recent meta-analysis, Hempel et al.8 assessed probiotics for both the prevention and the treatment of AAD and reported benefit. Those authors included 82 trials of significant heterogeneity: in addition to examining both prevention and treatment trials, they assessed trials involving both inpatients and outpatients, they evaluated all age groups, and they included 24 trials in which patients were receiving antibiotics for H. pylori eradication.
One of the strengths of our review was our emphasis on ensuring that comparable outcome definitions were used in the meta-analysis. We achieved this comparability by carefully selecting the study arms to include for trials with more than 2 intervention groups and by contacting primary authors for original data when needed. These actions mitigated some of the impact of the clinical heterogeneity among the trials. Another major strength was our focus on a specific patient population: inpatients. Reducing the incidence of CDI will improve individual patient outcomes while curtailing spread in the high-risk setting of hospitals. Thus, these results have implications for the health of other, non-infected inpatients. Finally, our meta-analysis showed that benefit was retained regardless of study quality, type of probiotic used, and duration of follow-up. However, the results were only significant for both AAD and CDI concurrently for the subgroups of good-quality studies, studies assessing Lactobacillus-based formulations, and studies in which the follow-up was less than 4 weeks.
Several limitations in the individual studies and in our meta-analysis merit discussion. A notable limitation among some of the more recent studies was the high baseline rates of AAD and CDI in the placebo arms. Three of the recent RCTs, all of which were assessed as being of good quality,13-15 reported AAD rates of 34%–44% and CDI rates of 16%–24% in the control groups. These high baseline event rates may have facilitated the detection of a significant effect size despite small sample sizes. The extent to which this result may have been influenced by different local practices in antimicrobial stewardship and environmental infection control is unknown, and thus their effect sizes may not be replicated in other settings where baseline rates of AAD and CDI are lower.
The included trials shared certain methodological issues that also limited broad interpretation of the results. Some of the studies had lower enrolment than planned for detecting the expected differences, a factor of particular importance for the negative trials.20,21 Almost all of the studies that we assessed excluded patients who might otherwise be considered candidates for a hospital-wide intervention like probiotics. For example, patients who had received a course of antibiotics on an outpatient basis in the weeks preceding trial enrolment were excluded to avoid inclusion of cases of community-associated CDI. Furthermore, patients with pre-existing gastrointestinal pathology were excluded to avoid inclusion of patients suffering from diarrhea not related to antibiotics. These steps may limit the interpretation of how probiotics will affect a more inclusive inpatient population. Two of the fair-quality studies18,21 reported possible probiotic under-dosing. A high rate of attrition (> 20%) was observed in 4 studies,22-24,26 which necessitated a poor quality rating.
Our meta-analysis also suffered from some important limitations. There was evidence of moderate publication bias (see Figure 3). Three trials were excluded because they were not in English.38-40 Furthermore, among all of the patients assessed for AAD, 1200 patients did not have end points for CDI.
We chose to convey outcome information using RDs and NNTs. We acknowledge the limitation of using NNTs to convey outcome information, given the clinical and statistical heterogeneity among the studies included in this review. Admittedly, NNT is difficult to interpret when such heterogeneity exists, and we therefore caution readers and decision-makers against using this information without putting it in the context of the study variability and considering the local prevalence of CDI in their respective populations of interest.
Conclusions
Our findings illuminate the benefits of probiotics in preventing both AAD and CDI in the specific patient population of adult inpatients requiring antibiotics. On the basis of the current review, probiotics can be recommended for such patients in the absence of contraindications; however, the prevalence of AAD and CDI should be taken into consideration before guidelines are developed. The literature does not clearly indicate a favoured choice of probiotic, although there is stronger evidence for Lactobacillus-based formulations.
Many health care providers have been hesitant to adopt probiotics in routine practice, despite impressive effect sizes. This may be because of the small sample sizes in the individual trials, the high baseline rates of AAD and CDI in the larger, more recent trials, the clinical and statistical heterogeneity between trials, and the publication bias seen in this and other meta-analyses. While there may be a signal toward clinical improvement with this intervention, future RCTs should strive to recruit more patients and to strengthen their power to help bring probiotics to the bedside. Other research that will add to our current knowledge in this area might address whether there is greater benefit with the use of combination therapy over single-species probiotic formulations. The hypothesis of a dose–response effect requires further validation.
Acknowledgments
We thank Elizabeth Uleryk, MLS, Director of Hospital Library and Archives, The Hospital for Sick Children, for her help in developing the search strategy, undertaking the search, and writing the section on literature search methods in online Appendix A.
Biographies
Reena Pattani, MD, is a resident in internal medicine at the University of Toronto, Toronto, Ontario.
Valerie A. Palda, MD, MSc, is an Associate Professor in the Department of Medicine and the Institute for Health Policy, Management and Evaluation, University of Toronto, Toronto, Ontario.
Stephen W. Hwang, MD, MPH, is an Associate Professor in the Department of Medicine, University of Toronto, and a Research Scientist at St. Michael’s Hospital, Toronto, Ontario.
Prakeshkumar S. Shah, MD, MSc, is an Associate Professor in the Department of Pediatrics and the Institute for Health Policy, Management and Evaluation, University of Toronto, Toronto, Ontario.
Appendices
Appendix A.
Search strategy
Appendix B.
Quality criteria
Footnotes
Competing interests: None declared.
Funding source: No external funding was received for this review.
Reena Pattani participated in designing the study methodology, contributed to data extraction and analysis, and was the principal writer of the manuscript. Valerie A. Palda conceived the project, outlined the qualitative study methods, and contributed to data extraction and analysis. Stephen W. Hwang assisted in designing the study methods and in extracting and analyzing the data. Prakeshkumar S. Shah outlined quantitative methods, conducted the quantitative analysis, and participated in writing the manuscript. All of the authors reviewed and edited drafts of the manuscript for important intellectual content, and each author approved the final version of the manuscript.
References
- 1.Valiquette Louis, Low Donald E, Pépin Jacques, McGeer Allison. Clostridium difficile infection in hospitals: a brewing storm. CMAJ. 2004 Jul 6;171(1):27–29. doi: 10.1503/cmaj.1040957. http://www.cmaj.ca/cgi/pmidlookup?view=long&pmid=15238490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurley Brian W, Nguyen Cuong C. The spectrum of pseudomembranous enterocolitis and antibiotic-associated diarrhea. Arch Intern Med. 2002 Oct 28;162(19):2177–2184. doi: 10.1001/archinte.162.19.2177. http://archinte.ama-assn.org/cgi/doi/10.1001/archinte.162.19.2177. [DOI] [PubMed] [Google Scholar]
- 3.Bauer Martijn P, van Dissel Jaap T. Alternative strategies for Clostridium difficile infection. Int J Antimicrob Agents. 2009;33 Suppl 1:S51–S56. doi: 10.1016/S0924-8579(09)70018-4. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett John G. A call to arms: the imperative for antimicrobial stewardship. Clin Infect Dis. 2011;53 Suppl 1:S41–S47. doi: 10.1093/cid/cir362. http://www.cid.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=21795727. [DOI] [PubMed] [Google Scholar]
- 5.Cremonini F, Di Caro S, Nista E C, Bartolozzi F, Capelli G, Gasbarrini G, Gasbarrini A. Meta-analysis: the effect of probiotic administration on antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2002;16(8):1461–1467. doi: 10.1046/j.1365-2036.2002.01318.x. http://doi.wiley.com/10.1046/j.1365-2036.2002.01318.x. [DOI] [PubMed] [Google Scholar]
- 6.McFarland Lynne V. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101(4):812–822. doi: 10.1111/j.1572-0241.2006.00465.x. http://www.nature.com/doifinder/10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 7.Dendukuri Nandini, Costa Vania, McGregor Maurice, Brophy James M. Probiotic therapy for the prevention and treatment of Clostridium difficile-associated diarrhea: a systematic review. CMAJ. 2005 Jul 19;173(2):167–170. doi: 10.1503/cmaj.050350. http://www.cmaj.ca/cgi/pmidlookup?view=long&pmid=16027434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hempel Susanne, Newberry Sydne J, Maher Alicia R, Wang Zhen, Miles Jeremy N V, Shanman Roberta, Johnsen Breanne, Shekelle Paul G. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012 May 9;307(18):1959–1969. doi: 10.1001/jama.2012.3507. http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2012.3507. [DOI] [PubMed] [Google Scholar]
- 9.Johnson Stuart, Maziade Pierre-Jean, McFarland Lynne V, Trick William, Donskey Curtis, Currie Brian, Low Donald E, Goldstein Ellie J C. Is primary prevention of Clostridium difficile infection possible with specific probiotics? Int J Infect Dis. 2012 Aug 3;16(11) doi: 10.1016/j.ijid.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Loo Vivian G, Bourgault Anne-Marie, Poirier Louise, Lamothe François, Michaud Sophie, Turgeon Nathalie, Toye Baldwin, Beaudoin Axelle, Frost Eric H, Gilca Rodica, Brassard Paul, Dendukuri Nandini, Béliveau Claire, Oughton Matthew, Brukner Ivan, Dascal Andre. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011 Nov 3;365(18):1693–1703. doi: 10.1056/NEJMoa1012413. http://www.nejm.org/doi/abs/10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–e130. http://www.openmedicine.ca/article/view/285/247. [PMC free article] [PubMed] [Google Scholar]
- 12.Harris R P, Helfand M, Woolf S H, Lohr K N, Mulrow C D, Teutsch S M, Atkins D Methods Work Group. Third US Preventive Services Task Force. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 Suppl):21–35. doi: 10.1016/S0749-3797(01)00261-6. http://www.scholaruniverse.com/ncbi-linkout?id=11306229. [DOI] [PubMed] [Google Scholar]
- 13.Gao Xing W, Mubasher Mohamed, Fang Chong Y, Reifer Cheryl, Miller Larry E. Dose–response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105(7):1636–1641. doi: 10.1038/ajg.2010.11. http://www.nature.com/doifinder/10.1038/ajg.2010.11. [DOI] [PubMed] [Google Scholar]
- 14.Hickson Mary, D'Souza Aloysius L, Muthu Nirmala, Rogers Thomas R, Want Susan, Rajkumar Chakravarthi, Bulpitt Christopher J. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335(7610):80. doi: 10.1136/bmj.39231.599815.55. http://www.bmj.com/cgi/doi/10.1136/bmj.39231.599815.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beausoleil M, Fortier N, Guénette S, L'ecuyer A, Savoie M, Franco M, Lachaine J, Weiss K. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol. 2007;21(11):732–736. doi: 10.1155/2007/720205. http://europepmc.org/abstract/MED/18026577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McFarland L V, Surawicz C M, Greenberg R N, Elmer G W, Moyer K A, Melcher S A, Bowen K E, Cox J L. Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol. 1995;90(3):439–448. http://www.scholaruniverse.com/ncbi-linkout?id=7872284. [PubMed] [Google Scholar]
- 17.Sampalis John, Psaradellis Eliofotisti, Rampakakis Emmanouil. Efficacy of BIO K+ CL1285 in the reduction of antibiotic-associated diarrhea - a placebo controlled double-blind randomized, multi-center study. Arch Med Sci. 2010 Mar 9;6(1):56–64. doi: 10.5114/aoms.2010.13508. http://europepmc.org/abstract/MED/22371721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Hyun J, Kim Jin Yong, Jung Sung Ae, Kim Seong Eun, Park Hye Sook, Jeong Yoolwon, Hong Sung P, Cheon Jae H, Kim Won H, Kim Hyo Jong, Ye Byong D, Yang Suk Kyun, Kim Sang Woo, Shin Sung Jae, Kim Hyun Soo, Sung Jae Kyu, Kim Eun Y. Effect of probiotic Lactobacillus (Lacidofil® cap) for the prevention of antibiotic-associated diarrhea: a prospective, randomized, double-blind, multicenter study. J Korean Med Sci. 2010;25(12):1784–1791. doi: 10.3346/jkms.2010.25.12.1784. http://jkms.org/DOIx.php?id=10.3346/jkms.2010.25.12.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plummer Sue, Weaver Mark A, Harris Janine C, Dee Phillipa, Hunter John. Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int Microbiol. 2004;7(1):59–62. http://www.im.microbios.org/25march04/09%20Plummer.pdf. [PubMed] [Google Scholar]
- 20.Thomas M R, Litin S C, Osmon D R, Corr A P, Weaver A L, Lohse C M. Lack of effect of Lactobacillus GG on antibiotic-associated diarrhea: a randomized, placebo-controlled trial. Mayo Clin Proc. 2001;76(9):883–889. doi: 10.4065/76.9.883. [DOI] [PubMed] [Google Scholar]
- 21.Lewis S J, Potts L F, Barry R E. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect. 1998;36(2):171–174. doi: 10.1016/S0163-4453(98)80008-X. http://linkinghub.elsevier.com/retrieve/pii/S016344539880008X. [DOI] [PubMed] [Google Scholar]
- 22.Pozzoni Pietro, Riva Alessia, Bellatorre Alessandro G, Amigoni Maria, Redaelli Elena, Ronchetti Anna, Stefani Mariangela, Tironi Rosangela, Molteni Edoardo E, Conte Dario, Casazza Giovanni, Colli Agostino. Saccharomyces boulardii for the prevention of antibiotic-associated diarrhea in adult hospitalized patients: a single-center, randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2012;107(6):922–931. doi: 10.1038/ajg.2012.56. [DOI] [PubMed] [Google Scholar]
- 23.Cimperman Lisa, Bayless Gina, Best Kathleen, Diligente Autumn, Mordarski Beth, Oster Melanie, Smith Meghann, Vatakis Felicia, Wiese Dawn, Steiber Alison, Katz Jeffry. A randomized, double-blind, placebo-controlled pilot study of Lactobacillus reuteri ATCC 55730 for the prevention of antibiotic-associated diarrhea in hospitalized adults. J Clin Gastroenterol. 2011;45(9):785–789. doi: 10.1097/MCG.0b013e3182166a42. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00004836-201110000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Wenus C, Goll R, Loken E B, Biong A S, Halvorsen D S, Florholmen J. Prevention of antibiotic-associated diarrhoea by a fermented probiotic milk drink. Eur J Clin Nutr. 2007 Mar 14;62(2):299–301. doi: 10.1038/sj.ejcn.1602718. http://www.nature.com/doifinder/10.1038/sj.ejcn.1602718. [DOI] [PubMed] [Google Scholar]
- 25.Can M, Beşirbellioglu B A, Avci I Y, Beker C M, Pahsa A. Prophylactic Saccharomyces boulardii in the prevention of antibiotic-associated diarrhea: a prospective study. Med Sci Monit. 2006;12(4):PI19–PI22. http://journals.indexcopernicus.com/ICinfoauthor.php?PMID=16572062. [PubMed] [Google Scholar]
- 26.Surawicz C M, Elmer G W, Speelman P, McFarland L V, Chinn J, van Belle G. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Gastroenterology. 1989;96(4):981–988. doi: 10.1016/0016-5085(89)91613-2. http://www.scholaruniverse.com/ncbi-linkout?id=2494098. [DOI] [PubMed] [Google Scholar]
- 27.Wunderlich P F, Braun L, Fumagalli I, D'Apuzzo V, Heim F, Karly M, Lodi R, Politta G, Vonbank F, Zeltner L. Double-blind report on the efficacy of lactic acid-producing Enterococcus SF68 in the prevention of antibiotic-associated diarrhoea and in the treatment of acute diarrhoea. J Int Med Res. 17(4):333–338. doi: 10.1177/030006058901700405. [DOI] [PubMed] [Google Scholar]
- 28.Gotz V, Romankiewicz J A, Moss J, Murray H W. Prophylaxis against ampicillin-associated diarrhea with a Lactobacillus preparation. Am J Hosp Pharm. 1979;36(6):754–757. [PubMed] [Google Scholar]
- 29.Bengmark S. Ecological control of the gastrointestinal tract: the role of probiotic flora. Gut. 1998;42(1):2–7. doi: 10.1136/gut.42.1.2. http://gut.bmj.com/cgi/doi/10.1136/gut.42.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly Ciaran P, Pothoulakis Charalabos, LaMont J Thomas. Clostridium difficile colitis. N Engl J Med. 1994 Jan 27;330(4):257–262. doi: 10.1056/NEJM199401273300406. http://www.scholaruniverse.com/ncbi-linkout?id=8043060. [DOI] [PubMed] [Google Scholar]
- 31.Rautio M, Jousimies-Somer H, Kauma H, Pietarinen I, Saxelin M, Tynkkynen S, Koskela M. Liver abscess due to a Lactobacillus rhamnosus strain indistinguishable from L. rhamnosus strain GG. Clin Infect Dis. 1999;28(5):1159–1160. doi: 10.1086/514766. http://cid.oxfordjournals.org/lookup/doi/10.1086/514766. [DOI] [PubMed] [Google Scholar]
- 32.Mackay Andrew D, Taylor Mark B, Kibbler Christopher C, Hamilton-Miller Jeremy M T. Lactobacillus endocarditis caused by a probiotic organism. Clin Microbiol Infect. 1999;5(5):290–292. doi: 10.1111/j.1469-0691.1999.tb00144.x. http://doi.wiley.com/10.1111/j.1469-0691.1999.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 33.Luong M L, Sareyyupoglu B, Nguyen M H, Silveira F P, Shields R K, Potoski B A, Pasculle W A, Clancy C J, Toyoda Y. Lactobacillus probiotic use in cardiothoracic transplant recipients: a link to invasive Lactobacillus infection. Transpl Infect Dis. 2010;12(6):561–564. doi: 10.1111/j.1399-3062.2010.00580.x. http://doi.wiley.com/10.1111/j.1399-3062.2010.00580.x. [DOI] [PubMed] [Google Scholar]
- 34.Gareau Mélanie G, Sherman Philip M, Walker W Allan. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol. 2010 Jul 27;7(9):503–514. doi: 10.1038/nrgastro.2010.117. http://www.nature.com/doifinder/10.1038/nrgastro.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.QuickStats: rates of Clostridium difficile infection among hospitalized patients aged ≥65 by age group—National Hospital Discharge Survey, United States, 1996–2009. [accessed 2012 Dec. 1];MMWR. 2011 60(34):1171. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6034a7.htm?s_cid=mm6034a7_w. [Google Scholar]
- 36.Cober Eric D, Malani Preeti N. Clostridium difficile infection in the “oldest” old: clinical outcomes in patients aged 80 and older. J Am Geriatr Soc. 2009;57(4):659–662. doi: 10.1111/j.1532-5415.2009.02182.x. [DOI] [PubMed] [Google Scholar]
- 37.Serrano Pablo E, Khuder Sadik A, Fath John J. Obesity as a risk factor for nosocomial infections in trauma patients. J Am Coll Surg. 2010;211(1):61–67. doi: 10.1016/j.jamcollsurg.2010.03.002. http://linkinghub.elsevier.com/retrieve/pii/S107275151000178X. [DOI] [PubMed] [Google Scholar]
- 38.Stein Gideon Y, Nanim Rayf, Karniel Eli, Moskowitz Ina, Zeidman Aliza. [Probiotics as prophylactic agents against antibiotic-associated diarrhea in hospitalized patients] Harefuah. 2007;146(7):520–522. Article in Hebrew. [PubMed] [Google Scholar]
- 39.Cots J M. [Consumption of probiotics can reduce the incidence of antibiotic-associated diarrhea] Form Med Contin Aten Prim. 2008;15(3):201. Article in Spanish. [Google Scholar]
- 40.Li D, Wang H, Tan M, Shao Y. [Use of probiotics for prevention of antibiotic-associated diarrhea in elderly patients] Chin J Gastroenterol. 2010;15(3):154–156. Article in Chinese. [Google Scholar]