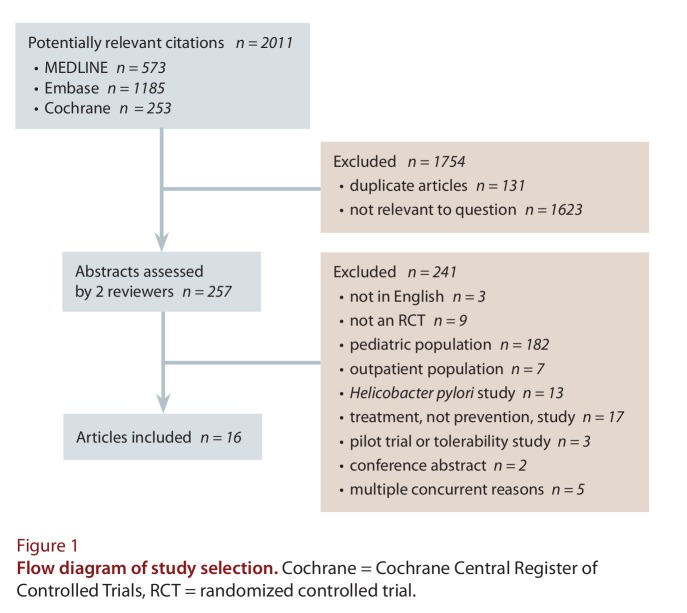
Abstract
Background
Both herpes zoster and malignancy are associated with immunosuppression. However, the association between herpes zoster and the subsequent diagnosis of malignancy is unclear. We undertook this study to assess whether a diagnosis of herpes zoster is a risk factor for subsequent malignancy.
Methods
For this matched retrospective cohort study, a physician billing database was used to identify individuals 18 years of age or older with a diagnosis of herpes zoster and no prior diagnosis of cancer or HIV infection. Individuals with a herpes zoster diagnosis were matched one-to-one to individuals without a herpes zoster diagnosis, and both groups were examined for up to 5 years for diagnosis of cancer.
Results
A total of 542 575 individuals with a diagnosis of herpes zoster were identified. Compared with matched controls, these patients were more likely (p < 0.001) to have a history of myocardial infarction, asthma, congestive heart failure, chronic obstructive pulmonary disease, diabetes mellitus, and hypertension. The incidence of cancer was significantly greater among individuals with herpes zoster than among those without herpes zoster, for both men and women and across all time intervals studied (up to 5 years). The greatest adjusted hazard ratio was seen 180 days after a herpes zoster diagnosis (1.19, 95% confidence interval 1.12–1.25); the hazard ratio decreased as the time from herpes zoster diagnosis increased. Lymphoma was the type of cancer with the greatest relative increase in incidence following diagnosis of herpes zoster.
Interpretation
There is a risk of malignancy following an episode of herpes zoster in both men and women and in all age groups 18 years and over. The risk is greatest during the first 180 days following the diagnosis of herpes zoster.
Herpes zoster, which presents as a painful vesicular rash occurring in a dermatomal distribution, results from reactivation of the varicella-zoster virus in the dorsal root ganglia.1,2 The rash is self-limited and resolves in 2 to 4 weeks.3 Age appears to be the key risk factor for the development of herpes zoster.4 A decrease in cell-mediated immunity is thought to be responsible for the increased incidence of varicella-zoster virus reactivation in older individuals.5 Patients with conditions resulting in impairment of cell-mediated immunity, such as lymphoma and HIV infection, and those receiving chemotherapy and steroids6 are at increased risk of herpes zoster.
Although it is known that both herpes zoster and malignancy are associated with immunosuppression,7 studies examining the association between herpes zoster and a subsequent diagnosis of cancer have had discordant results.8-13 Two studies showing the presence of an association examined the risk of hematological malignancies following herpes zoster.8,9 Similarly, Buntinx et al.12 examined a database of more than 300 000 patients and found a higher incidence of cancer following a herpes zoster diagnosis among all patients over 65 years of age and among women older than 65 years of age (but not among men of this age group). However, there was no difference in incidence in the first year after diagnosis of herpes zoster, which was interpreted as indicating that the herpes zoster diagnosis was not a marker of undiagnosed malignancy. Hence, Buntinx et al.12 recommended against screening patients with herpes zoster for underlying malignancy. Conversely, 3 studies10,11,13 showed no association between herpes zoster and a subsequent diagnosis of cancer. In particular, in a recent retrospective cohort study, Wang et al.13 found that overall cancer incidence among patients with herpes zoster was no different from the expected rate in the study population.
This article reports on what we believe to be the largest study to date examining whether a diagnosis of herpes zoster is a risk factor for subsequent diagnosis of cancer. More specifically, we assessed the risk of cancer following a diagnosis of herpes zoster in relation to age, sex, and type of malignancy.
Methods
A matched retrospective cohort design was used to study the subsequent diagnosis of cancer among patients with a diagnosis of herpes zoster.
Patients 18 years of age and older were eligible for inclusion in the study. Patients with a history of pre-existing cancer or HIV infection were excluded from the analysis.
Data relating to a diagnosis of herpes zoster were derived from the physician billings database in the Ontario Health Insurance Plan (OHIP) and were obtained through a comprehensive research agreement with the Ontario Ministry of Health and Long-Term Care. OHIP covers physician and hospital services for all Ontario residents, including approximately 94% of ambulatory physician visits in the province.14 Herpes zoster has a unique diagnostic code in the OHIP billing system (053). The exposed patients (cases) were those individuals for whom a first billing code for herpes zoster was submitted during the period of interest (1 Apr. 1993 to 31 Mar. 2010), whereas the unexposed controls were those individuals for whom a billing code for herpes zoster was not submitted during the study period.
The Ontario Cancer Registry was used to determine which patients had a diagnosis of cancer and, for those patients with cancer who also had a diagnosis of herpes zoster, whether the cancer diagnosis preceded or followed the herpes zoster diagnosis. The process of cancer registration in Ontario relies on records collected for other purposes, including hospital discharge and day surgery summaries that mention a diagnosis of cancer, pathology reports with any mention of cancer, records of patients referred to any of the 8 regional cancer centres in the province or the Princess Margaret Hospital in Toronto (the specialized institutions where Ontario cancer patients are treated), and death certificates with cancer recorded as the underlying cause of death. Approximately 400 000 records are submitted to the Ontario Cancer Registry each year. Cancer outcomes were identified until 31 Dec. 2010. Data were expressed in terms of person-years of follow-up, and the duration of follow-up ranged from a minimum of 9 months to a maximum of 5 years.
By definition, exposed cases had herpes zoster and unexposed controls did not. The controls were matched to cases by both age and sex. The date of diagnosis for an exposed case served as the baseline for matching the unexposed control. Simple proportions and means were used to compare the characteristics of exposed cases and unexposed controls. Time periods of 180 days, 1 year, 2 years, 3 years, 4 years, and 5 years were used to examine the relationship between cases and controls for the outcome of a cancer diagnosis using multivariate Cox proportional hazard models that controlled for urban v. rural setting, area-level income, and 6 comorbidities (acute myocardial infarction,15 asthma,16 congestive heart failure,17 chronic obstructive pulmonary disease [COPD],18 diabetes mellitus,19 and hypertension20). The graph of survival function versus survival time was inspected visually, to confirm that assumptions of the Cox proportional hazard models were met. Cases and controls were censored when cancer was diagnosed or at 5 years, whichever came first.
The study was approved by the Research Ethics Boards of St. Michael’s Hospital and Sunnybrook Health Sciences Centre in Toronto, Ontario.
Results
A total of 542 575 individuals received a diagnosis of herpes zoster from 1993 to 2010 and were included in the study, along with the same number of age- and sex-matched controls (see Table 1 for demographic characteristics). The mean age of cases and controls was 54.1 years, with the majority of cases (59.7%) occurring after age 49. The 2 groups were similar in terms of location of residence (urban v. rural) and income quintiles. Although the differences for these variables reached statistical significance, they were very small in absolute terms. The cases were more likely to have a history of myocardial infarction, asthma, congestive heart failure, COPD, diabetes, and hypertension (p < 0.001), but again the differences were slight.
Table 1.
Demographic characteristics of patients with and without a diagnosis of herpes zoster
The incidence of cancer was significantly greater among exposed cases than among unexposed controls across all time intervals studied (Table 2). The greatest adjusted hazard ratio (1.19, 95% confidence interval [CI] 1.12–1.25) was seen at 180 days after diagnosis of herpes zoster. The adjusted hazard ratio tended to decrease with increasing time from the diagnosis of herpes zoster.
Table 2.
Cumulative incidence of cancer from time of diagnosis of herpes zoster
For both men and women, the proportion of individuals in whom malignancy developed was greater among those with a prior diagnosis of herpes zoster than among those without herpes zoster. Similarly, for all age ranges studied, the risk of malignancy was greater among individuals with a prior episode of herpes zoster. For example, for the age groups 18–49 years, 50–64 years, 65–74 years, and 75 years and older, the relative increase in cancer risk following a diagnosis of herpes zoster was 0.24, 0.15, 0.13, and 0.14 per 100 person-years, respectively, at 1 year and 0.16, 0.10, 0.07, and 0.09 per 100 person-years, respectively, at 5 years.
The number of cancers diagnosed per 1000 person-years was greater among those with a diagnosis of herpes zoster for all time intervals and for all cancer types except colorectal cancer at 180 days and 4 years (Table 3). For all time periods, lymphoma was the type of cancer with the greatest relative increase in incidence following a herpes zoster diagnosis. The increase in risk was greatest (by 112%) at 180 days and remained high (by 46%) at 5 years. The greatest absolute increases in risk of malignancy following a herpes zoster diagnosis occurred for the combined category of “other” cancer types, followed by lymphoma, lung cancer, prostate cancer, and leukemia (with some variation in the exact order for different time periods).
Table 3.
Number and types of cancers in relation to time of diagnosis of herpes zoster in cases and controls
Interpretation
This matched retrospective cohort study examining the risk of malignancy following a diagnosis of herpes zoster is, to our knowledge, the largest published to date. The results demonstrate an association between herpes zoster and subsequent development of malignancy in both men and women and across all age categories studied. The greatest adjusted and unadjusted hazard ratios for cancer diagnosis following herpes zoster were seen at 180 days, with the hazard ratio persisting but declining over subsequent years. The increase in adjusted cancer risk following a herpes zoster diagnosis was 19% within the first 180 days after diagnosis and 11% at 1 year. Although this analysis demonstrated an increase in risk, the absolute increase was modest, at 1.34 per 1000 person-years at 1 year and 0.84 per 1000 person-years at 5 years. These values correspond to one additional cancer being diagnosed per 1000 people at 1 year after the diagnosis of herpes zoster and one additional cancer per 250 people at 5 years following diagnosis. The greatest relative increases in specific cancer types were for lymphoma and leukemia.
We also noted an association between the development of herpes zoster and the presence of comorbidities, namely myocardial infarction, asthma, congestive heart failure, COPD, diabetes, and hypertension. Previous studies have reported such an association for COPD,21 diabetes,22,23 and hypertension.22 Another study found an increased risk of undiagnosed diabetes (odds ratio 2.28, 95% CI 1.28–4.06) among patients presenting with herpes zoster diagnosis.24 It is possible that all of these conditions are associated with altered immunity, which leads to an increase in cases of herpes zoster. However, the exact mechanisms of the association have not been worked out.
A review of the literature revealed several studies documenting an association between diagnosis of herpes zoster and subsequent diagnosis of malignancy. In the earliest of these, Sørensen et al.25 compared the incidence of malignancy among patients admitted to hospital with herpes zoster with the expected rate of malignancy. The relative risk (RR) was reported as 1.2 (95% CI 1.1–1.2), with the risk being substantially elevated during the first year of follow-up (RR 1.3, 95% CI 1.1–1.5) and especially for hematological cancers (RR 3.4, 95% CI 2.3–4.9), specifically, non- Hodgkin’s lymphoma, multiple myeloma, and leukemia.
Buntinx et al.12 conducted a retrospective cohort study using a patient registry of 37 general practices in Belgium and found a statistically significant increase in cancer risk following a diagnosis of herpes zoster in all patients over the age of 65 (RR 1.85, 95% CI 1.18–2.90). When the data were stratified by sex, the increase was significant only for women (RR 2.65, 95% CI 1.43–4.90). There was no increased risk in the first year following diagnosis of herpes zoster (RR 1.75, 95% CI 0.82–3.75). Another retrospective cohort study26 revealed a 9.25-fold increase (95% CI 5.51–15.55) in risk of malignancy within the first year after a diagnosis of herpes zoster ophthalmicus. There were no significant differences in terms of cancer type.
In contrast to these findings, a recent retrospective cohort study did not support an association between herpes zoster and cancer. Wang et al.13 used the National Health Insurance Research Database of Taiwan to examine the incidence of cancer in 35 871 patients with herpes zoster. Patients with cancer were identified through applications for catastrophic illness certificates, and the rates of cancer in the study population were compared with national incidence rates. The authors concluded that a diagnosis of herpes zoster did not increase the risk of cancer overall (standardized incident ratio 0.99, 95% CI 0.93–1.06) but did increase the risk of multiple myeloma (standardized incident ratio 2.03, 95% CI 1.01–3.63) and of bone and soft-tissue cancers combined (standardized incident ratio 2.03, 95% CI 1.11–3.41).
Our study is among the first to use a population-based approach in examining the potential association between herpes zoster and cancer. It supports previous work showing that cancer occurs early after herpes zoster and that hematological cancers are most strongly implicated. Unlike other previous work, it did not reveal any differences between the sexes in cancer occurrence. We examined data for an extended period after the diagnosis of herpes zoster and found that although the risk diminished over time, it persisted for up to 5 years.
This research had some limitations. The data used in the study were collected for purposes other than research, and the OHIP diagnostic code for herpes zoster has not been validated. However, the Ontario Cancer Registry has undergone extensive validation.27 The large number of exposed cases and unexposed controls means that statistical significance may have been found even for small absolute differences. The main results did show an increase in cancer risk following herpes zoster diagnosis ranging between 8% and 19%, depending on the time following diagnosis. It is also possible that the increased risk of malignancy in the first 180 days following herpes zoster diagnosis could be due, in part, to greater clinician attention to the possibility of cancer immediately after the diagnosis of herpes zoster.
Conclusions
There is a risk that malignancy will develop following an episode of herpes zoster, and this risk is present for both men and women and for all age groups 18 years and over. The risk was greatest in the first 180 days following the diagnosis of herpes zoster and persisted for at least 5 years. Clinicians are encouraged to be vigilant for malignancy in patients presenting with herpes zoster; however, screening cannot be recommended because of the modest increase in risk and the lack of specificity with respect to cancer type.
Acknowledgments
The authors thank Morgan Slater for her assistance with editing and preparation for journal submission.
Biographies
Karl Iglar, MD, CCFP, is a Family Physician with the Department of Family and Community Medicine at St. Michael’s Hospital and an Associate Professor in the Department of Family and Community Medicine at the University of Toronto, Toronto, Ontario.
Alexander Kopp, BA, is a Senior Analyst with the Institute for Clinical Evaluative Sciences, Toronto, Ontario.
Richard H. Glazier, MD, MPH, FCFP, is a Senior Scientist at the Institute for Clinical Evaluative Sciences; a Professor in the Department of Family and Community Medicine and the Dalla Lana School of Public Health, University of Toronto; a Scientist at the Centre for Research on Inner City Health in the Keenan Research Centre of the Li Ka Shing Knowledge Institute, St. Michael’s Hospital; and a Clinician Scientist and Family Physician in the Department of Family and Community Medicine, St. Michael’s Hospital, Toronto, Ontario.
Footnotes
Competing interests: None declared.
Funding source: This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Karl Iglar, Alexander Kopp, and Richard Glazier designed the study protocol and were involved in analysis and interpretation of the data. Karl Iglar wrote the first draft of the manuscript, with assistance from Alexander Kopp and Richard Glazier, and all authors contributed to critically revising the manuscript. Karl Iglar is guarantor for the manuscript and takes responsibility for the contents and appropriateness of the reference list.
References
- 1.Mounsey Anne L, Matthew Leah G, Slawson David C. Herpes zoster and postherpetic neuralgia: prevention and management. Am Fam Physician. 2005 Sep 15;72(6):1075–1080. http://www.aafp.org/link_out?pmid=16190505. [PubMed] [Google Scholar]
- 2.High Kevin P. Preventing herpes zoster and postherpetic neuralgia through vaccination. J Fam Pract. 2007;56(10 Suppl A):51–57. http://www.scholaruniverse.com/ncbi-linkout?id=17949604. [PubMed] [Google Scholar]
- 3.Whitley Richard J. A 70-year-old woman with shingles: review of herpes zoster. JAMA. 2009 Jun 2;302(1):73–80. doi: 10.1001/jama.2009.822. http://www.scholaruniverse.com/ncbi-linkout?id=19491172. Errata in: JAMA 2009;302(17):1864. JAMA 2010;303(8):734. [DOI] [PubMed] [Google Scholar]
- 4.Gnann John W, Whitley Richard J. Clinical practice. Herpes zoster. N Engl J Med. 2002 Aug 1;347(5):340–346. doi: 10.1056/NEJMcp013211. http://www.nejm.org/doi/abs/10.1056/NEJMcp013211. [DOI] [PubMed] [Google Scholar]
- 5.Stankus S J, Dlugopolski M, Packer D. Management of herpes zoster (shingles) and postherpetic neuralgia. Am Fam Physician. 2000 Apr 15;61(8):2437–2444. http://www.aafp.org/link_out?pmid=10794584. [PubMed] [Google Scholar]
- 6.Wareham David W, Breuer Judith. Herpes zoster. BMJ. 2007 Jun 9;334(7605):1211–1215. doi: 10.1136/bmj.39206.571042.AE. http://www.bmj.com/cgi/doi/10.1136/bmj.39206.571042.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith J B, Fenske N A. Herpes zoster and internal malignancy. South Med J. 1995;88(11):1089–1092. doi: 10.1097/00007611-199511000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Anderson Lesley A, Landgren Ola, Engels Eric A. Common community acquired infections and subsequent risk of chronic lymphocytic leukaemia. Br J Haematol. 2009;147(4):444–449. doi: 10.1111/j.1365-2141.2009.07849.x. http://doi.wiley.com/10.1111/j.1365-2141.2009.07849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doody Michele M, Linet Martha S, Glass Andrew G, Friedman Gary D, Pottern Linda M, Boice John D, Jr, Fraumeni Joseph F. Leukemia, lymphoma, and multiple myeloma following selected medical conditions. Cancer Causes Control. 1992;3(5):449–456. doi: 10.1007/BF00051358. http://www.scholaruniverse.com/ncbi-linkout?id=1525326. [DOI] [PubMed] [Google Scholar]
- 10.Ragozzino Mark W, Melton L. Joseph, 3rd, Kurland Leonard T, Chu Chu P, Perry Harold O. Risk of cancer after herpes zoster: a population-based study. N Engl J Med. 1982 Aug 12;307(7):393–397. doi: 10.1056/NEJM198208123070701. [DOI] [PubMed] [Google Scholar]
- 11.Fueyo Michael A, Lookingbill Donald P. Herpes zoster and occult malignancy. J Am Acad Dermatol. 1984;11(3):480–482. doi: 10.1016/S0190-9622(84)70195-2. http://linkinghub.elsevier.com/retrieve/pii/S0190962284701952. [DOI] [PubMed] [Google Scholar]
- 12.Buntinx Frank, Wachana Richard, Bartholomeeusen Stefaan, Sweldens Kathleen, Geys Helena. Is herpes zoster a marker for occult or subsequent malignancy? Br J Gen Pract. 2005;55(511):102–107. http://europepmc.org/abstract/MED/15720930. [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Yu Ping, Liu Chia Jen, Hu Yu Wen, Chen Tzeng Ji, Lin Yi Tsung, Fung Chang Phone. Risk of cancer among patients with herpes zoster infection: a population-based study. CMAJ. 2012 Sep 17;184(15):E804–E809. doi: 10.1503/cmaj.120518. http://www.cmaj.ca/cgi/pmidlookup?view=long&pmid=22988158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goel V, Williams J I, Anderson G M, Blackstien-Hirsch P, Fooks C, Naylor C D, editors. Patterns of health care in Ontario. The ICES practice atlas. 2nd ed. Ottawa (ON): Canadian Medical Association; 1996. p. 340. [Google Scholar]
- 15.Tu Jack V, Austin Peter C, Walld Randy, Roos Leslie, Agras Jean, McDonald Kathryn M. Development and validation of the Ontario acute myocardial infarction mortality prediction rules. J Am Coll Cardiol. 2001;37(4):992–997. doi: 10.1016/S0735-1097(01)01109-3. http://www.scholaruniverse.com/ncbi-linkout?id=11263626. [DOI] [PubMed] [Google Scholar]
- 16.Gershon Andrea S, Wang Chengning, Guan Jun, Vasilevska-Ristovska Jovonka, Cicutto Lisa, To Teresa. Identifying patients with physician-diagnosed asthma in health administrative databases. Can Respir J. 2009;16(6):183–188. doi: 10.1155/2009/963098. http://europepmc.org/abstract/MED/20011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeung Darwin F, Boom Nicole K, Guo Helen, Lee Douglas S, Schultz Susan E, Tu Jack V. Trends in the incidence and outcomes of heart failure in Ontario, Canada: 1997 to 2007. CMAJ. 2012;184(14):E765–E773. doi: 10.1503/cmaj.111958. http://www.cmaj.ca/cgi/pmidlookup?view=long&pmid=22908143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gershon A S, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physician diagnosed COPD in health administrative databases. COPD. 2009;6(5):388–394. doi: 10.1080/15412550903140865. http://informahealthcare.com/doi/abs/10.1080/15412550903140865. [DOI] [PubMed] [Google Scholar]
- 19.Hux Janet E, Ivis Frank, Flintoft Virginia, Bica Adina. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512–516. doi: 10.2337/diacare.25.3.512. http://www.scholaruniverse.com/ncbi-linkout?id=11874939. [DOI] [PubMed] [Google Scholar]
- 20.Tu Karen, Campbell Norman R C, Chen Zhong Liang, Cauch-Dudek Karen J, McAlister Finlay A. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1(1):e18–e26. http://www.openmedicine.ca/article/view/17/23. [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Ya Wen, Chen Yi Hua, Wang Kuo Hsien, Wang Chen Yi, Lin Hui Wen. Risk of herpes zoster among patients with chronic obstructive pulmonary disease: a population-based study. CMAJ. 2011;183(5):e275–e280. doi: 10.1503/cmaj.101137. http://www.cmaj.ca/cgi/pmidlookup?view=long&pmid=21343261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hata A, Kuniyoshi M, Ohkusa Y. Risk of Herpes zoster in patients with underlying diseases: a retrospective hospital-based cohort study. Infection. 2011 Jul 29;39(6):537–544. doi: 10.1007/s15010-011-0162-0. http://link.springer.com/10.1007/s15010-011-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heymann A D, Chodick G, Karpati T, Kamer L, Kremer E, Green M S, Kokia E, Shalev V. Diabetes as a risk factor for herpes zoster infection: results of a population-based study in Israel. Infection. 2008 May 3;36(3):226–230. doi: 10.1007/s15010-007-6347-x. http://link.springer.com/10.1007/s15010-007-6347-x. [DOI] [PubMed] [Google Scholar]
- 24.Nassaji-Zavareh Mohammad, Taheri Ramin, Ghorbani Raheb, Aminian Maryam. Undiagnosed diabetes mellitus in patients with herpes zoster. Indian J Dermatol. 2008;53(3):119–121. doi: 10.4103/0019-5154.43211. http://www.e-ijd.org/text.asp?2008/53/3/119/43211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sørensen H T, Olsen J H, Jepsen P, Johnsen S P, Schønheyder H C, Mellemkjaer L. The risk and prognosis of cancer after hospitalisation for herpes zoster: a population-based follow-up study. Br J Cancer. 2004 Oct 4;91(7):1275–1279. doi: 10.1038/sj.bjc.6602120. http://dx.doi.org/10.1038/sj.bjc.6602120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho Jau Der, Xirasagar Sudha, Lin Herng Ching. Increased risk of a cancer diagnosis after herpes zoster ophthalmicus: a nationwide population-based study. Ophthalmology. 2011;118(6):1076–1081. doi: 10.1016/j.ophtha.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Hall Stephen, Schulze Karleen, Groome Patti, Mackillop William, Holowaty Eric. Using cancer registry data for survival studies: the example of the Ontario Cancer Registry. J Clin Epidemiol. 2006;59(1):67–76. doi: 10.1016/j.jclinepi.2005.05.001. http://linkinghub.elsevier.com/retrieve/pii/S089543560500185X. [DOI] [PubMed] [Google Scholar]