Abstract
Objective
Radiographic measures of the pathologic changes of knee osteoarthritis (OA) have shown modest associations with clinical pain. We sought to evaluate possible differences in quantitative sensory testing (QST) results and psychosocial distress profiles between knee OA patients with discordant versus congruent clinical pain reports relative to radiographic severity measures.
Methods
A total of 113 participants (66.7% women; mean ± SD age 61.05 ± 8.93 years) with knee OA participated in the study. Radiographic evidence of joint pathology was graded according to the Kellgren/ Lawrence scale. Central sensitization was indexed through quantitative sensory testing, including heat and pressure–pain thresholds, tonic suprathreshold pain (cold pressor test), and repeated phasic suprathreshold mechanical and thermal pain. Subgroups were constructed by dichotomizing clinical knee pain scores (median split) and knee OA grade scores (grades 1–2 versus 3–4), resulting in 4 groups: low pain/low knee OA grade (n = 24), high pain/high knee OA grade (n = 32), low pain/high knee OA grade (n = 27), and high pain/low knee OA grade (n = 30).
Results
Multivariate analyses revealed significantly heightened pain sensitivity in the high pain/low knee OA grade group, while the low pain/high knee OA grade group was less pain-sensitive. Group differences remained significant after adjusting for differences on psychosocial measures, as well as age, sex, and race.
Conclusion
The results suggest that central sensitization in knee OA is especially apparent among patients with reports of high levels of clinical pain in the absence of moderate-to-severe radiographic evidence of pathologic changes of knee OA.
Osteoarthritis (OA) is the most common form of arthritis and is characterized by joint degeneration and chronic, sometimes severely disabling pain. Standard objective assessment of pathologic changes in the joint is typically accomplished via radiography to evaluate the presence of osteophytes and joint space narrowing. Radiographic evidence, however, has been shown to have variable predictive validity as a marker of subjective clinical pain, with some population-based studies reporting weak correlations between the two (1–3) and others reporting strong correlations (4,5). The use of more advanced imaging techniques, such as magnetic resonance imaging (MRI), has not clarified the source of pain in OA (6). Some investigations have found that psychological factors, such as depression and anxiety, may partially explain the apparent discordance between objective measures and subjective pain reports (7). However, it is unlikely that such wide variability in population estimates can be attributed to psychological factors alone.
Theorists have therefore proffered that the discrepancy between pain and radiographic changes may be explained by the propensity of some OA patients to develop sensitized central nociceptive circuits that enhance pain during various states of peripheral tissue insult (8,9). This abnormality, known as central sensitization, is a maladaptive nociceptive process involving complex pain-amplifying neuroplastic alterations at multiple levels of the neuraxis (10). Since central sensitization is correlated with activation of neural circuits that are implicated in the descending facilitation of pain (11) and is therefore a risk factor for the development and maintenance of chronic pain (12), it is important to identify which patients exhibit abnormal responses to relevant painful stimuli.
Hip OA patients with referred pain have been shown to demonstrate hyperalgesia on quantitative sensory testing (QST) in the areas of referred pain, and these psychophysical responses correlate with functional MRI signals in areas associated with central pain modulation, including the anterior cingulate cortex (13). Knee OA patients have been shown to vary in local and diffuse sensitization on QST as a function of reports of clinical pain (14). Further, those reporting severe pain, but not those reporting mild pain, are more sensitive to local pressure stimulation than are healthy controls (14). Together, these findings show that central processes underlie a portion of the variability in the experience of pain in OA and suggest that simple clinical and experimental tools may be applied to identify those most at risk.
Recent reviews highlight the utility of multisite assessment of pain thresholds, assessment of responses to repeated noxious stimuli (e.g., temporal summation), and evaluation of sensitivity to tonic noxious stimulation as indices of sensitization within the central nervous system (15,16). However, to our knowledge, no study has yet investigated variances in response to QST between groups of knee OA patients who differ with regard to the relative congruence of clinical pain reports and radiographic disease severity. Woolf (16) has noted that the degree of sensitization in OA patients correlates with clinical pain reports but not with radiographic findings, and he suggested that further study of this potential mismatch may help to illuminate individual differences in OA-related symptoms. If subgroups of knee OA patients identified by common symptom profiles can be characterized as having more or less central sensitization, we may be able to better target clinical care, and we may gain a better understanding of factors that contribute to vulnerability or resilience to developing central sensitization over time.
The purpose of this study was to evaluate whether subgroups of knee OA patients defined by the relative congruence of clinical pain and radiographic severity differed on QST measures of central sensitization, after controlling for the potential contribution of psychological factors. We hypothesized that knee OA patients with self reports of elevated levels of clinical pain in the absence of moderate-to-severe radiographic evidence of pathologic changes of knee OA would be hypersensitive to QST measures of central sensitization.
PATIENTS AND METHODS
Study participants
The data from the current study were taken from a larger parent study designed to examine the efficacy of different psychosocial treatments for pain and insomnia in knee OA patients with and without insomnia (Smith MT, et al: manuscript in preparation). The data presented here were taken exclusively from the baseline assessment. Participants were 113 men (n = 38) and women (n = 75) who met the American College of Rheumatology (ACR) criteria for knee OA (17). Participants were recruited via advertisements in community media outlets and physicians’ offices. To be included in the study, participants had to meet the ACR criteria for knee OA, as diagnosed by a board certified rheumatologist (UJH) based on the patient’s history, physical examination, and radiographic (bilateral standing and semiflexion views) findings; have a score of at least 1 on the Kellgren/Lawrence scale in one or both knees; and have knee pain scored >2 on a 10-point scale on a near-daily basis (>4 days/week) for at least 6 months prior to entering the study, as determined during clinical interview.
Patients with serious medical illnesses, such as congestive heart failure, history of cerebrovascular accidents, cancer, or other chronic pain or rheumatic disorders, or joint replacement were excluded. Subjects were also excluded if they were diagnosed as having severe or unstable psychopathology, cognitive impairment/dementia, current substance abuse disorder, or positive findings on toxicology screening.
Study participants agreed to discontinue all pain-relieving and sedative medications 24 hours prior to pain testing. A majority of the study patients (76%) had been diagnosed as having insomnia, which is only slightly higher than the general OA population estimates (18).
Procedures
All participants completed an informed consent form, were administered a battery of questionnaires, and underwent clinical interviews and bilateral knee radiography. A subsequent visit included a knee examination conducted by a rheumatologist (UJH). Clinical pain was assessed with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (19). Knees were graded by a rheumatologist (UJH) using the Kellgren/Lawrence scale (scores ranging from 0 = normal to 4 = severe) (20). Symptoms of anxiety (using the State-Trait Anxiety Inventory) (21), depression (using the Center for Epidemiologic Studies Depression Scale) (22), pain catastrophizing (using the Pain Catastrophizing Scale) (23), and sleep disturbance (using the Pittsburgh Sleep Quality Index) (24) were measured via self report. Participants additionally underwent a series of quantitative sensory tests.
Quantitative sensory testing
Following precedent by other groups of investigators (15,16,25) and our own work in this field (26,27), we performed a multimodal assessment of pain responses to assess central sensitization. QST included sampling of pressure–pain thresholds (PPT) at affected and unaffected anatomic sites and responses to both repeated and sustained suprathreshold stimuli. PPT sampling was done first, followed by repeated phasic mechanical stimuli, repeated phasic thermal stimuli (order randomized for the repeated phasic stimuli), and finally, the cold pressor test (CPT). No participants dropped out of the study due to QST burden.
Central sensitization was operationalized in two ways (see ref. 9): as hypersensitivity to suprathreshold measures of repeated mechanical or thermal phasic pain, and as hypersensitivity to QST measures at unaffected anatomic sites distal to the index knee, including PPT at the trapezius and CPT. The index knee was the knee in which OA had been diagnosed. If OA was diagnosed in both knees, the participant was asked to rate their pain in each, and the knee that was rated as more painful was selected as the index knee for QST. If both knees were rated equally painful, the index knee for QST was selected randomly. To test group differences in the related phenomenon of pain inhibitory processing, or conditioned pain modulation (CPM) (28), we administered 2 competing noxious stimuli at distal anatomic sites (the hand and the trapezius).
Determination of the pressure–pain threshold
PPT was assessed via algometry (1-cm2 hard rubber probe; Somedic) 2 times at the trapezius muscle (bilaterally) and at the insertion point of the quadriceps (index knee), according to standard procedures (29). The mean PPT values (in kPa) were averaged across trials.
Determination of mechanical phasic pain
Pain ratings were gathered in response first to a single stimulus and then to a sequence of 10 stimuli of identically weighted (256 mN) punctate noxious probes applied on a flat contact area of 0.2 mm in diameter, separately, to the dorsal surface of the middle finger (nondominant hand) and the patella (index knee). The 10-stimulus train was delivered with an interstimulus interval of 1 second, which was guided by a metronome. Participants rated the stimuli from 0 (no pain) to 100 (worst pain imaginable). The short interstimulus interval is designed to induce temporal summation of pain, which is an indicator of central sensitization. Average mechanical pain ratings were calculated in order to index suprathreshold pain sensitivity to the mechanical phasic stimuli. Similar procedures assessing responses to repetitive suprathreshold noxious stimuli as an index of central sensitization have been used in healthy subjects (30) as well as to assess neuropathic pain (31,32). This procedure was conducted with both a 256-mN and a 512-mN probe. In an effort to reduce the number of measures included for analysis, we chose to use the lowest stimulus for which an increase in pain from the first to the tenth pulse was observed. The 256-mN probe produced such an increase and was therefore included in the present analysis.
Determination of thermal phasic pain
Pain ratings (0–100 scale) were gathered in response to each of 10 heat pulses of equal temperature (51°C) applied to the nondominant dorsal forearm by a 9-cm2 probe attached to a Medoc Contact Heat-Evoked Potential Stimulator (33). If a participant discontinued the test prior to the tenth pulse, the last pain rating was carried forward. To index suprathreshold pain sensitivity to the thermal phasic stimuli, an average of the 10 pain ratings was calculated for each participant. This procedure was conducted at both 49°C and 51°C. Again, we chose to include the lowest stimulus for which an increase in pain was observed from the first to the tenth pulse across all subjects. The 51°C, but not the 49°C, stimulus produced such an increase and was therefore included in the analysis of group differences in suprathreshold thermal phasic pain. This temperature has been used in prior studies of repeated thermal stimulation (34).
Determination of suprathreshold sensitivity to tonic pain
Pain ratings were assessed with the CPT. Participants immersed the nondominant hand in a circulating water bath maintained at ~4°C. A total of 2 immersions lasting for a maximum of 45 seconds were conducted. Participants were permitted to remove their hand prior to the completion of the trial if the pain became intolerable. Pain ratings on a scale of 0–100 were obtained immediately after participants removed their hand from the water. An average of pain ratings was calculated across the 2 trials.
Conditioned pain modulation
Two PPT readings (in kPa) were obtained bilaterally from the trapezius muscle immediately prior to the hand submersion test in the first CPT. Immediately upon hand withdrawal in the first CPT trial, a PPT reading was obtained on the trapezius muscle. The same procedure was conducted after the second CPT trial, obtaining a reading for the contralateral trapezius muscle. To index the CPM, the 2 PPT values obtained following each of the CPT trials were averaged and then divided by the average of the 2 baseline PPT readings. Resulting CPM index values >1 reflect pain inhibition through diffuse noxious inhibitory controls, which has been shown to be centrally mediated through lower brainstem functioning (28).
Statistical analysis
Subgroups were constructed according to a dichotomous division of WOMAC knee pain scores (median split: low pain ≤4.22 versus high pain >4.22) and Kellgren/Lawrence scores (grades 1–2 versus 3–4), resulting in 4 groups: low pain/low knee OA grade (n = 24), high pain/high knee OA grade (n = 32), low pain/high knee OA grade (n = 27), and high pain/low knee OA grade (n = 30). This grouping variable was then entered as a factor in 4 separate multivariate general linear models. Multivariate general linear modeling was chosen as the primary analytic framework because it allows for the testing of multiple dependent variables in a single model, which has the effect of partialling out covariance between the dependent variables and reducing Type I error. The first model tested group differences in the following psychosocial variables: pain catastrophizing, sleep disturbance, anxiety symptoms, and depression symptoms. The second model tested group differences in QST measures at affected sites proximal to the index knee: mechanical phasic pain at the patella and PPT at the quadriceps muscle. The third model tested group differences in QST measures at unaffected sites distal to the index knee: mechanical phasic pain at the finger, thermal phasic pain at the forearm, PPT at the trapezius, and CPT pain. A fourth univariate model tested group differences in CPM.
All models were run with age, race, and sex included as a priori covariates, since each of these variables has been shown in previous studies to explain individual differences in QST measures (35). Because of the high rate of insomnia in the sample, sleep disturbance and psychosocial variables were tested as covariates in all QST models. Significance of the multivariate tests was evaluated using Wilks’ λ. To minimize the chance of detecting spurious group differences, we used the “protected t-test” procedure, whereby tests of group differences in individual dependent variables within the multivariate set were interpreted only if the omnibus test was significant (36). Additionally, we corrected for multiple comparisons with Dunnett’s procedure (37), which applies a post hoc correction by comparing a reference group to all other groups. This correction method was appropriate because our hypotheses designated the high pain/low knee OA grade group a priori as the group of interest. Further, the protected t-test approach coupled with Dunnett’s procedure was preferable to the more conservative Bonferroni correction, which limits power, biases alpha, and increases Type II errors when measures are correlated (38). Finally, we present partial η2 estimates of effect size (i.e., percentage of variance explained) to aid in the interpretation of results. All analyses were conducted in SPSS version 19 software.
RESULTS
Demographic characteristics and descriptive statistics
Table 1 presents the demographic characteristics of the study sample of patients with knee OA. The majority of participants were female (66.7%) and Caucasian (62.4%), although African Americans were well-represented (35%). Seventy-six percent of the patients had comorbid insomnia, with the highest incidence (93.5%) observed among the high pain/low knee OA grade group and the lowest incidence (55.6%) observed among the low pain/high knee OA grade group.
Table 1.
Characteristics of the study patients with knee OA*
| Low pain/low knee OA grade (n = 24) | High pain/high knee OA grade (n = 32) | Low pain/high knee OA grade (n = 27) | High pain/low knee OA grade (n = 30) | Total (n = 113) | |
|---|---|---|---|---|---|
| Age, mean ± SD years | 62.04 ± 9.29 | 61.62 ± 9.63 | 63.81 ± 8.62 | 57.23 ± 7.07 | 61.05 ± 8.93 |
| % female | 68 | 79.4 | 48.1 | 67.7 | 66.7 |
| Race, % | |||||
| African American | 32 | 41.2 | 22.2 | 41.9 | 35 |
| Caucasian | 68 | 55.9 | 74.1 | 54.8 | 62.4 |
| % college graduates | 68 | 38.2 | 77.8 | 35.5 | 55.6 |
| Employment, % | |||||
| Employed | 52 | 26.4 | 37 | 51.6 | 41.1 |
| Retired | 28 | 52.9 | 51.9 | 29 | 41 |
| Unemployed | 20 | 20.6 | 11.1 | 19.3 | 17.9 |
| % with annual income >$50,000 | 56.5 | 45.1 | 59.2 | 25 | 45.8 |
| BMI, mean ± SD kg/m2 | 27.81 ± 4.11 | 33.59 ± 6.11 | 30.89 ± 5.82 | 30.68 ± 5.73 | 30.94 ± 5.85 |
| OA duration, mean ± SD years | 6.15 ± 5.59 | 6.91 ± 4.81 | 6.93 ± 5.46 | 6.00 ± 6.24 | 6.53 ± 5.43 |
| % with insomnia | 72 | 79.4 | 55.6 | 93.5 | 76.1 |
OA = osteoarthritis; BMI = body mass index.
Table 2 presents descriptive statistics for the primary study variables across the 4 pain/knee OA subgroups. Bivariate correlations of the primary study variables are shown in Table 3.
Table 2.
Descriptive statistics for primary study measures, by study group*
| Low pain/low knee OA grade | High pain/high knee OA grade | Low pain/high knee OA grade | High pain/low knee OA grade | |
|---|---|---|---|---|
| Pressure–pain threshold, trapezius, kPa | 395.51 ± 208.65 | 315.59 ± 110.69 | 434.58 ± 144.76 | 321.07 ± 120.44 |
| Cold pressor test, pain (range 0–100) | 69.85 ± 24.55 | 76.66 ± 27.10 | 68.80 ± 16.90 | 75.68 ± 22.51 |
| Mechanical phasic stimuli, finger (range 0–100) | 28.33 ± 28.77 | 31.00 ± 24.47 | 24.48 ± 18.25 | 44.31 ± 30.40 |
| Thermal phasic stimuli, arm (range 0–100) | 72.77 ± 23.84 | 70.64 ± 30.68 | 63.66 ± 21.50 | 83.65 ± 18.53 |
| Mechanical phasic stimuli, patella (range 0–100) | 30.54 ± 30.64 | 37.47 ± 29.84 | 22.32 ± 19.90 | 44.59 ± 33.33 |
| Pressure–pain threshold, quadriceps, kPa | 560.63 ± 250.29 | 444.84 ± 185.61 | 616.10 ± 225.13 | 439.07 ± 228.15 |
| Conditioned pain modulation index | 1.27 ± 0.25 | 1.27 ± 0.31 | 1.25 ± 0.23 | 1.23 ± 0.19 |
| Pain catastrophizing (range 0–56) | 12.04 ± 12.07 | 14.03 ± 11.24 | 8.52 ± 7.25 | 20.89 ± 12.11 |
| Sleep disturbance (range 0–21) | 9.08 ± 4.76 | 10.41 ± 4.56 | 7.44 ± 4.56 | 12.19 ± 4.74 |
| Anxiety symptoms (range 20–80) | 30.65 ± 8.83 | 33.36 ± 8.45 | 28.26 ± 7.69 | 35.77 ± 11.63 |
| Depression symptoms (range 0–60) | 10.17 ± 8.83 | 14.37 ± 8.87 | 6.93 ± 6.50 | 15.41 ± 9.46 |
Values are the mean ± SD. OA = osteoarthritis.
Table 3.
Correlations of primary study measures
| Measure
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| 1 Pressure–pain threshold, trapezius | 1 | −0.18 | −0.37* | −0.25* | −0.36* | 0.77* | −0.17 | −0.14 | −0.21† | −0.18 | −0.26* |
| 2 Cold pressor test, pain | 1 | 0.39* | 0.46* | 0.45* | −0.25* | −0.07 | 0.19† | 0.10 | 0.06 | 0.17 | |
| 3 Mechanical phasic stimuli, finger | 1 | 0.57* | 0.74* | −0.44* | 0.00 | 0.19† | 0.08 | 0.10 | 0.19† | ||
| 4 Thermal phasic stimuli, arm | 1 | 0.58* | −0.30* | 0.00 | 0.11 | 0.07 | 0.03 | 0.13 | |||
| 5 Mechanical phasic stimuli, patella | 1 | −0.41* | −0.06 | 0.19 | 0.15 | 0.15 | 0.14 | ||||
| 6 Pressure–pain threshold, quadriceps | 1 | −0.09 | −0.19 | −0.19 | −0.26* | −0.30* | |||||
| 7 Conditioned pain modulation | 1 | −0.06 | −0.01 | 0.05 | 0.03 | ||||||
| 8 Pain catastrophizing | 1 | 0.46* | 0.50* | 0.46* | |||||||
| 9 Sleep disturbance | 1 | 0.42* | 0.60* | ||||||||
| 10 Anxiety symptoms | 1 | 0.63* | |||||||||
| 11 Depression symptoms | 1 | ||||||||||
P < 0.01.
P < 0.05.
Pain/knee OA grade subgroup differences in psychosocial measures
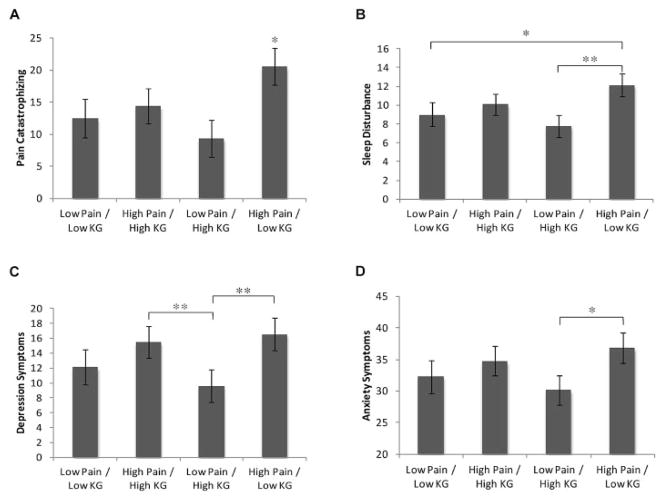
The omnibus multivariate test of pain/knee OA grade group membership on psychosocial function measures was significant (Wilks’ λ F[12,290] = 1.79, P = 0.049, partial η2 = 0.07), with significant effects on pain catastrophizing (F[3,109] = 4.61, P = 0.005, partial η2 = 0.12), sleep disturbance (F[3,109] = 4.13, P = 0.008, partial η2 = 0.11), and symptoms of depression (F[3,109] = 3.67, P = 0.015, partial η2 = 0.10), and a marginal, nonsignificant effect observed for symptoms of anxiety (P = 0.06).
The estimated marginal means for each group, with statistical significance of post hoc contrasts between groups, are shown in Figure 1. The high pain/low knee OA grade group reported significantly greater pain catastrophizing than all other groups, significantly greater sleep disturbance than both low pain groups, and significantly higher anxiety and depression symptoms than the low pain/high knee OA grade group. All contrasts survived Dunnett’s correction for multiple comparisons.
Figure 1.
Group differences on psychosocial variables as a function of high versus low pain and high versus low knee osteoarthritis grade (KG). Estimated marginal means for between-group differences in A, pain catastrophizing (measured with the Pain Catastrophizing Scale), B, sleep disturbance (measured with the Pittsburgh Sleep Quality Index), C, depression symptoms (measured with the Center for Epidemiologic Studies Depression Scale), and D, anxiety symptoms (measured with the State-Trait Anxiety Inventory) are presented. Values are the estimated marginal mean ± SEM. * = P < 0.05 for the indicated comparisons and versus all other groups in A; ** = P < 0.01 for the indicated comparisons.
Due to the significant between-group differences in sleep and psychosocial functioning, these variables were tested as covariates in the models for QST. None of the sleep or psychosocial functioning variables were statistically significant covariates in QST models, so they were removed from the final models.
Pain/knee OA grade subgroup differences in QST measures at affected sites
The results of the omnibus multivariate test of pain/knee OA grade group membership on QST measures at affected sites (i.e., proximal to the index knee) were not significant (Wilks’ λ F[6,190] = 1.64, P = 0.14). Neither of the between-subject tests of individual measures of PPT on the quadriceps muscle and repeated punctate probes on the patella was significant. Post hoc contrasts were not interpreted because of the failure of this omnibus model to reach significance.
Pain/knee OA grade subgroup differences in QST measures at unaffected sites
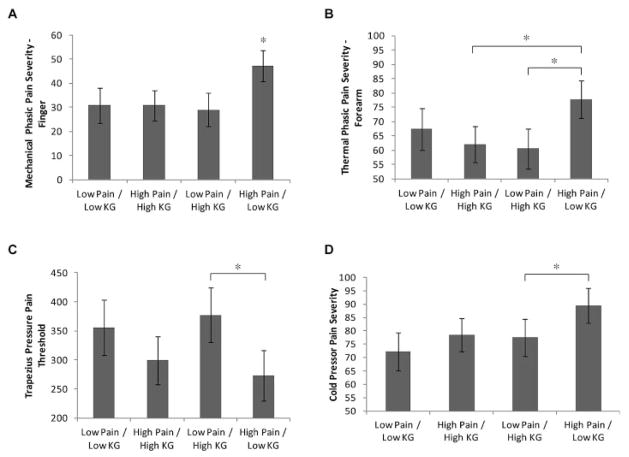
The results of the omnibus multivariate test of pain/knee OA grade group membership on QST measures at unaffected sites (i.e., distal to the index knee) were significant (Wilks’ λ F[12,230] = 1.82, P = 0.04, partial η2 = 0.06). Between-subject group effects were observed for mechanical phasic pain in the finger (F[3,98] = 3.45, P = 0.02, partial η2 = 0.10) and for thermal phasic pain in the forearm (F[3,98] = 2.95, P = 0.04, partial η2 = 0.09). The tests for group effects on cold pressor pain and trapezius PPT approached the a priori significance threshold (P = 0.07, for both comparisons).
The estimated marginal means, with statistical significance of post hoc contrasts between groups, are shown in Figure 2. Post hoc contrasts revealed that the high pain/low knee OA grade group had significantly greater pain in response to the mechanical phasic stimuli in the finger than each of the other groups and significantly more thermal phasic pain in the forearm than the low pain/high knee OA grade group and the high pain/high knee OA grade group. The high pain/low knee OA grade group also had a significantly lower trapezius muscle PPT and significantly higher pain ratings during cold pressor testing than the low pain/high knee OA grade group. After Dunnett’s correction for multiple comparisons, only the thermal phasic pain contrast with the high pain/high knee OA grade group fell beyond the threshold of significance (P = 0.06). Because group differences were observed in income, education, and employment status (Table 2), we conducted an additional post hoc analysis to determine if these variables accounted for variance in the group effects observed. Although income (P = 0.04) and education (P = 0.000) were both significant covariates, their inclusion in the overall model did not alter the significance of any of the between-group effects.
Figure 2.
Group differences on quantitative sensory testing measures of central sensitization at unaffected sites distal to the index knee. Groups were determined as a function of high versus low pain and high versus low knee osteoarthritis grade (KG). Estimated marginal means (± SEM) for between-group differences in A, mechanical phasic pain severity in the finger, B, thermal phasic pain severity in the forearm, C, pressure–pain threshold in the trapezius muscle, and D, cold pressor pain severity are shown. * = P < 0.05 for the indicated comparisons and versus all other groups in A.
Taken together, the data show that knee OA patients reporting high levels of clinical pain in the absence of moderate-to-severe radiographic knee OA exhibit hypersensitivity across threshold and supra-threshold QST measures at unaffected anatomic sites distal to the index knee, suggesting that this group may be more vulnerable to the development of central sensitization. Conversely, patients reporting low levels of clinical pain in the presence of moderate-to-severe radiographic evidence were less pain sensitive on most measures, suggesting that this group may be resilient to the development of central sensitization.
Pain/knee OA grade subgroup differences in conditioned pain modulation
The between-subjects effect of pain/knee OA grade group membership on CPM was not significant (P = 0.85).
DISCUSSION
In the current study, we sought to characterize the QST and psychosocial profiles of subgroups of knee OA patients defined according to the relative congruence of clinical pain and radiographic severity of knee OA. Several novel findings emerged from the analyses. Consistent with our hypotheses, patients with elevated levels of clinical pain in the absence of moderate-to-severe radiographic evidence of pathologic changes were consistently the most pain sensitive across measures distal to the index knee, suggesting evidence of central sensitization. The pattern of findings is evidence against the possibility that QST differences were simply a function of the reporting of high levels of clinical pain. If this were true, the 2 groups with high levels of pain should have been equivalent across measures. Instead, the high pain/low knee OA grade emerged as the most pain-sensitive group. Thus, these findings suggest that central sensitization may be an endophenotype marker of chronic pain in knee OA patients who report high levels of clinical pain in the absence of moderate-to-severe radiographic evidence of pathology, which may help to explain the often-noted phenomenon of minimal-to-modest relationship between self-reported pain and radiographic evidence of pathology in OA.
The group differences in QST measures were independent of psychosocial functioning. Although the high pain/low knee OA grade group reported elevated symptoms of anxiety, depression, and pain catastrophizing relative to the low pain groups, statistical adjustment for these differences did not alter the group effects on QST measures. It is now well-established that pain and emotions share common neurobiologic resources and are bidirectionally related in the course of daily life (39,40). Therefore, the potential for psychological functioning to explain central sensitization abnormalities in patients with discordant severities of clinical pain and radiographic knee OA may be better evaluated in a prospective study design through which changes in the strength of coupling of pain and cognitive/affective measures can be estimated over time.
Although controlling for sleep disturbance did not diminish the observed group effects, it is notable that 93% of the high pain/low knee OA grade group had comorbid insomnia, as compared to 56% of the low pain/high knee OA grade group. It is difficult to draw any firm conclusions from this finding because the sample was, in part, recruited for the presence of comorbid insomnia. One potential consideration not reflected in our statistical control of sleep disturbance is that other insomnia-related factors that could explain group differences in pain sensitivity, such as inflammation (41), may be at play. The results of the present study should therefore be replicated in an OA study designed without insomnia-specific recruitment strategies to determine if random cases of comorbid insomnia are similarly represented between groups and if QST profiles are altered should different distributions emerge.
The 4 subgroups did not differ significantly in measures of suprathreshold pain sensitivity at affected sites proximal to the index knee. Central sensitization is expected to be evident at both unaffected and affected areas in OA, and recent studies of knee OA patients documented hypersensitivity at anatomic sites corresponding to lesions discovered through imaging (14). However, our grouping system was novel and the findings cannot be directly compared to previous findings, which were based on QST differences between OA patients and healthy controls (see, for example, ref. 42), as a function of clinical pain alone (see, for example, ref. 14), or only at sites of referred pain (see, for example, ref. 13). Inspection of the group mean QST responses at affected sites does in fact suggest that the high pain/low knee OA grade group was hypersensitive relative to both of the low pain groups. Thus, the nonsignificant between-group effect appears to be driven by the similarity of the 2 high pain groups. More work is necessary to determine why the high pain/high knee OA grade group was especially sensitive at affected, but not unaffected, anatomic sites. It is possible that local factors not measured in this study, such as inflammation, introduced additional between-group variance to the QST response at affected sites. In future studies, a more precise pain sensitivity mapping technology, as described by Arendt-Nielsen et al (14), could be applied to more accurately evaluate the group differences we observed in the present study.
Contrary to the results on tests of pain sensitivity, the 4 subgroups were equivalent on the test of CPM. These findings are somewhat unexpected, given several recent studies documenting deficient pain inhibitory processing in patients with painful knee OA (14) and in patients with a variety of idiopathic pain disorders (43–45). It is not clear why the group differences from the present study favored measures of central pain facilitation over pain inhibition. Hypotheses accounting for this discrepancy, such as variability across studies in measurement techniques or neurobiologic system-specific effects for this particular set of subgroups (i.e., opioidergic versus glutamatergic), should be investigated in future studies.
The findings of the current study have potentially important clinical implications. It is of critical interest to the field to identify vulnerability and resilience factors that contribute to, or protect against, the transition from mild arthritis to severe arthritis. The finding that the high pain/low knee OA grade group was more pain sensitive than the other groups raises the question of whether they are in a transitional stage from mild to severe joint pathology. It would be important to know if the patterns of central sensitization observed in this group can be reversed with treatment (e.g., anticonvulsants; selective serotonin and norepinephrine reuptake inhibitors) and whether there is a particular window of opportunity during the course of disease progression when treatments are the most effective. One future direction of research, for example, could be to evaluate whether this subgroup responds differently to analgesic medications or other centrally acting agents relative to other OA patients. Similarly, it would be interesting to evaluate the medical management of low levels of clinical pain in the presence of severe radiographic knee OA and determine what clinical factors (e.g., preventative physical therapy) promote this group’s resilience over time. This group demonstrated relatively higher pain thresholds and lower ratings of phasic and tonic noxious stimuli applied at multiple body sites, as well as higher psychosocial functioning. Whether dimensions of neurobiologic resilience, such as a homeostatically balanced mesolimbic dopaminergic system activity (46), or psychosocial resilience, such as healthy affective regulation (see, for example, ref. 47), contribute to the pain response in this group remains to be determined and should also be taken up in future investigations.
Several limitations of this study deserve mention. First, the fact that these analyses were performed on a secondary data set limits our explanatory reach in accounting for the specific mechanisms associated with the effects reported here. On a related note, as part of the aims of the parent study, the majority of participants were recruited for the presence of comorbid insomnia. However, we view this as a minor limitation to the generality of the findings, since symptoms of insomnia are highly prevalent in OA, and epidemiologic estimates suggest that as many as 58% of OA patients in the general population experience significant sleep disturbance at least 3 nights per week (48).
The relatively small sample size may have limited power to detect effects, which may account for the lack of significant group effects on QST measures proximal to the index knee. Also owing to the small sample size, we chose to use a median split of WOMAC scores for grouping purposes, rather than select at the extreme ends of the scale. As a result, this may have underestimated the effect sizes due to the similarity of patients whose scores fall at or close to the median.
In conclusion, we identified a subgroup of knee OA patients who display abnormalities in pain processing that are consistent with central sensitization, as well as a subgroup of patients who may be resilient to these abnormalities despite prominent joint degeneration. The findings provide support for the notion that central sensitization is an endophenotype for chronic pain in knee OA and contributes to the ongoing debate surrounding the variable association of clinical pain and radiographic severity of knee OA.
Acknowledgments
Supported by the NIH (grants R01-AR-05487 and R01-AR-059410 to Dr. Smith and T32-NS-070201 to Dr. Finan).
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Smith had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Finan, Haque, Haythornthwaite, Smith.
Acquisition of data. Buenaver, Bounds, Hussain, Park, Haque, Campbell, Smith.
Analysis and interpretation of data. Finan, Edwards, Smith.
References
- 1.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513–7. [PubMed] [Google Scholar]
- 3.Lawrence JS, Bremner JM, Bier F. Osteoarthrosis: prevalence in the population and relationship between symptoms and x-ray changes. Ann Rheum Dis. 1966;25:1–24. [PMC free article] [PubMed] [Google Scholar]
- 4.Laxafoss E, Jacobsen S, Gosvig KK, Sonne-Holm S. Case definitions of knee osteoarthritis in 4,151 unselected subjects: relevance for epidemiological studies: the Copenhagen Osteoarthritis Study. Skeletal Radiol. 2010;39:859–66. doi: 10.1007/s00256-009-0856-x. [DOI] [PubMed] [Google Scholar]
- 5.Neogi T, Felson D, Niu J, Nevitt M, Lewis CE, Aliabadi P, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yusuf E, Kortekaas MC, Watt I, Huizinga TW, Kloppenburg M. Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann Rheum Dis. 2011;70:60–7. doi: 10.1136/ard.2010.131904. [DOI] [PubMed] [Google Scholar]
- 7.Creamer P, Hochberg MC. The relationship between psychosocial variables and pain reporting in osteoarthritis of the knee [review] Arthritis Care Res. 1998;11:60–5. doi: 10.1002/art.1790110110. [DOI] [PubMed] [Google Scholar]
- 8.Clauw DJ, Witter J. Pain and rheumatology: thinking outside the joint [editorial] Arthritis Rheum. 2009;60:321–4. doi: 10.1002/art.24326. [DOI] [PubMed] [Google Scholar]
- 9.Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther. 2011;13:211. doi: 10.1186/ar3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zambreanu L, Wise RG, Brooks JC, Iannetti GD, Tracey I. A role for the brainstem in central sensitisation in humans: evidence from functional magnetic resonance imaging. Pain. 2005;114:397–407. doi: 10.1016/j.pain.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev. 2004;27:729–37. doi: 10.1016/j.neubiorev.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61:1226–34. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- 14.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–81. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol. 2010;6:599–606. doi: 10.1038/nrrheum.2010.107. [DOI] [PubMed] [Google Scholar]
- 16.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 18.Power JD, Perruccio AV, Badley EM. Pain as a mediator of sleep problems in arthritis and other chronic conditions. Arthritis Rheum. 2005;53:911–9. doi: 10.1002/art.21584. [DOI] [PubMed] [Google Scholar]
- 19.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to anti-rheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 20.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spielberger CD, Gorusch RC, Lushene RE, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press; 1983. [Google Scholar]
- 22.Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 23.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:524–33. [Google Scholar]
- 24.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 25.Pavlakovic G, Petzke F. The role of quantitative sensory testing in the evaluation of musculoskeletal pain conditions. Curr Rheumatol Rep. 2010;12:455–61. doi: 10.1007/s11926-010-0131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards RR, Sarlani E, Wesselmann U, Fillingim RB. Quantitative assessment of experimental pain perception: multiple domains of clinical relevance. Pain. 2005;114:315–9. doi: 10.1016/j.pain.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Edwards RR, Wasan AD, Bingham CO, III, Bathon J, Haythornthwaite JA, Smith MT, et al. Enhanced reactivity to pain in patients with rheumatoid arthritis. Arthritis Res Ther. 2009;11:R61. doi: 10.1186/ar2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23:611–5. doi: 10.1097/ACO.0b013e32833c348b. [DOI] [PubMed] [Google Scholar]
- 29.Brennum J, Kjeldsen M, Jensen K, Jensen TS. Measurements of human pressure-pain thresholds on fingers and toes. Pain. 1989;38:211–7. doi: 10.1016/0304-3959(89)90240-6. [DOI] [PubMed] [Google Scholar]
- 30.Magerl W, Wilk SH, Treede RD. Secondary hyperalgesia and perceptual wind-up following intradermal injection of capsaicin in humans. Pain. 1998;74:257–68. doi: 10.1016/s0304-3959(97)00177-2. [DOI] [PubMed] [Google Scholar]
- 31.Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123:231–43. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 32.Vase L, Nikolajsen L, Christensen B, Egsgaard LL, Arendt-Nielsen L, Svensson P, et al. Cognitive-emotional sensitization contributes to wind-up–like pain in phantom limb pain patients. Pain. 2011;152:157–62. doi: 10.1016/j.pain.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Vierck CJ, Jr, Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- 34.Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain. 2006;22:730–7. doi: 10.1097/01.ajp.0000210914.72794.bc. [DOI] [PubMed] [Google Scholar]
- 35.Fillingim RB. Individual differences in pain responses. Curr Rheumatol Rep. 2005;7:342–7. doi: 10.1007/s11926-005-0018-7. [DOI] [PubMed] [Google Scholar]
- 36.Cohen J, Coehn P, West SG, Aiken LS. Applied multiple regression/ correlation analysis for the behavioral sciences. 3. Mahwah (NJ): Lawrence Erlbaum Associates; 2003. [Google Scholar]
- 37.Dunnett CW. New tables for multiple comparisons with a control. Biometrics. 1964;20:482–91. [Google Scholar]
- 38.Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Ecol. 2004;15:1044–5. [Google Scholar]
- 39.Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, et al. Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol. 2011;67:942–68. doi: 10.1002/jclp.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villemure C, Schweinhardt P. Supraspinal pain processing: distinct roles of emotion and attention. Neuroscientist. 2010;16:276–84. doi: 10.1177/1073858409359200. [DOI] [PubMed] [Google Scholar]
- 41.Smith MT, Quartana PJ, Okonkwo RM, Nasir A. Mechanisms by which sleep disturbance contributes to osteoarthritis pain: a conceptual model. Curr Pain Headache Rep. 2009;13:447–54. doi: 10.1007/s11916-009-0073-2. [DOI] [PubMed] [Google Scholar]
- 42.Graven-Nielsen T, Wodehouse T, Langford RM, Arendt-Nielsen L, Kidd BL. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum. 2012;64:2907–16. doi: 10.1002/art.34466. [DOI] [PubMed] [Google Scholar]
- 43.Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorders. Eur J Pain. 2009;13:1043–7. doi: 10.1016/j.ejpain.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114:295–302. doi: 10.1016/j.pain.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 45.Piche M, Arsenault M, Poitras P, Rainville P, Bouin M. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain. 2010;148:49–58. doi: 10.1016/j.pain.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Wood PB. Mesolimbic dopaminergic mechanisms and pain control. Pain. 2006;120:230–4. doi: 10.1016/j.pain.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Zautra AJ, Johnson LM, Davis MC. Positive affect as a source of resilience for women in chronic pain. J Consult Clin Psychol. 2005;73:212–20. doi: 10.1037/0022-006X.73.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilcox S, Brenes GA, Levine D, Sevick MA, Shumaker SA, Craven T. Factors related to sleep disturbance in older adults experiencing knee pain or knee pain with radiographic evidence of knee osteoarthritis. J Am Geriatr Soc. 2000;48:1241–51. doi: 10.1111/j.1532-5415.2000.tb02597.x. [DOI] [PubMed] [Google Scholar]