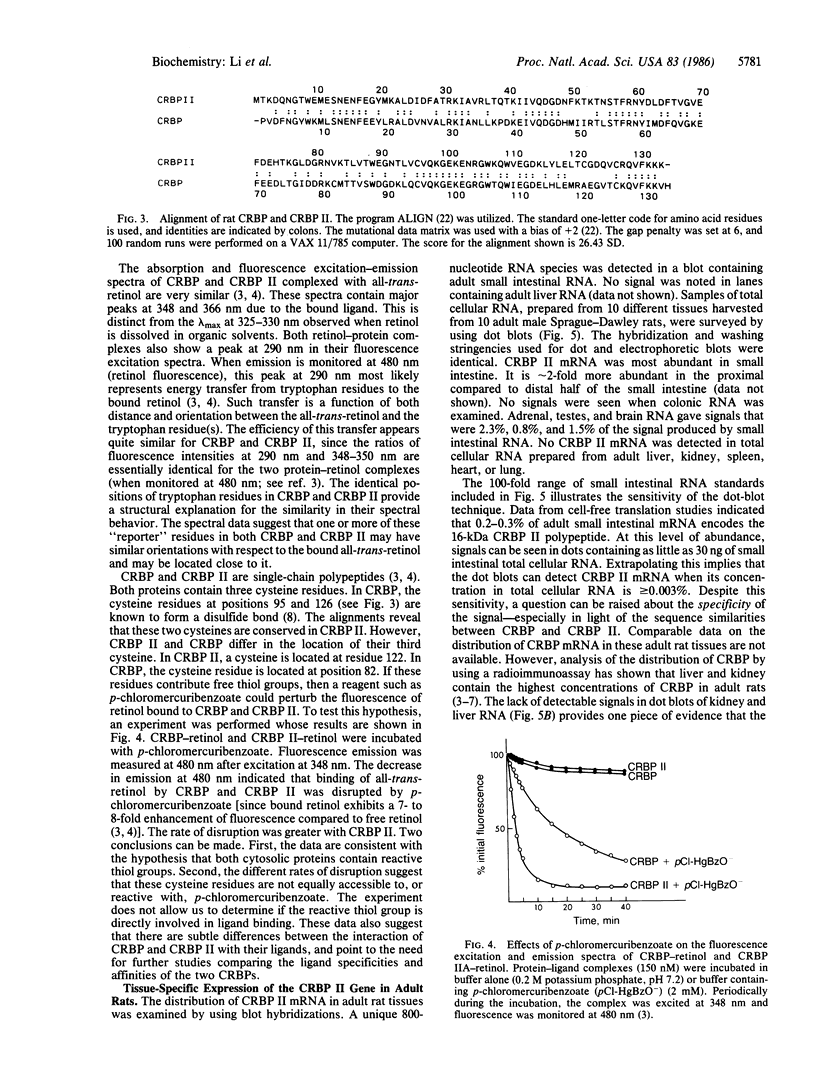
Abstract
The primary structure of rat cellular retinol-binding protein (CRBP) II has been determined from a cloned cDNA. Alignment of this 134-amino acid, 15,580-Da polypeptide with rat CRBP revealed that 75 of 133 comparable residues are identical. Both proteins contain four tryptophan residues, which occupy identical relative positions in the two primary structures, providing a structural explanation for their similar fluorescence spectra when complexed to retinol. Two of the three cysteines in each single-chain protein are comparably positioned. Both polypeptides contain reactive thiol groups, but the rate of disruption of CRBP II-retinol complexes by p-chloromercuribenzoate is greater than that of CRBP-retinol. The small intestine contains the highest concentrations of CRBP II mRNA in adult rats. CRBP II mRNA is first detectable in intestinal RNA during the 19th day of gestation, a time that corresponds to the appearance of an absorptive columnar epithelium. Unlike in intestine, a dramatic fall in liver CRBP II mRNA concentration occurs immediately after birth. The CRBP II gene remains quiescent in the liver during subsequent postnatal development. These data suggest that ligand-protein interactions may be somewhat different for the two rat CRBPs. They also support the concept that CRBP II plays a role in the intestinal absorption or esterification of retinol and suggest that changes in hepatic metabolism of vitamin A occur during development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boguski M. S., Elshourbagy N., Taylor J. M., Gordon J. I. Rat apolipoprotein A-IV contains 13 tandem repetitions of a 22-amino acid segment with amphipathic helical potential. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5021–5025. doi: 10.1073/pnas.81.16.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Crow J. A., Ong D. E. Cell-specific immunohistochemical localization of a cellular retinol-binding protein (type two) in the small intestine of rat. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4707–4711. doi: 10.1073/pnas.82.14.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshourbagy N. A., Boguski M. S., Liao W. S., Jefferson L. S., Gordon J. I., Taylor J. M. Expression of rat apolipoprotein A-IV and A-I genes: mRNA induction during development and in response to glucocorticoids and insulin. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8242–8246. doi: 10.1073/pnas.82.23.8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson U., Das K., Busch C., Nordlinder H., Rask L., Sundelin J., Sällström J., Peterson P. A. Cellular retinol-binding protein. Quantitation and distribution. J Biol Chem. 1984 Nov 10;259(21):13464–13470. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gordon J. I., Smith D. P., Andy R., Alpers D. H., Schonfeld G., Strauss A. W. The primary translation product of rat intestinal apolipoprotein A-I mRNA is an unusual preproprotein. J Biol Chem. 1982 Jan 25;257(2):971–978. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Herbst J. J., Sunshine P. Postnatal development of the small intestine of the rat. Changes in mucosal morphology at weaning. Pediatr Res. 1969 Jan;3(1):27–33. doi: 10.1203/00006450-196901000-00004. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Kato M., Blaner W. S., Mertz J. R., Das K., Kato K., Goodman D. S. Influence of retinoid nutritional status on cellular retinol- and cellular retinoic acid-binding protein concentrations in various rat tissues. J Biol Chem. 1985 Apr 25;260(8):4832–4838. [PubMed] [Google Scholar]
- Kylberg H. K., Ong D. E., Chytil F. Cellular retinol binding protein during postanal development of the rat small intestine. Biol Neonate. 1981;39(1-2):100–104. doi: 10.1159/000241399. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Ong D. E. A novel retinol-binding protein from rat. Purification and partial characterization. J Biol Chem. 1984 Feb 10;259(3):1476–1482. [PubMed] [Google Scholar]
- Rasmussen M., Petersen L. B., Norum K. R. Liver retinoids and retinol esterification in fetal and pregnant rats at term. Scand J Gastroenterol. 1985 Aug;20(6):696–700. doi: 10.3109/00365528509089197. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelin J., Anundi H., Trägårdh L., Eriksson U., Lind P., Ronne H., Peterson P. A., Rask L. The primary structure of rat liver cellular retinol-binding protein. J Biol Chem. 1985 May 25;260(10):6488–6493. [PubMed] [Google Scholar]
- Takahashi Y. I., Smith J. E., Goodman D. S. Vitamin A and retinol-binding protein metabolism during fetal development in the rat. Am J Physiol. 1977 Oct;233(4):E263–E272. doi: 10.1152/ajpendo.1977.233.4.E263. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trier J. S., Moxey P. C. Morphogenesis of the small intestine during fetal development. Ciba Found Symp. 1979 Jan 16;(70):3–29. doi: 10.1002/9780470720530.ch2. [DOI] [PubMed] [Google Scholar]
- Vahlquist A., Nilsson S. Vitamin A transfer to the fetus and to the amniotic fluid in rhesus monkey (Macaca mulatta). Ann Nutr Metab. 1984;28(6):321–333. doi: 10.1159/000176840. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]