Abstract
We combined viral vector delivery of human glial-derived neurotrophic factor (GDNF) with the grafting of dopamine (DA) precursor cells from fetal ventral mesencephalon (VM) to determine whether these strategies would improve the anti-Parkinson's effects in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated monkeys, an animal model for Parkinson's disease (PD). Both strategies have been reported as individually beneficial in animal models of PD, leading to clinical studies. GDNF delivery has also been reported to augment VM tissue implants, but no combined studies have been done in monkeys. Monkeys were treated with MPTP and placed into four balanced treatment groups receiving only recombinant adeno-associated virus serotype 5 (rAAV5)/hu-GDNF, only fetal DA precursor cells, both together, or a buffered saline solution (control). The combination of fetal precursors with rAAV5/hu-GDNF showed significantly higher striatal DA concentrations compared with the other treatments, but did not lead to greater functional improvement in this study. For the first time under identical conditions in primates, we show that all three treatments lead to improvement compared with control animals.
Introduction
When glial cell line-derived neurotrophic factor (GDNF) was first discovered, it seemed a natural candidate as a therapy for Parkinson's disease (PD). Studies in animals showed that the recombinant protein enhanced survival of midbrain dopamine (DA) neurons and caused sprouting of dopaminergic fibers, increasing neurite outgrowth and cell body size of tyrosine hydroxylase (TH)-positive neurons. It also protected against DA cell neurotoxicity from 6-hydroxydopamine, so long as it was injected close to, and soon after, the 6-hydroxydopamine administration.1,2,3,4,5 Despite these benefits, the protein does not cross the blood–brain barrier and requires invasive measures for delivery.
Subcutaneous pumps6 injecting the brain or cerebrospinal fluid were tried to increase the time frame over which the factor acts. Polymer microencapsulation of GDNF-producing cell lines secreted GDNF continuously, acting as minipumps which allowed nutrients to flow in and out due to selective permeability, but protected the cell from immune rejection.7 These pumps were used successfully in rodent models of PD8 and in nonhuman primate models of both PD8 and Huntington's disease.9 When combined with fetal mesencephalic grafts, the microcapsules increased implanted cell survival and caused outgrowth to be directed toward the capsules, but they also had a limited period of efficacy.
In vivo gene delivery using viral vectors offered a promising alternative to these techniques. GDNF injection using both lentiviral and recombinant adeno-associated viral vectors (rAAVs) has been shown to improve parkinsonism in rodent10,11,12 and primate13,14,15,16,17 models. These studies show that GDNF not only affects neurotransmission in intact adult DA neurons through (most commonly) increasing DA turnover and regulating TH expression;10,18 it also, often independently, causes cell regeneration and axonal sprouting.10,19,20 That is, the pharmacological effects on DA turnover and TH expression seem able to occur in the absence of sprouting, but not vice versa.
Ventral mesencephalic (VM) fetal DA precursor cells have been studied as replacements for DA cells lost in the course of PD. The majority of studies implanted grafts into the striatal target regions of the substantia nigra (SN). The grafted cells were shown to survive, differentiate into DA neurons, reinnerverate the striatum, release DA, and integrate into the host brain21,22 with 5–45% cell survival and behavioral evidence for functional recovery; see Rosenstein23 and Redmond24 for a review of these studies performed in rodents, primates, and humans. The recovery was often incomplete, however, and early clinical studies produced variable effects and some significant side effects, such as dyskinesia that conceivably could partially be attributed to the aberrant location of the grafts that were placed into the striatum instead of their physiological location in the SN.25
To produce more anatomic reconstruction of the DA system, we previously showed that the introduction of GDNF gene near grafted embryonic DA neurons in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated monkeys significantly increased their survival and outgrowth.15 Another study engrafted fetal VM cells into the SN and delivered GDNF into the striatum with an rAAV2/hu-GDNF vector and showed that SN grafts could reach the target areas, based upon retrograde labeling of the SN grafts with striatal injected fluorogold.26 These initial studies examined graft survival, directional outgrowth, and DA production, but no studies to date have examined functional effects of the combination of cellular injections with GDNF in nonhuman primate models. In this study, we examine the interactions between vector-delivered gene therapy with GDNF and cellular replacement therapies from both behavioral and biological perspectives. By injecting fetal cell grafts and/or viral vector-delivered GDNF into the putamen and caudate of subject monkeys, we compared their efficacy alone and in combination with an identical PD model and behavioral assessments.
Results
The monkeys were studied during a stable baseline period and then treated with standard doses of MPTP, assigned to four balanced treatment groups, and then injected bilaterally into the caudate and putamen with rAAV5/GDNF, fetal VM tissue, saline, or a combination of these. The four groups, each consisting of four monkeys, were FET (fetal tissue grafts only), FET/VEC (fetal tissue grafts plus vector-delivered GDNF), VEC (vector-delivered GDNF only), and SHAM (saline injected). The monkeys were observed over a period of 8 months after the cell or vector injections. Two monkeys (one from the FET group and one from the FET/VEC group) died of pneumonia within 2 weeks of surgery and therefore did not provide sufficient outcome data for behavioral analysis. Both were dropped from the final analysis. All of the remaining animals completed the study and were killed for histological and biochemical analyses postmortem.
Behavioral differences in parkinsonism between experimental groups
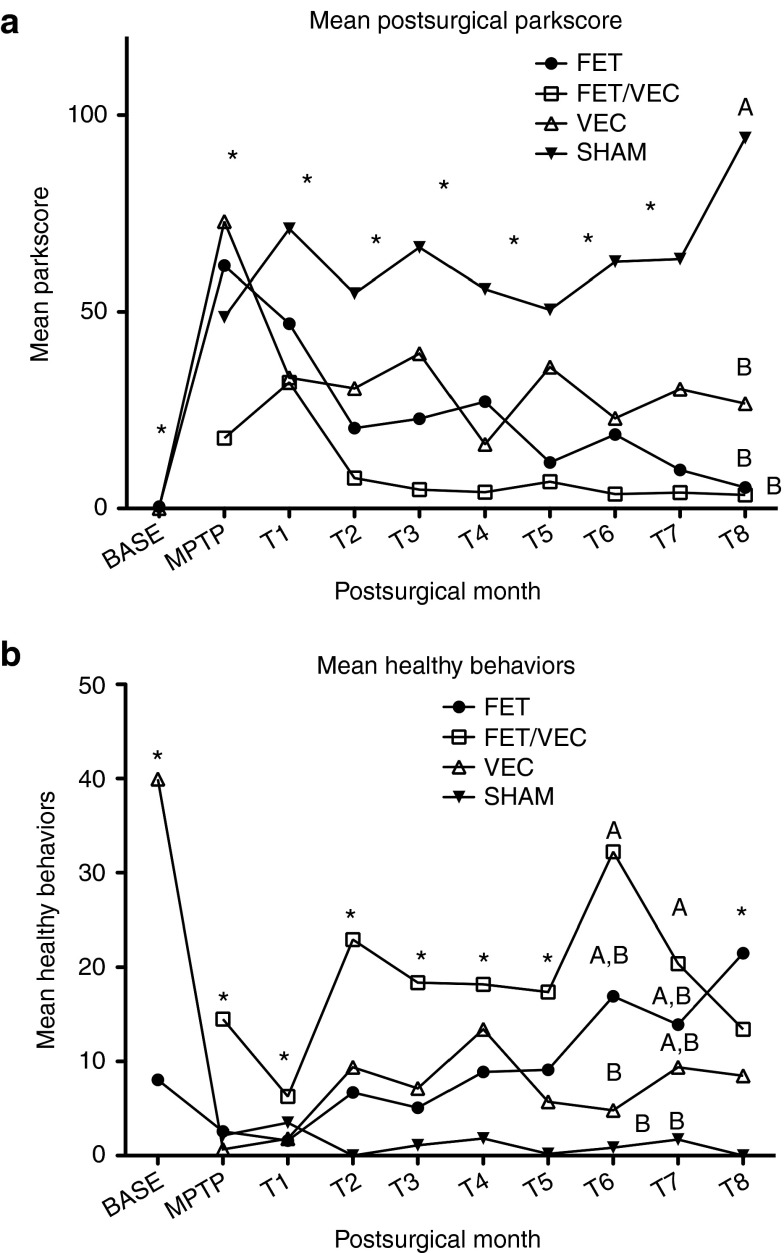
We found that each of the three treatments produced functional and behavioral improvements in MPTP-exposed parkinsonian monkeys compared with saline controls, but over the time period of the study, the combination treatment did not appear to be more effective than either treatment alone. Statistical significance of differences in behavior pre-and post-treatment (time) and between groups were analyzed using a two factor repeated measures analysis of variance, with post hoc tests using Student–Newman–Keuls at P < 0.05. There was a significant interaction between groups (the four treatments) and time (repeated measure, T0–T9) (F = 8.01, df = 25, 1406, P < 0.0001). For that reason, simple main effects were determined between groups and over time (see Figure 1a for the progression over time of parkinsonism across groups).
Figure 1.
Subject healthy and parkinsonian behavior throughout the study. (a) Mean Parkinson's factor score (“parkscore”) for monkeys before and after MPTP treatment and surgery. Higher scores are more parkinsonian. *Indicate no statistical significance between any of the groups during that month. A and B denote groups that are significantly different from each other based upon the analysis of variance (ANOVA) and post hoc Newman–Keuls test at P < 0.05. This occurred only at 8 months posttransplant (T8). All three active treatment groups, marked with B, are not different from each other, but are different from the SHAM group (marked A). A higher “parkscore” indicates more severe parkinsonism. (b) Sum factor “healthy behavior” scores for subjects before and after MPTP treatment and surgeries. *Indicate no statistical significance between groups during that month. A and B denote groups that are significantly different from each other based upon the ANOVA and post hoc Newman–Keuls test at P < 0.05. Groups with the same letters (A or B) are not different from each other, but are different from other letter. Higher scores represent more healthy behaviors. CONT, control group; FET, fetal tissue grafts only; FET+VEC, fetal tissue grafts plus vector-delivered GDNF; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; SHAM, saline-injected group; VEC, vector-delivered GDNF only.
Group differences were analyzed after MPTP and during each month after the experimental treatment (T0–T9). None of the groups' Parkinson's scores were different from the others after MPTP or at any other point after the surgical injections, except that the SHAM group was significantly higher than all of the other groups at T8, and the other groups were not different from each other (Figure 1a). Over time, the SHAM group became significantly worse than it had been during the prior periods. The FET group showed significant improvement over the MPTP and T1 period, with the final period (T8) not significantly different from the Baseline (F = 45.5, df = 9,377, P < 0.0001, and post hoc Newman–Keuls test, P < 0.05). The FET/VEC group also showed significant changes over time (F = 10.1, df = 8,242, P < 0.0001). All of the later measures were reduced from the peak parkinsonism seen at T1. Finally, the VEC alone group also showed significant changes over time (F = 62, df = 9,450, P < 0.0001). All the time periods were improved after the post-MPTP period, but did not return fully to baseline levels as the FET group did. Although the three treated groups were significantly different from the SHAM-treated group during the final 8th month period (T8) and were not statistically different from each other, the actual mean values suggest an interesting trend—SHAM = 94.15; VEC = 26.69; FET = 5.36; and FET/VEC = 3.47.
An unfortunate consequence of the death of one of the most severely affected FET/VEC animals was that the remaining animals in that group then had lower mean (less severe) “parkscores” during the MPTP period than the other three groups. However, these MPTP scores were not significantly different among any of these groups.
Behavioral differences in healthy behavior between groups
As with “parkscore,” group differences were analyzed for the MPTP period and during each month after the experimental treatment. Higher scores reflected greater amounts of normal healthy behaviors for this “healthy behavior” score, which is a sum of the frequency of several individual behaviors. The FET/VEC group had consistently higher scores than other groups through most of the experiment, and these scores were statistically significantly higher at T6 and T7. At T6 it shared that significance with the FET group, and at T7 with both other experimental groups (FET and VEC). At T6 both FET/VEC and FET were significantly different from control. And at T7 all experimental groups were significantly different from the SHAM group. At T8 FET/VEC dropped, for the first time, below the other groups and was no longer significantly different (Figure 1b). The SHAM group, on the other hand, stayed low throughout the entire experiment; their healthy behavior never improved significantly after MPTP treatment. The FET group showed significant improvement after T1, with the final period (T8) not significantly different from the baseline (F = 8.54, df = 9,376, P < 0.0001, and post hoc Newman–Keuls, P < 0.05). The FET/VEC group also showed significant changes over time (F = 3.93, df = 8,241, P < 0.0002). All of the later measures were improved after the time of the peak “parkscores” (and thus lowest healthy behavior score) seen at T1. Finally, the VEC alone group also showed significant changes over time (F = 39.3, df = 9,450, P < 0.0001). All the time periods were improved after the post-MPTP period, but did not return fully to baseline levels.
Post-treatment striatal GDNF and DA levels
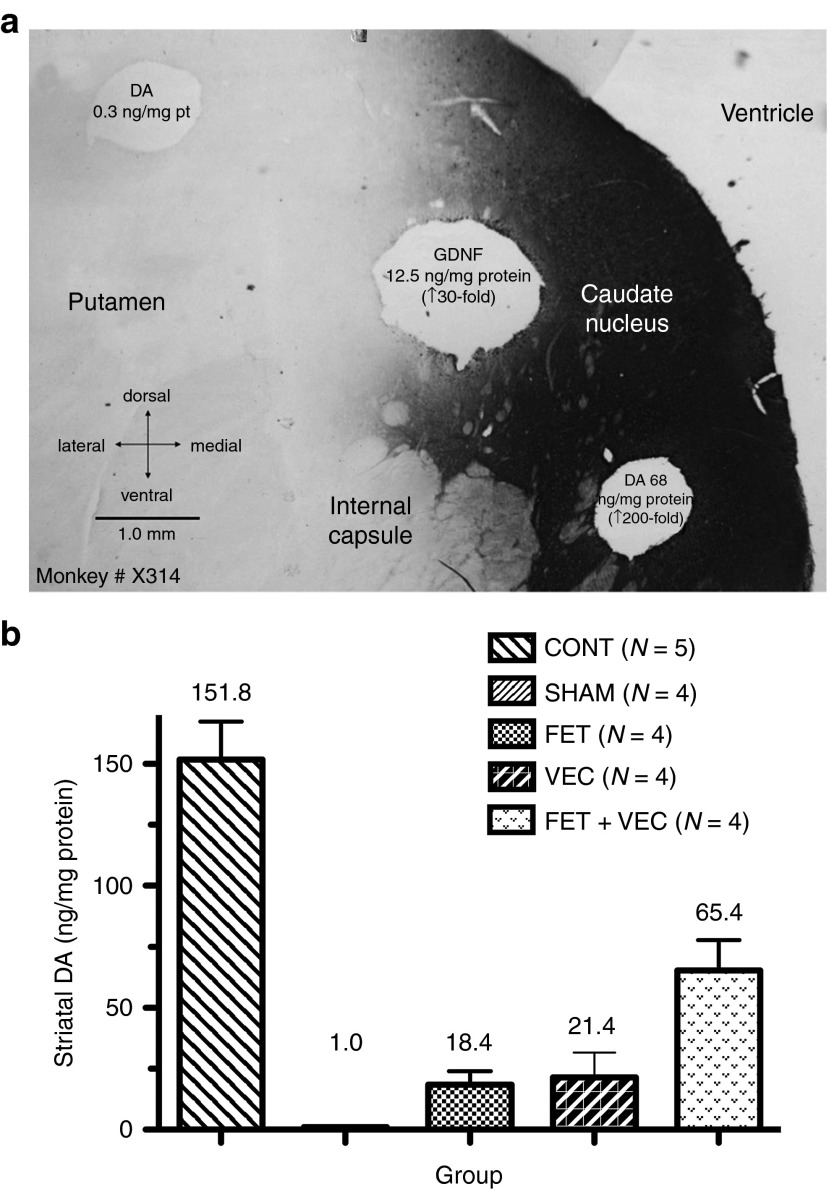
Punches were taken from representative areas of the striatum (through both the caudate and the putamen) for all subjects and then analyzed for DA and GDNF concentration (Figure 2a). To provide healthy control data, we analyzed the brains of five untreated monkeys (CONT group in Figure 2a). Striatal GDNF levels in monkeys that received rAAV5-hu-GDNF injections reached a concentration of 20–50 ng/mg protein in the vicinity of the injection site. The group that did not receive GDNF injections remained at normal levels of 0.2–0.3 ng/mg protein. Significant differences were determined by analysis of variance.
Figure 2.
Biological analyses of subject striata after sacrifice. (a) Tissue punches were removed from fresh brain slabs and assayed for dopamine (DA, by high-performance liquid chromatography) or GDNF (by ELISA); values are shown at the punch locations. Slabs were postfixed, and sections stained for GDNF immunoreactivity (gray to black). (b) Postsurgical and postmortem striatal mean DA concentrations in subject striata after treatment ± SEM. The striatal measures were the means of four individual measurements from the caudate and putamen and the number of animals analyzed is shown (N =). The mean values for each group are also shown. CONT, control group; FET, fetal tissue grafts only; FET+VEC, fetal tissue grafts plus vector-delivered GDNF; GDNF, glial-derived neurotrophic factor; SHAM, saline-injected group; VEC, vector-delivered GDNF only.
All experimental treatments resulted in elevated striatal DA concentrations compared with SHAM-treated MPTP monkeys; see Figure 2a,b for quantitative comparison. The FET–VEC group also exhibited significantly higher levels than either the VEC or FET group, but was still less than controls that were not treated with MPTP.
Across all groups for the subset of animals with all biochemical measurements, the mean striatal DA concentrations correlated significantly and negatively with both “parkscore” (Spearman's correlation coefficient r = −0.68; n = 10; P < 0.0289) and tremor (r = −0.70; n = 10; P < 0.0251) during the final month of the study. This was as expected from previous studies; the lower the DA concentration, the more parkinsonian the subjects regardless of the treatment group.
Histological analysis of grafts and GDNF expression
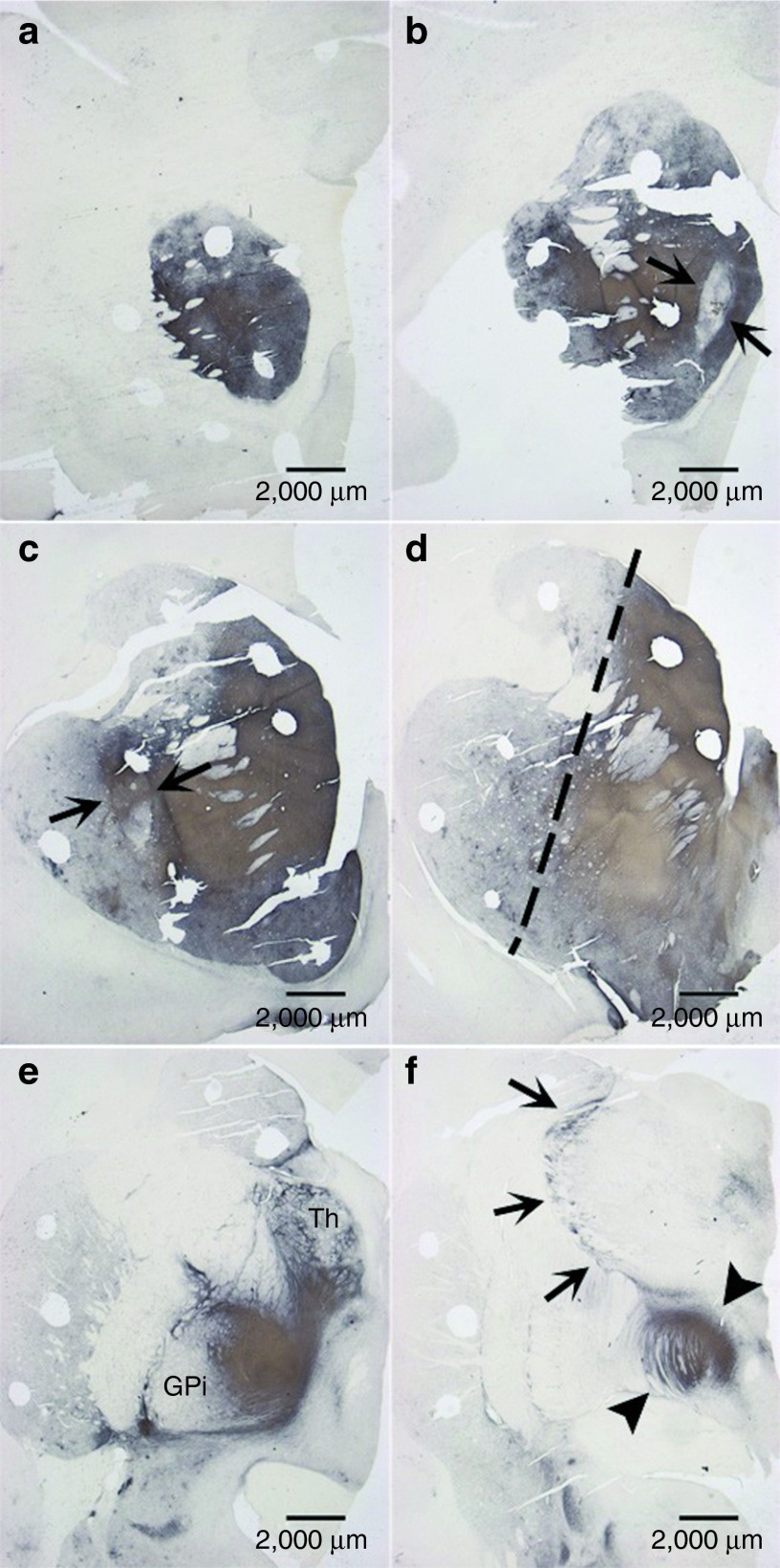
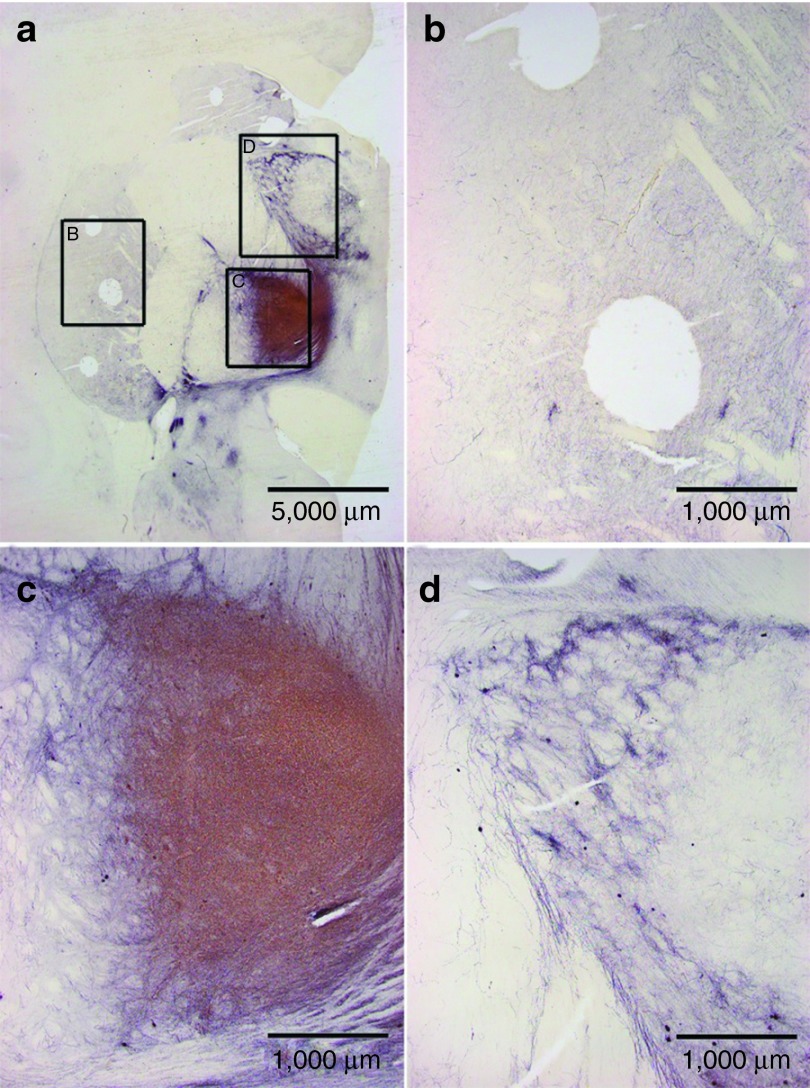
Analysis of grafts and the effects of vector GDNF were consistent with our prior studies26,27,28 and those of other investigators.5,29 These are illustrated best by two monkeys that were treated with both GDNF vector and fetal VM grafts (the FET/VEC group), monkey W538 (Figures 3 and 4) and monkey X604 (Figures 5 and 6). All of the monkeys receiving fetal VM grafts were confirmed to have surviving grafts, and consistent with prior studies showing GDNF expression for 2 years, GDNF expression was confirmed in these animals after 8 months. Overstaining of TH from aberrant sprouting is apparent in Figure 6c,d.
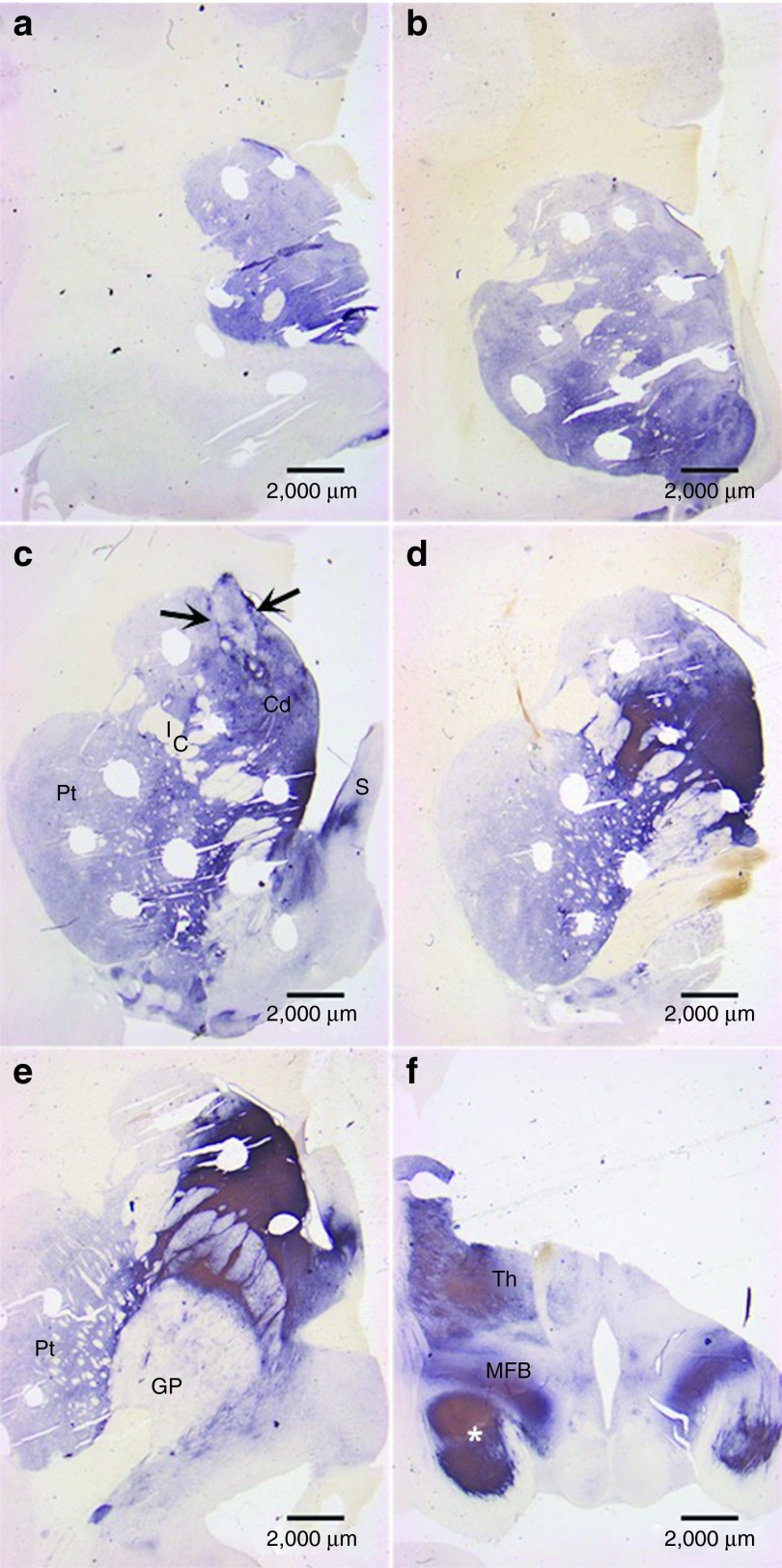
Figure 3.
Visualization of TH in subject striata via DAB staining. This scanning view depicts the pattern of tyrosine hydroxylase (TH) fiber stain seen throughout the neostriatum and adjacent structures from (a,b) rostral to (f) caudal levels through subject W538. The numerous oval holes in the tissue represent regions of micropunch dissections for biochemical determinations. (c) A prominent fetal cell graft (arrows). At this level, the neostriatum is divided by the anterior limb of the internal capsule (IC) into the caudate nucleus (Cd) and the putamen (Pt). A portion of the septum (S) is also visible. (d,e) TH fiber stain is densest in the caudal caudate nucleus as well as portions of the globus pallidus (GP) and thalamus (Th). Also noteworthy is the dense pattern of TH in the medial forebrain bundle (MFB) and adjacent portions of the temporal lobe (*) at the diencephalic level shown in f. These atypical patterns of TH fiber stain likely reflect a massive response from the residual dopamine neurons of the substantia nigra as opposed to a growth of fibers from the graft toward the mesencephalon.
Figure 4.
TH grafts and outgrowths in caudal part of striatum. (a) A level immediately caudal to that shown in Figure 3c reveals a large fetal cell graft (arrows) with heterogeneous patterns of tyrosine hydroxylase (TH)-positive neurons in the middle and ventral regions of the graft. Numerous TH-positive neurons (arrows) are seen to advantage in (b) and (c) while fiber outgrowth (arrows) from the graft is evident in (d). The size, shape, and presumptive dopamine neuron configuration of this graft is consistent with our earlier studies. What is different is the apparent gradient of TH stain in the caudate nucleus and putamen where it appears lighter in dorsomedial regions.
Figure 5.
TH fiber outgrowth in neostriatum and surrounding areas. This scanning view depicts the pattern of tyrosine hydroxylase (TH) fiber stain seen throughout the neostriatum and adjacent structures from (a) rostral to (f) caudal levels through animal X604. The density is greater than that of animal W538 and extends prominently and atypically into the (e) globus pallidus interna (GPi) and the thalamus (Th). (f) There is also an unusual pattern (arrows) external to the thalamus. Fetal cell grafts (arrows) are seen in the (b) caudate nucleus and (c) putamen. Noteworthy is (d) the prominent gradient of TH stain (dashed line) separating the dorsomedial from ventrolateral neostriatum. An unusual pattern of TH fibers (arrowheads) is also seen in the transition of the posterior limb of the internal capsule to the cerebral peduncle at a mesodiencephalic level in f.
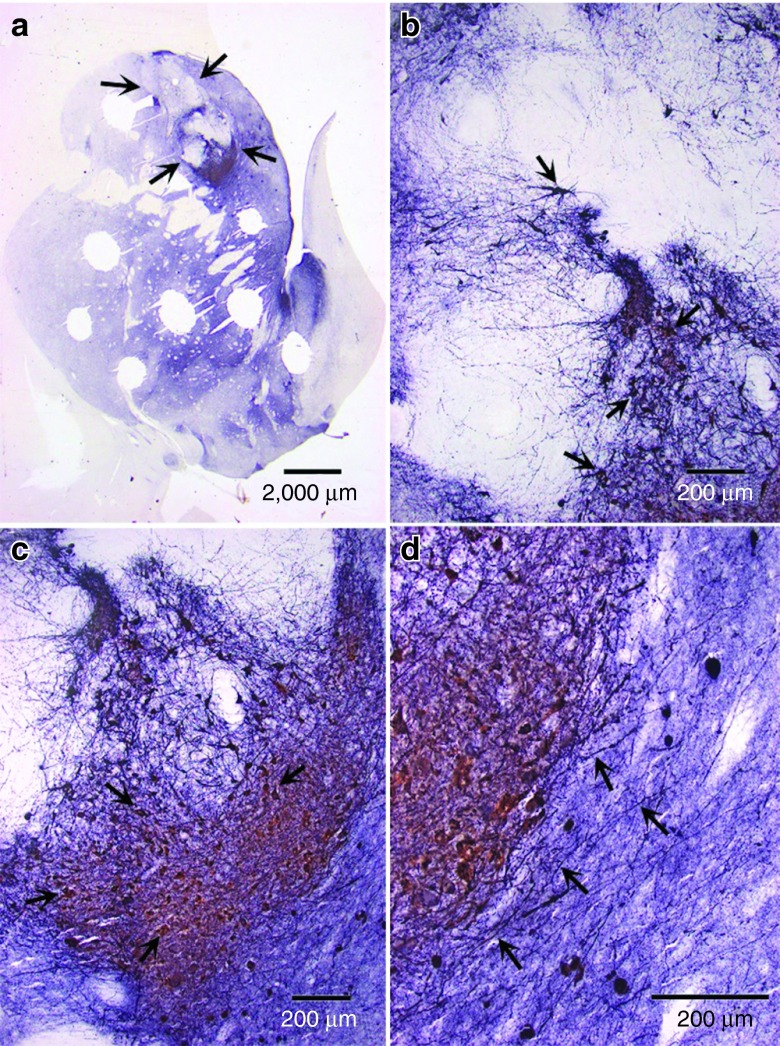
Figure 6.
Aberrant sprouting in sections of subject striatum. More detail is seen for (a) three regions; (b) the putamen, (c) globus pallidus interna, and (d) thalamus of subject X604 (FET/VEC group). The globus pallidus and thalamus of X604 show clear signs of overstaining from aberrant sprouting. Compare this to Figure 3, which shows a relatively consistent stain from subject W538; this monkey showed few signs of aberrant tyrosine hydroxylase fiber sprouting. FET/VEC, fetal tissue grafts plus vector-delivered GDNF.
Discussion
Behavioral differences across experimental groups
A large number of studies4,10,11,12,13,14,15,21,30 have shown improvement in parkinsonian model animals after GDNF overexpression or cell transplantation in MPTP parkinsonian monkeys. To our knowledge, this is the first study in nonhuman primates to study the combination of the two interventions and to compare all three treatments with a SHAM-injected control within the same study. Both surgical transplantation of fetal DA cells and overexpression of GDNF via fetal graft or viral vector alone improved behavior in the MPTP model of Parkinson's in monkeys as compared with a SHAM-injected group, but neither group restored DA concentrations to normal baseline (Figure 2b). The combination of both therapies (FET/VEC group) showed DA levels over twice as high as the individual treatment FET and VEC groups. However, these do not represent before and after measures of the same animal, and based upon the lower “parkscores” of the FET/VEC in the earliest time periods, it appears possible that these levels represent less severe parkinsonism in these monkeys from the beginning and not necessarily related to the injected treatments. With some exceptions due to related diseases (such as the two deaths from pneumonia), the monkeys remained healthy during the time period of the study. Body weight did not change in the groups treated with GDNF, contrary to a previous report of GDNF vector injections in aged but not MPTP-exposed monkeys.29
Increase of DA levels in the striatum
Higher DA concentrations in the striatum with GDNF plus cell transplantation are consistent with the current literature. GDNF has been shown to repair damaged DA neurons by inducing cellular regeneration and sprouting,10,11,19 and it seems to play an equally large part in neuroprotection, allowing developing neurons to grow without interference from toxic compounds such as MPTP. GDNF is preferentially expressed in high-growth rate areas of the infant brain, suggesting that it plays a role in protecting as well as stimulating growth.21,30 In addition, several studies have tested GDNF's neuroprotection after lesion or neurotoxin injection as their endpoint. They found that the factor is most effective when injected in repeated low doses rather than one large dose,31 suggesting that GDNF guides cell development continuously in addition to simply providing a boost to growth.
Given this role of GDNF in neuroprotection of developing cells, it is a reasonable hypothesis that combining it with cell transplantation might produce the largest effect on DA concentrations; it presumably acted as it would in the developing brain, allowing the newly implanted cells to grow in a protected environment as well as having direct effects on endogenous DA neurons.
Discrepancy between behavioral and biological improvements
Although the FET/VEC group showed a trend toward greater behavioral improvement than the FET and VEC groups alone, there was a somewhat surprising dissociation between biological and behavioral results with combined tissue/vector treatment. While the subjects' “parkscores” decreased fairly similarly, only sometimes showing a difference between the combined treatment (FET/VEC) group and the individual treatment (FET, VEC) groups, the difference in striatal DA concentrations was more apparent. This has been seen before, though; Georgievska et al. showed similar results in the SN of rats.10,11 The rats in that study were injected intrastriatally with GDNF via recombinant lentiviral vector, and then 4 weeks later with 6-hydroxydopamine. Analysis showed that both nigral DA neurons and their striatal fiber terminals were preserved in the rats, but they were not significantly different from controls in a number of motor tasks. Two possible causes were identified for this discrepancy: either (i) downregulation of TH in preserved DA terminals, or (ii) aberrant sprouting of TH-positive fibers in output nuclei of the basal ganglia.
The mechanisms of TH downregulation following GDNF treatments are not completely understood, but are fairly common after sustained treatment with the factor.32,33 It seems to be the brain's natural response to GDNF's stimulation of DA cell growth, as it is both time- and dose-dependent. It develops only after 6 weeks of continuous GDNF delivery and is most pronounced in animals where GDNF levels exceed 0.7 ng/mg tissue in the striatum. As shorter periods of GDNF delivery (up to 4 weeks) are associated with increased DA turnover and an upregulation—not downregulation—of DA synthesis,32,33 this trend is consistent with a time-dependent compensatory mechanism—continuous activation of DA neurons by GDNF is followed by a downregulation of the TH protein, thus restoring DA neuron activity to within the normal range.10
Aberrant sprouting is an unfortunate side effect of GDNF's encouragement of cell growth. Its induction of sprouting in damaged cells can heal the brain where it has been lesioned or suffered a toxic insult, but can also cause growth of projections in areas with little or no intrinsic DA innervation. While strategically placed GDNF injections in combination with embryonic cell grafts have been shown to cause beneficial axonal growth projecting from the graft location toward the GDNF injection site,26 some animals with GDNF injected into the SN directly have shown overt negative behavioral effects linked with extensive sprouting in and around the SN.5,12
It is interesting to note that Kirik et al. (2000), delivering GDNF via an rAAV vector, found functional recovery when GDNF was injected only into the striatum, but not when it was injected into either SN alone or both SN and striatum.12 Thus, it appears that overexpression of GDNF in the SN blocked the recovery that would have occurred from striatal injection. Along with these effects, there was extensive sprouting of TH-positive fibers in SN and surrounding areas—the entopeduncular nucleus and the ventral thalamus.
Unfortunately, since the biochemistry was measured postmortem, it is not possible to distinguish the effects of the treatments from possible variation in the extent of MPTP lesion in these groups. Although the groups were initially balanced by their degree of parkinsonism and were not statistically different from each other, the mean levels of parkinsonism appeared lower and hence the DA concentrations might have been higher in the FET/VEC group than the other groups initially. Another obvious reason for the failure to show a significant difference might be that the variability between animals was too great or sample size too small to achieve significance for a small effect.
The lack of significant association between biochemical effects of GDNF and behavioral recovery in our study may have been caused by the negative effects of GDNF overexpression in spite of the overall correlation between “parkscore” and DA concentrations. The previous results on aberrant sprouting12 were precisely reflected in the histology of the FET/VEC group. Subject X604 (FET/VEC) showed very little staining in the putamen but abnormally high amounts in the globus pallidus and thalamus (Figures 5 and 6; see particularly Figure 6 for a high-magnification view of these areas of interest). In contrast, W538 (also FET/VEC) showed a normal putamen and no aberrantly stained regions elsewhere in the striatum. This sprouting, combined with the sometimes delayed onset of MPTP-induced symptoms, could have halted the behavioral recovery of X604. While W538 showed its peak “parkscore” of 85.5 at T1 and decreased to 2.8 by T8, X604 showed its peak “parkscore” of 43.0 directly after MPTP treatment (time point MPTP) and decreased to 7.3 by T8. These are very large individual recoveries. The general effect of sprouting may explain the lack of significantly greater behavioral recovery in the FET/VEC group as a whole compared with the FET and VEC groups.
It is also possible that the site of placement of the GDNF or distribution of its effects was not uniform or in the right places to produce the most beneficial effects. Specifically, placement of the GDNF vector in this study was not in the target region aimed to elicit outgrowth as shown in our prior study,26 but may have prevented more widespread innervation from the grafted cells. Finally, it may be that the dissociation between having the highest DA levels but less impressive functional recovery is due to the fact that perhaps only 5–10% of normal striatal DA levels are sufficient for overtly normal motor performance in nonhuman primates.34 Compensatory mechanisms that operate following a lesion of the nigrostriatal DA pathway include increased release from residual neurons, reduced reuptake of released DA and upregulation of postsynaptic DA receptors.35 Exceeding 5–10% of the normal levels may not confer additional functional benefits. Similarly, both the FET group and the FET/VEC group's “parkscores” were so low that they could not easily go lower in the final month of the study and both appear much lower than the VEC and SHAM group. Even without statistical significance, it appears that receiving fetal grafts, with or without GDNF, was better than receiving GDNF vector alone or SHAM treatment.
Conclusions
This study addresses the potential benefits of combining gene and cellular therapy in a well known replacement model of PD in primates. The results of this study provide further evidence that both viral vector-delivered GDNF and intrastriatally injected fetal VM grafts reduce signs of PD behaviorally and biologically in monkeys. They also show, just as importantly, that there is much to understand about the interactions of these with the brain's natural systems. GDNF can cause very different effects depending on the dose used and the timeframe over which it is administered—it can restore damaged DA neurons dramatically, but can also cause uncontrolled sprouting of DA fibers into brain areas not intended for this cell type. And while it can clearly help cell transplants grow successfully, little is known about the precise dosage needed, the type of delivery to be used, and the injection site that provides the most benefit.
For future applications of GDNF delivery, especially as combined with stem cell-derived therapies, it will be important to determine how the GDNF behaves in different areas of the brain. We need to determine exactly where GDNF will provide the most beneficial effect on both endogenous neurons and injected tissue—so that it will provide the most substantial restoration of function without causing TH downregulation or disruptive DA fiber sprouting. Given the complex interactions seen just between striatum and SN, this may require testing a number of possibly unexpected sites. A great deal of progress has been made on these therapies so far, but the global picture of their effects is still far from complete.
Materials and Methods
Selection and care of monkeys for study. The monkeys were Chlorocebus sabaeus from the island of St Kitts, West Indies. The monkeys were fed recommended amounts of Harlan Teklad NIB Primate Diet (no. 8773, 20% protein; Harlan Teklad, Madison, WI) supplemented with locally grown fruits, given unrestricted access to water, and maintained in semi-outdoor enclosures that allowed ambient natural daylight at 17' 19” North latitude. All monkeys selected for the study were male and approximately age matched; while they were wild caught, and so exact age is impossible to tell, they were estimated to be in 5–15 years old, based upon weight and the absence of signs of old age. The protocol was approved by the Animal Care and Use Committees of Yale University and of St. Kitts Biomedical Research Foundation (Bourryeau Estate, St. Kitts-Nevis) where the animal studies were carried out.
MPTP treatment. Adult male monkeys were injected intramuscularly with five doses of MPTP HCl (RBI, Natick, MA) given over a 5-day period (total dose 2.25 mg/kg) and were each observed twice a day, three times a week, for a month before surgery, and during the follow-up period. Details of administration can be found in ref. 36.
Observation of subjects. Trained blinded observers scored and rated the behavior and motor movements of each monkey individually during two daily observation periods performed 5 days a week. From these quantitative time-sampled and rated assessments of 29 behaviors, a parkinsonian summary score was derived based on a previous principle component factor analysis of 55,000 observations of 80 monkeys with varying signs of parkinsonism or normal daily behaviors. The parkinsonian summary score (“parkscore”)37 contains both time-based quantitation of Parkinson-related behaviors and qualitative behaviors scored from 0 to 5, with 0 being “normal” and 5 being severely parkinsonian. The quintiles of a large group of monkeys exposed to MPTP are 0–7.3 = asymptomatic; 7.3–14.3 = mild; 14.3–26.4 = moderate; 27–60.6 = severe; and 60.6–98.3 = very severe. Inter-rater reliability was assessed once a week, and the observers achieved a coefficient of concordance (Kendall's) >0.95 on all behaviors. The protocol for observing the monkeys and the details of the measurements have been thoroughly tested and described in detail previously.38,39 Additional quantitatively scored normal behaviors are combined to assess healthy behaviors (labeled “healthy behavior”) that are very similar to the Unified Parkinson's Disease Rating Subscale (UPDRS) “activities of daily living,” which is used to assess function in human Parkinson's patients.40
Synthesis of rAAV5-hu-GDNF. AAV virus production was previously described using three-plasmid co-transfection in 293 cells. The virus titer was determined by Southern dot blot or quantitative PCR.41,42,43
Determination of optimal viral vector serotype. In order to distinguish between six serotypes (rAAV-hu serotypes 1–6) available for viral vector work at that time, each of the vectors was used to deliver green fluorescent protein to the caudate and the SN of the monkey brain, and it was determined through a variety of measures, including intensity of fluorescence and infected volume, that the rAAV5-hu viral vector would be the most effective for delivery of GDNF to the monkey brain.27
Evaluating the effectiveness of three gene delivery systems for GDNF. The viral vectors AAV2 and AAV5 were compared with the lentivirus EIAV, which possessed the advantage of having a larger carrying capacity than the AAV vectors. Results were obtained by measuring the number of cells transfected and the volume of tissue with induced GDNF expression. rAAV5 showed the largest volume of transfection in the primate brain with duration of expression up to 2 years, compared with rAAV2 and EIAV.
Treatment groups. Monkeys were selected for further study after MPTP treatment based upon a moderate-to-severe level of parkinsonism determined from the behavioral scores (monkeys with lower levels do not have room for improvement or are known to improve spontaneously). These monkeys were then assigned to four balanced treatment groups each having moderate-to-severe “parkscores”.
All monkeys were treated 1 month after MPTP administration and were injected in the same stereotaxic coordinates in the posterior caudate and putamen bilaterally. The measurements used for the target regions of the brain were as follows (in mm): caudate: anteroposterior: 19.1, 21.1, 23.1, lateral: 4, vertical relative to ear bar zero: 19; putamen: anteroposterior: 19.1, 21.1, 22.1, lateral: 10 (anterior), 10.5 (posterior), vertical relative to ear bar zero: 18.5.
The four treatment groups were as follows:
FET group: five monkeys had phosphate-buffered saline and small pieces of solid fetal VM tissue injected;
FET/VEC group: four monkeys had rAAV5-hu-GDNF vector and solid small pieces of fetal tissue injected;
VEC group: four monkeys had rAAV5-hu-GDNF vector injected;
SHAM group: four monkeys had 15 μl phosphate-buffered saline injected at each site.
Surgical procedures. Details of the fetal tissue dissection and implantation44 and the vector injections (AAV5-mediated GDNF insertion)45 have been previously described. In brief, the animals were anesthetized with ketamine (10 mg/kg intramuscularly) and sodium pentobarbital (15–25 mg/kg intravenously), and mounted into a stereotactic frame using sterile technique. Monkeys in the FET/VEC group received 20 μl of rAAV5-hu-GDNF (Lot no. AV2959; University of North Carolina, Chapel Hill, NC) vector at a concentration of 1 × 1012 virus genomes/ml injected bilaterally into the posterior caudate, and solid small pieces of fresh fetal tissue injected bilaterally into the anterior caudate and posterior putamen. Monkeys in the FET group received bilateral small solid grafts of VM tissue into the same locations in the anterior caudate and posterior putamen. Twenty microliters of rAAV5-hu-GDNF vector at a concentration of 1 × 1012 virus genomes/ml were injected bilaterally into the posterior caudate of monkeys in the VEC group via a 22-gauge needle attached to a Hamilton syringe (Hamilton, Reno, NV) driven by a microprofusion pump (Stoelting Instruments, Wood Dale, IL) at a rate of 1 μl/minute.
After the day of transplanting (TDAY = T0), observations were averaged into 1-month periods (T1, T2…T9) until the animals were killed for neurochemical and histological analyses at the end of post-transplant month nine (T9).
Histological analyses. A free-floating set of tissue from each brain was immunostained for TH-ir using methods that have been published.46 Briefly, TH-ir was identified using a primary antibody (1:1,000 overnight at room temperature, MAB-318; Chemicon, Temecula, CA) with the ABC technique using a Vectastain Elite kit (Vector Labs, Burlingame, CA) and visualized with a diaminobenzidine reaction (0.05% diaminobenzidine and 0.002% hydrogen peroxide).47 Sections were mounted onto glass slides and coverslipped. The anatomical interpretations were done without the knowledge of the behavioral outcomes. Illustrations were provided from the group which had both fetal grafts and GDNF injections, since these were the most complex.
Biochemical analyses. Concentration of DA was measured in tissue punches removed from the dorsal and ventral caudate nucleus and from the dorsal and ventral putamen, using the method described previously.48 Briefly, this involved purification of tissue extracts on an alumina column and separation of eluted catechols by reverse-phase isocratic high-performance liquid chromatography with electrochemical detection. DA was quantified with respect to internal and external standards, and the mean concentration in the four punches expressed as ng per mg protein.
Additional tissue punches taken from the same regions of caudate and putamen were devoted to measurement of GDNF concentration. Tissue was sonicated in lysis buffer, and GDNF levels in the supernatant quantified using a sensitive and selective ELISA (catalog no. G7621; Promega, Madison, WI).
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke (1 U01 NS46028), the Axion Research Foundation, and the St. Kitts Biomedical Research Foundation. We thank Ricaldo Pike, DVM, Steve Whittaker, Ernell Nisbett, Clive R. Wilson, Xavier Morton, Shervin Liddie, O'Neal Whattley, and other staff at St. Kitts Biomedical for their outstanding contributions to this study. We also thank Barbara Blanchard and Jeremy Bober for the excellent histology, and Feng-Pei Chen for excellent technical assistance in biochemical measurements. We also thank Maryanne Johnson and Joanne Simiola for their tireless administrative efforts in support of research. This work was carried out at Yale University in New Haven, Connecticut, USA, as well as at St. Kitts Biomedical Research Foundation on St. Christopher and Nevis (“St. Kitts”), West Indies. The authors declared no conflict of interest.
References
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Kearns CM, Cass WA, Smoot K, Kryscio R, Gash DM. GDNF protection against 6-OHDA: time dependence and requirement for protein synthesis. J Neurosci. 1997;17:7111–7118. doi: 10.1523/JNEUROSCI.17-18-07111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AM, Opacka-Juffry J, Blunt SB. Long-term protection of the rat nigrostriatal dopaminergic system by glial cell line-derived neurotrophic factor against 6-hydroxydopamine in vivo. Eur J Neurosci. 1998;10:57–63. doi: 10.1046/j.1460-9568.1998.00016.x. [DOI] [PubMed] [Google Scholar]
- Sauer H, Rosenblad C, Björklund A. Glial cell line-derived neurotrophic factor but not transforming growth factor beta 3 prevents delayed degeneration of nigral dopaminergic neurons following striatal 6-hydroxydopamine lesion. Proc Natl Acad Sci USA. 1995;92:8935–8939. doi: 10.1073/pnas.92.19.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Björklund A. Preservation of a functional nigrostriatal dopamine pathway by GDNF in the intrastriatal 6-OHDA lesion model depends on the site of administration of the trophic factor. Eur J Neurosci. 2000;12:3871–3882. doi: 10.1046/j.1460-9568.2000.00274.x. [DOI] [PubMed] [Google Scholar]
- Ai Y, Markesbery W, Zhang Z, Grondin R, Elseberry D, Gerhardt GA, et al. Intraputamenal infusion of GDNF in aged rhesus monkeys: distribution and dopaminergic effects. J Comp Neurol. 2003;461:250–261. doi: 10.1002/cne.10689. [DOI] [PubMed] [Google Scholar]
- Lysaght MJ, Aebischer P. Encapsulated cells as therapy. Sci Am. 1999;280:76–82. doi: 10.1038/scientificamerican0499-76. [DOI] [PubMed] [Google Scholar]
- Sautter J, Tseng JL, Braguglia D, Aebischer P, Spenger C, Seiler RW, et al. Implants of polymer-encapsulated genetically modified cells releasing glial cell line-derived neurotrophic factor improve survival, growth, and function of fetal dopaminergic grafts. Exp Neurol. 1998;149:230–236. doi: 10.1006/exnr.1997.6718. [DOI] [PubMed] [Google Scholar]
- Mittoux V, Joseph JM, Conde F, Palfi S, Dautry C, Poyot T, et al. Restoration of cognitive and motor functions by ciliary neurotrophic factor in a primate model of Huntington's disease. Hum Gene Ther. 2000;11:1177–1187. doi: 10.1089/10430340050015220. [DOI] [PubMed] [Google Scholar]
- Georgievska B, Kirik D, Björklund A. Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induces time- and dose-dependent downregulation of tyrosine hydroxylase in the intact nigrostriatal dopamine system. J Neurosci. 2004;24:6437–6445. doi: 10.1523/JNEUROSCI.1122-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgievska B, Kirik D, Björklund A. Aberrant sprouting and downregulation of tyrosine hydroxylase in lesioned nigrostriatal dopamine neurons induced by long-lasting overexpression of glial cell line derived neurotrophic factor in the striatum by lentiviral gene transfer. Exp Neurol. 2002;177:461–474. doi: 10.1006/exnr.2002.8006. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson's model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Eslamboli A, Baker HF, Ridley RM, Annett LE. Sensorimotor deficits in a unilateral intrastriatal 6-OHDA partial lesion model of Parkinson's disease in marmoset monkeys. Exp Neurol. 2003;183:418–429. doi: 10.1016/s0014-4886(03)00139-0. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Redmond DE, Jr, Leranth C, Bjugstad KB, Sladek JR, Jr, Collier TJ, et al. AAV2-mediated gene transfer of GDNF to the striatum of MPTP monkeys enhances the survival and outgrowth of co-implanted fetal dopamine neurons. Exp Neurol. 2008;211:252–258. doi: 10.1016/j.expneurol.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling JL, Kells AP, Pivirotto P, Beyer J, Bringas J, Federoff HJ, et al. Functional effects of AAV2-GDNF on the dopaminergic nigrostriatal pathway in parkinsonian rhesus monkeys. Hum Gene Ther. 2009;20:511–518. doi: 10.1089/hum.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LC, Eberling J, Pivirotto P, Hadaczek P, Federoff HJ, Forsayeth J, et al. Clinically relevant effects of convection-enhanced delivery of AAV2-GDNF on the dopaminergic nigrostriatal pathway in aged rhesus monkeys. Hum Gene Ther. 2009;20:497–510. doi: 10.1089/hum.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblad C, Georgievska B, Kirik D. Long-term striatal overexpression of GDNF selectively downregulates tyrosine hydroxylase in the intact nigrostriatal dopamine system. Eur J Neurosci. 2003;17:260–270. doi: 10.1046/j.1460-9568.2003.02456.x. [DOI] [PubMed] [Google Scholar]
- Batchelor PE, Wills TE, Hewa AP, Porritt MJ, Howells DW. Stimulation of axonal sprouting by trophic factors immobilized within the wound core. Brain Res. 2008;1209:49–56. doi: 10.1016/j.brainres.2008.02.098. [DOI] [PubMed] [Google Scholar]
- Georgievska B, Kirik D, Rosenblad C, Lundberg C, Björklund A. Neuroprotection in the rat Parkinson model by intrastriatal GDNF gene transfer using a lentiviral vector. Neuroreport. 2002;13:75–82. doi: 10.1097/00001756-200201210-00019. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Freeman TB, Snow BJ, Vingerhoets FJ, Mufson EJ, Sanberg PR, et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson's disease. N Engl J Med. 1995;332:1118–1124. doi: 10.1056/NEJM199504273321702. [DOI] [PubMed] [Google Scholar]
- Mendez I, Sanchez-Pernaute R, Cooper O, Viñuela A, Ferrari D, Björklund L, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease. Brain. 2005;128 Pt 7:1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein JM. Why do neural transplants survive? An examination of some metabolic and pathophysiological considerations in neural transplantation. Exp Neurol. 1995;133:1–6. doi: 10.1006/exnr.1995.1001. [DOI] [PubMed] [Google Scholar]
- Redmond DE., Jr Cellular replacement therapy for Parkinson's disease–where we are today. Neuroscientist. 2002;8:457–488. doi: 10.1177/107385802237703. [DOI] [PubMed] [Google Scholar]
- Ma Y, Feigin A, Dhawan V, Fukuda M, Shi Q, Greene P, et al. Dyskinesia after fetal cell transplantation for parkinsonism: a PET study. Ann Neurol. 2002;52:628–634. doi: 10.1002/ana.10359. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Jr, Elsworth JD, Roth RH, Leranth C, Collier TJ, Blanchard B, et al. Embryonic substantia nigra grafts in the mesencephalon send neurites to the host striatum in non-human primate after overexpression of GDNF. J Comp Neurol. 2009;515:31–40. doi: 10.1002/cne.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markakis EA, Vives KP, Bober J, Leichtle S, Leranth C, Beecham J, et al. Comparative transduction efficiency of AAV vector serotypes 1-6 in the substantia nigra and striatum of the primate brain. Mol Ther. 2010;18:588–593. doi: 10.1038/mt.2009.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek JR, Jr, Bjugstad KB, Collier TJ, Bundock EA, Blanchard BC, Elsworth JD, et al. Embryonic substantia nigra grafts show directional outgrowth to cografted striatal grafts and potential for pathway reconstruction in nonhuman primate. Cell Transplant. 2008;17:427–444. [PubMed] [Google Scholar]
- Su X, Kells AP, Huang EJ, Lee HS, Hadaczek P, Beyer J, et al. Safety evaluation of AAV2-GDNF gene transfer into the dopaminergic nigrostriatal pathway in aged and parkinsonian rhesus monkeys. Hum Gene Ther. 2009;20:1627–1640. doi: 10.1089/hum.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Strömberg I, Björklund L, Johansson M, Tomac A, Collins F, Olson L, et al. Glial cell line-derived neurotrophic factor is expressed in the developing but not adult striatum and stimulates developing dopamine neurons in vivo. Exp Neurol. 1993;124:401–412. doi: 10.1006/exnr.1993.1214. [DOI] [PubMed] [Google Scholar]
- Martin D, Miller G, Cullen T, Fischer N, Dix D, Russell D. Intranigral or intrastriatal injections of GDNF: effects on monoamine levels and behavior in rats. Eur J Pharmacol. 1996;317:247–256. doi: 10.1016/s0014-2999(96)00756-x. [DOI] [PubMed] [Google Scholar]
- Hudson J, Granholm AC, Gerhardt GA, Henry MA, Hoffman A, Biddle P, et al. Glial cell line-derived neurotrophic factor augments midbrain dopaminergic circuits in vivo. Brain Res Bull. 1995;36:425–432. doi: 10.1016/0361-9230(94)00224-o. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Taylor JR, Sladek JR, Jr, Collier TJ, Redmond DE, Jr, Roth RH. Striatal dopaminergic correlates of stable parkinsonism and degree of recovery in old-world primates one year after MPTP treatment. Neuroscience. 2000;95:399–408. doi: 10.1016/s0306-4522(99)00437-6. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–296. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]
- Lawrence MS, Redmond DE., Jr MPTP lesions and dopaminergic drugs alter eye blink rate in African green monkeys. Pharmacol Biochem Behav. 1991;38:869–874. doi: 10.1016/0091-3057(91)90255-z. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Roth RH, Sladek JR, Jr, Redmond DE., Jr1994Behavioral effects of MPTP administration in the vervet monkey: a primate model of Parkinson's disease. Woodruff ML, Nonneman AJ.eds). Toxin-Induced Models of Neurological Disorders Plenum Press; New York; pp. 139–174. [Google Scholar]
- Taylor JR, Elsworth JD, Roth RH, Sladek JR, Jr, Collier TJ, Redmond DE., Jr Grafting of fetal substantia nigra to striatum reverses behavioral deficits induced by MPTP in primates: a comparison with other types of grafts as controls. Exp Brain Res. 1991;85:335–348. doi: 10.1007/BF00229411. [DOI] [PubMed] [Google Scholar]
- Redmond DE., Jr2011 Behavioral assessment in the African green monkey after MPTP administration. Lane E, Dunnett S.eds). Neuromethods: Animal Models of Movement Disorders Springer Science: New York [Google Scholar]
- Fahn S, Elton RL.Members of the UPDRS Development Committee 1987Unified Parkinson's disease rating scale. Fahn S, Marsden CD, Calne DB, Goldstein M.eds). Recent Developments in Parkinson's Disease vol. 2Macmillan Healthcare Information; Florham Park, NJ; pp. 153–164. [Google Scholar]
- Choi VW, Asokan A, Haberman RA, McCown TJ, Samulski RJ. Production of recombinant adeno-associated viral vectors and use for in vitro and in vivo administration. Curr Protoc Neurosci. 2006;Chapter 4:Unit 4.17. doi: 10.1002/0471142301.ns0417s35. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek JR, Jr, Elsworth JD, Taylor JR, Roth RH, Redmond DE., Jr1995Techniques for neural transplantation in non-human primates. Ricordi C, Landes RG.eds). Techniques for Neural Transplantation CRC Press; Austin, TX; pp 391–408. [Google Scholar]
- During MJ, Samulski RJ, Elsworth JD, Kaplitt MG, Leone P, Xiao X, et al. In vivo expression of therapeutic human genes for dopamine production in the caudates of MPTP-treated monkeys using an AAV vector. Gene Ther. 1998;5:820–827. doi: 10.1038/sj.gt.3300650. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Redmond DE, Jr, Roth RH, Elsworth JD. Development of A9/A10 dopamine neurons during the second and third trimesters in the African green monkey. J Comp Neurol. 2005;488:215–223. doi: 10.1002/cne.20599. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin–biotin–peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Roth RH, Redmond DE, Elsworth JD. Impact of methamphetamine on dopamine neurons in primates is dependent on age: implications for development of Parkinson's disease. Neuroscience. 2011;189:277–285. doi: 10.1016/j.neuroscience.2011.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]