Abstract
Cytokine-induced killer (CIK) cells raised interest for use in cellular antitumor therapy due to their capability to recognize and destroy autologous tumor cells in a HLA-independent fashion. The antitumor attack of CIK cells, predominantly consisting of terminally differentiated CD8+CD56+ cells, can be improved by redirecting by a chimeric antigen receptor (CAR) that recognizes the tumor cell and triggers CIK cell activation. The requirements for CIK cell activation were, however, so far less explored and are likely to be different from those of “younger” T cells. We revealed that CD28 and OX40 CARs produced higher interferon- secretion as compared with the first-generation ζ-CAR; CD28-ζ and the third-generation CD28-ζ–OX40 CAR, however, performed similar in modulating most CIK cell effector functions. Compared with the CD28-ζ CAR, however, the CD28-ζ–OX40 CAR accelerated terminal maturation of CD56+ CIK cells producing high frequencies in activation-induced cell death (AICD) and reduced antitumor efficiency in vivo. Consequently, CD28-ζ CAR CIK cells of CD56− phenotype were superior in redirected tumor cell elimination. CAR-mediated CIK cell activation also increased antigen-independent target cell lysis; the CD28-ζ CAR was more efficient than the CD28-ζ–OX40 CAR. Translated into therapeutic strategies, CAR-redirected CIK cells benefit from CD28 costimulation; “super-costimulation” by the CD28-ζ–OX40 CAR, however, performed less in antitumor efficacy due to increased AICD.
Introduction
Cytokine-induced killer (CIK) cells are generated ex vivo from peripheral blood mononuclear cells by propagating in the presence of interferon-γ (IFN-), an agonistic anti-CD3 monoclonal antibody (mAb) and interleukin-2 (IL-2).1 Upon 2–3 weeks of culture, the expanded cells express natural-killer cell markers to a variable extent in addition to classical T-cell markers, express in high numbers the CD3+CD56+ phenotype, and resemble tumor infiltrating lymphocytes.2 CIK cells have an extraordinary cytolytic potential, recognize and destroy autologous tumor cells in a HLA-independent fashion, proliferate more rapidly than tumor infiltrating lymphocytes and lymphokine-activated T killer cells, and preferentially migrate into the periphery.3 Due to these properties, CIK cells are of particular interest for their use in cell therapy of malignant diseases. The antitumor efficacy of CIK cells is moreover underlined by the observation that adoptive transfer of CIK cells improved short-term, but not long-term, survival of patients.4 The short-term effect may be due to the short survival of CIK cells, because those cells are predominantly composed of terminally differentiated T (TEMRA) cells5, which are prone to spontaneous apoptosis3, substantially limiting the long-term efficacy in the fight against cancer.
Recent clinical trials impressively demonstrate the antitumor activity of adoptively transferred T cells with redirected specificity.6,7 The strategy is based on ex vivo engineering T cells with an antibody-derived chimeric antigen receptor (CAR), which in contrast to the T-cell receptor, consists of one polypeptide chain with an extracellular single-chain fragment of variable region (scFv) antibody for binding and the intracellular CD3ζ chain for T-cell activation. By using an antibody-like targeting chimeric receptor, engineered T cells can be redirected toward nearly every epitope on the target cell surface for which an antibody is available. The therapeutic efficacy of such CAR T cells is, however, often limited by inefficient cytolysis or short-term persistence after adoptive transfer.8,9,10 Costimulation provided by a CD28 signaling domain in a second-generation CAR substantially improved the efficacy of redirected T cells in an antitumor attack; other costimuli differentially modulate the T-cell effector functions in a specific fashion.11 Combined costimulation by the so-called third-generation CAR with early CD28 and late costimulation by OX40 or 4-1BB promotes T-effector memory cell differentiation and protects the cells from apoptosis.12
The observations provide the rationale to take advantage of CAR-provided costimulatory signals to counteract activation-induced cell death (AICD) and to improve the antitumor activity of CIK cells. The requirements of CIK cells to prevent AICD are, however, so far poorly understood; in particular, no comparative analysis was performed to dissect the impact of different costimuli in sustaining the CAR-redirected CIK cell antitumor attack. We addressed the issue in a thoroughly controlled situation by engineering CIK cells with first-, second-, and third-generation CARs of the same format and with the same specificity for the carcinoembryonic antigen (CEA). The CAR harbors either a CD28-ζ or a combined CD28-ζ–OX40 intracellular signaling domain. Upon CAR stimulation, CIK cells acquired a CD56+ phenotype, which was most rapidly induced by OX40 and less induced by CD28 costimulation or even without costimulation, and entered apoptosis which could not be prevented by OX40 or CD28. Consequently, the CD28-ζ CAR was superior to the “super-stimulatory” CD28-ζ–OX40 CAR, and CD28-ζ CIK cells with CD56− phenotype showed more robust CAR-mediated and CAR-independent tumor cell lysis.
Results
CAR-redirected CIK cells show antigen-specific tumor cell killing
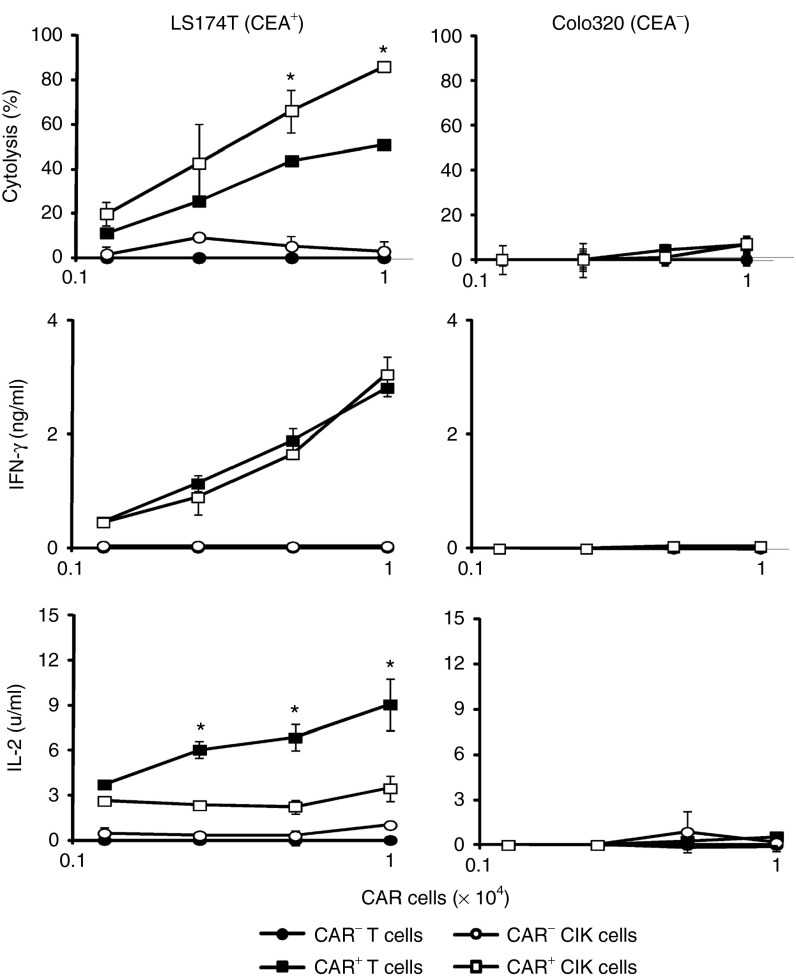
CIK cells were derived ex vivo from peripheral blood lymphocytes by stimulation with IFN-, anti-CD3 antibody and propagated in the presence of IL-2 as described.1 These cells displayed a mixed T–natural-killer cell phenotype and were predominantly composed of CD8+ T cells which express CD56 in 10–70% of the cells. Since CIK cells attack tumor cells in a major histocompatibility complex-unrestricted, antigen-independent fashion,1,13 we asked in a thoroughly controlled side-by-side comparison how tumor cell killing is improved when CIK cells are redirected in an antigen-specific fashion by a CAR. Peripheral blood T cells were activated by anti-CD3/CD28 and IL-2 stimulation for comparison. CIK cells and stimulated T cells from the same donor were engineered with a CD28-ζ signaling CAR with specificity for CEA. The CAR was expressed with similar efficiencies in T cells and CIK cells (Supplementary Figure S1). CAR-mediated activation was monitored by recording secreted cytokines and target cell killing upon coincubation with CEA+ and CEA− tumor cells. As summarized in Figure 1, both T cells and CIK cells were specifically redirected against CEA+ tumor cells producing efficient tumor cell killing; CAR-redirected CIK cells lysed target cells more efficiently than CAR T cells. CEA− Colo320 cells were not attacked by CIK or T cells demonstrating the antigen specificity of the targeting CAR. Although the killing activity was substantially different, CIK cells secreted the same amount of IFN-γ as did T cells but produced less amounts of IL-2.
Figure 1.
Chimeric antigen receptor (CAR)-redirected activation of T cells and cytokine-induced killer (CIK) cells. T cells and CIK cells from the same healthy donor were engineered with a carcinoembryonic antigen (CEA)-specific CD28-ζ CAR and coincubated (2.5 × 103 − 2 × 104 cells/well) for 48 hours with CEA+ LS 174T and CEA− Colo320 target cells (2 × 104 cells/well), respectively. Cytolysis was determined by an XTT-based colorimetric assay and culture supernatants were analyzed for interferon- (IFN-γ) and interleukin-2 (IL-2) by enzyme-linked immunosorbent assay. Numbers represent mean values of triplicates ± SD. A representative assay is shown. Statistical analyses were performed using Student's t-test. *P < 0.05.
CD28 and OX40 costimulation increased cellular activation of CIK cells and IFN- secretion
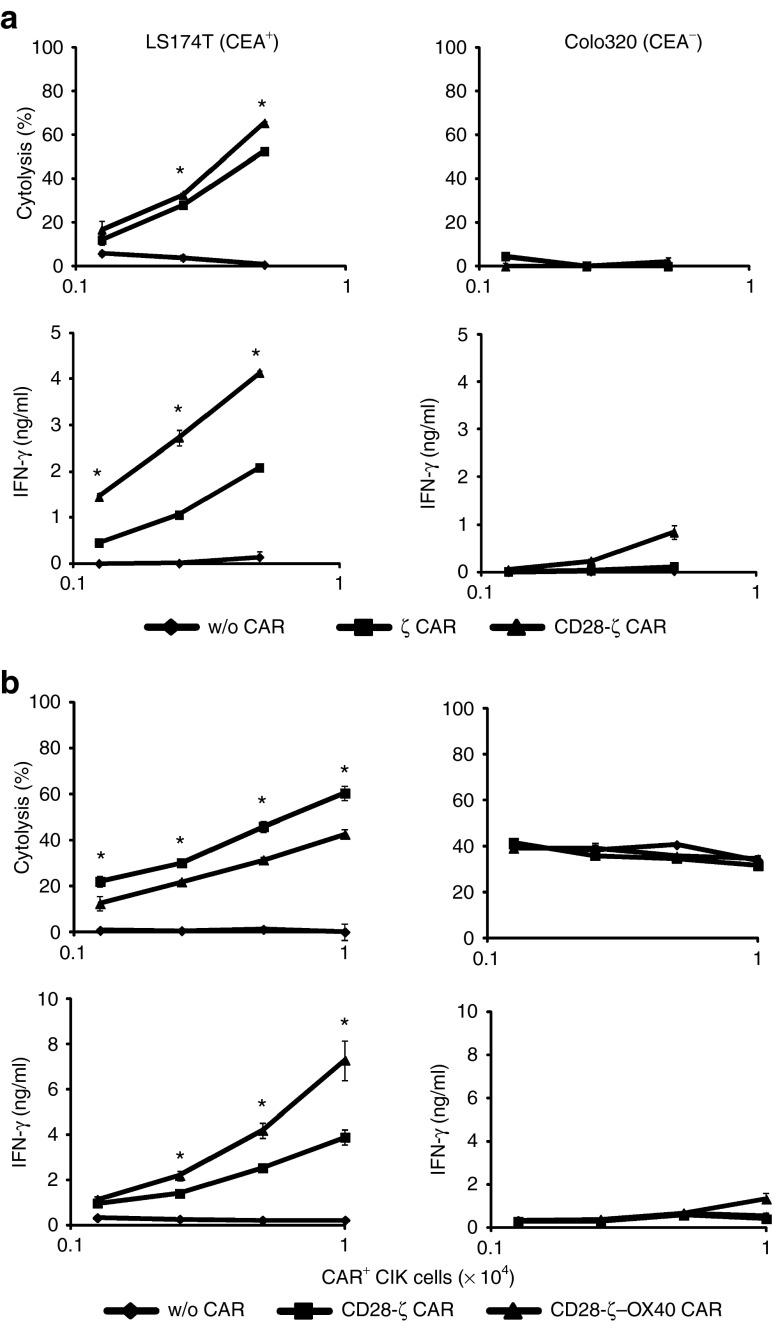
We explored the impact of CAR signaling on specific tumor cell killing and IFN-γ secretion of redirected CIK cells. CIK cells were induced in vitro and engineered to express the ζ, CD28-ζ and CD28-ζ–OX40 CAR, respectively, each redirecting CIK cells toward CEA+ tumor cells. As summarized in Figure 2, engineered CIK cells with either CAR lysed CEA+ target cells in a dose-dependent fashion, but not CEA− cells. The CD28-ζ CAR was slightly more effective in redirecting CIK cell killing than the ζ or CD28-ζ–OX40 CAR (Figures 2a,b). Improved target cell lysis was not due to impaired signaling because the CD28-ζ CAR induced higher IFN-γ secretion than the ζ-CAR (Figure 2a) but was less efficient than the CD28-ζ–OX40 CAR (Figure 2b).
Figure 2.
ζ, CD28-ζ, and CD28-ζ–OX40 CAR redirected CIK cells efficiently kill tumor cells in vitro. Cytokine-induced killer (CIK) cells were engineered with the ζ, CD28-ζ, and CD28-ζ–OX40 CAR, and cocultivated (1.25 − 10 × 103 CAR+ cells/well) with CEA+ LS174T or CEA− Colo320 tumor cells (each 2 × 104 cells/well) for 48 hours. Cytolysis was determined by an XTT-based colorimetric assay and supernatants were analyzed for interferon- (IFN-γ) by enzyme-linked immunosorbent assay. Side-by-side comparison of (a) ζ and CD28-ζ CAR engineered CIK cells and (b) CD28-ζ and CD28-ζ–OX40 engineered CIK cells. Numbers represent mean values of triplicates ± SD. A representative assay is shown. Statistical analyses were performed using Student's t-test. *P < 0.05. CAR, chimeric antigen receptor; CEA, carcinoembryonic antigen.
Combined CD28–OX40 costimulation accelerates maturation to CD56+ CIK cells that are prone to apoptosis and cannot be rescued by OX40 costimulation
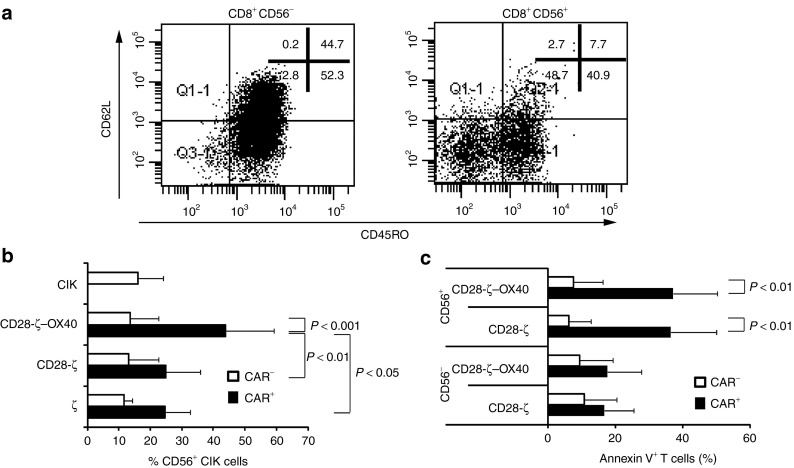
CD56+ cells in the CIK cell population may represent terminally differentiated T cells which accumulate to high numbers. To address this issue, we isolated CD8+CD56+ CIK cells and stimulated these cells via CD3 in the presence of IL-2 for additional 7 days. CD56+ CIK cells were predominantly of CD62L−CD45RO− phenotype, which is also indicative for terminally differentiated T cells (Figure 3a). By contrast, CD8+CD56− CIK cells were mainly composed of CD45RO+ and comprise a substantial number of CD62L+ cells indicating less-matured cells. As matured T cells, including CD62L− effector memory T cells, benefit in survival by a third-generation CAR with combined CD28 and OX40 stimulation,12 we asked whether CIK cells may also benefit from CD28-ζ–OX40 CAR signaling. We engineered CIK cells from healthy donors to express the anti-CEA CAR with ζ, CD28-ζ, and CD28-ζ–OX40 endodomains and stimulated engineered cells by their CAR utilizing a CAR-specific anti-idiotypic mAb. As summarized in Figure 3b, CAR-mediated CIK cell activation produced CD56 upregulation that was significantly enhanced by CD28-ζ–OX40 CAR with the additional OX40 costimulation as compared with the CD28-ζ CAR. CD56 upregulation was specifically enhanced by OX40 costimulation because CD28 costimulation has no impact on CD56 expression as compared with ζ-CAR signaling only. To rule out that the observed differences were due to different CAR expression, we compared the expression of the first-generation with the second- and third-generation CARs in several CIK cell batches (n = 6). The density of the CD28-ζ CAR and the CD28-ζ-OX40 CAR on the cell surface was similar, but significantly higher than that of the ζ CAR. The number of CAR-engineered CIK cells was, however, the same indicating that the observed differences between CD28-ζ and CD28-ζ-OX40 CAR CIKs were unlikely due to a potentially different CAR expression (Supplementary Figure S2). Taken together, combined CD28–OX40 costimulation increased the number of CIK cells with a CD56+ phenotype of terminally differentiated cells.
Figure 3.
CD56+ cytokine-induced killer (CIK) cells are terminally differentiated T cells that are induced by OX40 costimulation and prone to activation-induced cell death. (a) CIK cells were generated as described in Materials and Methods. After 14 days of culture, cells were separated into CD56+ and CD56− cells and stained for CD45RO and CD62L, respectively. A representative experiment out of three is shown. (b) CIK cells from healthy donors were grafted with the ζ (n = 5), CD28-ζ (n = 12), or CD28-ζ–OX40 CAR (n = 12) and cultivated for 3 days in the presence of the anti-idiotypic monoclonal antibody (mAb) BW2064 (1 µg/ml) and an antimouse immunoglobulin G1 (IgG1) (0.5 µg/ml) antibody for CAR crosslinking. Cells were stained for CD56 and CAR expression and analyzed by flow cytometry. Nonmodified CIK cells were stained for control (n = 4). Numbers represent the mean value ± SD. Statistical analyses were performed using Student's t-test. (c) CIK cells from six healthy donors were engineered with the CD28-ζ or CD28-ζ–OX40 CAR and cultivated for 3 days in the presence of the anti-idiotypic mAb BW2064 (1 µg/ml) and an antimouse IgG1 (0.5 µg/ml) antibody for CAR crosslinking. Cells were stained for CD56 and CAR expression and apoptotic cells were identified by annexin V expression. Numbers represent mean value ± SD. P values were determined by Student's t-test. CAR, chimeric antigen receptor.
We asked whether CD28–OX40 costimulation also protects CD56+ CIK cell from AICD and apoptosis. We engineered CD56− and CD56+ subset CIK cells with the CD28-ζ and CD28-ζ–OX40 CAR, and stimulated these cells by the CAR-specific anti-idiotypic mAb. Annexin V staining revealed that CAR-induced apoptosis of CD56+ CIK cells was substantially higher than those of CD56− cells (Figure 3c) irrespective of CD28 or combined CD28–OX40 costimulation by the grafted CAR. CD56− CIK cells, by contrast, appeared to be more robust to AICD because the number of apoptotic cells after CAR stimulation did not increase substantially. CAR signaling mediated apoptosis of CD56+ cells because CD56+ cells without CAR showed no increase in the number of apoptotic cells. Taken together, CD28-ζ–OX40 CAR stimulation enhanced the number of terminally differentiated CD56+ CIK cells but neither CD28 nor OX40 costimulation protected the cells from AICD.
Combined CD28–OX40 costimulation decreased the antitumor efficacy of CAR-redirected CIK cells in vivo.
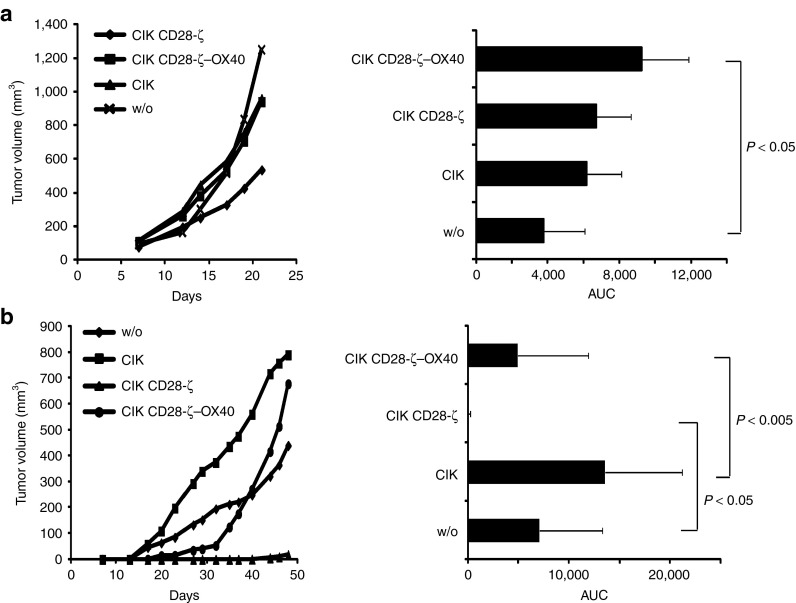
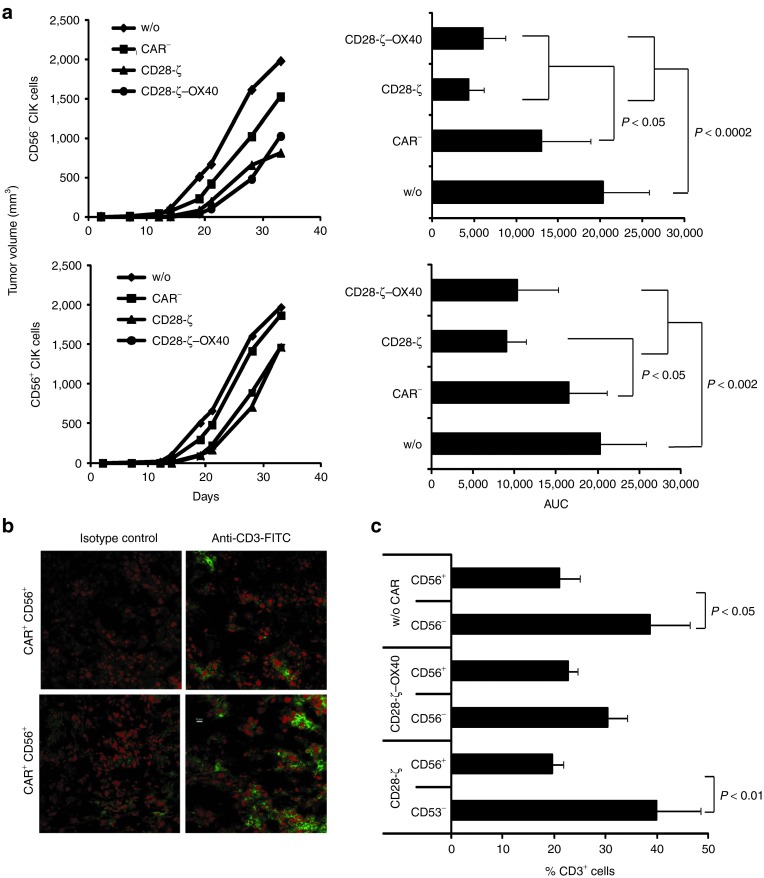
We asked whether CARs with different costimulation moieties may mediate tumor cell killing by CIK cells in vivo to a different extent. To address this issue, we grafted immune-deficient mice with CEA+ C15A3 tumor cells. After 7 days, when tumors were clearly visible established (2–4 mm in diameter) CD28-ζ- and CD28-ζ–OX40 CAR-engineered CIK cells were intravenously injected and tumor growth was recorded. Strikingly, only CIK cells with the CD28-ζ CAR delayed tumor progression, whereas CIK cells with the CD28-ζ–OX40 CAR or nonmodified CIK cells did not (Figure 4a). Different homing capacities of the engineered CIK cells with the respective CAR into the target tissue may contribute to the effect. To rule this out, we coinjected CEA+ tumor cells together with CAR+ CIK cells into immune-deficient mice and recorded tumor progression. Moreover, CAR CIK cells produced substantially better antitumor cell efficacy in vivo when redirected by the CD28-ζ CAR than by the CD28-ζ–OX40 CAR (Figure 4b). CD28-ζ CAR-redirected CIK cells more efficiently suppressed tumor growth than CD28-ζ–OX40 CAR CIK cells, the latter, however, still more efficiently than nonmodified CIK cells.
Figure 4.
OX40 costimulation reduced antitumor efficacy of chimeric antigen receptor (CAR)-redirected cytokine-induced killer (CIK) cells. (a) Rag−/− common γ-chain−/− mice were engrafted subcutaneously with CEA+ C15A3 tumor cells (1 × 106 cells/mouse). When tumors were clearly established (~7 days after injection), CAR-engineered CIK cells were intravenously injected (5 × 106 cells/mouse; 4–7 animals/group). The number of CD28-ζ and CD28-ζ–OX40 engineered CIK cells was 16.5 and 19.2%, respectively. (b) Rag−/− common γ-chain−/− mice were coinjected with CEA+ C15A3 tumor cells (1 × 106 cells/mouse) and CIK cells with or without CAR (2.5 × 105 cells/mouse; 5–7 animals/group). The numbers of injected T cells were adjusted to equal numbers by adding nontransduced CIK cells. Tumor growth was recorded every 2–3 days and the volume was determined using a digital caliper. Mean values are shown; area under the curve (AUC) was calculated and P values were determined using Student's t-test. CEA, carcinoembryonic antigen.
The CD28-ζ and CD28-ζ–OX40 CAR redirected purified CD56− and CD56+ CIK cells with similar efficiency.
CD28-ζ–OX40 CAR–redirected CIK cells produced efficient tumor cell killing in vitro while less antitumor activity in vivo. We asked whether the dramatically reduced antitumor activity of CD28-ζ–OX40 as compared with CD28-ζ CAR-redirected CIK cells is due to the increased number of terminally matured CD56+ CIK cells after long-term CD28-ζ–OX40 CAR stimulation. To test the antitumor activity of CD56+ and CD56− CAR CIK cells, we engineered CIK cells with the respective CAR and isolated the CAR+ CD56+ and CD56− CIK cell subsets to high purity; the respective CIK cell subsets without CAR obtained from the same batch served as controls (Supplementary Figure S3). The CIK cell subsets were coinjected together with CEA+ tumor cells into immune-deficient mice and tumor outgrowth was recorded (Figure 5a). No differences in the antitumor cell efficacy were observed when CD56− CIK cells with CD28-ζ or CD28-ζ–OX40 CAR were used; only minor differences between the two CARs were recorded when engineered CD56+ CIK cells were applied (Figure 5a). We conclude that the different antitumor efficacies are rather due to the CIK cell subsets than due to the different CAR signaling moieties. Isolated CIK cell subsets were redirected by CD28-ζ and CD28-ζ–OX40 CARs with similar efficacy. CAR-redirected CD56+ CIK cells were, however, less efficient than CD56− CIK cells in tumor cell elimination irrespective of the CAR-provided costimulation. As the CD28-ζ–OX40 CAR promotes maturation of CIK cells toward the CD56+ CIK cell subset, which is prone to high frequencies in AICD, the antitumor cell efficacy is less compared with CD28-ζ CAR-redirected CIK cells.
Figure 5.
CD28-ζ and CD28-ζ–OX40 CAR redirect CD56+ and CD56− CIK cells with similar efficiency. (a) Rag−/− common γ-chain−/− mice were coinjected with CEA+ C15A3 tumor cells (1 × 106 cells/mouse) and sorted cytokine-induced killer (CIK) cells with or without CAR (2.5 × 105 cells/mouse; 5–7 animals/group). Tumor volume was recorded every 2–3 days. Mean values are shown; area under the curve (AUC) was calculated and P values were determined using Student's t-test. (b,c) Cryosections of explanted tumors were stained with an Alexa-Fluor 488 conjugated antihuman CD3 monoclonal antibody (mAb) and analyzed by confocal microscopy as described in Materials and Methods. (b) A representative tissue section of a tumor treated with CD28-ζ CAR CIK cells is shown. (c) The numbers of CD3+ T cells in the tumor tissue were determined as described in Material and Methods. P values were determined using Student's t-test. CAR, chimeric antigen receptor; CEA, carcinoembryonic antigen.
To confirm the results, we screened the tumors for persisting T cells. Figure 5b exemplarily demonstrates immune fluorescence staining of CD28-ζ CAR-engineered CD56+ and CD56− tumor infiltrating T cells. Quantitative recoding (Figure 5c) revealed higher numbers of persisting CD56− than CD56+ T cells, which is in accordance with the observed differences in the efficacy of tumor suppression.
CAR signaling impacts NKG2D-mediated target cell lysis by CIK cells
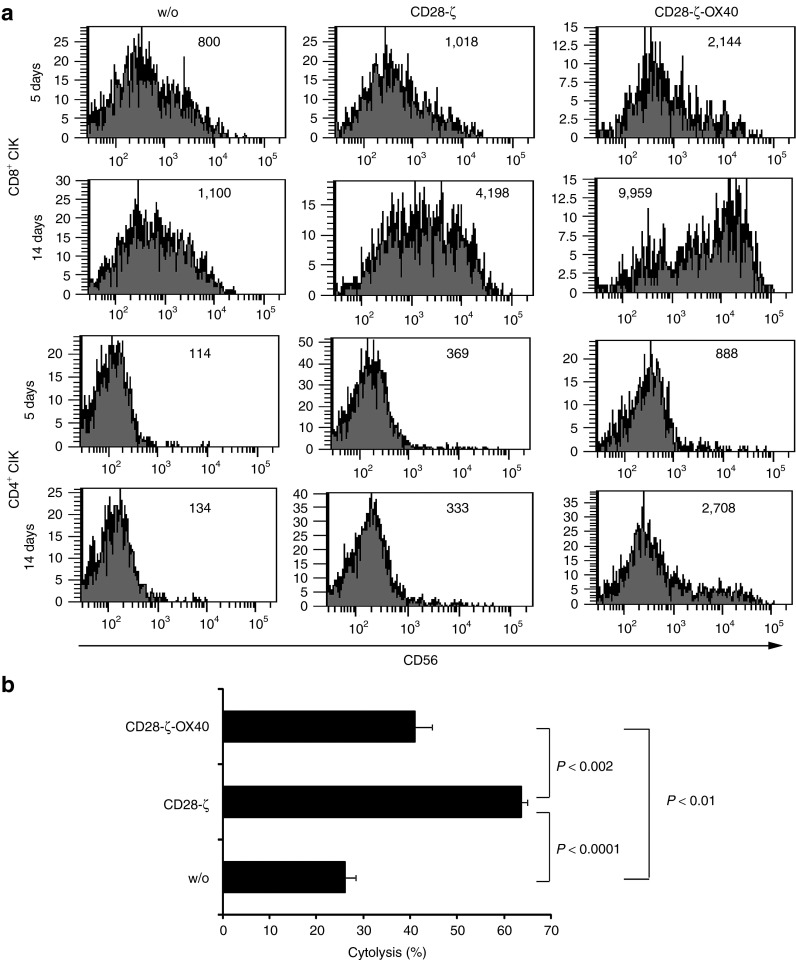
An outstanding property of CIK cells is to lyse tumor cells in a HLA-independent fashion14; NKG2D is crucial for this process.15 We defined the impact of second- and third-generation CAR signaling on NKG2D-mediated, CAR-independent tumor cell lysis utilizing Daudi cells as targets. Daudi cells are susceptible to NKG2D-mediated lysis but are not attacked by other natural-killer cell–associated targeting receptors.16 Of note, Daudi cells are not attacked by the engineered anti-CEA CAR cells. We modified CIK cells with the CD28-ζ and CD28-ζ–OX40 CAR, respectively, isolated CAR CIK cells by cell sorting and stimulated CAR CIK cells by the anti-idiotypic mAb BW2064 for 5–14 days in the presence of IL-2. Flow cytometric analyses revealed upregulated CD56 in CD8+ CIK cells upon CD28-ζ CAR signaling; additional OX40 costimulation by the CD28-ζ–OX40 CAR moreover increased CD56 expression (Figure 6a). By contrast, CD4+ CIK cells showed only minor alterations in the CD56 expression after CD28-ζ–OX40 CAR stimulation in this period of time. The CD4+:CD8+ T-cell ratio did not substantially change (not shown). CD56 upregulation was dependent of CAR stimulation and was not observed in CD8+ or CD4+ CIK cells without CAR. We coincubated long-term stimulated CAR-engineered CIK cells with Daudi cells and recorded target cell lysis. As summarized in Figure 6b, CD28-ζ CAR CIK cells were more effective in Daudi cell killing than CD28-ζ–OX40 CAR CIK cells with the additional OX40 costimulation; CAR-stimulated CIK cells were, however, more effective than CIK cells without CAR stimulation.
Figure 6.
Chimeric antigen receptor (CAR) signaling improves CAR- and HLA-independent lysis of Daudi tumor cells. (a) Cytokine-induced killer (CIK) cells were engineered with the CD28-ζ and CD28-ζ–OX40 CAR, and CAR expressing cells were isolated by cell sorting. CAR CIK cells were cultivated on plates coated with the CAR-specific anti-idiotypic monoclonal antibody (mAb) BW2064 (1 µg/ml coating concentration). After 5 and 14 days of culture, cells were stained for CD4, CD8, and CD56 expression and analyzed by flow cytometry. Numbers represent the mean fluorescence intensity. (b) Cells that were cultivated for 14 days on anti-idiotypic mAb-coated surfaces were recovered and cocultivated with carboxyfluorescein diacetate succinamidyl ester-labeled Daudi cells (2 × 104 cells/well) for 24 hours. Cells were stained with 7-aminoactinomycin D (0.5 µg/ml) and the numbers of life and dead cells were determined by flow cytometry. Numbers represent mean values ± SD. P values were determined using the Student's t-test.
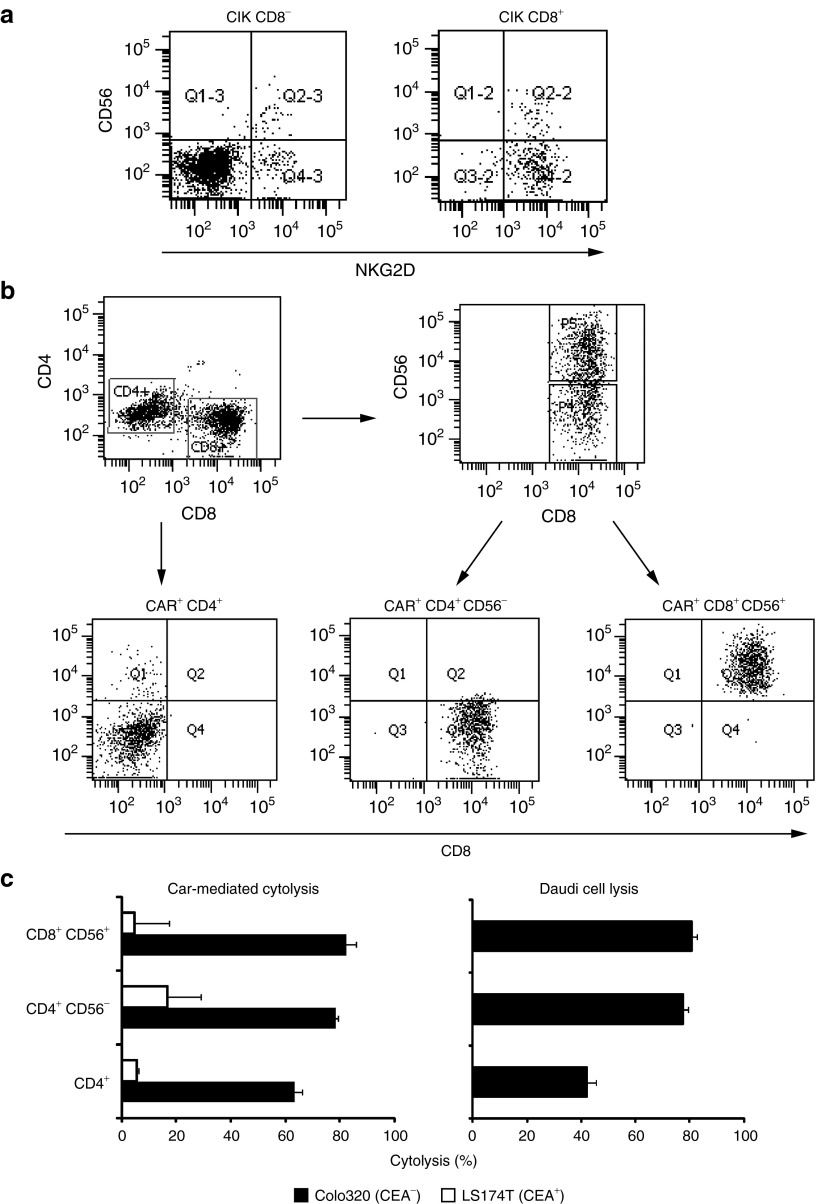
NKG2D was expressed at similar levels on nearly all CD8+CD56+ T cells and the majority of CD8+CD56− CIK cells; most CD8−CD56− CIK cells, however, lacked NKG2D (Figure 7a). As CD28-ζ–OX40 CAR signaling favors the accumulation of CD56+ CIK cells, we asked whether the different efficiency in the attack toward Daudi cells upon CAR signaling is due to these CIK cell subsets. To record CAR-mediated and CAR-independent antitumor activity, CIK cells were engineered with the CD28-ζ CAR, sorted for CAR expression, and stimulated by their CAR for 21 days by incubation with the anti-idiotypic mAb as described earlier. CAR+ CIK cells were further sorted into CD4+, CD8+CD56−, and CD8+CD56+ cells (Figure 7b) and cytotoxicity against CEA+ LS174T tumor cells, which is CAR mediated, and killing of CEA− Daudi cells, which is NKG2D mediated, were recorded. CEA− Colo320 tumor cells served as additional control for CAR specificity. As summarized in Figure 7c, all CAR+ CIK cell subsets killed CEA+ target cells with similar efficiency in vitro; CEA− cells were not lysed indicating the redirected specificity of the CAR. By contrast, cytolysis of Daudi target cells was predominantly mediated by CD8+ CIK cells; both CD8+CD56− and CD8+CD56+ CIK cells were equally effective. The data indicate that the less-matured CD56− CIK cells mediate HLA-independent target cell killing as effective as the highly matured CD56+ CIK cells.
Figure 7.
Antigen-redirected and antigen-independent target cell killing is mediated by CAR+ CD56+ and CD56− CIK cells with similar efficiency. (a) CD8+CD56−and CD8+CD56+ CIK cells express NKG2D with similar frequency. CIK cells were sorted into CD8+ and CD8− cells and analyzed for NKG2D and CD56 expression, respectively. (b) Purified CD28-ζ CAR CIK cells were cultivated for 14 days on anti-idiotypic monoclonal antibody-coated plates and sorted into CD4+, CD8+CDC56−, and CD8+CD56+ CIK cells by flow cytometry-based procedures. (c) Fractionated CAR+ CIK cells were cocultivated at an effector-to-tumor (E:T) cell ratio of 1:5 with CEA+ LS174T or CEA− Colo 320 tumor cells (each 2.5 x 104 cells/well) or at an E:T ratio of 1:1 with carboxyfluorescein diacetate succinamidyl ester-labeled Daudi cells (2.5 x 104 cells/well) for 24 hours. Cytolysis of LS174T and Colo 320 cells was determined by an XTT-based colorimetric assay. To determine Daudi cell killing cells were stained with 7-aminoactinomycin D (0.5 µg/ml) and the number of life and dead cells was determined by flow cytometry. The assay was performed in triplicate. Numbers represent mean values ± SD. CAR, chimeric antigen receptor; CEA, carcinoembryonic antigen; CIK, cytokine-induced killer.
In summary, our data demonstrate that “super-stimulation” of CIK cells by a third-generation CAR combining the CD28 early and OX40 late costimulation resulted in accelerated maturation of cells with a CD56+ phenotype which are prone to AICD and which in consequence substantially decreases CAR- and NKG2D-mediated target cell lysis.
Discussion
Cell therapy with ex vivo-propagated CIK cells has shown to be effective in the treatment of malignant diseases due to the HLA-independent recognition of tumor cells and the high killing capacity. The antitumor activity was further improved by engineering the cells with CARs which redirect CIK cells antigen-specifically against tumor cells.17,18,19 Cell therapy with CAR-modified CIK cells thereby aims to combine antigen-redirected and antigen-independent target cell recognition in the fight against cancer. CAR signaling to activate CIK cells upon contact to target cells benefits from appropriate costimulation in addition to the CD3ζ signal19 (see Figure 2) which, however, requires different prerequisites as the cells progress in differentiation. This is of particular relevance as our in-depth analyses revealed that CIK cells are heterogeneous in cell populations and also contain terminally differentiated cells with a CD56+ CD62L− CD45RO− TEMRA phenotype (see Figure 3). The observation is in accordance with a recent report.5 We recently demonstrated that CD28 cosignaling in particular improves secretion of proinflammatory cytokines including IL-2 and prolongs T-cell survival, whereas OX40 costimulation potentiates cytolysis in the absence of IL-2 and improves differentiation and survival of CCR7− and CD62L− effector memory cells.12,20,21 As matured CIK cells predominantly exhibit a TEMRA-like phenotype, we asked whether CIK cells may also benefit from extensive costimulation. Combined CD28–OX40 costimulation increased the number of CIK cells with the CD56+ phenotype, but these cells were more prone to AICD than CD56− cells and CD28–OX40 costimulation did not rescue CD56+ CIK cells from AICD. By contrast, the CD28 CAR produced less CD56 expression and a substantially lower number of TEMRA-like CIK cells which results in a more robust antitumor response. The differences in the CIK cell response are not due to reduced signaling by the CD28-ζ–OX40 CAR because (i) CIK cells stimulated by this CAR produced higher amounts of IFN- than by the CD28-ζ CAR and (ii) both CARs redirected CIK cells with similar efficiency when sorted CD56− and CD56+ CIK cell subsets were used (see Figure 5). There are, however, substantial differences in the CAR-induced CIK cell maturation which likely account for the observed differences; “super-stimulation” by CD28-ζ–OX40 produces more CD56+ TEMRA CIK cells with reduced survival and antitumor activity. This is in accordance with lower numbers of CD56+ CIK persisting in tumor tissues as compared with CD56− CIK cells (see Figure 5). Taking this effect into account, the CD28-ζ CAR is more efficient in redirecting CIK cells than the third-generation CD28-ζ–OX40 CAR. These data moreover indicate that the requirements of appropriate costimulation for “younger” effector memory T cells differ substantially from those of terminally differentiated T cells such as CD56+CIK cells.
CD56+ CIK cells were reported to be the most effective CIK cell population in tumor cell elimination; contrary observations, however, were also reported.13,14,15,22,23 In this article, we demonstrate that CAR-mediated activation improves CAR-independent CIK cell recognition of target cells as exemplarily demonstrated for killing of Daudi cells; moreover, CIK cells stimulated by the CD28-ζ CAR were superior as compared with those stimulated by the CD28-ζ–OX40 CAR. CAR+CD56− CIK cells expressed NKG2D, the predominant mediator of CIK cell killing,16 to a similar extent of that of CD56+ CIK cells; both CIK cell subsets were equally efficient in Daudi cell killing in the short term. OX40 costimulation strongly upregulated CD56 expression producing high numbers of TEMRA-like CIK cells which, although highly cytolytic, are also highly susceptible to AICD. In the long term, the increased rate of apoptosis of CD56+ CIK cells substantially reduces the antitumor efficacy as particularly observed upon CD28-ζ–OX40 costimulation. Our data are, therefore, not contradictory to reports which describe CD56+ CIK cells as the most effective subset in CIK cell therapy.13,22 The apparent discrepancies can be explained at least in part by substantial differences in the context in which CIK cells are activated. The antigen-independent killer-cell–like reactivity is independent of TCR signaling, whereas the CAR-redirected activation recruits the TCR-associated activation machinery of the “conventional” T cells. Terminally matured CD56+ CIK cells recognize their targets preferentially in a TCR- and antigen-independent fashion, whereas less-matured CD56− cells still have high levels of TCR downstream signaling molecules in place which can be recruited by CAR signaling. On the other hand, accelerated CIK cell maturation and AICD will reduce unintended off-target activation and damage to healthy tissues. The functional dichotomy of both CIK cell subsets is furthermore supported by a lower alloreactivity of CD56+ CIK cells as compared with CD56− CIK cells.22,24
Taken together, the requirements for activation of both CIK cell subsets and their consequences for modulating CIK cell function are obviously different. While less-matured CD56− CIK cells better respond to CAR signaling, which utilizes the TCR signaling pathway, terminal maturation- and activation-induced cell death are induced resulting in reduced antigen-specific antitumor reactivity. CAR-mediated “super-stimulation” by combined CD28 and OX40 costimulation furthermore accelerated maturation of CIK cells to CD56+ cells; less costimulation by CD28 only is therefore more suitable to prolong the CAR-mediated CIK cell response. Accordingly, the signaling requirements of CAR-engineered T cells are different from those of engineered CIK cells with limited costimulation being best for a sustained antitumor response of adoptively transferred CAR CIK cells.
Materials and Methods
Cell lines and reagents. 293T cells are human embryonic kidney cells that express the SV40 large T antigen.25 The CEA-expressing colon carcinoma cell line LS174T (ATCC CCL 188), the CEA-negative cell line Colo320 (ATCC CCL 220.1), and the Burkitt's lymphoma cell line Daudi (ATCC CCL-213) were obtained from ATCC (Rockville, MD). The anti-BW431/26 anti-idiotypic mAb BW2064/36 was described earlier.26 OKT3 (ATCC CRL 8001) is a hybridoma cell line that produces the anti-CD3 mAb OKT3. All cell lines were cultured in Roswell Park Memorial Institute 1640 medium, 10% (v/v) fetal calf serum (all Life Technologies, Paisly, UK). MAbs were affinity purified from hybridoma supernatants utilizing goat antimouse immunoglobulin G (IgG) 2a and IG1 antibodies, (Southern Biotechnology, Birmingham, AL) which were immobilized on N-hydroxy-succinimid-ester -activated sepharose (Amersham Biosciences, Freiburg, Germany). The goat antihuman IgG antibody and the biotin-, FITC-, or PE-conjugated F(ab′)2 derivatives were purchased from Southern Biotechnology. The anti-CD3-FITC or PE mAb UCHT-1 and the anti-CD8-FITC mAb DK25 were purchased from Dako (Eching, Germany); the anti-CD8-PECy7 mAb RPA-T8 and the anti-CD45RO-FITC mAb UCHL-1 were purchased from BD Pharmingen (Heidelberg, Germany); the anti-CD4-PerCP mAb EDU-2, the anti-CD62L-APC mAb LT-TD180, and the anti-CD56-PE mAb MEM-188 were purchased from Immunotools (Friesoythe, Germany); and the anti-NKG2D-APC mAb BAT221 was purchased from Miltenyi (Bergisch-Gladbach, Germany). The antihuman IFN- mAb NIB42 and the biotinylated antihuman IFN- mAb 4S.B3, the anti-IL-2 mAb 5344.111 and the biotinylated antihuman IL-2 B33-2 mAb, were purchased from BD Bioscience (San Diego, CA).
Generation and expression of recombinant anti-CEA CARs. The engineering of the retroviral expression cassettes for the BW431/26-scFv-Fc-ζ, BW431/26-scFv-Fc-CD28-ζ, and the BW431/26-scFv-Fc-CD28-ζ-OX40 CARs were described in detail.12,27,28 Retroviral transduction of T cells with recombinant receptors was described in detail elsewhere25,29 and receptor expression was monitored by flow cytometry.
Generation of CIK cells. Peripheral blood lymphocytes were isolated from blood of healthy volunteers by density centrifugation. CIK cells were induced by cultivation for 24 hours in Roswell Park Memorial Institute 1640 medium supplemented with 10% (v/v) fetal calf serum and 10 ng/ml IFN-γ (Imunkin, Boehringer Ingelheim, Ingelheim, Germany). After 24 hours, OKT3 antibody (100 ng/ml) and IL-2 (500 U/ml) were added. Cells were further cultured for 2–3 weeks and fresh medium with IL-2 (500 U/ml) was supplemented every 3 days. For comparison, peripheral blood lymphocytes were cultivated for 72 hours in the presence of anti-CD3 and anti-CD28 mAbs (OKT3, 15E8; 100 ng/ml each) and IL-2 (500 U/ml), respectively and propagated in the presence of IL-2 (500 U/ml).
Immunofluorescence analysis and cell sorting. CAR-engineered CIK cells and T cells, respectively, were stained with PE- or FITC-conjugated F(ab′)2 antihuman IgG1 antibodies (0.5 µg/ml) and the FITC- or PE-conjugated anti-CD3 mAb and identified by flow cytometry using a FACSCanto (Becton Dickinson, Mountain View, CA) cytofluorometer equipped with the FACS-Diva research software (Becton Dickinson). To isolate subpopulations, CIK cells were labeled with fluorochrome-conjugated antibodies. Cell sorting was performed utilizing a FACSAria (Becton Dickinson) cell sorter equipped with the FACS-Diva research software (Becton Dickinson).
CAR-mediated activation of engineered CIK cells. CIK cells were engineered with anti-CEA CARs and cultivated in microtiter plates (Nunc Omnitray, Roskilde, Denmark), precoated with purified anti-idiotypic mAb BW2064/36 (1 µg/ml), for 5–14 days in the presence of 50 U/ml IL-2. In a second set of experiments CAR-engineered CIK or T cells (1.25 × 103 − 1 × 104 cells/well) were cocultivated for 48 hours in 96-well round bottom plates with CEA+ LS174T and CEA− Colo320 tumor cells (each 2.5 × 104 cells/well). After 48 hours, culture supernatants were analyzed for IFN-γ and IL-2 by enzyme-linked immunosorbent assay. Briefly, cytokines in the supernatants were bound to the solid phase capture antibodies (each 1 µg/ml) and detected by the biotinylated detection antibodies (each 0.5 µg/ml). The reaction product was visualized by a peroxidase–streptavidin conjugate (1:10,000) and ABTS. Specific cytotoxicity of engineered T cells against target cells was monitored by an 2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetrazolium-5-Carboxanilide (XTT) XTT-based colorimetric assay.30 Briefly, CAR-engineered T cells were coincubated with tumor cells for 48 hours. The number of engineered T cells was adjusted to same numbers by adding nonmodified T cells. Viability of tumor cells was monitored using the “Cell Proliferation Kit II” (Roche Diagnostics, Mannheim, Germany) and reduction of XTT to formazane by viable tumor cells was monitored colorimetrically. Values were calculated as means of six wells containing tumor cells only subtracted by the mean background level of wells containing Roswell Park Memorial Institute 1640 medium, 10% (v/v) fetal calf serum. Nonspecific formation of formazane due to the presence of effector cells was determined from triplicate wells containing effector cells in same numbers as in the corresponding experimental wells. The number of viable tumor cells was calculated as follows: viability (%) = [optical density(experimental wells − corresponding number of effector cells)]/[optical density(tumor cells without effectors−medium)] × 100. Cytotoxicity (%) was defined as 100 – viability (%).
Nonspecific lysis of Daudi cells. TCR- and CAR-independent lysis of Daudi target cells was determined as follows: CAR CIK cells were isolated by cell sorting and cultivated for 15 days on plates that were precoated with the anti-idiotypic mAb as described earlier. Daudi cells were labeled for 5 min at 4°C with 1.25 µM carboxyfluorescein diacetate succinimidyl ester (molecular probes, life technologies, Darmstadt, Germany), washed extensively and cocultivated in 96-round-bottom plates (5 × 104/well) with CAR-activated CIK cells (5 × 104/well). After 24 hours, cells were recovered, stained with 7-aminoactinomycin D (0.5 µg/ml), and analyzed by flow cytometry. The cytometer was set to time count for 30 seconds and the number of life cells was determined. Viability was calculated as follows: viability (%) = (number of life Daudi cells with effector cells)/ (number of life Daudi cells without effector cells) × 100. Cytotoxicity (%) was defined as 100 – viability (%).
Activation-induced cell death. CAR-engineered T cells (2.5 × 106 total cells) were cultivated in the presence of 0.5 µg/ml of the anti-idiotypic mAb BW2064 and 0.25 µg/ml of a goat antimouse IgG antibody (Southern Biotechnology), respectively. After 72 hours, cells were removed and stained for CAR and CD56 expression with a FITC-conjugated F(ab′)2 antihuman IgG1 (Southern Biotechnology) and PE-conjugated anti-CD56 antibody (Immunotools, Friesoythe, Germany), respectively. Apoptotic cells were recorded by staining with APC-conjugated Annexin V (Immunotools) and 7-aminoactinomycin D, respectively. T cells were analyzed by flow cytometry and the number of CD56+ CAR+Annexin V+ and CD56+CAR−AnnexinV+ cells were determined.
CAR-mediated suppression of tumor growth. Rag−/− common −/− mice (Charles River, Sulzfeld, Germany) (4–8 animals/group) were subcutaneously transplanted with CEA+ C15A3 tumor cells (1 × 106 cells/animal) and CAR-modified CIK cells (1 × 106/animal). The number of totally injected CIK cells was adjusted to same numbers by adding nonmodified CIK cells. Alternatively, sorted CIK cell populations were applied (2.5 × 105/animal). CIK cells without CAR served as the control. Tumor growth was recorded every 2–3 days and tumor volumes were determined. Area under the curve was recorded as described31 and statistical significance between groups was determined by Student's t-test.
Immune histological analyses. Cryostat sections from tumor tissues were fixed with acetone for 10 min at −20°C. Slides were first incubated with “Fc receptor block” reagent for 15 minutes, followed by “Background Buster” for 30 minutes (both Innovex Biosciences, Richmond, CA) to block nonspecific binding. Slides were stained with an Alexa-488-conjugated anti-CD3 (HIT3A) (Biolegend, London, UK). Specificity of staining was assayed by incubation with the respective isotype-matched control antibodies. Stained sections were mounted with “Immunoselect” Antifading Mounting Medium supplemented with propidium iodide for nuclear staining (Dianova, Hamburg, Germany). One slide out of a series of tissue sections was routinely stained with haematoxylin–eosin (Carl Roth, Karlsruhe, Germany) to confirm histology. Stained sections were recorded by laser scan microscopy (Olympus Fluoview FV 1000 microscope equipped with FV10-ASW 3.0 software; Olympus, Hamburg, Germany). Three typical regions of each section were selected and the numbers of CD3+ cells and total cells were counted. The percentage of CD3+ cells was determined as follows: CD3+ (%) = (number of CD3+ cells / number of total cells) × 100.
SUPPLEMENTARY MATERIAL Figure S1. T cells and CIK cells express the same CAR with similar efficacy. Figure S2. Expression analyses of different CARs in CIK cells. Figure S3. Isolation of CAR CIK cell subpopulations.
Acknowledgments
We thank Birgit Hops, Nicole Hoffmann, and Dana Chrobok for their excellent technical assistance. This work was supported by the Deutsch José Carreras-Leukämie Stiftung, Munich.
Supplementary Material
References
- Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174:139–149. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donia M, Junker N, Ellebaek E, Andersen MH, Straten PT, Svane IM.2011Characterization and comparison of “Standard” and “Young” tumor infiltrating lymphocytes for adoptive cell therapy at a Danish Translational Research Institution. Scand J Immunolepub ahead of print). [DOI] [PubMed]
- Nishimura R, Baker J, Beilhack A, Zeiser R, Olson JA, Sega EI, et al. In vivo trafficking and survival of cytokine-induced killer cells resulting in minimal GVHD with retention of antitumor activity. Blood. 2008;112:2563–2574. doi: 10.1182/blood-2007-06-092817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Zhang Z, Tang L, Xu YC, Xie ZM, Gu XF, et al. Cytokine-induced killer cells in the treatment of patients with solid carcinomas: a systematic review and pooled analysis. Cytotherapy. 2012;14:483–493. doi: 10.3109/14653249.2011.649185. [DOI] [PubMed] [Google Scholar]
- Franceschetti M, Pievani A, Borleri G, Vago L, Fleischhauer K, Golay J, et al. Cytokine-induced killer cells are terminally differentiated activated CD8 cytotoxic T-EMRA lymphocytes. Exp Hematol. 2009;37:616–628.e2. doi: 10.1016/j.exphem.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizée G, Cantu MA, Hwu P. Less yin, more yang: confronting the barriers to cancer immunotherapy. Clin Cancer Res. 2007;13 18 Pt 1:5250–5255. doi: 10.1158/1078-0432.CCR-07-1722. [DOI] [PubMed] [Google Scholar]
- Rossig C, Bollard CM, Nuchtern JG, Rooney CM, Brenner MK. Epstein-Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood. 2002;99:2009–2016. doi: 10.1182/blood.v99.6.2009. [DOI] [PubMed] [Google Scholar]
- Shrikant P, Mescher MF. Control of syngeneic tumor growth by activation of CD8+ T cells: efficacy is limited by migration away from the site and induction of nonresponsiveness. J Immunol. 1999;162:2858–2866. [PubMed] [Google Scholar]
- Hombach AA, Holzinger A, Abken H. The Weal and Woe of Costimulation in the Adoptive Therapy of Cancer with Chimeric Antigen Receptor (CAR)-Redirected T Cells. Curr Mol Med. 2013;13:1079–1088. doi: 10.2174/1566524011313070003. [DOI] [PubMed] [Google Scholar]
- Hombach AA, Abken H. Costimulation by chimeric antigen receptors revisited the T cell antitumor response benefits from combined CD28-OX40 signalling. Int J Cancer. 2011;129:2935–2944. doi: 10.1002/ijc.25960. [DOI] [PubMed] [Google Scholar]
- Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–1696. [PubMed] [Google Scholar]
- Mesiano G, Todorovic M, Gammaitoni L, Leuci V, Giraudo Diego L, Carnevale-Schianca F, et al. Cytokine-induced killer (CIK) cells as feasible and effective adoptive immunotherapy for the treatment of solid tumors. Expert Opin Biol Ther. 2012;12:673–684. doi: 10.1517/14712598.2012.675323. [DOI] [PubMed] [Google Scholar]
- Verneris MR, Karami M, Baker J, Jayaswal A, Negrin RS. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood. 2004;103:3065–3072. doi: 10.1182/blood-2003-06-2125. [DOI] [PubMed] [Google Scholar]
- Pende D, Cantoni C, Rivera P, Vitale M, Castriconi R, Marcenaro S, et al. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur J Immunol. 2001;31:1076–1086. doi: 10.1002/1521-4141(200104)31:4<1076::aid-immu1076>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Marin V, Pizzitola I, Agostoni V, Attianese GM, Finney H, Lawson A, et al. Cytokine-induced killer cells for cell therapy of acute myeloid leukemia: improvement of their immune activity by expression of CD33-specific chimeric receptors. Haematologica. 2010;95:2144–2152. doi: 10.3324/haematol.2010.026310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzitola I, Agostoni V, Cribioli E, Pule M, Rousseau R, Finney H, et al. In vitro comparison of three different chimeric receptor-modified effector T-cell populations for leukemia cell therapy. J Immunother. 2011;34:469–479. doi: 10.1097/CJI.0b013e31821e763b. [DOI] [PubMed] [Google Scholar]
- Schlimper C, Hombach AA, Abken H, Schmidt-Wolf IG. Improved activation toward primary colorectal cancer cells by antigen-specific targeting autologous cytokine-induced killer cells. Clin Dev Immunol. 2012;2012:238924. doi: 10.1155/2012/238924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach AA, Heiders J, Foppe M, Chmielewski M, Abken H. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology. 2012;1:458–466. doi: 10.4161/onci.19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach AA, Chmielewski M, Rappl G, Abken H. Adoptive immunotherapy with redirected T cells produces CCR7- cells that are trapped in the periphery and benefit from combined CD28-OX40 costimulation. Hum Gene Ther. 2013;24:259–269. doi: 10.1089/hum.2012.247. [DOI] [PubMed] [Google Scholar]
- Sangiolo D, Martinuzzi E, Todorovic M, Vitaggio K, Vallario A, Jordaney N, et al. Alloreactivity and anti-tumor activity segregate within two distinct subsets of cytokine-induced killer (CIK) cells: implications for their infusion across major HLA barriers. Int Immunol. 2008;20:841–848. doi: 10.1093/intimm/dxn042. [DOI] [PubMed] [Google Scholar]
- Rettinger E, Kuçi S, Naumann I, Becker P, Kreyenberg H, Anzaghe M, et al. The cytotoxic potential of interleukin-15-stimulated cytokine-induced killer cells against leukemia cells. Cytotherapy. 2012;14:91–103. doi: 10.3109/14653249.2011.613931. [DOI] [PubMed] [Google Scholar]
- Todorovic M, Mesiano G, Gammaitoni L, Leuci V, Giraudo Diego L, Cammarata C, et al. Ex vivo allogeneic stimulation significantly improves expansion of cytokine-induced killer cells without increasing their alloreactivity across HLA barriers. J Immunother. 2012;35:579–586. doi: 10.1097/CJI.0b013e31826b1fd9. [DOI] [PubMed] [Google Scholar]
- Weijtens ME, Willemsen RA, Hart EH, Bolhuis RL. A retroviral vector system ‘STITCH' in combination with an optimized single chain antibody chimeric receptor gene structure allows efficient gene transduction and expression in human T lymphocytes. Gene Ther. 1998;5:1195–1203. doi: 10.1038/sj.gt.3300696. [DOI] [PubMed] [Google Scholar]
- Kaulen H, Seemann G, Bosslet K, Schwaeble W, Dippold W. Humanized anti-carcinoembryonic antigen antibody: strategies to enhance human tumor cell killing. Year Immunol. 1993;7:106–109. [PubMed] [Google Scholar]
- Hombach A, Sent D, Schneider C, Heuser C, Koch D, Pohl C, et al. T-cell activation by recombinant receptors: CD28 costimulation is required for interleukin 2 secretion and receptor-mediated T-cell proliferation but does not affect receptor-mediated target cell lysis. Cancer Res. 2001;61:1976–1982. [PubMed] [Google Scholar]
- Hombach A, Schlimper C, Sievers E, Frank S, Schild HH, Sauerbruch T, et al. A recombinant anti-carcinoembryonic antigen immunoreceptor with combined CD3zeta-CD28 signalling targets T cells from colorectal cancer patients against their tumour cells. Gut. 2006;55:1156–1164. doi: 10.1136/gut.2005.076208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach A, Wieczarkowiecz A, Marquardt T, Heuser C, Usai L, Pohl C, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001;167:6123–6131. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- Jost LM, Kirkwood JM, Whiteside TL. Improved short- and long-term XTT-based colorimetric cellular cytotoxicity assay for melanoma and other tumor cells. J Immunol Methods. 1992;147:153–165. doi: 10.1016/s0022-1759(12)80003-2. [DOI] [PubMed] [Google Scholar]
- Duan F, Simeone S, Wu R, Grady J, Mandoiu I, Srivastava PK. Area under the curve as a tool to measure kinetics of tumor growth in experimental animals. J Immunol Methods. 2012;382:224–228. doi: 10.1016/j.jim.2012.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.