Abstract
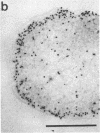
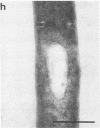
Antibodies were raised against homogeneous preparations of component C of the methylreductase system from Methanococcus voltae and Methanobacterium thermoautotrophicum. Cells of these organisms were fixed with paraformaldehyde and/or glutaraldehyde, sectioned, and labeled with antibodies and colloidal gold-labeled protein A. In M. voltae the gold particles were predominantly located in the vicinity of the cytoplasmic membrane. In rare cases a similar result was obtained also with M. thermoautotrophicum. However, in all but a few of the ultrathin sections of this bacterium, the label was randomly distributed in the cell interior. If one assumes a reliable fixation of all cell components, these results would suggest that the two distantly related methanogens studied have distinctive patterns for the localization of component C. The results with M. voltae are in agreement with recent findings that the methylreductase system is involved in the generation of a proton-motive force at the membrane.
Keywords: methanogen, methane, Archaeobacteria, nickel tetrapyrrole
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendayan M., Roth J., Perrelet A., Orci L. Quantitative immunocytochemical localization of pancreatic secretory proteins in subcellular compartments of the rat acinar cell. J Histochem Cytochem. 1980 Feb;28(2):149–160. doi: 10.1177/28.2.7354212. [DOI] [PubMed] [Google Scholar]
- Blaut M., Gottschalk G. Coupling of ATP synthesis and methane formation from methanol and molecular hydrogen in Methanosarcina barkeri. Eur J Biochem. 1984 May 15;141(1):217–222. doi: 10.1111/j.1432-1033.1984.tb08178.x. [DOI] [PubMed] [Google Scholar]
- Brada D., Roth J. "Golden blot"--detection of polyclonal and monoclonal antibodies bound to antigens on nitrocellulose by protein A-gold complexes. Anal Biochem. 1984 Oct;142(1):79–83. doi: 10.1016/0003-2697(84)90518-9. [DOI] [PubMed] [Google Scholar]
- Crider B. P., Carper S. W., Lancaster J. R. Electron transfer-driven ATP synthesis in Methanococcus voltae is not dependent on a proton electrochemical gradient. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6793–6796. doi: 10.1073/pnas.82.20.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Component C of the methylreductase system of Methanobacterium. J Biol Chem. 1981 May 10;256(9):4259–4262. [PubMed] [Google Scholar]
- Hippe H., Caspari D., Fiebig K., Gottschalk G. Utilization of trimethylamine and other N-methyl compounds for growth and methane formation by Methanosarcina barkeri. Proc Natl Acad Sci U S A. 1979 Jan;76(1):494–498. doi: 10.1073/pnas.76.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohring G. W., Mayer F., Mayer H. Immunoelectron microscopic localization of the restriction endonuclease EcoRI in Escherichia coli BS 5. Eur J Cell Biol. 1985 May;37:1–6. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nagle D. P., Jr, Wolfe R. S. Component A of the methyl coenzyme M methylreductase system of Methanobacterium: resolution into four components. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2151–2155. doi: 10.1073/pnas.80.8.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde M., Mayer F., Meyer O. Immunocytochemical localization of carbon monoxide oxidase in Pseudomonas carboxydovorans. The enzyme is attached to the inner aspect of the cytoplasmic membrane. J Biol Chem. 1984 Dec 10;259(23):14788–14792. [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Schechter I., Schechter B., Sela M. Combining sites of antibodies with L-alanine and D-alanine peptide specificity and the effect of serum proteolytic activity on their estimation. Biochim Biophys Acta. 1966 Oct 31;127(2):438–456. doi: 10.1016/0304-4165(66)90398-9. [DOI] [PubMed] [Google Scholar]
- Schönheit P., Beimborn D. B. ATP synthesis in Methanobacterium thermoautotrophicum coupled to CH4 formation from H2 and CO2 in the apparent absence of an electrochemical proton potential across the cytoplasmic membrane. Eur J Biochem. 1985 May 2;148(3):545–550. doi: 10.1111/j.1432-1033.1985.tb08874.x. [DOI] [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. Structure and methylation of coenzyme M(HSCH2CH2SO3). J Biol Chem. 1974 Aug 10;249(15):4879–4885. [PubMed] [Google Scholar]
- Tommassen J., Leunissen J., van Damme-Jongsten M., Overduin P. Failure of E. coli K-12 to transport PhoE-LacZ hybrid proteins out of the cytoplasm. EMBO J. 1985 Apr;4(4):1041–1047. doi: 10.1002/j.1460-2075.1985.tb03736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTON K. W., SCOTT P. J. ESTIMATION OF THE LOW-DENSITY (BETA) LIPOPROTEINS OF SERUM IN HEALTH AND DISEASE USING LARGE MOLECULAR WEIGHT DEXTRAN SULPHATE. J Clin Pathol. 1964 Nov;17:627–643. doi: 10.1136/jcp.17.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]