Abstract
The strongest risk factor for developing Alzheimer's Disease (AD) is age. Here, we study the relationship between ageing and AD using a systems biology approach that employs a Drosophila (fruitfly) model of AD in which the flies overexpress the human Aβ42 peptide. We identified 712 genes that are differentially expressed between control and Aβ-expressing flies. We further divided these genes according to how they change over the animal's lifetime and discovered that the AD-related gene expression signature is age-independent. We have identified a number of differentially expressed pathways that are likely to play an important role in the disease, including oxidative stress and innate immunity. In particular, we uncovered two new modifiers of the Aβ phenotype, namely Sod3 and PGRP-SC1b.
The phenomenon of protein aggregation has been associated with a variety of human disorders that affect large sections of the population worldwide1,2,3. These disorders, which include Alzheimer's, Parkinson's, type II diabetes, and the spongiform encephalopathies, are rapidly becoming one of the most important groups of pathologies worldwide in terms of both incidence and social costs.
Alzheimers disease (AD) is the leading cause of dementia in the human population. At least for familial AD, mutations that result in the generation of aggregation-prone isoforms of the amyloid β peptide are sufficient to cause amyloid plaques in the brain and the clinical features of the disease. Mature amyloid plaques are always seen in AD; however it is thought that precursor conformers, termed Aβ oligomers, are of primary importance in the pathology. It is becoming evident that, while the neuronal injury in AD is initiated by the accumulation of neurotoxic aggregates of Aβ peptide, these then give rise to a complex network of downstream events, (including aggregation of the tau protein) that culminate in neurodegeneration3. Neither the complete list of pathways involved in disease progression nor the causal chain of events that unites them is clear. It is therefore becoming increasingly apparent that a paradigm shift is required in order to describe and rationalise this complexity. It may simply not be possible to understand sporadic AD as the result of perturbations to a single pathway, where a single cause leads to the effect. Rather, the disorder should be studied as a system; that is, a change in the homeostatic equilibrium of many pathways4.
Ageing is a physiological process rather than just a chronological one. It is accompanied by changes in the steady-state mRNA levels of a number of genes and in the levels of many proteins that are involved in a variety of physiological processes5. A component of ageing is the collapse of cellular protein homeostasis and this process is thought to underpin the increasing incidence of protein aggregation diseases in the elderly4,6. Indeed, age-related changes in gene transcription lead to decreased quality control functions with age7. Late onset Alzheimer's disease accounts for the overwhelming majority8 of disease cases, making age the strongest risk factor for developing the disease.
It follows that, in order to elucidate the underlying causes that trigger AD and affect its development, it is necessary to investigate the changes in AD as a function of age. Here, we have used a Drosophila model of AD9 to study how various cellular pathways (as measured by transcription profiling) change with age and AD. In this model, the secreted human Aβ42 peptide is expressed specifically in the central nervous system of Drosophila melanogaster. The model recapitulates many of the pathologies observed in human AD, including Aβ accumulation, and premature death9,10,12. In the present study, we have used two versions of this model. For the transcriptomics study, we expressed the wild type Aβ42 coding sequence whereas, for gene-specific RNAi knock-down or over-expression experiments, we extended our study by validating the original observations in flies expressing the familial AD-linked Arctic (E22G) variant of Aβ42. We investigated the changes in transcriptome profiles over time for both control flies and those expressing Aβ42. The use of such an early onset model allowed us to distinguish between changes in gene expression due to AD and those due to ageing.
Results
Transcriptome analysis of AD and control flies over time
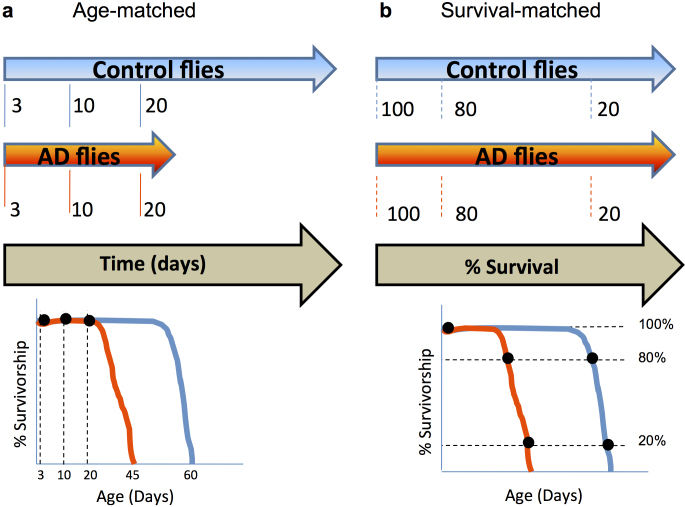
To investigate the differences between the processes of ageing and AD we used microarrays to measure changes in gene expression over time in control and Aβ42-expressing flies (hereafter referred to as Aβ flies). The Aβ flies used in these experiments carried 2 copies of a transgene expressing human Aβ42 (elavGAL4 > UAS-Aβ42), the 2 × Aβ42 model. Aβ flies have a much shorter lifespan with a median survival (50% flies still alive) of 23 vs. 63 days in control flies (see Supplementary Fig. S1, for the climbing, survival, and molecular phenotypes of Aβ flies used in our experiments). Therefore, in order to clearly distinguish Aβ and age-related changes in gene expression, we compared Aβ and control flies using a two-pronged strategy. In the first experiment, we age-matched flies according to their chronological age and extracted RNA samples from fly heads at days 3, 10 and 20 for both Aβ flies and the control cohort (Fig. 1). At these time points the survival of the flies is approximately 100% and so we can match samples from flies according to their chronological age.
Figure 1. Schematic representation of the experimental design.
(a) RNA samples are extracted from both control and AD flies at the same time in days. (b) RNA samples are extracted from both AD and control flies at the same % survival, which is a different time in days for each after 100% survival.
Beyond day 20, mortality in Aβ flies begins to increase and it is conceivable that increasing mortality itself could be associated with changes in gene expression. To compare gene expression changes associated with the increase in mortality in Aβ flies with those associated with normal ageing, we continued to extract RNA samples from Aβ and control flies but at different times such that the % survival of each group was the same (80% and 20% survival, this corresponded to days 21, and 25 in Aβ flies and days 56, and 68 for control flies). We used this data for a separate analysis of the gene expression changes over time according to survival. All RNA samples were extracted from one cohort of flies (see Methods) to reduce the effect of biological variability, but the age-matched and survival-matched samples were analysed separately and we refer to them as separate experiments. The day 3 (100% survival) sample is common to both analyses.
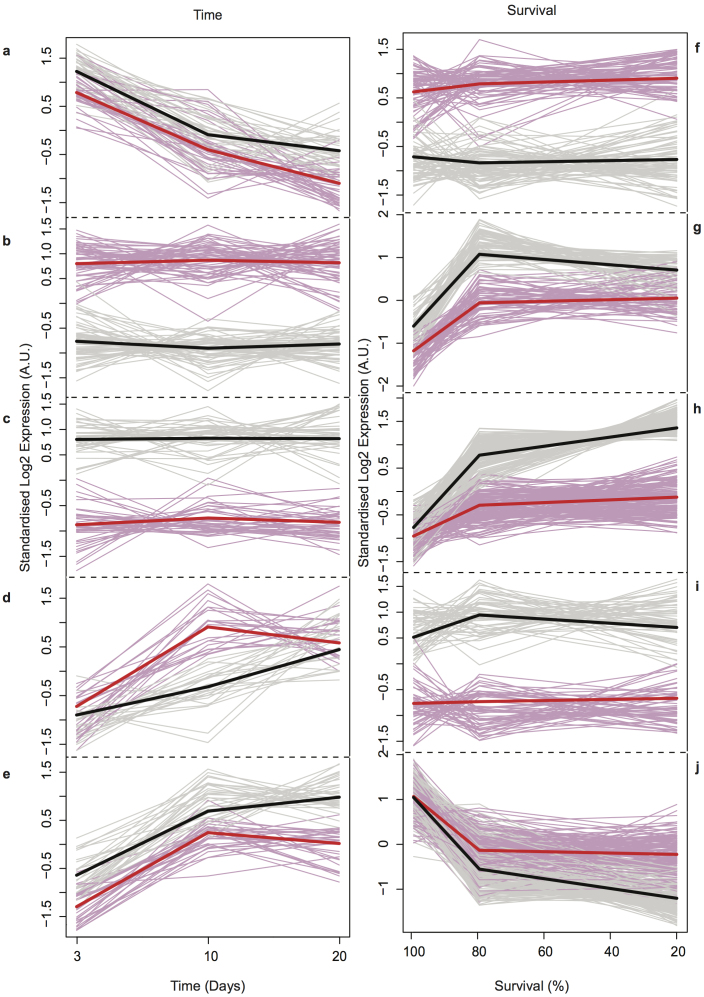
Analysis of the gene expression profiles from the two experiments (see Methods for details) identified 233 (day-matched) and 636 (survival-matched) differentially expressed genes, with a total of 712 genes combined (and an overlap of 157 genes, see Supplementary Table S1 online). We clustered average expression levels of the significantly differentially expressed genes using fuzzy c-means clustering (R mfuzz package)12,13 for each of the two experiments (Fig. 2: “a–e” day-matched and “f–j” survival-matched).
Figure 2. Clustering of changes in gene expression in Control and Aβ flies.
(a–j). Clustering of gene expression changes in Control (black) and AD (red) using Mfuzz. Bold lines represent cluster centroids, thin lines are average expression profiles for all cluster members plotted against equivalent time points (a–e) based on day (days 3, 10 and 20 for both Aβ and control flies, all approximately 100% survival) and (f–j) based on % survival of the flies (days 3, 21 and 25 (Aβ flies) or days 3, 56 and 68 (control flies), corresponding to 100%, 80% and 20% survival in each case).
In each experiment, we identify two categories of clusters. The first category of clusters (for both experiments), represents genes that are differentially up- or down- regulated in Aβ flies compared to controls, but do not change with time (Clusters “b”, “c”, “f” and “i”; 69, 47, 95 and 85 genes respectively – note that as fuzzy clustering is used, the number of genes assigned to each cluster does not total the number of significant genes). These genes presumably represent a direct response of the flies to the Aβ aggregation insult. By contrast the second category of expression profile clusters, for both experiments, represents genes with expression profiles that change over time (clusters “a”, “d”, “e”, “g”, “h” and “j” - 46, 35, 39, 121, 169, and 171 genes respectively). Based on these clusters, it appears that very few genes change expression over time in Aβ flies compared to controls, and that changes over time are more pronounced in control flies.
We therefore analysed the expression profiles of all genes present on the array for Aβ and control flies separately (see Methods) to identify all genes that change expression significantly over time in each group. For Aβ flies we identified 144 genes, whereas for control flies we identified 612 (with an overlap of 90 genes, Supplementary Table 1 online). For both Aβ and control flies, the genes changing over time were involved in similar pathways based on enriched Gene Ontology (GO) terms, in particular immune response and metabolic processes (data not shown). Therefore we conclude that the dysregulation is gene specific rather than pathway specific. For each of these genes, we tested the correlation between gene expression level and percent survival. Of the 144 genes whose expression level changes significantly over time in Aβ flies, there were only four (IM23, CG14933, CG7830, CG8036) whose expression level correlated with survival (Pearson's Product Moment Correlation Coefficient (ρ), 0.8 ≤ ρ ≤ −0.8). There is little information available on the function of these four genes, so we are unable to explain why the expression of these genes in particular correlates with the decrease in survival. Based on the fact that only 144 genes change expression over time in Aβ flies, we conclude that the transcriptional response to Aβ expression in our fly model is mostly not age dependent.
By contrast, 195 out of 612 genes changing expression correlated with the decreased survival in control flies using the same threshold. Of the four genes whose expression correlated with survival in Aβ flies, two were also correlated in controls (CG14933 and IM23) and the expression of both genes increased over time for both Aβ and control cohorts. We therefore conclude that there is no common mortality signature (i.e. genes that change in expression over time in both AD and control cohorts and whose expression levels correlated with survival in both), and that the gene expression changes occurring with normal ageing are distinct from those associated with Aβ expression in Drosophila. At the transcriptional level, the Aβ expression-associated signature is a constant change in the relative level of transcription of a certain set of genes over all time points measured, rather than the signature of ageing that manifests as a change in the level of transcription over time.
The 612 genes whose expression changes significantly over time in our control flies constitute a transcriptional signature of normal ageing in control flies. We compared this list of genes to a previous ageing study in Drosophila14. 44% (282/612) of genes changing expression with age in our control flies were also identified as ageing-related genes by Landis et al14, significantly more than expected by chance (hypergeometric test for enrichment, p < 1 × 10e-19). Furthermore, 63% of our 195 ageing signature genes for which expression level correlated with decreased survival with age were identified as ageing genes in the same study14 (hypergeometric test for enrichment, p < 1 × 10e-22). An analysis of the correlated genes for the control cohort using the Flymine database15 revealed that the pathways in which these genes are involved are significantly enriched in a number of pathways and GO annotations related to xenobiotic metabolism, glutathione metabolism and immune response pathways16,17. This is consistent with current theories of ageing19,20,18. The transcriptional changes associated with ageing in our control flies are therefore consistent with expected changes with age in Drosophila, both at the single gene and pathway level. The fact that our analysis produces expected results for control flies lends weight to the conclusions drawn from our analysis of the Aβ flies.
We performed Principal Components Analysis (PCA) of the expression data (Supplementary Fig. S2) and found that, while across the first three sample points Aβ and control groups cluster together, in the final two (where samples did not have the same age in days) they do not, confirming that the downstream effects of Aβ expression in this model are not similar to normal ageing, at least at the level of transcription. Supplementary Table S1 online lists all the differentially expressed genes, what method determined their significance, the cluster to which they belong for both experiments, their Pearson correlation with survival and their normalised expression level (see Methods) for each data point and for each of the replicates.
Oxidative stress-related changes in gene expression with AD
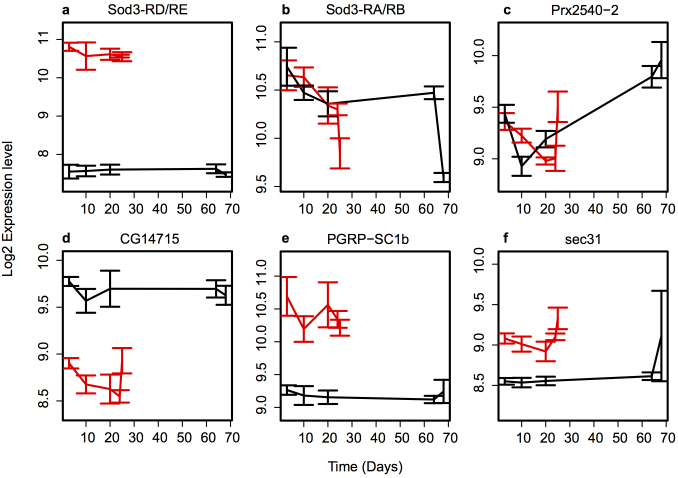
The transcript most highly up-regulated (~8-fold; Fig. 3a) in Aβ flies in our experiments was Sod3 (CG9027) which encodes an extracellular Cu/Zn superoxide dismutase21. In Drosophila there are 4 Sod3 transcripts (and 3 protein products), and two of these, Sod3-RD and Sod3-RE are specifically up-regulated in the Aβ flies (see Fig. 3a for Sod3-RD/RE and Fig. 3b for Sod3-RA/RB profiles). Superoxide dismutase enzymes have recently been linked to inflammation, with Sod3 proposed to contribute to this process both by scavenging free radicals and also, more directly, by affecting immune responses and signal initiation22. Interestingly, in 2009, before Sod3 had been discovered in flies21, Rival et al10 showed that overexpression of Sod1 was associated with a decrease in lifespan in Aβ flies.
Figure 3. Expression profiles of individual genes in Control and Aβ flies.
Gene expression profiles of RNA from heads from Control (black) and Aβ (red) female mated flies at 6 time points during adulthood. Time points correspond to days 3, 10 and 20 (100% survival) in both control and Aβ flies and at 56 (21), and 68 (25) days in Control (Aβ) flies that correspond to 80% and 20% survival, respectively. Average gene expression levels of four biological replicates (except time point 3 and 5 which are the average of 3 biological replicates, see Methods) ± s.e.m. plotted. (a) Sod3-RD and Sod3-RE (b) Sod3-RA and Sod3-RB (c) Prx2540-2 (d) CG14715 (e) PGRP-SC1b (f) sec31.
Cyp6a20, encoding a cytochrome P450 enzyme, was another gene with significantly altered expression in Aβ flies compared to control flies. We found that Cyp6a20 expression was significantly reduced in Aβ flies in an age- independent manner (see Supplementary Table S1 for expression data). Cyp6a20 was also identified in a genetic screen as a modifier of the survival phenotype in Aβ flies10 and cytochrome P450s have previously been identified as being a group of enzymes that are up-regulated with age24,23. On the other hand, genes such as Prx2540-2 (Fig. 3c), encoding peroxidases involved in the clearance of hydrogen peroxide25, appear to change with time in control flies. This change was not observed in Aβ flies. Increased expression with age in control, but not Aβ, flies was also observed for genes involved in glutathione metabolism, for example several of the glutathione-S-transferases increased expression over time only in control flies (Supplementary Table S1). Interestingly, one member of this family, GstE9, did increase expression over time in Aβ flies. We again conclude that the same processes are important in Aβ toxicity and ageing, but with different specific genes affected by each process.
Immune response-related changes in gene expression with AD
Two important processes that showed significantly altered gene expression profiles in our AD model are the innate immunity and defence response pathways. Many genes in these pathways normally become up-regulated during ageing23,26. In control flies, increased expression with age was observed for genes involved in the antibacterial humoral response14, such as Dpt, CecA1, CecA2 and Drs. Similar changes were not observed in Aβ flies. For example, immune response genes, such as that encoding the proteoglycan recognition protein PGRP-SC1b were significantly altered in our experiment (Fig. 3e), with increased transcript levels in Aβ flies vs. controls at all time points. PGRP-SC1b is a catalytic PGRP that is likely to be involved in down-regulation of the Imd innate immunity pathway in response to injury27.
Cellular transport and chaperone related changes in gene expression with AD
Intracellular transport, specifically endocytic processing, has been implicated in AD using GWA studies for sporadic AD28. In our study, sec31, a gene coding for an essential component of the COPII coat for ER to Golgi transport29, was increased in expression in Aβ flies compared to controls (Fig. 3f). CG14715, another gene implicated in intracellular transport and protein folding, encodes the Drosophila ortholog of FKBP2/FKBP13, a prolyl-isomerase thought to function as an ER chaperone31,30. The expression of CG14715 was down-regulated in an age-independent manner in the Aβ flies (Fig. 3d).
Identifying modifiers of the Aβ phenotype using gene-specific RNAi
Our microarray analysis identified significant expression changes (between control and Aβ flies) in 712 genes. These genes are potential modifiers of the Aβ phenotype. In order to determine whether the observed expression changes are relevant to the onset or development of the disease or whether they are simply correlative, we manipulated the levels of four of these genes in the Drosophila AD model (Data summarised in Table 1). We chose two genes that are highly up-regulated in an age-independent manner in Aβ flies: Sod3, which was identified as the most differentially expressed gene between the Aβ and control flies (~8 fold increased levels in Aβ flies, Fig. 3a) and PGRP-SC1b (~2 fold increased levels in Aβ flies, Fig. 3e). These genes are involved in oxidative stress and innate immunity, respectively. We also investigated sec31, encoding a protein involved in intracellular transport (~1.5 fold increased levels in Aβ flies, Fig. 3f) and CG14715, coding for a putative ER chaperone (~1.7 fold decreased levels in Aβ flies, Fig. 3d).
Table 1. Differentially expressed genes selected for follow up experiments and summary of results.
| Gene | Function | Expression Profile | Effect of RNAi in this work |
|---|---|---|---|
| Sod3-RD, Sod3-RE | Extracellular Cu/Zn superoxide dismutase21,22,42 | Over-expressed in Aβ flies. Fig. 3a | Improved climbing and survival in Aβarc flies. No effect on survival of control flies, slight increase in climbing performance. Figure 4 |
| PGRP-SC1b | Innate immune response27 | Over-expressed in Aβ flies. Fig. 3e | Improved climbing and survival in Aβarc flies. No effect in control flies. Figure 5 |
| sec31 | ER to Golgi transport29 | Under-expressed in Aβ flies. Fig. 3f | Increased survival of Aβarc flies, no effect on climbing ability. No effect on survival or climbing performance in control flies. Supplementary Figure S6 |
| CG14715 | Intracellular transport and protein folding31,30 | Over-expressed in Aβ flies, Fig. 3d | Negative effect on climbing and survival in both control and Aβarc flies. Supplementary Figure S5 |
In these experiments, we used a modified version of the fly AD model (elavGAL4 > UAS- Aβ42arc) in which flies expressed a single copy of the Aβ peptide containing the familial E22G (arctic) mutation that increases the aggregation propensity of the Aβ42 peptide32. It has been shown previously11,32,33 that these models are equivalent and that their effect on the flies' lifespan is proportional to the aggregation propensity of the Aβ variant. Luheshi et al. (2010) investigated the effect of mutations (including the arctic mutation) in the sequence of Aβ42 and showed that the aggregation propensity and in vivo toxicity as quantified by locomotor and survival assays were correlated. Finally, as shown below, the two models show similar changes in expression of specific genes. Although, we cannot exclude the possibility that the brain pathology due to expression of the alternative Aβ transgenes is different, this evidence suggests that they have similar effects.
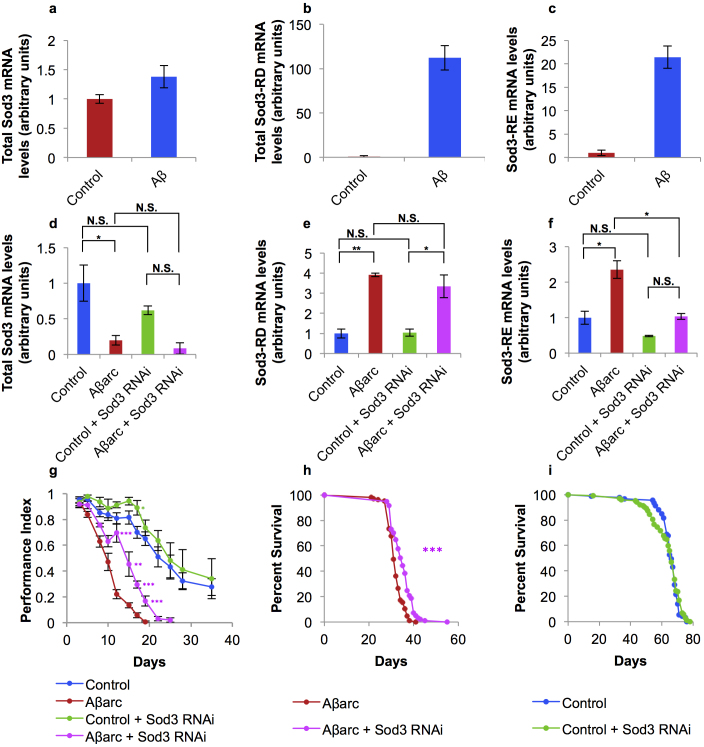
We confirmed the up-regulation of Sod3 using qRT-PCR. Sod3 has 4 alternative transcripts. Increases in Sod3 mRNA levels in the microarray experiment (neuronal expression of two copies of Aβ42) were specific to two transcripts, Sod3-RD and Sod3-RE. qRT-PCR quantification of total Sod3 levels suggested a 50% increase in Sod3 transcription in the heads of Aβ flies at day 20 (Fig. 4a). However, transcript-specific primers showed a much larger increase in Sod3-RD (~100-fold) and Sod3-RE levels (~20-fold) confirming the array data (Fig. 4b, 4c). In Aβarc flies (expressing neuronal Aβ42arc peptide), total Sod3 mRNA levels were actually decreased (Fig. 4d). However as in the 2 × Aβ42 flies, both Sod3-RD and Sod3-RE mRNA levels were significantly increased in heads from Aβarc flies (Fig. 4e, 4f). Thus, both Aβ and Aβarc flies show a specific increase in the levels of Sod3-RD and Sod3–RE transcripts.
Figure 4. Sod3 levels in Aβ flies and effects of Sod3 RNAi on Sod3 expression, locomotion and survival.
(a–c). Levels of Sod3 transcripts in head RNA from Aβ (elavGAL4 > UAS-Aβ42 × 2) and control (elavGAL4/+) female mated flies at day 20 measured by qRT-PCR and plotted relative to Act5C mRNA levels, in arbitrary units, represented relative to levels in control (set to 1). (a) Total Sod3 mRNA levels (primers against A, B, D, E common transcript). (b) Sod3-RD mRNA levels. (c) Sod3-RE mRNA levels. (d–f), Levels of Sod3 transcripts in head RNA from Control (elavGAL4/+), Aβarc (elavGAL4 > UAS-Aβ42arc), Control + Sod3 RNAi and Aβarc + Sod3 RNAi female mated flies at day 7 measured by qRT-PCR and plotted relative to Act5C mRNA levels, in arbitrary units, represented relative to mRNA levels in Control (set to 1). qRT-PCR analysis: n = 3 replicates, mean ± s.e.m. plotted. mRNA levels between groups compared using two-tailed Student's t-test (f-test for equal variance). (d) Total Sod3 mRNA levels. (e) Sod3-RD mRNA levels. (f) Sod3-RE mRNA levels. (g) Climbing performance of Control (elavGAL4/+), Aβarc (elavGAL4 > UAS-Aβ42arc), Control + Sod3 RNAi and Aβarc + Sod3 RNAi mated females at different time points at 24°C. n = 3 (3 replicates, 10 flies/replicate). Performance indices (see Methods) between Control and Control + Sod3 RNAi and Aβarc and Aβarc + Sod3 RNAi were compared at each time-point using two-tailed Student's t-test (f-test for equal variance). (h) Survival curves of Aβarc (n = 105, median = 31, P < 0.0001 vs. Control) and Aβarc + Sod3 RNAi (n = 97, median survival = 35, P < 0.0001 vs. Aβarc) mated females at 25°C. (i) Survival curves of Control (n = 93; median survival = 66) and Control + Sod3 RNAi (n = 102; m = 66, P = 0.5280 vs. Control) mated females at 25°C. Comparison of survival curves was carried out using the Log-rank test. P values: *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
As Sod3 transcript levels are also increased in Aβarc flies, we tested whether reducing Sod3 levels using RNAi could ameliorate the Aβ phenotype. Ubiquitous RNAi against Sod3 resulted in effective knock-down of total Sod3 expression levels in head mRNA (down to 10% of control) and substantial knock-down of Sod3-RD and Sod3-RE expression levels (down to 30–40% of control: Supplementary Fig. S3), confirming that indeed the Sod3-RNAi line is substantially knocking down Sod3 transcript levels.
Targeting Sod3 RNAi specifically to the nervous system resulted in a consistent (though not statistically significant) decrease in total Sod3 RNA levels in head RNA from both Sod3 RNAi control and Aβarc flies (vs. non-RNAi controls, Fig. 4d–f). Since Sod3 levels were measured from whole head RNA extracts, which also include other cell types apart from neurons, our measurement of total head Sod3 mRNA is likely to be an underestimate of the actual knock-down in the nervous system.
Sod3 RNAi improved climbing (Fig. 4g and Supplementary Fig. S3) and survival in AD flies; median survival for Aβarc Sod3-RNAi flies was 35 days vs. 31 days for control Aβarc flies (Log-rank test, P < 0.0001: Fig. 4h and Supplementary Fig. S3), and this appeared to correlate with significantly decreased Sod3-RE mRNA levels (Fig. 4f). Ubiquitous knock-down of Sod3 has previously been reported to be detrimental for Drosophila lifespan21. However, we did not observe any significant effect of nervous system-specific Sod3-RNAi in control (i.e. no Aβarc) flies (Fig. 4g and 4i and Supplementary Fig. S3). Thus, ablating Sod3 up-regulation in Aβarc flies ameliorates the Aβ phenotype.
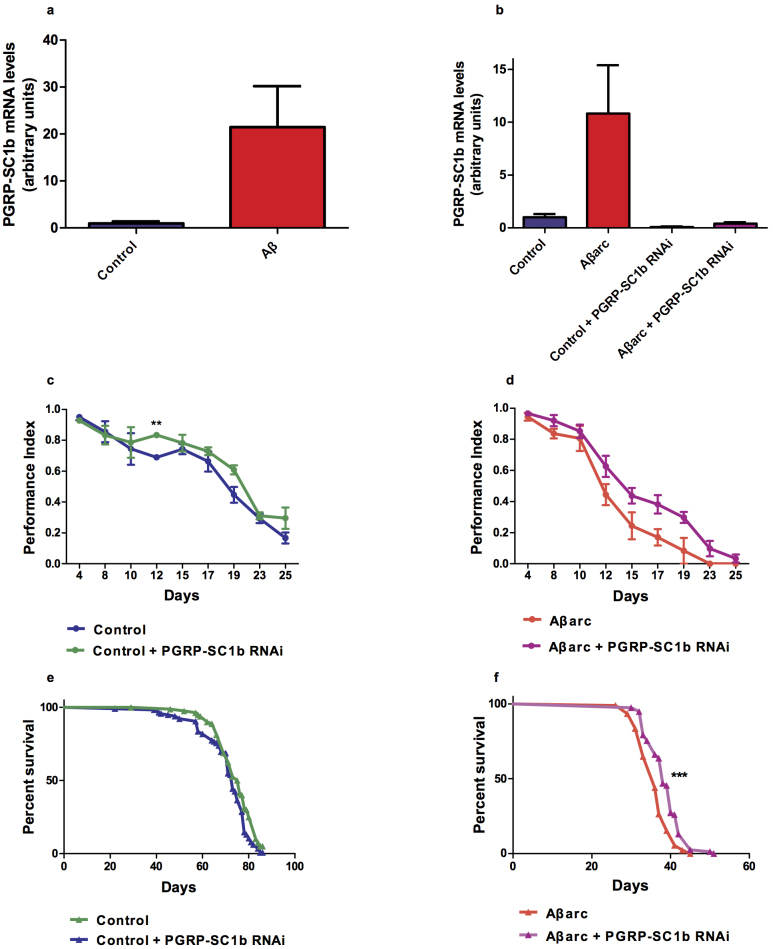
We next took a similar approach to investigating PGRP-SC1b. In the microarray study, PGRP-SC1b RNA levels were increased ~2-fold in Aβ flies (Fig. 3e); moreover, qRT-PCR, showed a similar increase (Fig. 5a). In Aβarc flies (expressing neuronal Aβ42arc peptide), PGRP-SC1b RNA levels were increased ~10-fold in head RNA (compared to control flies, Fig. 5b). Ubiquitous RNAi against PGRP-SC1b resulted in effective knock-down of PGRP-SC1b expression levels (down to 30% of control: Supplementary Fig. S5). Targeting RNAi against PGRP-SC1b to the nervous system resulted in improved climbing (Fig. 5d) and survival (median survival for Aβarc PGRP-SC1b RNAi flies was 38 days vs. 36 days for Aβarc, Log-rank test, P < 0.0001, Fig. 5f) flies compared to their (non-RNAi) controls. However, we did not observe any significant effect of nervous system-specific PGRP-SC1b-RNAi in control (i.e. no Aβarc) flies (Fig. 5c, 5e and Supplementary Fig. S4).
Figure 5. Effects of PGRP-SC1b RNAi on PGRP-SC1b expression, locomotion and survival.
(a) PGRP-SC1b mRNA levels in head RNA from Aβ (elavGAL4 > UAS-Aβ42 × 2) and control (elavGAL4/+) mated female flies at day 20 measured by qRT-PCR and plotted relative to Act5C mRNA levels, in arbitrary units, represented relative to mRNA levels in elavGAL4/+ flies (set to 1). n = 3 replicates, mean ± s.e.m. plotted. mRNA levels between groups compared using two-tailed Student's t-test (f-test for equal variance). (b) Levels of PGRP-SC1b mRNA in head RNA from Control (elavGAL4/+), Aβarc (elavGAL4>UAS-Ab42arc), Control + PGRP-Sc1b RNAi, and Aβarc + PGRP-SC1b RNAi from flies at day 7 measured by qRT-PCR. n = 3 replicates, mean ± s.e.m. plotted. mRNA levels between groups compared using two-tailed Student's t- test (f-test for equal variance). (c) Climbing performance of Control, and Control + PGRP-Sc1b RNAi, at different time points at 24°C. Performance indices were compared at each time-point using two-tailed Student's t-test (f-test for equal variance). (d) Climbing performance of Aβarc and Aβarc + PGRP-SC1b RNAi at different time points at 24°C. Performance indices were compared at each time-point using two-tailed Student's t-test (f-test for equal variance). (e) Survival curves of Control (n = 119, median = 73) and Control + PGRP-SC1b RNAi (n = 77, m = 74.5, P = 0.1825 vs. Control) female flies at 25°C. (f) Survival curves of Aβarc (n = 91, median = 36, P < 0.0001 vs. Control) and Aβarc + PGRP-SC1b RNAi (n = 77, m = 38, P < 0.0001 vs. Aβarc) female flies at 25°C. Comparison of survival curves was carried out using the Log-rank test. P values: *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
RNAi against CG14715 (which encodes a prolyl-isomerase thought to function as an ER chaperone31,30) in the fly nervous system of Aβarc flies resulted in a significant increase in survival (median survival for two Aβarc CG14715 RNAi lines was 34 days vs. 31 days for Aβarc, Log-rank test, P < 0.0001; Supplementary Fig. S5) but no improvement in climbing ability (compared to non-RNAi controls; Supplementary Fig. S6). By contrast, overexpression of CG14715, in Aβarc flies, resulted in a significant increase in climbing ability in early adulthood but had no effect on survival (Supplementary Fig. S5). In control (non-Aβarc) flies, RNAi knock-down or overexpression of CG14715 resulted in either no effect or a slight deficit in climbing ability; neither treatment affected survival (Supplementary Fig. S5). Thus manipulating CG14715 expression can modify the Aβ phenotype, but the effect is complex.
Overexpression or RNAi knock-down of sec31 in the fly nervous system resulted in decreased climbing ability and longevity in control (non-Aβarc) and either no, or a negative, effect in Aβarc flies. (Supplementary Fig. S6). The sec31 protein is an essential component of the COPII tracking complex29 so it likely that sec31 levels are critical for normal cellular function. Thus it is unclear whether sec31 has any specific modifying effect on the Aβ phenotype.
These results suggest that modifying expression of Sod3, PGRP-SC1b and CG14715 in Aβ flies can suppress the locomotor and survival defects associated with toxic Aβ42 expression.
Discussion
In this study, we have used time-course transcriptomic analysis to identify 712 D. melanogaster genes that are differentially expressed between Aβ-expressing and control flies. Our results suggest that Aβ-expressing flies are more similar to young than to old control flies. Since Aβ and control flies remained transcriptionally distinct as their mortality increased, we therefore conclude that the expression of Aβ in this model does not equate to an increased rate of ageing. Cluster analysis revealed that differentially expressed genes can be separated into those that change expression over time and those whose expression is constant. These results are consistent with the very aggressive nature of the model in which very high levels of Aβ are expressed at all times. We suggest that any change at the molecular level that correlates with the phenotype in the Aβ flies lies downstream of the transcriptome and that, by day 3, the Aβ flies are already in an essentially “terminal” transcriptional state. In this state, certain pathways such as the oxidative stress response pathway appear to be dysregulated and drive degeneration, which is expressed as both decreased locomotor activity and increased mortality.
We have further investigated some of the genes that were consistently over expressed in Aβ flies and we identified two modifiers of Aβ toxicity: Sod3 and PGRP-SC1b. In particular we found that increased levels of two Sod3 transcripts in Aβ flies were not accompanied by a compensatory increase in expression of either catalase or peroxidases. An imbalance in the relative levels of these three types of enzymes may result in an increased level of toxic H2O2 in Aβ flies, contributing to disease pathology. The expression of an RNAi for Sod3 resulted in a reduction in mRNA levels for at least one Sod3 transcript, and was accompanied by improved locomotor ability and survival in Aβ, but not control, flies. This suggests that decreasing Sod3 enzyme levels in our Aβ flies alleviated a toxic H2O2 overload. Rival et al10, found similar results regarding the toxicity of H2O2. In particular, they observed increased survival of Aβ flies when a dominant negative mutant Sod1 was expressed and reduced survival when wild-type Sod1 was expressed. By contrast, they found that the median survival was increased when Cat (encoding catalase) was overexpressed, suggesting that overproduction of H2O2 by the Sod1 enzyme can overwhelm catalase resulting in toxicity and a decrease in lifespan of the Aβ flies. Moreover, a previous study34 in C. elegans showed that loss of the sod-4 gene, (the C. elegans ortholog of Sod3) had no effect on lifespan in wild-type worms, but increased the survival of daf-2 (insulin receptor) mutants. Doonan and colleagues34 suggested that the SOD-4 enzyme may be generating H2O2, which acts as a signalling molecule and activates IIS (insulin/IGF-like signalling) by inactivating redox-sensitive phosphatases35; consequently, its loss in IIS mutants would enhance their long-lived phenotype. Increased H2O2 in Aβ flies could also act as a signal. Thus, by reducing Sod3 enzyme levels, we would decrease both the toxic overload and affect the signalling role of H2O2 to enhance lifespan. There have also been a number of reports on the activation of autophagy by H2O2 through the PI3K/Beclin1 and the PI3K/Akt/mTOR pathways36. In the case of PGRP-SC1b, it is not clear why RNAi results in an increase in lifespan and improved locomotor abilities in AD flies. We speculate that it could be due to a dysregulation of the pathway, similar to what we observed for Sod3.
The comparative analysis of gene expression between AD and ageing revealed changes at the single gene level, rather than the pathway level. Nevertheless, if the individual genes that are regulated very differently in Aβ flies vs. wild-type controls are considered, a number of important inferences may be made concerning AD. One particular example is the oxidative stress pathway, which is up-regulated with age in control flies (in our experiment and Landis et al)14. In the wild-type fly, manipulating cellular antioxidant defences (using transgenes) is not necessarily beneficial or detrimental to the health of the organism18. In other words, physiological levels of ROS can be dealt with by the insect's powerful enzymatic and non-enzymatic detoxification routes. However, mitochondrial dysfunction is observed in AD and this may be exacerbated with age37; therefore it is possible that ROS generated as a consequence of this mitochondrial dysfunction overwhelms cellular detoxification pathways.
It is becoming clear that the cascade of events that originates from the aggregation of Aβ and tau involves major stress response pathways, and all these stress pathways appear to be inter-related. However, their co-regulation and inter-relationships have been poorly characterised to date. In this study, we observed the dysregulation of a number of genes that belong to pathways that appear to be related, even though we were not able to assess their precise regulatory relationship. In all, this study has allowed us to investigate the processes that change in Aβ flies and dissect these from the processes that change with normal ageing. We observed a large number of dysregulated processes in AD flies. In particular, we highlight a number of genes involved in redox stress, innate immune response and pathogen defense response and intracellular transport. We have shown that either knocking-down or over-expressing some of these genes increased lifespan and improved locomotor (climbing) ability in Aβ flies compared to control flies. This suggests that the processes of oxidative stress and the immune response are likely to play an important role in the disease. The insights into time/age-dependent gene expression levels in AD that have been gained using an insect model may prove valuable in the design of strategies to combat this economically and socially important disease.
Methods
Fly stocks and maintenance
The UAS-Aβ42-51D and UAS-Aβ42arc-51D flies were generated using the PhiC31 method as previously described38. ElavGAL4C155 and tubGAL4 were used for neuronal-specific expression and ubiquitous expression of transgenes respectively. For microarray experiments, w1118, elavGAL4 > UAS-Aβ42-51D (×2 copies) flies and controls w1118, elavGAL4/+; 51D (×2 copies, empty insertion site) were used. For RNAi experiments, we replaced UAS-Aβ42-51D (×2 copies) flies with flies carrying a single UAS-Aβ42arc-51D transgene. The presence of one, as opposed to two, transgenes in this line facilitated the use of the other transgenes required in these experiments. Aβ42 carrying the E22G (arctic) mutation was used, since a single copy of the wildtype Aβ42 transgene was found not to result in a significant locomotor or survival defects in our experiments. The phenotypes caused by expression of the wild-type Aβ transgene and the arctic Aβ transgene are quantitatively and qualitatively similar, as can be seen from the relative effects of each transgene on locomotor performance and survival (Supplementary Fig. S1). Median survival for the arctic model is 31 days in the Aβ flies compared to 24 days in the 2 × Aβ42 model and 66 days in control flies. For the RNAi experiments, w1118, elavGAL4 > UAS-Aβ42arc-51D flies were used and controls, w1118, elavGAL4/+;51D (empty insertion site). In these experiments, female virgin w1118; 51D or w1118; UAS-Aβ42arc-51D flies were crossed to w1118, elavGAL4 > UAS-RNAi males. In the PGRP-SC1b experiments (RNAi insertion on chromosome X), female virgin w1118, UAS-PGRP-SC1b RNAi flies were mated to w1118, elavGAL4 > UAS-Aβ42arc-51D/CyO or w1118, elavGAL4; 51D/CyO flies to generate the experimental flies. RNAi and over-expression lines were obtained from the VDRC or Bloomington stock centers. The lines used were Sod3-RNAi (VDRC# 37793, w1118; P{GD4801}v37793), PGRP-SC1b RNAi (VDRC #51237, w1118, p{GD5490} v51237), sec31-RNAi (VDRC #35867, w1118; P{GD13867}v35867), sec31-OE (Bloomington #22308, y1 w67c23; P{w[+mC] y[+mDint2] = EPgy2}sec31 [EY19759]), CG14715 RNAi, (VDRC #104124 w1118; P{KK104150}VIE-260B; #12828, w1118; P{GD4788}v12828), CG14715 OE (Bloomington #32608, w1118; P{w[+mC] = EP}CG14715 [G6908]). All stocks were backcrossed for at least 6 generations into the w1118 background prior to carrying out the experiments. Flies were raised and maintained on cornmeal medium (87.5 g/l dextrose, 87.5 g/l maize, 19 g/l yeast). Stocks were maintained and experiments were conducted at 25C on a 12:12 hours light/dark cycle at constant humidity.
Microarray methods
RNA was extracted from 50 fly heads according to standard Trizol (Invitrogen, Paisley, UK) protocols. FlyChip_long_oligonucleotide_003 (FL003) - INDAC (Flychip, University of Cambridge, http://www.flychip.org.uk/) microarray chips were used for the expression analysis (GEO Platform GPL14121). These chips have been used extensively for Drosophila expression profiling and include 14,444 transcript-specific oligonucleotides (70mers) and various controls. Samples were labelled and hybridised according to standardised protocols (http://www.flychip.org.uk/protocols/). Four replicates were used for each sample (w1118, elavGAL4 > UAS-Aβ42-51D (×2 copies) flies and controls w1118, elavGAL4/+; 51D (×2 copies, empty insertion site) and five time points. Time points used corresponded to days 3,10 and 20 (100% survival in both Aβ and control flies) and days 21, 25 (Aβ flies) or days 56, and 68 (control flies), corresponding to 80% and 20% survival in each case) Samples were matched on arrays by time point. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE48681 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE48681).
Analysis of microarray data
The raw data were filtered to remove any probes that were rejected in over 50% of samples and were quantile-normalised across all arrays using Limma39. One array (hybridised to a time point 3 sample) was removed from further analysis at this stage. Any missing values were imputed using the impute package (R package version 1.32.0. http://CRAN.R-project.org/package=impute). Differentially expressed genes were identified using two methods. Firstly, Limma was used to fit a linear model to the entire time course and genes identified as significantly differentially expressed were those with an F statistic p-value < 0.05 following FDR correction. Secondly, the maSigPro40 package was used to identify genes with significantly (p < 0.05 after FDR correction) different changes in expression over time. For both methods, the samples were matched by % survival. The results from maSigPro and Limma analysis were combined and the expression of each significant gene averaged over all replicates and standardised (to have a mean of 0 and standard deviation of 1). These data, matched by % survival, were then clustered using the R package Mfuzz13, which implements fuzzy c-means clustering12. Two clustering parameters are required; the fuzzifier m and the number of clusters c. The appropriate value of m was determined using the Mfuzz function “m.estimate”, c was determined by examining the effect of c on the minimum centroid distance13,41.
maSigPro40 was used to identify genes changing expression over time in Aβ and control flies separately (p < 0.05 after FDR correction). Each of these genes was tested for correlation with % survival using a Pearson Product Moment Correlation Coefficient in R.
Lifespan
Flies were reared at standard density, allowed to mate for 24 h, sorted by sex, and then transferred to experimental vials at a density of ten female flies per vial. Flies were transferred to fresh vials three times a week, and deaths were scored three to five times a week. Lifespan data were subjected to survival analysis (Log-rank tests) using GraphPad Prism 5 Software (GraphPad Software, Inc).
Locomotor/climbing assays
The locomotor ability of the flies was assessed in a 1 min negative geotaxis assay as previously described9. Ten flies were placed in a plastic 25-ml pipette and knocked to the bottom of the pipette. The number reaching the 10 ml line of the pipette (ntop) and the number remaining at the bottom (below the 2-ml line) (nbottom), after 1 min, were measured. The performance (mobility) index was then calculated as (ntop − nbottom + ntotal)/2ntotal. Three to four replicates were used per genotype. Climbing in each pipette was assessed three times and the average performance index for each pipette calculated. Assays were carried out in a well-lit room at a temperature of 23–24°C. The mean of the independent biological replicates for each genotype was plotted with the s.e.m.. Two-tailed Student's t-tests (f-test for equal variance) were used to identify significant differences at specific time points.
qRT-PCR
Total RNA was extracted from 20 adult heads per genotype using standard Trizol (Invitrogen, Paisley, UK) protocols. RNA was DNase-treated (Fermentas, Thermo Scientific, UK) and cDNA was prepared using oligo-d(T) primers and a Promega Reverse transcription kit (#A3500) according to the manufacturer's protocol (Promega, Southampton, UK). qRT-PCR was performed using a Biorad iQ machine and KAPA (KK4608) SYBR green PCR master mix (Biorad, Hemel Hempstead, UK). Relative quantities of transcripts were determined using the relative ΔΔCt method and normalised to Act5C. Two to five independent RNA extractions were used for each genotype. Primer sequences are available upon request.
Author Contributions
M.E.G., G.F. and D.C.C. designed the project. M.E.G., H.B., B.F., D.M.B. and E.B. carried out experimental work. D.M.B., G.F. and B.F. carried out bioinformatics analysis with the guidance of S.G.O. G.F., M.E.G., D.M.B., S.G.O., S.R., D.C.C. and H.A.B. wrote the paper.
Supplementary Material
Supplementary Info
Table S1
Acknowledgments
This work was funded by an Alzheimer's Research UK fellowship to M.E.G. and an ARUK pilot grant (ART-PPG2010A-2) to M.E.G., G.F. and D.C.C. The work of D.M.B., G.F. and S.G.O. was supported by the Wellcome Trust/MRC (grant code: 089703/Z/09/Z). D.C.C. was also supported by the MRC (grant code: G0700990), Wellcome Trust/MRC (grant code: 082604/2/07/Z), and Alzheimer's Research UK (grant code: ART-SRF2010-2). The authors thank Drs M. Landgraff and M. Oswald for critical reading of the manuscript.
References
- Dobson C. M. Protein misfolding, evolution and disease. Trends in Biochemical sci 24, 329–332 (1999). [DOI] [PubMed] [Google Scholar]
- Chiti F. & Dobson C. M. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 75, 333–366 (2006). [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Folding proteins in fatal ways. Nature 426, 900–904 (2003). [DOI] [PubMed] [Google Scholar]
- Balch W. E., Morimoto R. I., Dillin A. & Kelly J. W. Adapting proteostasis for disease intervention. Science 319, 916–919 (2008). [DOI] [PubMed] [Google Scholar]
- Craig T., Smelick C. & de Magalhaes J. P. The Digital Ageing Atlas: http://ageing-map.org (2010–2013).
- Lopez-Otin C., Blasco M. A., Partridge L., Serrano M. & Kroemer G. The hallmarks of aging. Cell 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal M., Sandoval H., Zhang K., Bayat V. & Bellen H. J. Probing mechanisms that underlie human neurodegenerative diseases in Drosophila. Annu Rev Genet 46, 371–396 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M., P M. & Guerchet M. World Alzheimer Report. (2013). [Google Scholar]
- Crowther D. C. et al. Intraneuronal Abeta, non-amyloid aggregates and neurodegeneration in a Drosophila model of Alzheimer's disease. Neuroscience 132, 123–135 (2005). [DOI] [PubMed] [Google Scholar]
- Rival T. et al. Fenton chemistry and oxidative stress mediate the toxicity of the beta-amyloid peptide in a Drosophila model of Alzheimer's disease. Eur J Neurosci 29, 1335–1347 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luheshi L. M. et al. Systematic in vivo analysis of the intrinsic determinants of amyloid Beta pathogenicity. PLoS Biol 5, e290 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futschik M. E. & Carlisle B. Noise-robust soft clustering of gene expression time-course data. J Bioinform Comput Biol 3, 965–988 (2005). [DOI] [PubMed] [Google Scholar]
- Kumar L. & M E. F. Mfuzz: a software package for soft clustering of microarray data. Bioinformation 2, 5–7 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis G., Shen J. & Tower J. Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster. Aging (Albany NY) 4, 768–789 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyne R. et al. FlyMine: an integrated database for Drosophila and Anopheles genomics. Genome Biology 8, R129 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio E., Spellman P. T., Tzou P., Rubin G. M. & Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. Embo J 21, 2568–2579 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saisawang C., Wongsantichon J. & Ketterman A. J. A preliminary characterization of the cytosolic glutathione transferase proteome from Drosophila melanogaster. Biochem J 442, 181–190 (2012). [DOI] [PubMed] [Google Scholar]
- Gems D. & Partridge L. Genetics of longevity in model organisms: debates and paradigm shifts. Annu Rev Physiol 75, 621–644 (2013). [DOI] [PubMed] [Google Scholar]
- Sohal R. S. & Orr W. C. The redox stress hypothesis of aging. Free Radical Biology Med 52, 539–555 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee J. J. et al. Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biology 8, R132 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung I., Kim T. Y. & Kim-Ha J. Identification of Drosophila SOD3 and its protective role against phototoxic damage to cells. FEBS letters 585, 1973–1978 (2011). [DOI] [PubMed] [Google Scholar]
- Kwon M. J., Kim B., Lee Y. S. & Kim T. Y. Role of superoxide dismutase 3 in skin inflammation. J Dermatol Sci 67, 81–87 (2012). [DOI] [PubMed] [Google Scholar]
- Pletcher S. D. et al. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr Biol 12, 712–723 (2002). [DOI] [PubMed] [Google Scholar]
- Landis G. N. et al. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc Natl Acad Sci U S A 101, 7663–7668 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radyuk S. N., Klichko V. I., Spinola B., Sohal R. S. & Orr W. C. The peroxiredoxin gene family in Drosophila melanogaster. Free Radic Biology Med 31, 1090–1100 (2001). [DOI] [PubMed] [Google Scholar]
- Landis G., Shen J. & Tower J. Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster. Aging (Albany NY) 4, 768–789 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes J. C., Welchman D. P., Poidevin M. & Lemaitre B. Negative regulation by amidase PGRPs shapes the Drosophila antibacterial response and protects the fly from innocuous infection. Immunity 35, 770–779 (2011). [DOI] [PubMed] [Google Scholar]
- Harold D. et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nature Genetics 41, 1088–1093 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B. L. et al. Mammalian homologues of yeast sec31p. An ubiquitously expressed form is localized to endoplasmic reticulum (ER) exit sites and is essential for ER-Golgi transport. The J Biol Chem 275, 13597–13604 (2000). [DOI] [PubMed] [Google Scholar]
- Bush K. T., Hendrickson B. A. & Nigam S. K. Induction of the FK506-binding protein, FKBP13, under conditions which misfold proteins in the endoplasmic reticulum. Biochem J 303 (Pt 3), 705–708 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla P. I. et al. Interaction of FK506-binding protein 13 with brefeldin A-inhibited guanine nucleotide-exchange protein 1 (BIG1): effects of FK506. Proc Natl Acad Sci U S A 100, 2322–2327 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsberth C. et al. The ‘Arctic’ APP mutation (E693G) causes Alzheimer's disease by enhanced Abeta protofibril formation. Nat Neurosci 4, 887–893 (2001). [DOI] [PubMed] [Google Scholar]
- Brorsson A. C. et al. Intrinsic determinants of neurotoxic aggregate formation by the amyloid beta peptide. Biophys J 98, 1677–1684 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doonan R. et al. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev 22, 3236–3241 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B. J., Mahadev K. & Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes 54, 311–321 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. et al. Oxidative stress induces parallel autophagy and mitochondria dysfunction in human glioma U251 cells. Toxicol Sci 110, 376–388 (2009). [DOI] [PubMed] [Google Scholar]
- Muller W. E., Eckert A., Kurz C., Eckert G. P. & Leuner K. Mitochondrial dysfunction: common final pathway in brain aging and Alzheimer's disease--therapeutic aspects. Mol Neurobiol 41, 159–171 (2010). [DOI] [PubMed] [Google Scholar]
- Jahn T. R. et al. Detection of early locomotor abnormalities in a Drosophila model of Alzheimer's disease. J Neurosci Methods 197, 186–189 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth G. K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3, Article3 (2004). [DOI] [PubMed] [Google Scholar]
- Conesa A., Nueda M. J., Ferrer A. & Talon M. maSigPro: a method to identify significantly differential expression profiles in time-course microarray experiments. Bioinformatics 22, 1096–1102 (2006). [DOI] [PubMed] [Google Scholar]
- Schwammle V. & Jensen O. N. A simple and fast method to determine the parameters for fuzzy c-means cluster analysis. Bioinformatics 26, 2841–2848 (2010). [DOI] [PubMed] [Google Scholar]
- Jang Y. S., Lee M. H., Lee S. H. & Bae K. Cu/Zn superoxide dismutase is differentially regulated in period gene-mutant mice. Biochem Biophys Res Commun 409, 22–27 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Info
Table S1