Abstract
Controllable synthesis and surface engineering of nanomaterials are of strategic importance for tailoring their properties. Here, we demonstrate that the synthesis and surface adjustment of highly stable hierarchical of SnO2 nanospheres can be realized by biomineralization, vulcanization and oxidation techniques. Furthermore, we reveal that the highly stable hierarchical SnO2 nanospheres ensure a remarkable sensitivity towards NO gas with fast response and recovery due to their high crystallinity and special structure. Such technique acquiring highly stable hierarchical SnO2 nanospheres offers promising potential for future practical applications in monitoring the emission from waste incinerators and combustion process of fossil fuels.
Rational design and control over the morphology and the functional properties of inorganic nanostructures have been a long-standing subject in the development of bottom-up device fabrication processes1. In the past decade, tin oxide (SnO2) has been extensively applied in the fields of lithium-ion batteries (LIBs), gas sensors, light-emitting diodes (LEDs), solar cell, catalysis and so on owing to its excellent electrical and optical properties2,3,4,5,6. These properties are usually strongly dependent on SnO2 nanostructures. Thus, tremendous effort has been focused on the synthesis of SnO2 nanostructures such as nanocrystals (NCs)7, nanoboxes2, nanorods (NRs)8, nanowires (NWs)9, nanobelts (NBs)10, nanoplates (NPs)11, hollow nanospheres (HNSs)12, nanorod-bundles (NRBs)13, etc. Although many sophisticated synthetic methods have been developed so far, it is still a challenge to manipulate or adjust the structure and morphology of SnO2 nanostructures.
Bio-inspired mineralization has been proved to be an innovative strategy to the fabrication of functional crystals with complex forms which mimic nature14, and has been expanded to the design and preparation of nonbiological minerals15. In recent years, some pioneering research on the synthesis of many functional materials or their precursors employing the bio-inspired mineralization methods has been reported16,17,18,19,20,21. For example, Wu et al. synthesized the calcite superstructures with two different platonic shapes (pseudo-dodecahedral and pseudo-octahedral structures) by a polymer-controlled crystallization method via a cooperative assembly process of primary nanocrystals and mesocrystal formation16. Rana and his coworkers reported a polyamine-mediated bio-inspired synthesis methodology for preparing Ag/AgCl/ZnO composite materials with enhanced photocatalytic activity in a single step at room temperature and neutral pH20. Recently, Yu group synthesized the hierarchical FeOOH nanostructure array films constructed by different nanosized building blocks at the air–water interface via a bio-inspired gas–liquid diffusion method21. These excellent results inspire us to explore new bio--inspired mineralization route to synthesize SnO2 with some promising properties. In addition, in regard of bio-inspired synthesis of SnO2, several interesting work also have been conducted. Su and co-workers successfully used biomolecules in pollen coats to guide the self-assembly of SnO2-precursors22, and also fabricated the interwoven tubular hierarchy of SnO2 films using a biotemplate eggshell membrane combined sol–gel approach23. More recently, Tremel group employed a surface-bound spermine to promote the fast deposition of homogeneous, thin and transparent biomimetic SnO2 coatings on glass surfaces with high photoactivity24. These reports urgently drive us to develop a mild, facile, and environmentally benign approach to prepare SnO2 crystals with specific morphologies and nanostructures that will endow them with larger scale applications.
Structural manipulation of nanocrystals has been of significant interest as a way to tune their properties for enhanced performance in various applications25,26,27,28,29. To date, some methods have been used to change the surface properties of nanocrystals, such as sub-structure, composites, surface ligand exchange, et al.25,26,27,28,29,30,31,32. For example, Lou et al. reported that α-Fe2O3 microboxes with hierarchically structured shells can be synthesized simply by annealing Prussian blue (PB) microcubes at different temperatures25. Tessler group demonstrated tuning of the electronic level positions with respect to the vacuum level in colloidal InAs nanocrystals using surface ligand exchange26. Jasieniak and Mulvaney used the successive ion layer adhesion and reaction (SILAR) protocol to manipulate the surface stoichiometry of CdSe NCs from Cd-rich to Se-rich27. These interesting results encourage us to look for new techniques to manipulate the surface of SnO2 crystals for obtaining enhanced properties. For the modification of SnO2 surfaces, there also exist some reported examples. For example, Zhang et al. synthesized the SnS2/SnO2 nanocomposites with tunable SnO2 contents via in situ hydrothermal oxidation of SnS2 nanoparticles in 0.375–4.5 mass% H2O2 aqueous solutions at 180°C for 0–12 h30. Chen group reported that carbon-coated SnO2 hollow microspheres synthesized with the assistance of d-glucose demonstrated higher reversible capacity and good cycling performance than that of pure SnO231. Kim and his co-workers reported Bimetallic Pd/Pt nanoparticles functionalized networked SnO2 nanowires via a sequential γ-ray radiolysis can obtain a fast response and recovery ability in their NO2-sensing performance32. Therefore, it is very meaningful to tune the properties of SnO2 nanostructures via an effective structure manipulation.
Herein, we report a facile, green biomineralization process to synthesize stable hierarchical SnO2 nanospheres (SnO2 HNSs-1) using bio-inspired hydrothermal method with the assistance of tris(hydroxymethyl) aminomethane (THAM). The structure and composition of the as-obtained SnO2 nanospheres can be further adjusted by vulcanization and oxidation techniques, after which the hierarchical S-doped SnO2 nanospheres (SnO2 HNSs-2) and novel hierarchical SnO2 nanospheres (SnO2 HNSs-3) can be easily produced, respectively. Structural characterizations were undertaken to investigate the correlation between the effects of biomineralization, vulcanization as well as oxidation processes on the structures and composition of the SnO2 HNSs. Notably, gas sensors based on such stable SnO2 HNSs exhibit the higher sensitivity towards NO gas with fast response and recovery. It is expected that the as-prepared SnO2 HNSs-3 are promisingly applied to monitor the NO gas emission from waste incinerators and combustion process of fossil fuels.
Results
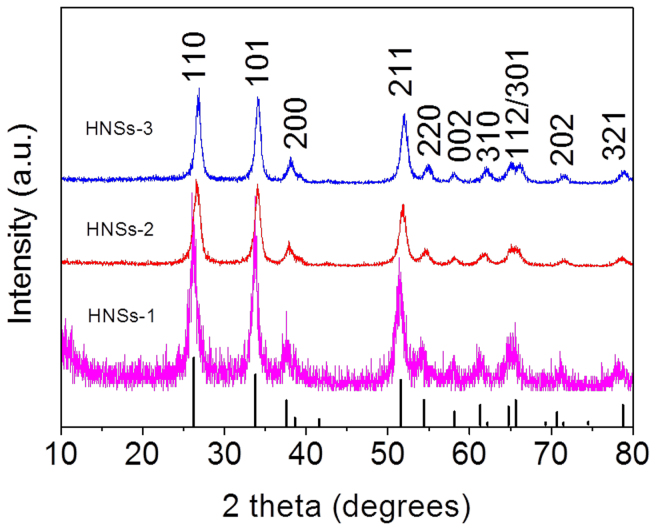
We have synthesized the three SnO2 HNSs with different BET surface area and pore size using biomineralization, vulcanization and oxidation techniques. Firstly, we synthesized the stable SnO2 HNSs-1 using bio-inspired hydrothermal method in the presence of THAM. Then, the as-synthesized SnO2 HNSs-1 was modified by vulcanization method with TAA, and converted into the hierarchical S-doped SnO2 nanospheres (SnO2 HNSs-2). The as-obtained SnO2 HNSs-2 was further oxidized in air at 500°C, and converted into the SnO2 HNSs-3. The phase and structures of the three SnO2 samples were characterized by X-ray powder diffraction (XRD). As shown in Figure 1, all of the diffraction peaks in the patterns for all the samples can be indexed to the tetragonal phase of SnO2 (JCPDS no. 41-1445; a = 0.47 nm, c = 0.32 nm), and the broad nature of the XRD peaks indicates the presence of very small crystallites of SnO2. Notably, there is no SnS2 peak in the XRD pattern for SnO2 HNSs-2, which may be due to the low content or low crystalline of SnS2 in the SnO2 HNSs-2. Energy dispersive X-ray spectroscopy (EDS) results (Figure S1a and b) confirm that there is the existence of a low amount of sulfur element in the SnO2 HNSs-2 (S, w 0.2%) and not any sulfur element in the SnO2 HNSs-3, respectively.
Figure 1. XRD patterns for the three SnO2 HNSs.
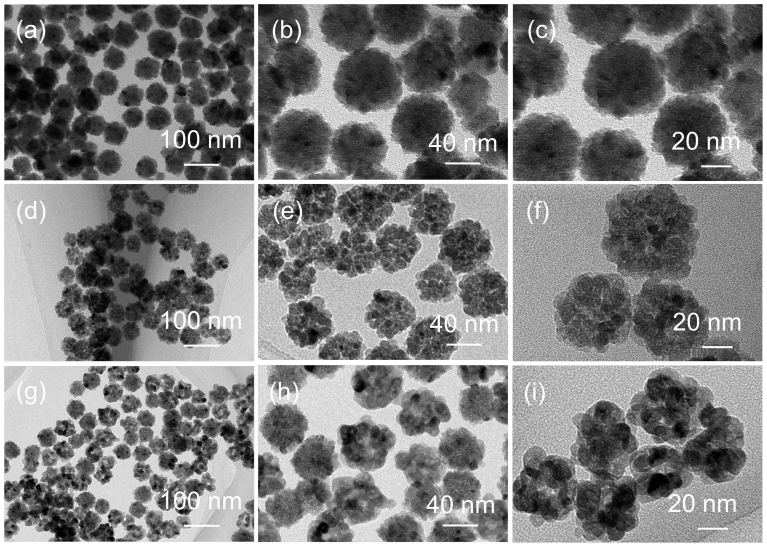
Transmission electron micro-scope (TEM) images show the morphology and structural analysis of the three SnO2 HNSs (Figure 2). Figure 2a, d and g show the low-magnification TEM images of the SnO2 HNSs-1, SnO2 HNSs-2 and SnO2 HNSs-3, respectively. In Figure 1a, d and g, one can find that all the three samples are well dispersed, without any aggregation, and they have a similar diameter of about 40 nm. In the high TEM images of SnO2 HNSs (Figure 2b, e and h), they clearly show that interparticle distance of the SnO2 HNSs-2 is largest among the three samples. This is proved to be effective for us to tailor the microstructures with the vulcanization technique. To further investigate the higher resolution TEM images in Figure 2c, f and i, we found that the nanoparticles of the SnO2 HNSs increased and nanoparticles of the SnO2 HNSs-3 were biggest after the vulcanization and oxidation treatments. It gives a clear proof for the effectiveness of our methods of structural control over the hierarchical SnO2 nanospheres. Moreover, the textural properties of the SnO2 HNSs were further investigated by measuring adsorption–desorption isotherms of nitrogen at 77 K, as shown in Figure S2. The Brunauer–Emmett–Teller (BET) specific surface areas (SBET) for the SnO2 HNSs-1, SnO2 HNSs-2 and SnO2 HNSs-3 are 24, 72.3 and 35.6 m2/g, respectively. Moreover, their pore size distributions have also been changed largely. These results further confirm the important effects of the vulcanization and oxidation treatments on the structures of the SnO2 HNSs.
Figure 2. TEM images for the three SnO2 HNSs.
(a–c) SnO2 HNSs-1; (d–f) SnO2 HNSs-2; (g–i) SnO2 HNSs-3.
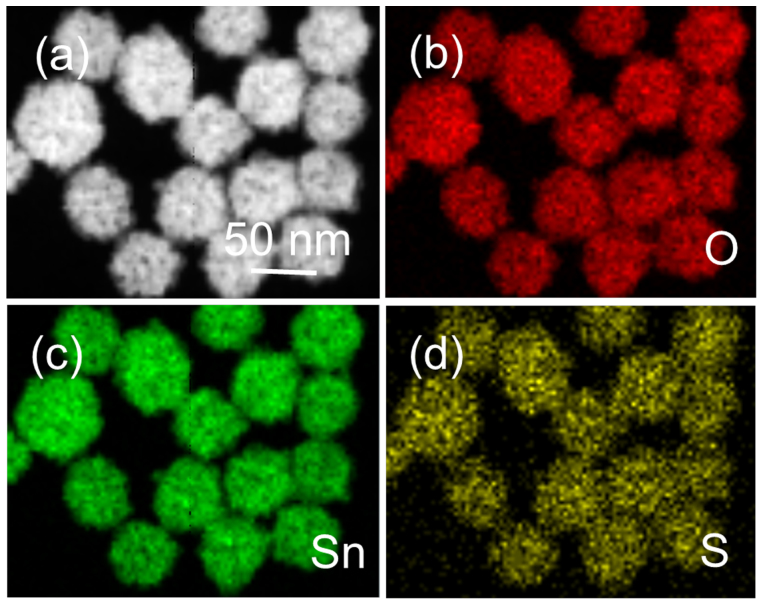
The composition and elemental distribution of the as-synthesized SnO2 HNSs-2 were further mapped through EDS by displaying the integrated intensity of oxygen, tin and sulfur signals as a function of the beam position when operating the TEM in scanning mode (STEM). An HAADF-STEM image of the SnO2 HNSs-2 is shown in Figure 3a, which reveals the sample is almost monodisperse. The results shown in Figure 3b–d reveal that the three elements of O, Sn, and S are distributed very homogeneously in nanospheres and exhibit no apparent element separation or aggregation. Moreover, the Sn/S ratio measured from several nanospheres with EDS analysis show an average ratio of 70.96:0.24. This ratio is extremely high, similar to EDS results from SEM. It indicates that the content of S is relatively low in the SnO2 HNSs-2.
Figure 3. STEM-EDS elemental maps of the SnO2 HNSs-2.
(a) HAADF-STEM image, (b) O elemental map, (c) Sn elemental map, and (d) S elemental map.
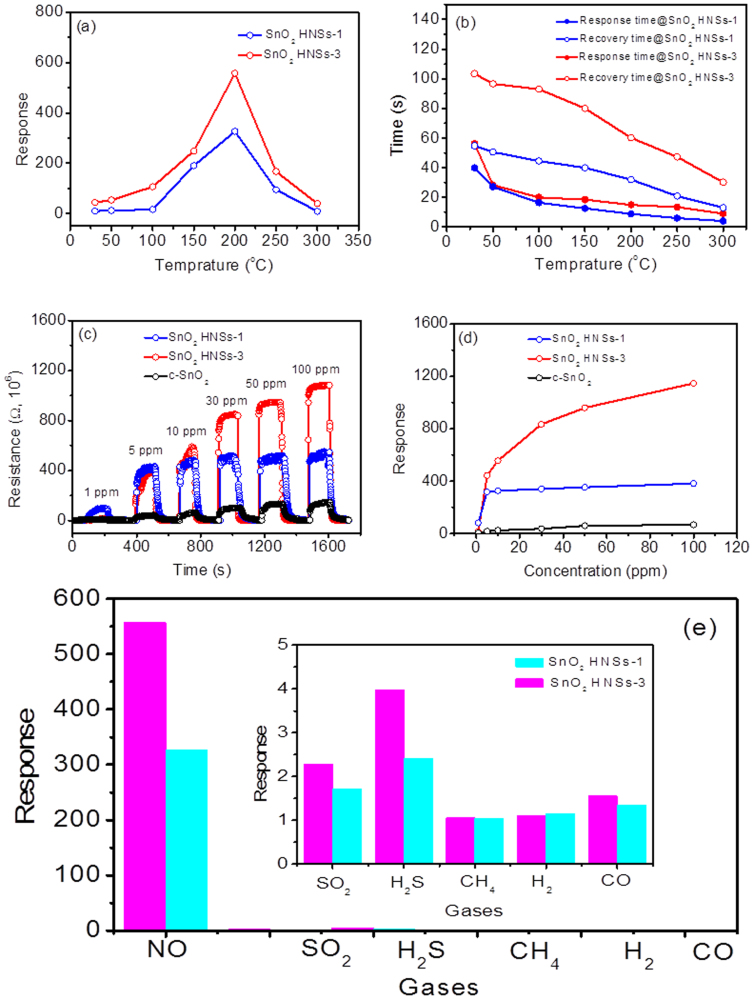
Prompted by the porous appearance of SnO2 HNSs, we believed that these SnO2 HNSs must have potentially excellent gas-sensing performance as other sensing materials. NO gas is well-known as a highly toxic gas emitted from vehicles and industrial processes, thus it has become a studied target for semiconductor sensors. Thus, we studied the NO-sensing performance of the SnO2 HNSs-1 and SnO2 HNSs-3 (SnO2 HNSs-2 is not good sensing material due to the existence of sulfur, which has been proved). The optimized working temperature was first confirmed by the following experiments. We have screened the sensitivity-temperature profile of two SnO2 sensors from 25 to 300°C, and found that the highest sensitivity towards 10 ppm NO gas for both of SnO2 sensors could be obtained at 200°C, as shown in Figure 4a. The sensitivity of SnO2 HNSs-3 is obviously superior to that of SnO2 HNSs-1. Figure 4b further shows the response and recovery-time profiles of SnO2 sensors towards 10 ppm NO gas, respectively. In Figure 4b, one can find that the response and recovery time of SnO2 sensors become short when the temperature increases. Although the sensitivity of SnO2 HNSs-3 is higher, its response and recovery is slower than these of SnO2 HNSs-1. The response and recovery times of SnO2 HNSs-3 are about 60 and 15 s, respectively. These data is better than those in reported sensor based on SnOx–SWNT composite nanowires33. Considering the above factors, thus, in the following section the optimal operating temperature for maximizing NO gas sensitivity was set at 200°C for further sensing analysis.
Figure 4. Gas-sensing performance for the SnO2 HNSs-1 and HNSs-3.
(a) sensitivity and (b) response and recovery time of two sensors to 10 ppm NO gas at different working temperature; (c) The real-time response curve of three sensors to NO gas with increased concentration at a working temperature of 200°C; (d) The relationship between the sensitivity and the NO concentration; (e) Sensor response to various gases with 10 ppm at 200°C.
To investigate the response towards the various NO gas concentrations, the real-time response experiment was conducted. Figure 4c shows the real-time response and recovery curves of the three sensing materials (SnO2 HNSs-1, SnO2 HNSs-3 and commercial SnO2 (c-SnO2)) for the NO gas concentrations from 1 ppm to 100 ppm. In Figure 4c, one can find that the sensitivities for three SnO2 sensors increase with NO gas concentrations increasing. However, the response increase for SnO2 HNSs-1 and c-SnO2 is extremely slow, which may be attributed to easy saturation to NO gas, and thus it limits the testing range. It also indicates that SnO2 HNSs-3 has a strong response to NO gas in a wide range. In Figure 4d, the sensitivities of SnO2 HNSs-3 sensor are shown to be also higher than that of SnO2 HNSs-1 sensor, and increase with the NO concentration increasing from 1 ppm to 100 ppm. The sensitivities of SnO2 HNSs-3 sensor towards 1, 10 and 100 ppm NO gas are about 14, 557 and 1147, respectively. The sensitivity of SnO2 HNSs-3 sensor can be comparable to those of the reported NO sensors34,35. Finally, we studied the gas-selectivity of the SnO2 HNSs sensors through comparing the sensitivities towards various gases with the concentration of 10 ppm (Figure 4e). Figure 4e clearly shows that both of two SnO2 HNSs sensors have higher sensitivities towards NO gas than the reducing gases (H2S, CH4, H2 and CO). Due to its high sensitivity and selectivity, the SnO2 HNSs-3 sensor is promising to be used to detect NO gas in future.
Discussion
The above-mentioned methods are very effective for us to adjust the microstructures and porous characteristics of the SnO2 HNSs. In this work, some interesting chemical reactions were involved in the synthetic processes. The chemical processes are composed of the liquid-liquid, liquid-solid and gas-solid phase reactions. For the liquid-liquid phase reaction, Sn4+ ions were firstly hydrolyzed into SnO2 in aqueous solution with THAM, which plays the important role in the formation of SnO2 HNSs assembled by small nanocrystals as a mineralizer (see Figure S3 in the SI); for the solid-liquid phase reaction, SnO2 were partly vulcanized into S-doped SnO2 in aqueous solution with TAA; for the gas-solid phase reaction, the S-doped SnO2 HNSs were further oxidized to SnO2 by oxygen in air at 500°C. Moreover, with the above-mentioned reactions, two important processes should be emphasized to illustrate us the crystal growth processes and microstructure change of SnO2 HNSs. One is the Ostwald ripening process, during which SnO2 nanospheres were formed in the assistance of THAM, and S-doped SnO2 was formed with the vulcanization of SnO2. Another is the recrystallization process, during which S-doped SnO2 was completely oxidized into SnO2 at high temperature. Due to the effective influence of above-mentioned processes, these methods should be extended to adjust the structure and composite of other materials for optimizing their properties or performance.
The SnO2 HNSs-3 sensor displays fast response and recovery as well as high sensitivity towards NO gas. Therefore, it is necessary to research the NO-sensing mechanism of SnO2 HNSs and analyze the potential reasons. It is widely reported that the change in resistance for SnO2-based sensors is mainly caused by the adsorption and desorption of gas molecules on the surface of the sensing structure9. It is well-known that tin oxide is an n-type semiconductor originating from the oxygen vacancy in the non-stoichiometric SnOx. When NO gas is adsorbed on the surface of SnOx as an oxidizing molecule, capturing electrons from SnOx, and thus, the consequent decrease in the number of electrons in SnOx causes an increase of resistance of the sensors33. Epifani and coauthors suggested that the bridging vacancies, generated by heat treatment at 500°C, can enhance the charge transfer from the surface to the adsorbate36. Another model of the significant electron transfer to the adatoms suggested by Maiti et al. can also well explain an orders-of-magnitude drop in electrical conductance for the oxidizing gas (NO)37. For the sensors described here, we believe that the high sensitivity towards NO gas can be attributed to the intrinsically small grain size and high surface-to-volume ratios associated with the hierarchical nanospheres9. The network of interconnected pores in the sensor facilitates both the analyte and the background gas to access all the surfaces of SnO2 nanoparticles contained in the sensing unit. Due to the unique porous structure and high SBET of SnO2 HNSs-3, more NO gas could be adsorbed onto the semiconductor surface, where subsequent reaction with surface oxygen species (O2ads−) causes the reduction in the electron concentration, resulting in the obvious increase in the sensor resistance33,34. Therefore, the excellent response of SnO2 HNSs-3 to NO gas could be attributable to the unique porous structure and high SBET, for facilitating the electron transfer onto the surface of SnO2 nanoparticles.
In summary, we have successfully synthesized, and adjusted the structure and composition of hierarchical SnO2 nanospheres through biomineralization, vulcanization and oxidation processes, respectively. The control over the structure and composite of nanospheres was found to be largely influence its gas-sensing properties and performance through changing the porous characteristics and composition. The as-tailored SnO2 nanospheres have presented excellent performance towards NO gas and found the potential application of detecting automobile exhaust. We hope that our findings will pave the way for tailored design of other complicated gas-sensing materials in sensors.
Methods
Materials
All the reagents for synthesis and preparation were of analytic grade and used as received without further purification. Na2SnO3·3H2O and TAA were purchased from Aldrich. THAM was provided by Beijing Chemical Factory.
Biomineralization of highly stable hierarchical SnO2 nanospheres
In a typical biomineralization process, 0.27 g of Na2SnO3·3H2O and 0.2 g of THAM were first dissolved into 35 ml of distilled H2O, and then transferred into a 40 mL Teflon-lined stainless-steel autoclave at 120°C for 10 h. Finally, the obtained white sample was rinsed with deionized water, pure ethanol and dried in ambient conditions. In order to investigate the effect of THAM on the morphology of final products, a series of controlled experiments were also conducted, and the amount of THAM is 0, 0.05 and 0.5 g, respectively.
Slight vulcanization of hierarchical SnO2 nanospheres and its oxidation
In order to vulcanize the as-synthesized SnO2 nanospheres and further oxidize it, we carried the experiment as followings: Typically, 0.05 g of the as-synthesized SnO2 nanospheres and 0.025 g of thioacetamide (TAA) were first dissolved into 35 ml of distilled H2O, and then transferred into a 40 mL Teflon-lined stainless-steel autoclave at 180°C for 10 h. Finally, the obtained yellow sample was rinsed with deionized water, pure ethanol and dried in ambient conditions. The as-obtained yell sample was calcined in air at 500°C.
Characterization
The morphology and structural characteristics were observed using X-ray diffraction (XRD, Rigaku D/max 2500 diffractometer), high-resolution transmission electron microscopy (HRTEM, JEOL 2010), nitrogen adsorption–desorption isotherms (ASAP 2020 nitrogen adsorption apparatus) and high-angle annular dark field scanning TEM (HAADF-STEM) images (JEOL JEM-2100F electron microscope). The Brunauer–Emmett–Teller (BET) specific surface areas (SBET) were calculated using the BET equation. Desorption isotherm was used to determine the pore size distribution using the Barret–Joyner–Halender (BJH) method.
Sensor measurements
The fabrication and testing principles of the gas sensor are similar to that described in our previous reports. Firstly, the SnO2 samples were mixed with terpineol to form a paste and then coated onto the outside surface of an alumina tube with a diameter of 1 mm and a length of 5 mm. A platinum coil through the tube was employed as a heater to control the operating temperature. To improve their stability and repeatability, the gas sensors were aged at 300°C for 10 h in air. Here, the sensing properties of the sensors were measured by a NS-4003 series gas-sensing measurement system (China Zhong-Ke Micro-nano IOT, Internet of Things, Ltd.). The relative humidity (RH) is about 45%. The response and recovery times were defined as the time required for a change of the resistance to reach 90% of the equilibrium value after injecting and that for removing the detected gas, respectively. When air and ppm-level target gas were flowed through the sensor element, the corresponding steady-state resistances of the sensor in air (Rair) and in the air–gas mixture (Rgas) were recorded, respectively. The sensor gas response for oxidizing gas (NO) is defined as the ratio of Rgas/Rair, while the response for reducing gas (H2S, H2, CO or CH4) is defined as the ratio of Rair/Rgas38.
Author Contributions
J.M.M., Z.X. and T.H.W. proposed and designed the experiments. L.W. and Y.J.C. carried out the synthetic experiments and conducted the characterization. L.W., Y.J.C. and L.B.C. performed the HRTEM, SEM characterization and structural analysis. L.W. and Q.H.L. analysed the data. L.W., J.M.M., Z.X. and T.H.W. wrote the manuscript. All the authors participated in discussions of the research.
Supplementary Material
Supporting Information
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 21103046, 21373081, 51302079 and 61376073) and the Young Teachers' Growth Plan of Hunan University (Grant No. 2012-118).
References
- Joo J. et al. Face-selective electrostatic control of hydrothermal zinc oxide nanowire synthesis. Nature Mater. 10, 596–601 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Y. et al. Fast formation of SnO2 nanoboxes with enhanced lithium storage capability. J. Am. Soc. Chem. 133, 4738–4741 (2011). [DOI] [PubMed] [Google Scholar]
- Wang B. et al. Fabrication of a SnO2 nanowire gas sensor and sensor performance for hydrogen. J. Phys. Chem. C 112, 6643–6647 (2008). [Google Scholar]
- Li Y. F. et al. Realizing a SnO2-based ultraviolet light-emitting diode via breaking the dipole-forbidden rule. NPG Asia Mater. 4, e30 (2012). [Google Scholar]
- Birkel A. et al. Highly efficient and stable dye-sensitized solar cells based on SnO2 nanocrystals prepared by microwave-assisted synthesis. Energy Environ. Sci. 5, 5392–5400 (2012). [Google Scholar]
- Liu G. B. et al. Selective oxidation of dimethyl ether to methyl formate over trifunctional MoO3–SnO2 catalyst under mild conditions. Green Chem. 15, 1501–1504 (2013). [Google Scholar]
- Chen Y. J. et al. Gram-scale synthesis of ultrasmall SnO2 nanocrystals with an excellent electrochemical performance. Nanoscale 5, 3262–3265 (2013). [DOI] [PubMed] [Google Scholar]
- Xi G. C. & Ye J. H. Ultrathin SnO2 nanorods: template- and surfactant-free solution phase synthesis, growth mechanism, optical, gas-sensing, and surface adsorption properties. Inorg. Chem. 49, 2302–2309 (2011). [DOI] [PubMed] [Google Scholar]
- Wang Y. L. et al. A solution-phase, precursor route to polycrystalline SnO2 nanowires that can be used for gas sensing under ambient conditions. J. Am. Chem. Soc. 125, 16176–16177 (2003). [DOI] [PubMed] [Google Scholar]
- Kolmakov A. et al. Enhanced gas sensing by individual SnO2 nanowires and nanobelts functionalized with Pd catalyst particles. Nano Lett. 5, 667–673 (2004). [DOI] [PubMed] [Google Scholar]
- Wang C. et al. Large-scale synthesis of SnO2 nanosheets with high lithium storage capacity. J. Am. Chem. Soc. 132, 46–47 (2010). [DOI] [PubMed] [Google Scholar]
- Lou X. W. et al. Preparation of SnO2/carbon composite hollow spheres and their lithium storage properties. Chem. Mater. 20, 6562–6566 (2008). [Google Scholar]
- Wang Q. H. et al. Shape-controlled synthesis of porous SnO2 nanostructures via morphological conserved transformation from SnC2O4 precursor approach. Nano-Micro Lett. 3, 34–42 (2011). [Google Scholar]
- Aizenberg J. New nanofabrication strategies: Inspired by biomineralization. MRS Bull. 35, 323–330 (2010). [Google Scholar]
- Kim S. J. & Park C. B. Bio-inspired synthesis of minerals for energy, environment, and medicinal Applications. Adv. Funct. Mater. 23, 10–25 (2013). [Google Scholar]
- Song R. Q. et al. Calcite crystals with platonic shapes and minimal Surfaces. Angew. Chem. Int. Ed. 48, 395–399 (2009). [DOI] [PubMed] [Google Scholar]
- Tao A. R. et al. Bio-inspired nanofabrication of barium titanate. J. Mater. Chem. 20, 7916–7923 (2010). [Google Scholar]
- Kim Y. Y. et al. Bio-inspired synthesis and mechanical properties of calcite-polymer particle composites. Adv. Mater. 22, 2082–2086 (2010). [DOI] [PubMed] [Google Scholar]
- Xiao J. W. & Yang S. H. Biomimetic synthesis, hierarchical assembly and mechanical properties of calcite/chitosan composites in a three-dimensional chitosan scaffold. Adv. Eng. Mater. 13, B32–B40 (2011). [Google Scholar]
- Begum G. et al. Controlled orientation in a bio-inspired assembly of Ag/AgCl/ZnO nanostructures enables enhancement in visible-light-induced photocatalytic performance. Chem. Eur. J. 18, 6847–6853 (2012). [DOI] [PubMed] [Google Scholar]
- Liu L. et al. Bio-inspired fabrication of hierarchical FeOOH nanostructure array films at the air-water interface, their hydrophobicity and application for water treatment. ACS Nano 7, 1368–1378 (2013). [DOI] [PubMed] [Google Scholar]
- Song F. et al. 3D hierarchical porous SnO2 derived from self-assembled biological systems for superior gas sensing application. J. Mater. Chem. 22, 1121–1126 (2012). [Google Scholar]
- Dong Q. et al. Fabrication and gas sensitivity of SnO2 hierarchical films with interwoven tubular conformation by a biotemplate-directed sol–gel technique. Nanotechnology 17, 3968–3972 (2006). [Google Scholar]
- André R. et al. Self-cleaning antimicrobial surfaces by bio-enabled growth of SnO2 coatings on glass. Nanoscale 5, 3447–3456 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang L. et al. Formation of Fe2O3 microboxes with hierarchical shell structures from metal organic frameworks and their lithium storage properties. J. Am. Chem. Soc. 134, 17388–17391 (2012). [DOI] [PubMed] [Google Scholar]
- Soreni-Harari M. et al. Tuning energetic levels in nanocrystal quantum dots through surface manipulations. Nano Lett. 8, 678–684 (2008). [DOI] [PubMed] [Google Scholar]
- Jasieniak J. & Mulvaney P. From Cd-rich to Se-rich - the manipulation of CdSe nanocrystal surface stoichiometry. J. Am. Chem. Soc. 129, 2841–2848 (2007). [DOI] [PubMed] [Google Scholar]
- Yu W. W. et al. Forming biocompatible and nonaggregated nanocrystals in water using amphiphilic polymers. J. Am. Chem. Soc. 129, 2871–2879 (2007). [DOI] [PubMed] [Google Scholar]
- Rivesta J. B. & Jain P. K. Cation exchange on the nanoscale: an emerging technique for new material synthesis, device fabrication, and chemical sensing. Chem. Soc. Rev. 42, 89–96 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang Y. C. et al. High-performance visible-light-driven SnS2/SnO2 nanocomposite photocatalyst prepared via in situ hydrothermal oxidation of SnS2 nanoparticles. ACS Appl. Mater. Interfaces, 3, 1528–1537 (2011). [DOI] [PubMed] [Google Scholar]
- Guo X. W. et al. Lithium storage in carbon-coated SnO2 by conversion reaction. J. Power Sources 226, 75–81 (2013). [Google Scholar]
- Choi S. W. et al. Bimetallic Pd/Pt nanoparticle-functionalized SnO2 nanowires for fast response and recovery to NO2. Sensor. Actuat. B-Chem. 181, 446–453 (2013). [Google Scholar]
- Hoa N. D. et al. Nanowire structured SnOx–SWNT composites: High performance sensor for NOx detection. Sens. Actuators B: Chem. 142, 253–259 (2009). [Google Scholar]
- Sayago I. et al. The effect of additives in tin oxide on the sensitivity and selectivity to NOx and CO. Sens. Actuators B: Chem. 26–27, 19–23 (1995). [Google Scholar]
- Lin C. Y. et al. Fabrication of NOx gas sensors using In2O3–ZnO composite films. Sens. Actuators B: Chem. 146, 28–34 (2010). [Google Scholar]
- Epifani M. et al. The role of surface oxygen vacancies in the NO2 sensing properties of SnO2 nanocrystals. J. Phys. Chem. C 112, 19540–19546 (2008). [Google Scholar]
- Maiti A. et al. SnO2 nanoribbons as NO2 sensors: insights from first principles calculations. Nano Lett. 3, 1025–1028 (2003). [Google Scholar]
- Fan F. Y. et al. Synthesis and gas sensing properties to NO2 of ZnO nanoparticles. Sensor. Actuat. B-Chem. 185, 377–382 (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information