Abstract
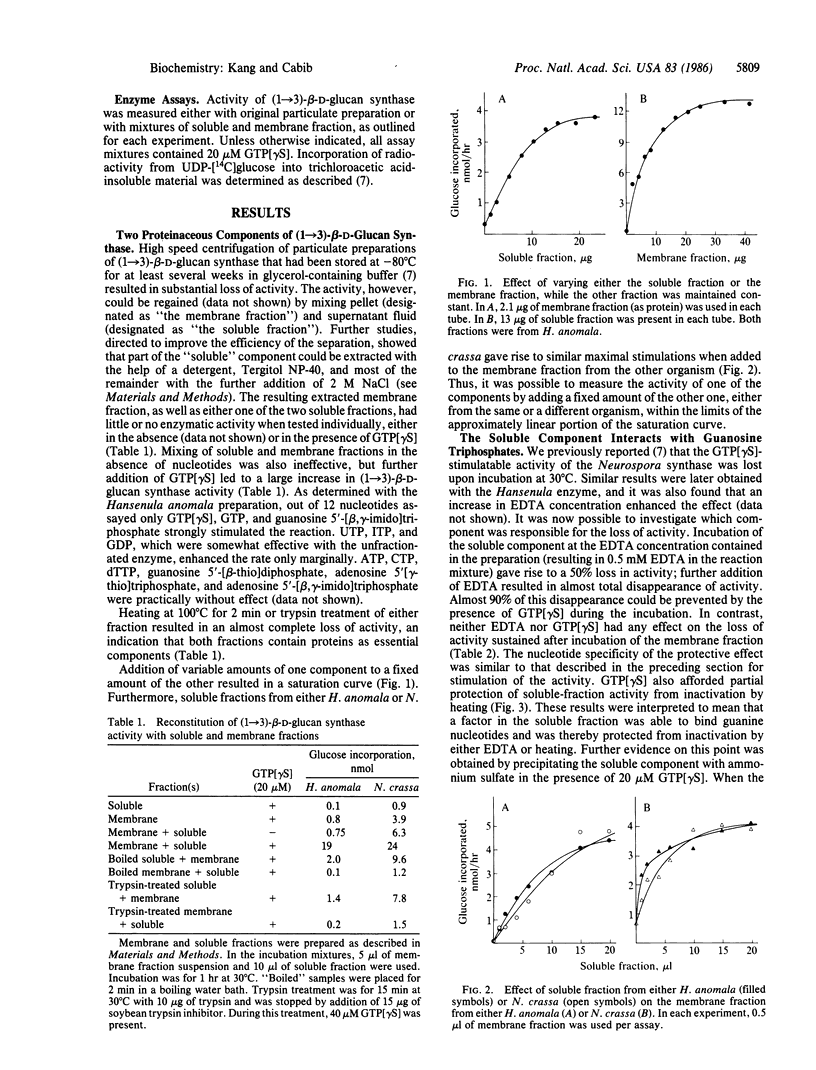
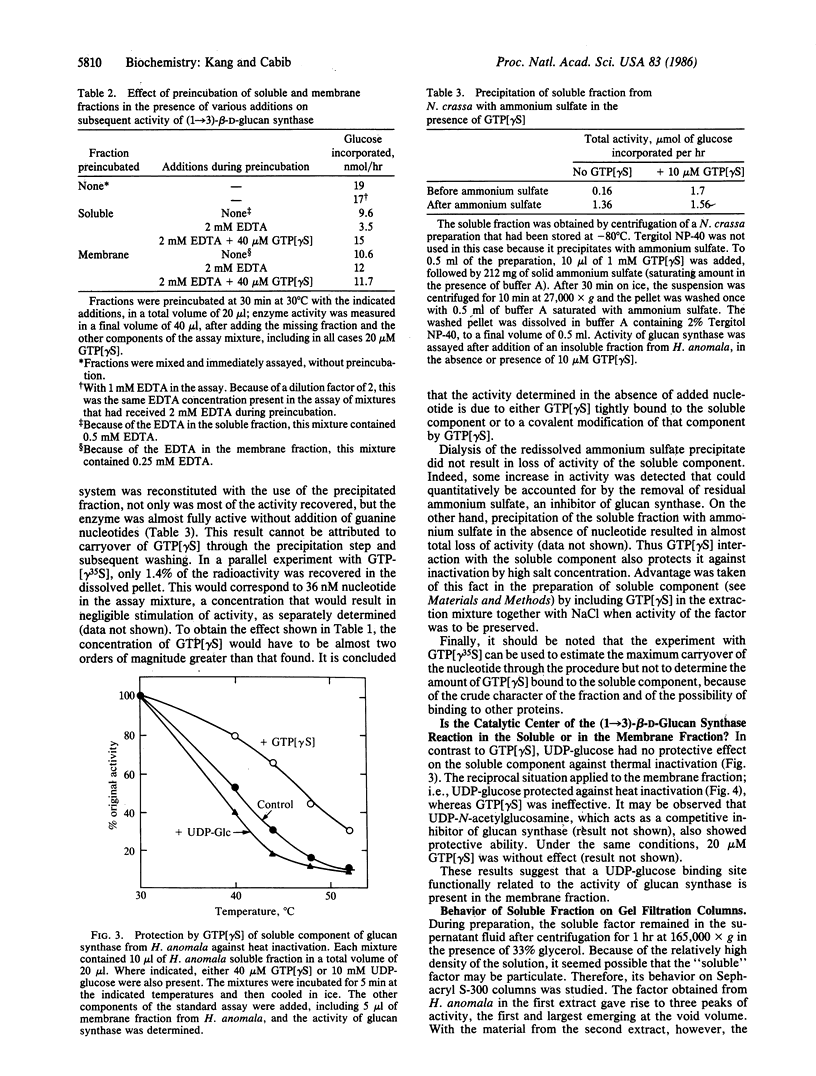
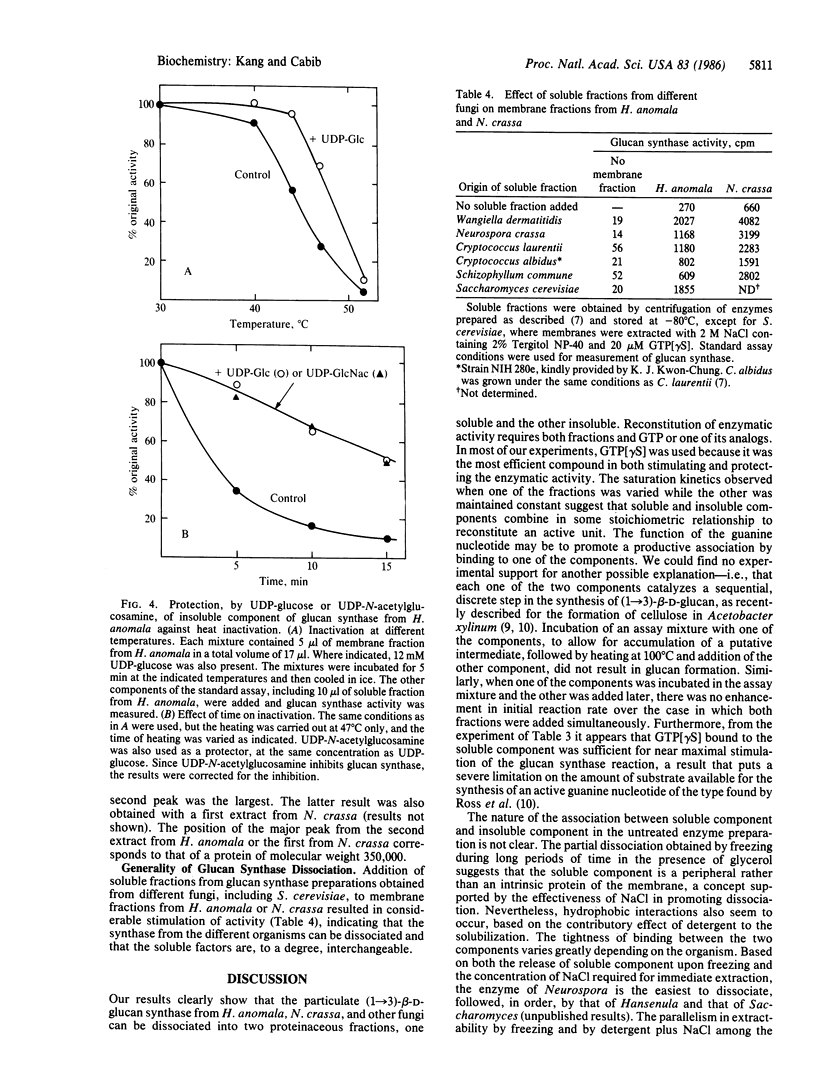
By treatment with detergent and NaCl, particulate (1----3)-beta-D-glucan synthase (EC 2.4.1.34) from Hansenula anomala or Neurospora crassa was dissociated into a "soluble fraction" and a "membrane fraction." Each fraction alone was almost inactive, but enzymatic activity could be reconstituted by mixing the two fractions and adding GTP or one of its analogs. Based on their lability to heat and to incubation with trypsin, the activity in both fractions is proteinaceous. The active component in the soluble fraction appears to bind guanosine 5'-[gamma-thio]triphosphate (GTP[gamma S]), since it was specifically protected by this nucleotide against heat inactivation and against inactivation in the presence of EDTA. Furthermore, precipitation of the soluble component with ammonium sulfate in the presence of GTP[gamma S] gave rise to a fraction that was highly active in the absence of added nucleotide, indicating either tight binding or covalent interaction between GTP[gamma S] and the soluble component. The membrane fraction probably contains the catalytic moiety, because it was partially protected against heat inactivation by the substrate, UDP-glucose. Soluble fractions that stimulated membrane fractions from H. anomala and N. crassa were obtained from several other fungi, including Saccharomyces cerevisiae. We propose that the soluble fraction contains a GTP-binding protein that modulates the biosynthesis of (1----3)-beta-D-glucan of fungal cell walls and probably has a major role in the regulation of cell wall morphogenesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y., Cohen R., Benziman M., Delmer D. Solubilization of the UDP-glucose:1,4-beta-D-glucan 4-beta-D-glucosyltransferase (cellulose synthase) from Acetobacter xylinum. A comparison of regulatory properties with those of the membrane-bound form of the enzyme. J Biol Chem. 1983 Apr 10;258(7):4419–4423. [PubMed] [Google Scholar]
- Cabib E., Roberts R., Bowers B. Synthesis of the yeast cell wall and its regulation. Annu Rev Biochem. 1982;51:763–793. doi: 10.1146/annurev.bi.51.070182.003555. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Notario V., Kawai H., Cabib E. Interaction between yeast beta-(1 goes to 3)glucan synthetase and activating phosphorylated compounds. A kinetic study. J Biol Chem. 1982 Feb 25;257(4):1902–1905. [PubMed] [Google Scholar]
- Shematek E. M., Braatz J. A., Cabib E. Biosynthesis of the yeast cell wall. I. Preparation and properties of beta-(1 leads to 3)glucan synthetase. J Biol Chem. 1980 Feb 10;255(3):888–894. [PubMed] [Google Scholar]
- Shematek E. M., Cabib E. Biosynthesis of the yeast cell wall. II. Regulation of beta-(1 leads to 3)glucan synthetase by ATP and GTP. J Biol Chem. 1980 Feb 10;255(3):895–902. [PubMed] [Google Scholar]
- Szaniszlo P. J., Kang M. S., Cabib E. Stimulation of beta(1----3)glucan synthetase of various fungi by nucleoside triphosphates: generalized regulatory mechanism for cell wall biosynthesis. J Bacteriol. 1985 Mar;161(3):1188–1194. doi: 10.1128/jb.161.3.1188-1194.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagaya M., Nakano K., Fukui T. A new affinity labeling reagent for the active site of glycogen synthase. Uridine diphosphopyridoxal. J Biol Chem. 1985 Jun 10;260(11):6670–6676. [PubMed] [Google Scholar]
- Zlotnik H., Fernandez M. P., Bowers B., Cabib E. Saccharomyces cerevisiae mannoproteins form an external cell wall layer that determines wall porosity. J Bacteriol. 1984 Sep;159(3):1018–1026. doi: 10.1128/jb.159.3.1018-1026.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



