Abstract
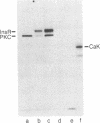
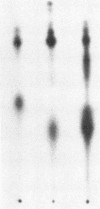
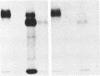
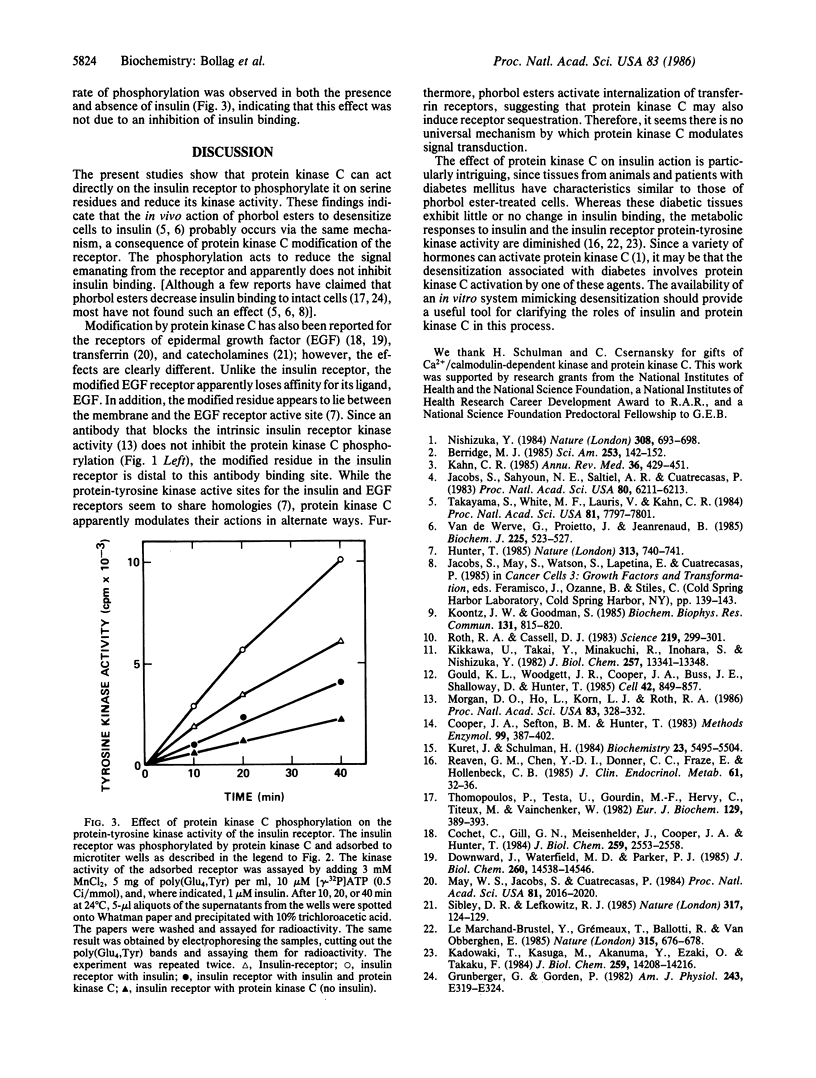
The beta subunit of purified insulin receptor is phosphorylated on a serine residue by purified preparations of protein kinase C (ATP: protein phosphotransferase, EC 2.7.1.37). This phosphorylation is inhibited by antibodies to protein kinase C and stimulated by phospholipids, diacylglycerol, and Ca2+. The phosphorylation of the receptor by protein kinase C does not affect its insulin-binding activity but does inhibit by 65% the receptor's intrinsic tyrosine-specific protein kinase activity (ATP: protein-tyrosine O-phosphotransferase, EC 2.7.1.112). These results indicate that activators of protein kinase C, such as phorbol esters, desensitize cells to insulin by direct protein kinase C action on the insulin receptor.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. The molecular basis of communication within the cell. Sci Am. 1985 Oct;253(4):142–152. doi: 10.1038/scientificamerican1085-142. [DOI] [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Downward J., Waterfield M. D., Parker P. J. Autophosphorylation and protein kinase C phosphorylation of the epidermal growth factor receptor. Effect on tyrosine kinase activity and ligand binding affinity. J Biol Chem. 1985 Nov 25;260(27):14538–14546. [PubMed] [Google Scholar]
- Gould K. L., Woodgett J. R., Cooper J. A., Buss J. E., Shalloway D., Hunter T. Protein kinase C phosphorylates pp60src at a novel site. Cell. 1985 Oct;42(3):849–857. doi: 10.1016/0092-8674(85)90281-8. [DOI] [PubMed] [Google Scholar]
- Grunberger G., Gorden P. Affinity alteration of insulin receptor induced by a phorbol ester. Am J Physiol. 1982 Oct;243(4):E319–E324. doi: 10.1152/ajpendo.1982.243.4.E319. [DOI] [PubMed] [Google Scholar]
- Hunter T. Cell-surface proteins. At last the insulin receptor. 1985 Feb 28-Mar 6Nature. 313(6005):740–741. doi: 10.1038/313740a0. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Sahyoun N. E., Saltiel A. R., Cuatrecasas P. Phorbol esters stimulate the phosphorylation of receptors for insulin and somatomedin C. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6211–6213. doi: 10.1073/pnas.80.20.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T., Kasuga M., Akanuma Y., Ezaki O., Takaku F. Decreased autophosphorylation of the insulin receptor-kinase in streptozotocin-diabetic rats. J Biol Chem. 1984 Nov 25;259(22):14208–14216. [PubMed] [Google Scholar]
- Kahn C. R. The molecular mechanism of insulin action. Annu Rev Med. 1985;36:429–451. doi: 10.1146/annurev.me.36.020185.002241. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Koontz J. W., Goodman S. Phorbol ester inhibition of hormonal induction of tyrosine aminotransferase. Biochem Biophys Res Commun. 1985 Sep 16;131(2):815–820. doi: 10.1016/0006-291x(85)91312-9. [DOI] [PubMed] [Google Scholar]
- Kuret J., Schulman H. Purification and characterization of a Ca2+/calmodulin-dependent protein kinase from rat brain. Biochemistry. 1984 Nov 6;23(23):5495–5504. doi: 10.1021/bi00318a018. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Grémeaux T., Ballotti R., Van Obberghen E. Insulin receptor tyrosine kinase is defective in skeletal muscle of insulin-resistant obese mice. Nature. 1985 Jun 20;315(6021):676–679. doi: 10.1038/315676a0. [DOI] [PubMed] [Google Scholar]
- May W. S., Jacobs S., Cuatrecasas P. Association of phorbol ester-induced hyperphosphorylation and reversible regulation of transferrin membrane receptors in HL60 cells. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2016–2020. doi: 10.1073/pnas.81.7.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O., Ho L., Korn L. J., Roth R. A. Insulin action is blocked by a monoclonal antibody that inhibits the insulin receptor kinase. Proc Natl Acad Sci U S A. 1986 Jan;83(2):328–332. doi: 10.1073/pnas.83.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Chen Y. D., Donner C. C., Fraze E., Hollenbeck C. B. How insulin resistant are patients with noninsulin-dependent diabetes mellitus? J Clin Endocrinol Metab. 1985 Jul;61(1):32–36. doi: 10.1210/jcem-61-1-32. [DOI] [PubMed] [Google Scholar]
- Roth R. A., Cassell D. J. Insulin receptor: evidence that it is a protein kinase. Science. 1983 Jan 21;219(4582):299–301. doi: 10.1126/science.6849137. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Takayama S., White M. F., Lauris V., Kahn C. R. Phorbol esters modulate insulin receptor phosphorylation and insulin action in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7797–7801. doi: 10.1073/pnas.81.24.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos P., Testa U., Gourdin M. F., Hervy C., Titeux M., Vainchenker W. Inhibition of insulin receptor binding by phorbol esters. Eur J Biochem. 1982 Dec 15;129(2):389–393. doi: 10.1111/j.1432-1033.1982.tb07062.x. [DOI] [PubMed] [Google Scholar]
- van de Werve G., Proietto J., Jeanrenaud B. Tumour-promoting phorbol esters increase basal and inhibit insulin-stimulated lipogenesis in rat adipocytes without decreasing insulin binding. Biochem J. 1985 Jan 15;225(2):523–527. doi: 10.1042/bj2250523. [DOI] [PMC free article] [PubMed] [Google Scholar]