Abstract
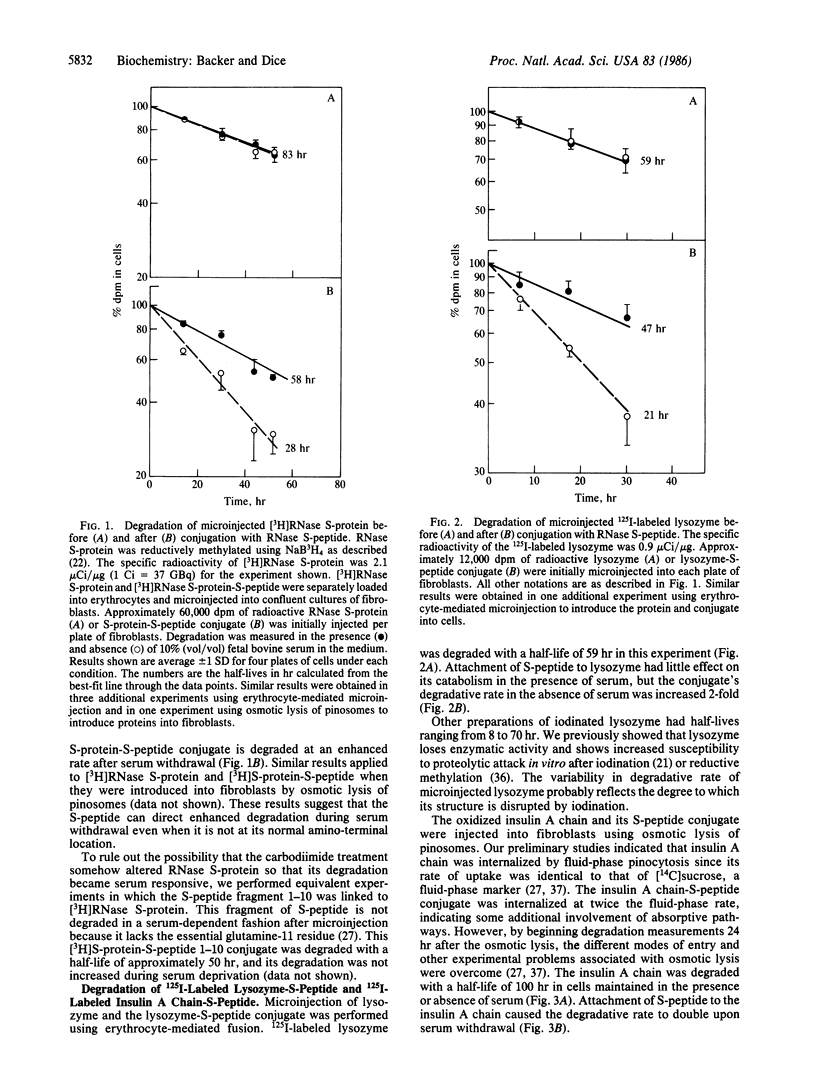
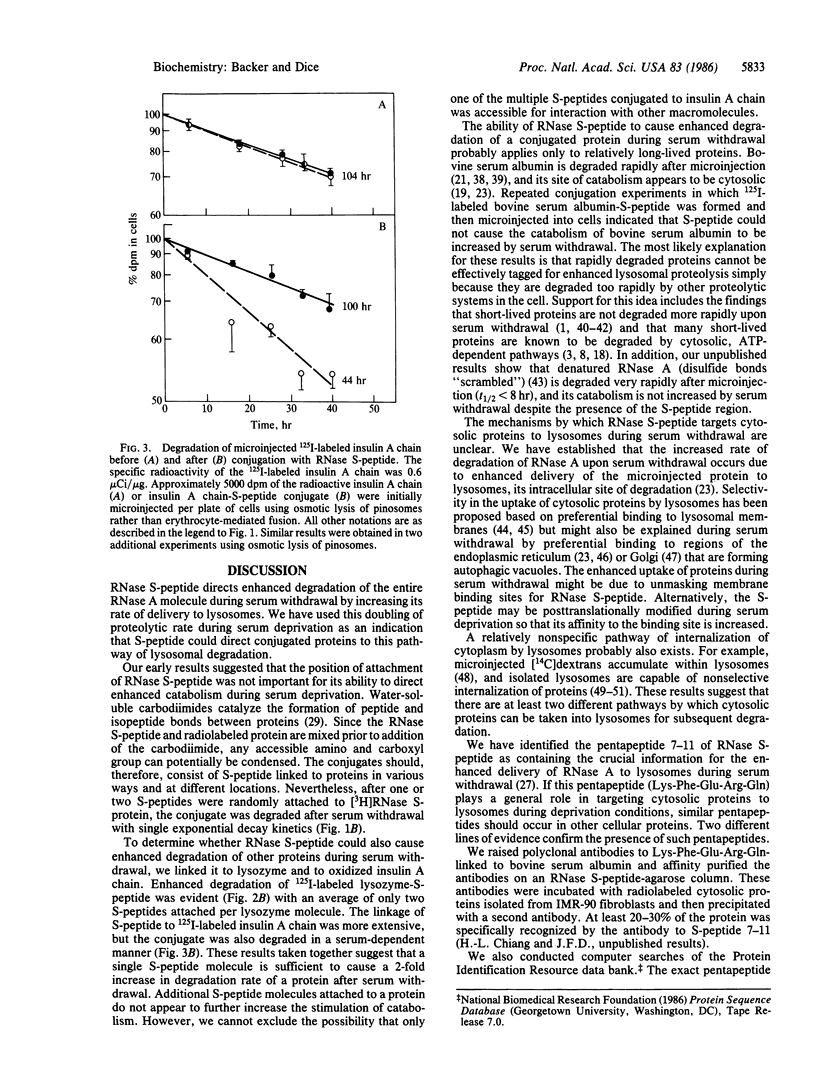
The amino-terminal 20 amino acids are required for microinjected ribonuclease A (RNase A) to be taken up by lysosomes and degraded at an enhanced rate during serum withdrawal. We used water-soluble carbodiimides to covalently attach the RNase S-peptide (residues 1-20) to [3H]RNase S-protein (residues 21-124) at unspecified locations. We then measured catabolism of the [3H]S-protein-S-peptide conjugate after its microinjection into human diploid fibroblasts. The attached S-peptide caused the degradation of S-protein to be enhanced 2-fold in the absence of serum. Control experiments showed that degradation of [3H]RNase S-protein remained unresponsive to serum after conjugation with the inactive fragment, RNase S-peptide (residues 1-10). Covalent attachment of RNase S-peptide had a similar effect on the catabolism of two other proteins. Degradation rates of microinjected 125I-labeled lysozyme and 125I-labeled insulin A chain are normally unresponsive to serum withdrawal. However, breakdown rates of microinjected 125I-labeled lysozyme-S-peptide and 125I-labeled insulin A chain-S-peptide conjugates were increased 2-fold during serum deprivation. We suggest that RNase S-peptide acts as a "single sequence" that directs cytosolic proteins to lysosomes through a pathway that is activated by deprivation conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLENDE J. E., RICHARDS F. M. The action of trypsin on ribonuclease-S. Biochemistry. 1962 Mar;1:295–304. doi: 10.1021/bi00908a017. [DOI] [PubMed] [Google Scholar]
- Ahlberg J., Berkenstam A., Henell F., Glaumann H. Degradation of short and long lived proteins in isolated rat liver lysosomes. Effects of pH, temperature, and proteolytic inhibitors. J Biol Chem. 1985 May 10;260(9):5847–5854. [PubMed] [Google Scholar]
- Ahlberg J., Marzella L., Glaumann H. Uptake and degradation of proteins by isolated rat liver lysosomes. Suggestion of a microautophagic pathway of proteolysis. Lab Invest. 1982 Dec;47(6):523–532. [PubMed] [Google Scholar]
- Amenta J. S., Brocher S. C. Mechanisms of protein turnover in cultured cells. Life Sci. 1981 Mar 16;28(11):1195–1208. doi: 10.1016/0024-3205(81)90444-6. [DOI] [PubMed] [Google Scholar]
- Amenta J. S., Hlivko T. J., McBee A. G., Shinozuka H., Brocher S. Specific inhibition by NH4CL of autophagy-associated proteloysis in cultured fibroblasts. Exp Cell Res. 1978 Sep;115(2):357–366. doi: 10.1016/0014-4827(78)90289-6. [DOI] [PubMed] [Google Scholar]
- Amenta J. S., Sargus M. J., Brocher S. C. Protein synthesis and degradation in growth regulation in rat embryo fibroblasts: role of fast-turnover and slow-turnover protein. J Cell Physiol. 1980 Oct;105(1):51–61. doi: 10.1002/jcp.1041050108. [DOI] [PubMed] [Google Scholar]
- Auteri J. S., Okada A., Bochaki V., Dice J. F. Regulation of intracellular protein degradation in IMR-90 human diploid fibroblasts. J Cell Physiol. 1983 May;115(2):167–174. doi: 10.1002/jcp.1041150210. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Bourret L., Dice J. F. Regulation of catabolism of microinjected ribonuclease A requires the amino-terminal 20 amino acids. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2166–2170. doi: 10.1073/pnas.80.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman E., Walters D. E., Allerhand A. Structure and dynamic behavior of the oligosaccharide side chain of bovine pancreatic ribonuclease B. Application of carbon 13 nuclear magnetic resonance spectroscopy. J Biol Chem. 1981 Apr 25;256(8):3853–3857. [PubMed] [Google Scholar]
- Bigelow S., Hough R., Rechsteiner M. The selective degradation of injected proteins occurs principally in the cytosol rather than in lysosomes. Cell. 1981 Jul;25(1):83–93. doi: 10.1016/0092-8674(81)90233-6. [DOI] [PubMed] [Google Scholar]
- Chin D. T., Kuehl L., Rechsteiner M. Conjugation of ubiquitin to denatured hemoglobin is proportional to the rate of hemoglobin degradation in HeLa cells. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5857–5861. doi: 10.1073/pnas.79.19.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Finley D., Varshavsky A. The ubiquitin-mediated proteolytic pathway and mechanisms of energy-dependent intracellular protein degradation. J Cell Biochem. 1984;24(1):27–53. doi: 10.1002/jcb.240240104. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Finley D., Varshavsky A. Ubiquitin dependence of selective protein degradation demonstrated in the mammalian cell cycle mutant ts85. Cell. 1984 May;37(1):57–66. doi: 10.1016/0092-8674(84)90300-3. [DOI] [PubMed] [Google Scholar]
- Davidson S. J., Hughes W. L., Barnwell A. Renal protein absorption into sub-cellular particles. I. Studies with intact kidneys and fractionated homogenates. Exp Cell Res. 1971 Jul;67(1):171–187. doi: 10.1016/0014-4827(71)90633-1. [DOI] [PubMed] [Google Scholar]
- Dean R. T. Lysosomes and membrane recycling. A hypothesis. Biochem J. 1977 Dec 15;168(3):603–605. doi: 10.1042/bj1680603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T. Macrophage protein turnover. Evidence for lysosomal participation in basal proteolysis. Biochem J. 1979 May 15;180(2):339–345. doi: 10.1042/bj1800339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T. Modes of access of macromolecules to the lysosomal interior. Biochem Soc Trans. 1984 Dec;12(6):911–913. doi: 10.1042/bst0120911. [DOI] [PubMed] [Google Scholar]
- Desautels M., Goldberg A. L. Liver mitochondria contain an ATP-dependent, vanadate-sensitive pathway for the degradation of proteins. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1869–1873. doi: 10.1073/pnas.79.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice J. F. Altered degradation of proteins microinjected into senescent human fibroblasts. J Biol Chem. 1982 Dec 25;257(24):14624–14627. [PubMed] [Google Scholar]
- Dice J. F., Chiang H. L., Spencer E. P., Backer J. M. Regulation of catabolism of microinjected ribonuclease A. Identification of residues 7-11 as the essential pentapeptide. J Biol Chem. 1986 May 25;261(15):6853–6859. [PubMed] [Google Scholar]
- Dice J. F., Dehlinger P. J., Schimke R. T. Studies on the correlation between size and relative degradation rate of soluble proteins. J Biol Chem. 1973 Jun 25;248(12):4220–4228. [PubMed] [Google Scholar]
- Etlinger J. D., Goldberg A. L. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977 Jan;74(1):54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis G. L., Ballard F. J. Distribution and partial purification of a liver membrane protein capable of inactivating cytosol enzymes. Biochem J. 1980 Feb 15;186(2):571–579. doi: 10.1042/bj1860571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendil K. B. Autophagy of metabolically inert substances injected into fibroblasts in culture. Exp Cell Res. 1981 Sep;135(1):157–166. doi: 10.1016/0014-4827(81)90308-6. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
- Hershko A., Eytan E., Ciechanover A., Haas A. L. Immunochemical analysis of the turnover of ubiquitin-protein conjugates in intact cells. Relationship to the breakdown of abnormal proteins. J Biol Chem. 1982 Dec 10;257(23):13964–13970. [PubMed] [Google Scholar]
- Hoare D. G., Koshland D. E., Jr A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967 May 25;242(10):2447–2453. [PubMed] [Google Scholar]
- Hough R., Rechsteiner M. Effects of temperature on the degradation of proteins in rabbit reticulocyte lysates and after injection into HeLa cells. Proc Natl Acad Sci U S A. 1984 Jan;81(1):90–94. doi: 10.1073/pnas.81.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALNITSKY G., HUMMEL J. P., DIERKS C. [Some factors which affect the enzymatic digestion of ribonucleic acid]. J Biol Chem. 1959 Jun;234(6):1512–1516. [PubMed] [Google Scholar]
- Kato I., Anfinsen C. B. Purification of synthetic ribonuclease S-peptide derivatives by specific complex formation on columns of ribonuclease S-protein bound to agarose. J Biol Chem. 1969 Nov 10;244(21):5849–5855. [PubMed] [Google Scholar]
- Katznelson R., Kulka R. G. Degradation of microinjected methylated and unmethylated proteins in hepatoma tissue culture cells. J Biol Chem. 1983 Aug 25;258(16):9597–9600. [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levit S., Berger A. Ribonuclease S-peptide. A model for molecular recognition. J Biol Chem. 1976 Mar 10;251(5):1333–1339. [PubMed] [Google Scholar]
- McElligott M. A., Dice J. F. Erythrocyte-mediated microinjection, a technique to study protein degradation in muscle cells. Biochem J. 1983 Dec 15;216(3):559–566. doi: 10.1042/bj2160559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott M. A., Dice J. F. Microinjection of cultured cells using red-cell-mediated fusion and osmotic lysis of pinosomes: a review of methods and applications. Biosci Rep. 1984 Jun;4(6):451–466. doi: 10.1007/BF01122221. [DOI] [PubMed] [Google Scholar]
- McElligott M. A., Dice J. F. Microinjection of cultured cells using red-cell-mediated fusion and osmotic lysis of pinosomes: a review of methods and applications. Biosci Rep. 1984 Jun;4(6):451–466. doi: 10.1007/BF01122221. [DOI] [PubMed] [Google Scholar]
- McElligott M. A., Miao P., Dice J. F. Lysosomal degradation of ribonuclease A and ribonuclease S-protein microinjected into the cytosol of human fibroblasts. J Biol Chem. 1985 Oct 5;260(22):11986–11993. [PubMed] [Google Scholar]
- Mortimore G. E., Hutson N. J., Surmacz C. A. Quantitative correlation between proteolysis and macro- and microautophagy in mouse hepatocytes during starvation and refeeding. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2179–2183. doi: 10.1073/pnas.80.8.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimore G. E., Pösö A. R. Lysosomal pathways in hepatic protein degradation: regulatory role of amino acids. Fed Proc. 1984 Apr;43(5):1289–1294. [PubMed] [Google Scholar]
- Neely A. N., Cox J. R., Fortney J. A., Schworer C. M., Mortimore G. E. Alterations of lysosomal size and density during rat liver perfusion. Suppression by insulin and amino acids. J Biol Chem. 1977 Oct 10;252(19):6948–6954. [PubMed] [Google Scholar]
- Neff N. T., Bourret L., Miao P., Dice J. F. Degradation of proteins microinjected into IMR-90 human diploid fibroblasts. J Cell Biol. 1981 Oct;91(1):184–194. doi: 10.1083/jcb.91.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland P. A., Dice J. F. Red blood cell-mediated microinjection: methodological considerations. Anal Biochem. 1985 Oct;150(1):214–220. doi: 10.1016/0003-2697(85)90461-0. [DOI] [PubMed] [Google Scholar]
- Okada C. Y., Rechsteiner M. Introduction of macromolecules into cultured mammalian cells by osmotic lysis of pinocytic vesicles. Cell. 1982 May;29(1):33–41. doi: 10.1016/0092-8674(82)90087-3. [DOI] [PubMed] [Google Scholar]
- Pfeifer U. Inhibition by insulin of the formation of autophagic vacuoles in rat liver. A morphometric approach to the kinetics of intracellular degradation by autophagy. J Cell Biol. 1978 Jul;78(1):152–167. doi: 10.1083/jcb.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDS F. M., VITHAYATHIL P. J. The preparation of subtilisn-modified ribonuclease and the separation of the peptide and protein components. J Biol Chem. 1959 Jun;234(6):1459–1465. [PubMed] [Google Scholar]
- Schlegel R. A., Rechsteiner M. C. Red cell-mediated microinjection of macromolecules into mammalian cells. Methods Cell Biol. 1978;20:341–354. doi: 10.1016/s0091-679x(08)62026-9. [DOI] [PubMed] [Google Scholar]
- Ward W. F., Cox J. R., Mortimore G. E. Lysosomal sequestration of intracellular protein as a regulatory step in hepatic proteolysis. J Biol Chem. 1977 Oct 10;252(19):6955–6961. [PubMed] [Google Scholar]
- Winkler J. R., Segal H. L. Swainsonine inhibits glycoprotein degradation by isolated rat liver lysosomes. J Biol Chem. 1984 Dec 25;259(24):15369–15372. [PubMed] [Google Scholar]
- Zavortink M., Thacher T., Rechsteiner M. Degradation of proteins microinjected into cultured mammalian cells. J Cell Physiol. 1979 Jul;100(1):175–185. doi: 10.1002/jcp.1041000118. [DOI] [PubMed] [Google Scholar]


