Abstract
Objective
The objective was to examine the effectiveness of a self-help treatment as a first line primary care intervention for binge eating disorder (BED) in obese patients. This study compared the effectiveness of a usual care plus self-help version of cognitive behavioral therapy (shCBT) to usual care (UC) only in ethnically/racially diverse obese patients with BED in primary care settings in an urban center.
Method
48 obese patients with BED were randomly assigned to either shCBT (N=24) or UC (N=24) for four months. Independent assessments were performed monthly throughout treatment and at post-treatment.
Results
Binge-eating remission rates did not differ significantly between shCBT (25%) and UC (8.3%) at post-treatment. Mixed models of binge eating frequency determined using the Eating Disorder Examination (EDE) revealed significant decreases for both conditions but that shCBT and UC did not differ. Mixed models of binge eating frequency from repeated monthly EDE-questionnaire assessments revealed a significant treatment-by-time interaction indicating that shCBT had significant reductions whereas UC did not during the four-month treatments. Mixed models revealed no differences between groups on associated eating disorder psychopathology or depression. No weight loss was observed in either condition.
Conclusions
Our findings suggest that pure self-help CBT did not show effectiveness relative to usual care for treating BED in obese patients in primary care. Thus, self-help CBT may not have utility as a front-line intervention for BED for obese patients in primary care and future studies should test guided-self-help methods for delivering CBT in primary care generalist settings.
Keywords: obesity, binge eating, eating disorders, treatment, primary care
Binge-eating disorder (BED) is defined by recurrent binge eating (eating unusually large quantities of food accompanied by feelings of loss of control), marked distress, and the absence of inappropriate weight compensatory behaviors that characterize bulimia nervosa. The disorder is prevalent and is associated with obesity (Hudson, Hiripi, Pope, & Kessler, 2007), elevated risk for medical (Johnson, Spitzer, & Williams, 2001), and psychiatric co-morbidity (Grilo, White, & Masheb, 2009). BED has diagnostic validity, is distinct from other eating disorders (Grilo, Crosby, et al., 2009) and obesity (Grilo, Hrabosby, et al., 2008), and is a formal eating disorder in the DSM-5 (American Psychiatric Association, 2013).
Research has found that certain psychological treatments are effective for BED (Wilson, Grilo, & Vitousek 2007). Of these, cognitive-behavioral therapy (CBT), is the most widely studied and best-established treatment for BED (NICE, 2004; Wilson et al., 2007). CBT has demonstrated “treatment specificity” (Grilo, Masheb, & Wilson, 2005) and durable outcomes for 12-months (Grilo, Crosby, Wilson, & Masheb, 2013; Grilo, Masheb et al., 2011) through 48-months (Hilbert et al. 2012) following treatment in specialist clinics.
Unfortunately, despite the existence of empirically-supported CBT methods for BED and other eating disorders (Wilson et al., 2007), only a small number of individuals with eating/weight concerns receive mental health services (Marques et al., 2011), and even fewer receive treatments with documented effectiveness (Hart et al., 2011; Wilson & Zanberg, 2012). There is a shortage of clinicians with specialized training in CBT (Kazdin & Blase, 2011; Shafran et al., 2009) in general, and this is particularly the case for eating disorders (Hart et al., 2011; Mussell et al., 2000). Furthermore, research suggests that even clinicians who describe themselves as delivering CBT-based interventions for disordered eating do not follow most key aspects of empirically-supported CBT (Tobin, Banker, Weisberg, & Bowers, 2007; Waller, Stringer, & Meyer, 2012). Thus, one of the most pressing research needs facing the eating disorder field is for research on greater dissemination of effective treatment methods (Shafran et al., 2009); Wilson & Zandberg, 2012).
In an effort to address the need for dissemination of effective interventions, initial treatment studies with various forms of guided self-help and “pure” self-help CBT have shown promise for addressing BED (NICE, 2004; Sysko & Walsh, 2008; Wilson & Zandberg, 2012). Controlled trials have found that “guided” self-help CBT – that is, with some form of facilitation or guidance by a clinician – has efficacy for BED across diverse clinical and community settings (see critical reviews by Sysko & Walsh, 2008, and Wilson & Zandberg, 2012), with one controlled trial documenting “treatment-specificity” for guided self-help CBT versus guided self-help behavioral weight loss (Grilo & Masheb, 2005). Much less research, however, has examined “pure” self-help CBT - that is, self-help that is purely self-directed and without guidance from a clinician. While inspection of findings across studies suggests that pure self-help tends to be less beneficial than guided self-help (Sysko & Walsh, 2008; Wilson & Zandberg, 2012), only three studies that have directly tested pure self-help CBT for BED against no-self-help (i.e., wait-list) and these have yielded mixed results. Carter and Fairburn (1998) and Peterson and colleagues (1998) found that pure self-help CBT was superior to wait-list control in trials performed with a community-based sample and in a specialty clinic, respectively. More recently, however, in a larger trial Peterson and colleagues (2009) found that self-help CBT was not superior to wait-list control in a trial performed at a specialty clinic. Thus, further research is needed on the effectiveness of self-help CBT methods for BED across diverse settings (Wilson & Zandberg, 2012).
The existing treatment literature for BED is based mostly on trials performed in specialist research clinics and findings may not generalize adequately due to potential confounds associated with various clinic biases (Grilo, Lozano, & Masheb, 2005) or to more diverse patient groups comprising different ethnic/racial minorities (Franko et al., 2012). For example, African-American and Hispanic groups are vastly under-represented in the existing treatment literature for BED (Franko et al., 2012) with participation rates that are much lower than expected based on prevalence rates reported in epidemiological studies (Alegria et al., 2007; Marques et al., 2011). The three RCTs testing pure self-help CBT for BED consisted of 97% (Carter & Fairburn, 1998), 96.1% (Peterson et al., 1998), and 96.5% (Peterson et al., 2009) white participants. Furthermore, studies have found that minority groups with eating disorders have lower mental health utilization rates than whites and receive most of their health care from generalist or primary care settings rather than specialists (Marques et al., 2011).
The present study was designed to provide new information about the effectiveness of (pure) self-help CBT – initiated by generalist clinicians – as a potential first-step intervention method in primary care. BED is associated with increased health-care service utilization in general primary care settings (Johnson, Spitzer, & Williams, 2001) but binge eating problems are infrequently identified by general healthcare providers (Mond, Myers, Crosby, Hay, & Mitchell, 2010). In addition to gaining a better understanding of the effectiveness of pure self-help CBT and extending research into generalist medical settings, there is a pressing need for treatment research to include more diverse patient groups (Franko et al., 2012). Thus, this RCT was designed to provide information about the effectiveness of self-help CBT amongst a diverse sample of patients with BED in a highly relevant generalist medical setting.
Methods
Participants
Participants were 48 consecutively evaluated obese patients who exceeded DSM-5 criteria for BED and were randomized to treatment. The participants were respondents for a treatment study for weight loss and binge eating being performed in primary care in a large university-based medical health-care center in an urban setting. Participants were recruited using posters and flyers placed throughout primary care office settings in addition to “word-of-mouth” and referrals initiated by primary care physicians; we did not use newspaper or other media to recruit. Participants were required to be obese (body mass index (BMI) ≥ 30) and exceed proposed DSM-5 criteria for BED such that the stricter duration criteria of 6 months from the DSM-IV-TR was used, as opposed to 3 months1.
Recruitment for the treatment study was intended to enhance generalizability by utilizing relatively few exclusionary criteria. Exclusion criteria included BMI ≥ 50, over 65 years of age, current antidepressant therapy, current weight loss treatment or current use of medications known to influence eating/weight, select severe psychiatric problems (schizophrenia, bipolar disorder, and current substance use disorder), severe medical problems (e.g., cardiac disease), and uncontrolled liver disease, hypertension, thyroid disease, or diabetes. The study had full IRB review and approval and all participants provided written informed consent.
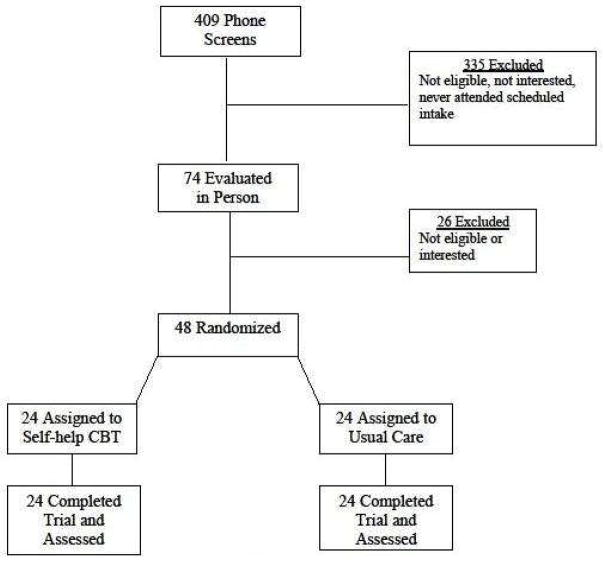
Figure 1 summarizes the flow of participants throughout the study. Four hundred and nine individuals were screened by telephone and 74 who passed screening (most excluded because they were not binge eating) were scheduled for in-person assessments to determine eligibility. Of these, 48 individuals were interested in participating, met eligibility requirements, completed baseline assessments, and were randomly assigned to one of the two treatment conditions that lasted four months. Of the N=48 participants, 34 met “full” DSM-IV-TR criteria for BED (N=17 were randomized to shCBT and N=17 were randomized to UC). The remaining N=14 met “subthreshold” DSM-IV-TR criteria for BED (greater than once-weekly binge eating frequency with a duration of at least six months) and N=7 were randomized to shCBT and N=7 were randomized to UC.
Figure 1.
Flow of participants throughout the study
Overall, participants had a mean age of 45.8 years (SD = 11.0) and a mean BMI of 37.62 kg/m2 (SD = 4.79). Seventy-nine percent (N=38) were female. The majority of participants comprised members of minority groups; 46% (N=22) were Caucasian, 35% (N=17) were African-American, 6% (N=3) were Hispanic-American, and 13% (N=6) considered themselves of “other” minority/ethnic groups (e.g., bi- or multi-racial).
Diagnostic Assessments and Repeated Measures
Diagnostic and assessment procedures were performed by trained doctoral-level research-clinicians. BED and co-existing DSM-IV psychiatric disorder diagnoses were based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P; First, Spitzer, Gibbon, & Williams, 1996). Participants were given $150 for completing post-treatment assessments.
Eating Disorder Examination Interview (EDE; Fairburn & Cooper 1993), a semi-structured, investigator-based interview, was administered to confirm the EDE diagnosis and to characterize baseline levels of binge eating frequency and eating disorder psychopathology. The EDE focuses on the previous 28 days except for diagnostic items, which are rated for DSM-based duration stipulations. The EDE assesses the frequency of objective bulimic episodes (OBE; i.e., binge-eating defined as unusually large quantities of food with a subjective sense of loss of control), which corresponds to the DSM-based definition of binge-eating. The EDE also generates a total global score reflecting overall severity, which reflects the mean of four subscales. The EDE has well-established inter-rater and test-retest reliability (Grilo, Masheb, Lozano-Blanco, & Barry, 2004) and validity (Grilo, Masheb, & Wilson, 2001). Evaluators for this study participated in an inter-rater reliability study of 34 cases not included in this treatment trial; intra-class correlation coefficients (ICC) for OBE episodes (.83), OBE days (.90), and EDE global score (0.93) were excellent.
Eating Disorder Examination-Questionnaire (EDE-Q; Fairburn & Beglin, 1994), the self-report version of the EDE, was administered at baseline, monthly during treatment, and at post-treatment. The repeated administration of this brief and non-intrusive self-report version was intended to obtain change data during the four months. Research has documented that the EDE-Q performs well as a measure of change in treatment trials (Sysko, Walsh, & Fairburn, 2005) and that among patients with BED it converges adequately with the EDE (Grilo et al., 2001; Grilo, Masheb, & Wilson, 2001b) and has good test-retest reliability (Reas, Grilo, & Masheb, 2006).
Beck Depression Inventory (BDI; Beck & Steer, 1987), the 21-item version, is a well-established self-report (Beck, Steer, & Garbin, 1998) measure of depression levels and symptoms. The BDI was given at baseline, monthly during treatment, and at post-treatment.
Knowledge Questionnaire (KQ; Carter & Fairburn, 1998) is a 15-item measure that was adapted and shortened to a 10-item version for this study (by deleting the purging items relevant for bulimia nervosa). This 10-item true-false questionnaire was used to assess patients’ knowledge of the educational content of the shCBT (Fairburn, 1995) patient manual described below. Scores ranged from 0–10, with higher scores reflecting greater knowledge.
Weight and height were measured at baseline and weight was measured monthly throughout treatment and at post-treatment using a large capacity digital scale. BMI was calculated from these measurements.
Randomization to Treatments and Maintaining Treatment Blindness
Participants were randomly assigned to either shCBT (N=24) or UC (N=24) for four months within the primary care settings. Randomization to treatment assignment occurred in the exact order following completion of all assessments and medical approval and was performed independently from the investigators based on a randomization schedule. Randomization was stratified by DSM diagnosis of BED (i.e., full DSM-IV-TR research criteria requiring twice-weekly binge-eating for six months versus the proposed DSM-5 criteria requiring once weekly binge eating that we expanded to a required duration of six months). Within each stratum, randomization was performed in blocks of 12 to obviate any secular trends. The repeated assessments, including the EDE re-administered at post-treatment, were performed by independent evaluators who were kept blinded to the treatment condition. All the assessments were performed separately at our research clinic (i.e., not in primary care) in order to foster the independence of assessments from the treatment and to facilitate honest reporting.
Self-Help Cognitive-Behavioral Therapy (shCBT)
Half the patients were randomly assigned to receive shCBT (in addition to UC described below). shCBT involved being given Overcoming Binge Eating (Fairburn, 1995) a self-help program which follows the professional CBT program (Fairburn, Marcus, & Wilson, 1993) and is considered to be the treatment of choice for BED (NICE, 2004; Wilson et al., 2007). This is the same patient self-help manual used in the Carter and Fairburn (1998) community-based effectiveness trial with non-specialist clinicians and in controlled trials at specialist centers using guided-self-help CBT (e.g., Grilo & Masheb, 2005; Grilo, Masheb, & Salant, 2005; see review by Sysko & Walsh (2008)). The patient self-help manual (Fairburn, 1995) has 3 stages. The first stage consists of: presentation of the CBT model including the structure, goals, and methods; education regarding binge eating, dieting, and health; introduction of self-monitoring techniques; and introduction of graded behavioral techniques for establishing normalized eating patterns. The second stage consists of maintaining the normalized eating and self-monitoring procedures and integrates cognitive restructuring procedures and the development of coping skills for triggers of maladaptive eating. The third stage focuses on consolidating progress, maintenance of changes, and relapse prevention methods. The manual provides guidance as to when to move on to the next step of the program.
Primary care physicians, who did not have any specific training as mental health professionals or with eating disorders, instructed the participants assigned to shCBT to read and follow the self-help CBT manual. Physicians were given a brief script to focus on key details of the study. The primary care physicians (PCP): (1) introduced the formal start to the treatment study; (2) noted there would be a 50% chance of receiving a self-help book in addition to usual care; (3) indicated the treatment period would be four months; and (4) noted they would receive monthly assessments during treatment and a final post-treatment assessment after four months. The PCP then opened the sealed packet and informed the patient if they receive the self-help book and UC, or UC without shCBT. Patients receiving shCBT additionally received a copy of the book and the following one time information from the PCP: (1) instructions to read both parts of the book with part I providing background information about binge eating and part II providing the self-help program; and (2) encouragement to follow suggestions for record keeping and goal setting as those seem to be key ingredients for treatment effectiveness.
Usual Care (UC)
All patients received UC, but half the patients were randomly assigned to receive only UC (without shCBT). In UC, patients were instructed to follow whatever advice and treatment recommended by their primary care physicians. All participants had existing relationships with primary care settings although the nature of the on-going naturalistic treatments likely differed across patients and primary care physicians. The PCP (and patients’ other healthcare providers) – i.e., the “usual care” - operated independently of the research team and were free to make recommendations and/or offer treatments, although patients were asked to refrain from seeking commercial self-help programs. Since UC was neither “enhanced” nor influenced by the research team, this condition approximates so-called routine care that patients would receive if they were not involved in this treatment study. We note UC is technically distinct from both TAU (which implies that most patients receive a particular treatment) and “standard of care” (which implies that patients receive evidence-based or guideline-compliant care), which are often confused and infrequently actually followed (Freedland et al., 2011). UC has served as the comparison in major treatment trials testing interventions for psychiatric conditions in primary care (Alexopoulos et al., 2009) and for combined psychiatric-medical conditions (Davidson et al., 2010; Rollman et al., 2009). As described by Freedland and colleagues (2011), UC does not indicate or suggest that patients receive a specific intervention for the problem (i.e., BED in obese patients, in the present study) but rather that patients might receive a broad range of possible interventions.
Statistical Analyses
Analyses designed to compare treatments were performed for all randomized patients (intent-to-treat). Baseline characteristics (demographic, psychiatric, and clinical variables) for the treatment groups were compared using chi-square analyses for categorical variables and t-tests for continuous measures.
The primary treatment outcome variable was binge eating. Binge eating was analyzed in several complementary ways. First, rates of “remission” from binge eating (defined as zero binges (OBEs)) during the previous 28 days (as assessed using the EDE interview at post-treatment) were compared between the two conditions using chi-square analyses. Second, “frequency” of binge eating assessed using two different methods (OBEs during the previous 28 days assessed with the EDE-Q at monthly intervals and OBEs during the previous 28 days assessed with the EDE interview at post-treatment) was analyzed with mixed models (SAS PROC MIXED) utilizing all available data throughout the study, to compare the two treatment conditions.
Secondary outcome variables included continuous measures of eating disorder psychopathology (EDE global score, EDE-Q global score), depression levels (BDI score), and BMI which were compared between treatments using mixed models. Except for the EDE global score, which was measured at baseline and post-treatment only, each of the continuous measures was obtained monthly throughout the course of treatment and at post-treatment.
In each of the mixed models analyses, fixed effects of treatment condition, time (with the relevant time points for each measure as described above), the interaction of treatment by time, and random subject-level effects were considered. Distributions of all data were examined and transformations were applied if necessary to satisfy model assumptions (e.g., OBE (binge), EDE global and the BDI data were square-root transformed) although the tables show untransformed raw values. For each model, different variance-covariance structures (unstructured, autoregressive with and without heterogeneous variances, compound symmetry with and without heterogeneous variances) were evaluated and the best-fitting structure was selected based on Schwartz Bayesian criterion (BIC). When significant interactions were detected, post-hoc comparisons among least squared means were performed to explain the significant effects. Significance level was fixed at 0.05 level and post-hoc comparisons were adjusted using Bonferroni correction.
Results
Randomization and Patient Characteristics
Of the 48 randomized patients, 24 received shCBT and 24 did not. All participants completed post-treatment assessments (Figure 1). Treatment groups did not differ significantly in demographic or psychiatric variables (Table 1) or on pretreatment levels of any outcome variables (Table 2).
Table 1.
Demographic and Clinical Characteristics of the 48 Randomized Patients
| Variable | Overall (N= 48) | shCBT (N= 24) | Usual Care (N= 24) | Test statistic | p value |
|---|---|---|---|---|---|
| Age, mean (SD) | 45.8 (11.0) | 45.0 (11.8) | 46.5 (10.2) | t(46)=0.47 | .64 |
| Female, N (%) | 38 (79.2) | 21 (87.5) | 17 (70.8) | X2(1)=2.02 | .16 |
| Ethnicity, N (%) | |||||
| Caucasian | 22 (45.8) | 11 (45.8) | 11 (45.8) | X2(3)=4.47 | .22 |
| African-American | 17 (35.4) | 6 (25.0) | 11 (45.8) | ||
| Hispanic-American | 3 (6.3) | 2 (8.4) | 1 (4.2) | ||
| Other | 6 (12.5) | 5 (20.8) | 1 (4.2) | ||
| Education, N (%)a | X2(1)=0.54 | .46 | |||
| College degree | 23 (48.9) | 13 (54.2) | 10 (43.5) | ||
| No college degree | 24 (51.1) | 11 (45.8) | 13 (56.5) | ||
| DSM-IV co-morbidity, N (%) | |||||
| Mood disorders | 24 (50.0) | 10 (41.7) | 14 (58.3) | X2(1)=1.33 | .25 |
| Anxiety disorders | 24 (50.0) | 13 (54.2) | 11 (45.8) | X2(1)=0.33 | .56 |
| Substance use disorders | 10 (20.8) | 4 (16.7) | 6 (25.0) | X2(1)=0.51 | .48 |
| Age onset BED, mean (SD)b | 24.7 (13.8) | 25.8 (13.9) | 23.5 (13.9) | t(42)= −0.56 | .58 |
Note: shCBT = self-help-Cognitive behavioral therapy. SD = standard deviation. N = number. BED = binge eating disorder.
denotes N=47;
denotes N=44; Test statistic = chi-square for categorical variables and t-tests for dimensional variables. P values are for two-tailed tests.
Table 2.
Clinical Variables Across the Two Treatments at Pre- and Post-Treatment
| Variable | Pre-treatment | Post-treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| shCBT | Usual Care | shCBT | Usual Care | Effect Size | |||||
| M | SD | M | SD | M | SD | M | SD | ||
| EDE OBE/month | 15.13 | 7.60 | 16.08 | 8.86 | 5.75 | 5.94 | 6.50 | 7.22 | 0.24 |
| EDE Global Score | 2.61 | 0.80 | 2.34 | 0.83 | 1.97 | 0.58 | 1.99 | 0.71 | 0.38 |
| EDE-Q OBE/month | 13.83 | 8.65 | 9.74 | 7.11 | 4.54 | 5.01 | 8.21 | 9.36 | 0.90 |
| EDE-Q Global Score | 3.58 | 0.95 | 3.17 | 0.86 | 3.02 | 0.88 | 2.81 | 0.89 | 0.34 |
| BDI Depression | 14.57 | 8.48 | 16.09 | 8.61 | 8.88 | 7.67 | 11.96 | 7.38 | 0.13 |
| Body mass index | 38.01 | 5.36 | 37.22 | 4.22 | 37.45 | 5.34 | 37.42 | 4.44 | 0.54 |
Note: shCBT = self-help-Cognitive behavioral therapy. M = mean. SD = standard deviation. Effect size calculated as Cohen’s d for the group differences in changes from baseline to post-treatment. EDE = Eating Disorder Examination. EDE-Q = Eating Disorder Examination Questionnaire version; OBE = objective bulimic episodes. BDI = Beck Depression Inventory. Analyses revealed no significant group differences at baseline.
Binge Eating Remission and Frequency
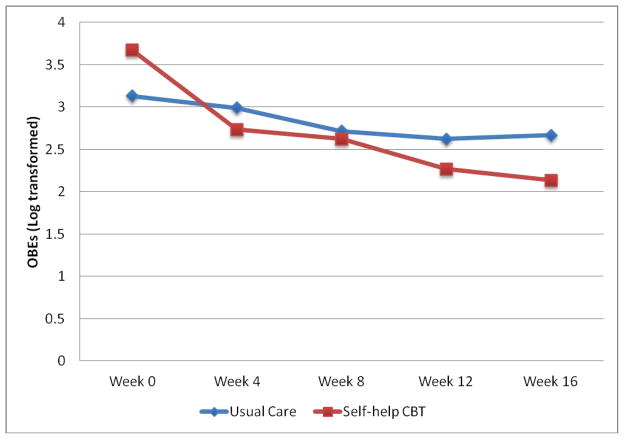
Remission rates at post-treatment (defined as zero OBEs (binge episodes) during the past month based on the EDE interview) were 25% (N = 6 of 24) for shCBT versus 8.3% (N = 2 of 24) for UC; these rates did not differ significantly across treatments (X2(1) = 2.40, p = 0.12; Fisher’s exact test p = 0.24; OR = 3.67, p = 0.14, 95% CI = 0.66–20.42). Table 2 summarizes binge frequency based on both the EDE and the EDE-Q assessment methods for the two conditions at post-treatment. Figure 2 summarizes the binge eating frequency data based on monthly EDE-Q assessments throughout treatment and at posttreatment.
Figure 2.
Monthly frequency of binge eating by participants in the two treatment conditions (self-help CBT and Usual Care). The data shown are based on estimated marginal means (derived from mixed models analyses) for all N=48 patients based on the Eating Disorder Examination – Questionnaire version given during the repeated monthly assessments.
Mixed models analysis of the EDE-based data (see Table 2) revealed a significant time effect indicating improvements in binge eating (F(1,46) = 89.75, p < 0.0001). Both groups had significant decreases in binge eating frequency (F(1,46) = 53.47, p<0.0001, for the shCBT and F(1,46)=37.03, p<0.0001, for UC); however, the two groups did not differ significantly in their improvements over time (F(1,46) = 0.75, p = 0.39).
Mixed models analysis of the repeated EDE-Q assessments (see Figure 2) revealed a significant time effect for the frequency of binge eating (F(4,181) = 10.88, p < 0.0001) and a significant group by time effect (F(4,181) = 2.81, p = 0.03). The shCBT group improved significantly over time (F(4,181) = 12.25, p < 0.0001) whereas the UC group did not change significantly over time (F(4,181) = 1.63, p = 0.17).
Associated Eating Disorder Psychopathology, Depression, and BMI
Mixed models analysis of the EDE global score (see Table 2) revealed a significant time effect indicating improvements (F(1,46) = 21.14, p < 0.0001). Both groups had significant reductions in eating disorder psychopathology (F(1,46)=17.6, p = 0.0001, for shCBT and F(1,46) = 5.34, p = 0.025, for UC); however, the two groups did not differ significantly in their improvements over time (F(1,46) = 1.77, p = 0.19). Mixed models analysis of the repeated assessments of EDE-Q global score revealed the same pattern of findings as for the EDE post-treatment assessment. A significant time effect was observed for EDE-Q global (F(4,181) = 8.75, p < 0.0001); both groups had significant reductions (F(1,181)=17.39, p<.0001, for shCBT and F(1,181) = 7.08, p = 0.001, for UC); however, the two groups did not differ significantly in their improvements over time (F(4,181) = 0.96, p = 0.43).
Mixed models analysis of the repeated BDI scores revealed a significant time effect indicating reductions in depression levels (F(4,181) = 10.80, p < 0.0001); both groups had significant reductions (F(4,181) = 7.8, p < 0.0001, for shCBT and F(4,181) = 3.78, p = 0.001, for UC); however, the two groups did not differ significantly in their improvements over time (F(4,181) = 0.74, p = 0.57).
Mixed models of monthly BMI data revealed no significant time effects (F(4,180) = 0.70, p = 0.59) and no significant group by time effect (F(4,180) = 1.02, p = 0.40).
Exploratory Analysis of Knowledge Scores
Exploratory analyses of the Knowledge Questionnaire revealed no significant differences between groups on total scores either at baseline M = 3.3 (SD = 1.9) for shCBT versus M = 3.4 (SD = 2.1) for UC; t(45) = 0.25, p = 0.81 or at posttreatment (M = 4.6 (SD = 1.9) for shCBT versus M = 3.8 (SD = 2.0) for UC; t(45) = 1.51, p=0.14). Paired samples t-tests revealed significant improvements in Knowledge Questionnaire scores from baseline to post-treatment for shCBT (t(22) = −3.40, p = 0.003) but not for UC (t(22) = −0.74, p = 0.47).
Discussion
The aim of this study was to examine the effectiveness of a pure self-help cognitive behavioral intervention (i.e., without guidance) as a first line intervention for obese patients with BED in urban primary care settings serving ethnically/racially diverse patients. Our comparison of shCBT (along with usual care) to UC (usual care without shCBT) over the four-month study period revealed little to no significant differences over time. Binge-eating remission rates were not statistically significantly greater for the shCBT (25%) versus UC (8%). Analyses of binge-eating frequency assessed using two complementary assessments (EDE interview pre- and post and EDEQ monthly assessments) revealed significant overall decreases. Analyses of the EDE binge frequency data revealed significant decreases for both conditions but that the two conditions did not differ significantly. In contrast, mixed models analyses of the repeated monthly EDEQ binge eating data revealed an advantage for shCBT: shCBT reduced binge-eating frequency significantly whereas UC did not. Mixed models analyses of associated eating disorder psychopathology and depression revealed significant reductions but no differences between conditions. Analyses of BMI revealed no changes over time, which is consistent with CBT outcomes regardless of delivery method (Wilson et al., 2007). Collectively, these findings suggest that shCBT (plus usual care) did not show effectiveness relative to usual care for treating BED in obese patients seen in primary care.
The 25% binge-eating remission rate for shCBT in this study falls in-between the 17.9% remission rate for shCBT reported by Peterson et al (2009) in the specialty clinic settings and the 43% remission rate reported by Carter and Fairburn (1998) in the community-based setting. Our observed remission rate for shCBT is roughly comparable to the considerable range reported by various trials comparing it to guided-self-help methods for BED and “mixed” BED and bulimia nervosa patient groups (see Sysko & Walsh, 2008). The 8.3% remission rate observed for UC in our study closely parallels the 10.1% and 8% remission rates reported by the Peterson et al (2009) and Carter and Fairburn (1998) studies respectively for their wait-list control groups. Our findings regarding the general lack of relative effectiveness for shCBT relative to UC for BED in obese patients in primary care is consistent with the large Peterson et al (2009) trial performed in a specialty clinic in which self-help CBT was not superior to wait-list control. These findings contrast with the Peterson et al (1998) trial at a specialty clinic and the Carter & Fairburn (1998) trial in a community based setting which found that self-help CBT was superior to wait-list controls. Comparisons across these four trials can only be made cautiously as UC is a distinct comparison condition from wait-list control (see Freedland et al., 2011), generalist and specialist treatment settings and providers differ, and our patient group had much greater ethnic/racial diversity than the other there trials.
As context for our findings, we note several potential limitations to consider and to inform future work. First, we did not obtain any systematic empirical data regarding either the PCPs’ “level of comfort” or “adherence” to the suggested script and the approach taken with patients participating in the trial. It is certainly possible, for example, that the study introduced some possible degree of artificiality or interpersonal concern. If this occurred, however, there would be no reason to suspect that the two conditions would be impacted differently and thus it would be unlikely to bias the analyses testing for differences between shCBT and UC. Conversely, given the well-documented anti-obesity attitudes and feelings of frustration and ineffectiveness in treating obesity which are pervasive among physicians (e.g., Ferrante et al., 2009; Sabin et al., 2012; see review by Puhl & Heuner 2009), it is possible that the study script provided structure and served to enhance confidence and empathic communication.
We did not collect direct data regarding patients’ compliance with reading the self-help book. Such data would have been informative for addressing the more limited question of whether the self-help CBT book helps when or if patients actually read it. We did, however, obtain relevant related information in the form of changes in “knowledge” about binge eating from pre- to post-treatment. Those exploratory analyses revealed that patients in the shCBT, but not in the UC condition had statistical significant improvements in knowledge during the study although the two conditions did not differ significantly at post-treatment. Such data suggests some degree of compliance with reading the self-help book. We emphasize, however, that the scores on the Knowledge Questionnaire were quite modest – which suggests that some degree of clinician guidance is likely needed to enhance knowledge and/or use of the CBT methods. This speculation seems consistent with the finding by Carter & Fairburn (1998) that 68% of patients receiving gshCBT versus only 5% of patients following pure shCBT reported trying to follow the entire program.
We acknowledge the possible limitation of limited statistical power of our study to detect significant smaller differences between treatments. For example, the three-fold relative advantage of shCBT over UC for binge remission rates (odds ratio of 3.67 which approached statistical significance) and the one significant group by time effect indicating that shCBT had significant reductions in binge eating (on the EDEQ) over time whereas the UC group did not, might suggest a possible “signal” for shCBT that could be tested in future studies with larger samples. The EDEQ results which differed slightly from the EDE results may simply reflect greater effect size for the EDEQ-based data and perhaps greater power afforded by the repeated monthly EDEQ assessments (versus just pre- and post-treatment EDE assessments) or simply that these two assessment methods do not converge precisely (Grilo et al., 2001). We note, however, that the magnitude of our clinical findings for shCBT and UC - which closely parallel those reported by Peterson et al (2009) in a specialty clinic - are considerably dampened relative to what trials generally report for guided-self-help methods for delivering CBT to patients with BED (Grilo & Masheb, 2005; Grilo, Masheb, & Salant, 2005; Wilson et al., 2010; see reviews by Sysko & Walsh, 2008; Wilson & Zandberg, 2012). A final limitation is that the study did not include a follow-up assessment to determine the durability or maintenance of the outcomes.
In closing, we offer the following thoughts about the implications for treatment and research of binge eating in primary care. As a broader context for our findings, we note the striking discrepancy in findings between two RCTs testing self-help CBT methods and fluoxetine for bulimia nervosa performed in specialty clinic (Mitchell et al. 2001) versus in primary care (Walsh, Fairburn, Mickley, Sysko, & Parides, 2004). Whereas Mitchell and colleagues (2001) reported that both shCBT and fluoxetine were effective, Walsh and colleagues (2004) found that guided-self-help CBT and fluoxetine were both associated with very high dropout and poor outcomes. Walsh and colleagues (2004) reported that patients with bulimia nervosa receiving fluoxetine had statistically greater reductions in binge eating and vomiting (though the reductions were not clinically meaningful and only 15.9% achieved remission) than those receiving placebo whereas gshCBT did not differ significant from no-gshCBT. Thus, our findings – which raise concerns about the utility of pure shCBT as a front line intervention for BED for obese patients in primary care - suggest the need for future studies to test guided-self-help methods for delivering CBT. Similarly, the few available data for bulimia nervosa (Walsh et al., 2004) suggest caution in believing that outcomes achieved in specialist settings will translate well to primary care. An important area for research will involve not just scalability of interventions but also training methods for delivering the interventions (Wilson & Zandberg, 2012).
Highlights.
RCT testing self-help CBT for binge eating disorder in primary care
Ethnically- and racially-diverse obese patients
Self-help CBT did not show effectiveness relative to usual care
Future studies should test guided-self-help
Acknowledgments
This research was supported by National Institutes of Health grant R01 DK073542. Dr. Grilo was also supported by NIH grant K24 DK070052. Dr. Barnes was also supported by NIH grant K23 DK092279.
Footnotes
When we first designed this RCT, the DSM-5 criteria for BED were well researched but not yet finalized. To be able to address both criteria for DSM-IV-TR and the likely criteria for DSM-5, we included both the longer duration criteria (>6 months) and both cut-points for the frequency criteria (i.e., >twice weekly and >once weekly). This provided us the ability to stratify randomization by meeting “full” criteria for DSM-IV-TR frequency (frequency at least twice weekly OBE) and “subthreshold” (frequency at least once weekly) which was consistent with the literature at the time and ultimately allowed us to exceed DSM-5 criteria.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alegria M, Woo M, Cao Z, Torres M, Meng XL, Striegel-Moore RH. Prevalence and correlates of eating disorders in Latinos in the United States. International Journal of Eating Disorders. 2007;40(suppl):S15–21. doi: 10.1002/eat.20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Reynolds CF, III, Bruce ML, et al. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. American Journal of Psychiatry. 166:882–890. doi: 10.1176/appi.ajp.2009.08121779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Association; 2013. (DSM-5) [Google Scholar]
- Beck AT, Steer R, Garbin M. Psychometric properties of the Beck Depression Inventory: 25 years of evaluation. Clinical Psychology Review. 1998;8:77–100. [Google Scholar]
- Beck AT, Steer R. Manual for revised Beck Depression Inventory. New York: Psychological Corporation; 1987. [Google Scholar]
- Carter JC, Fairburn CG. Cognitive-behavioral self-self for binge eating disorder: A controlled effectiveness study. Journal of Consulting and Clinical Psychology. 1998;66:616–623. doi: 10.1037//0022-006x.66.4.616. [DOI] [PubMed] [Google Scholar]
- Davidson KW, Rieckmann N, Clemow L, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Archives Internal Medicine. 2010;170:600–608. doi: 10.1001/archinternmed.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn CG. Overcoming binge eating. New York: Guilford Press; 1995. [Google Scholar]
- Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? International Journal of Eating Disorders. 1994;16:363–370. [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z. The Eating Disorder Examination. In: Fairburn CG, Wilson GT, editors. Binge eating: nature, assessment, and treatment. 12. New York: Guilford Press; 1993. pp. 317–360. [Google Scholar]
- Fairburn CG, Marcus MD, Wilson GT. Cognitive-behavioral therapy for binge eating and bulimia nervosa: a comprehensive treatment manual. In: Fairburn CG, Wilson GT, editors. Binge eating: nature, assessment, and treatment. New York: Guilford Press; 1993. pp. 361–404. [Google Scholar]
- Ferrante JM, Piasecki AK, Ohman-Strickland PA, Crabtree BF. Family physicians’ practices and attitudes regarding care of extremely obese patients. Obesity. 2009;17:1710–1716. doi: 10.1038/oby.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders - patient Edition (SCID-I/P, Version 2.0) New York: New York State Psychiatric Institute; 1996. [Google Scholar]
- Franko DL, Thompson-Brenner H, Thompson DR, et al. Racial/ethnic differences in adults in randomized clinical trials of binge eating disorder. Journal of Consulting and Clinical Psychology. 2012;80:186–195. doi: 10.1037/a0026700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedland KE, Mohr DC, Davidson K, Schwartz JE. Usual and unusual care: existing practice control groups in randomized controlled trials of behavioral interventions. Psychosomatic Medicine. 2011;73:323–335. doi: 10.1097/PSY.0b013e318218e1fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Crosby RD, Masheb RM, White MA, Peterson CB, Wonderlich SA, Engel SG, Crow SJ, Mitchell JE. Overvaluation of shape and weight in binge eating disorder, bulimia nervosa, and sub-threshold bulimia nervosa. Behaviour Research and Therapy. 2009;47:692–696. doi: 10.1016/j.brat.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Crosby RD, Wilson GT, Masheb RM. 12-Month follow-up of fluoxetine and cognitive behavioral therapy for binge eating disorder. Journal of Consulting and Clinical Psychology. 2012;80:1108–1113. doi: 10.1037/a0030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Hrabosky JI, White MA, Allison KC, Stunkard AJ, Masheb RM. Overvaluation of shape and weight in binge eating disorder and overweight controls: refinement of BED as a diagnostic construct. Journal of Abnormal Psychology. 2008;117:414–419. doi: 10.1037/0021-843X.117.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Lozano C, Masheb RM. Ethnicity and sampling bias in binge eating disorder. International Journal of Eating Disorders. 2005;38:257–262. doi: 10.1002/eat.20183. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM. A randomized controlled comparison of guided self-help cognitive behavioral therapy and behavioral weight loss for binge eating disorder. Behaviour Research and Therapy. 2005;43:1509–1525. doi: 10.1016/j.brat.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, Lozano-Blanco C, Barry DT. Reliability of the Eating Disorder Examination in patients with binge eating disorder. International Journal of Eating Disorders. 2004;35:80–85. doi: 10.1002/eat.10238. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, Salant SL. Cognitive behavioral therapy guided self-help and orlistat for the treatment of binge eating disorder: a randomized double-blind placebo-controlled trial. Biological Psychiatry. 2005;57:1193–1201. doi: 10.1016/j.biopsych.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, Wilson GT. A comparison of different methods for assessing the features of eating disorders in patients with binge eating disorder. Journal of Consulting and Clinical Psychology. 2001;69:317–322. doi: 10.1037//0022-006x.69.2.317. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, Wilson GT. Different methods for assessing the features of eating disorders in patients with binge eating disorder: a replication. Obesity Research. 2001b;9:418–422. doi: 10.1038/oby.2001.55. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, Wilson GT. Efficacy of cognitive behavioral therapy and fluoxetine for the treatment of binge eating disorder: a randomized double-blind placebo-controlled comparison. Biological Psychiatry. 2005;57:301–309. doi: 10.1016/j.biopsych.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Grilo CM, White MA, Masheb RM. DSM-IV psychiatric disorder comorbidity and its correlates in binge eating disorder. International Journal of Eating Disorders. 2009;42:228–234. doi: 10.1002/eat.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart LM, Granillo MT, Jorm AF, Paxton SJ. Unmet need for treatment in the eating disorders: a systematic review of eating disorder specific treatment seeking among community cases. Clinical Psychology Review. 2011;31:727–735. doi: 10.1016/j.cpr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Hilbert A, Bishop M, Stein R, Wilfley DE. Long-term efficacy of psychological treatments for binge eating disorder. British Journal of Psychiatry. 2012;200:232–237. doi: 10.1192/bjp.bp.110.089664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survery Replication. Biological Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JG, Spitzer RL, Williams JB. Health problems, impairment and illnesses associated with bulimia nervosa and binge eating disorder among primary care and obstetric gynaecology patients. Psychological Medicine. 2001;31:1455–66. doi: 10.1017/s0033291701004640. [DOI] [PubMed] [Google Scholar]
- Kazdin AE, Blase SL. Rebooting psychotherapy research and practice to reduce the burden of mental illness. Perspectives on Psychological Science. 2011;6:21–37. doi: 10.1177/1745691610393527. [DOI] [PubMed] [Google Scholar]
- Marques L, Alegria M, Becker AE, Chen CN, Fang A, Chosak A, Diniz JB. Comparative prevalence, correlates of impairment, and service utilization for eating disorders across US ethnic groups: implications for reducing ethnic disparities in health care access for eating disorders. International Journal of Eating Disorders. 2011;44:412–420. doi: 10.1002/eat.20787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JE, Fletcher L, Hanson K, Mussell MP, Seim H, Crosby RD, Al-Banna M. The relative efficacy of fluoxetine and manual-based self–help in the treatment of outpatients with bulimia nervosa. Journal of Clinical Psychopharmacology. 2001;21:298–304. doi: 10.1097/00004714-200106000-00008. [DOI] [PubMed] [Google Scholar]
- Mond JM, Myers TC, Crosby RD, Hay PJ, Mitchell JE. Bulimic eating disorders in primary care: hidden mortality still? Journal of Clinical Psychology in Medical Settings. 2010;17:56–63. doi: 10.1007/s10880-009-9180-9. [DOI] [PubMed] [Google Scholar]
- National Institute for Clinical Excellence (NICE) Eating Disorders – Core Interventions in the treatment and management of anorexia nervosa, bulimia nervosa, related eating disorders. London: NICE; 2004. NICE Clinical Guideline No. 9. [PubMed] [Google Scholar]
- Peterson CB, Mitchell JE, Crow SJ, Crosby RD, Wonderlich SA. The efficacy of self-help group treatment and therapist-led group treatment for binge eating disorder. American Journal of Psychiatry. 2009;166:1347–1354. doi: 10.1176/appi.ajp.2009.09030345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CB, Mitchell JE, Engbloom S, Nugent S, Mussell MP, Miller JP. Group cognitive-behavioral treatment of binge eating disorder: a comparison of therapist-led versus self-help formats. International Journal of Eating Disorders. 1998;24:125–136. doi: 10.1002/(sici)1098-108x(199809)24:2<125::aid-eat2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Puhl R, Heuer CA. The stigma of obesity: a review and update. Obesity. 2009;17:941–964. doi: 10.1038/oby.2008.636. [DOI] [PubMed] [Google Scholar]
- Reas DL, Grilo CM, Masheb RM. Reliability of the Eating Disorder Examination-Questionnaire in patients with binge eating disorder. Behaviour Research and Therapy. 2006;44:43–51. doi: 10.1016/j.brat.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Rollman BL, Belnap BH, LeMenager MS, et al. Telephone-delivered collaborative care for treating post-CABG depression: a randomized controlled trial. Journal of the American Medical Association. 302:2095–2103. doi: 10.1001/jama.2009.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin JA, Marini M, Nosek BA. Implicit and explicit anti-fat bias among a large sample of medical doctors by BMI, race/ethnicity, and gender. PLoS One. 2012;7:e48448. doi: 10.1371/journal.pone.0048448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafran R, Clark DM, Fairburn CG, et al. Mind the gap: improving the dissemination and implementation of CBT. Behaviour Research and Therapy. 2009;47:902–909. doi: 10.1016/j.brat.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Sysko R, Walsh BT. A critical evaluation of the efficacy of self-help interventions for the treatment of bulimia nervosa and binge-eating disorder. International Journal of Eating Disorders. 2008;41:97–112. doi: 10.1002/eat.20475. [DOI] [PubMed] [Google Scholar]
- Sysko R, Walsh BT, Fairburn CG. Eating Disorder Examination – Questionnaire as a measure of change in patients with bulimia nervosa. International Journal of Eating Disorders. 2005;37:100–106. doi: 10.1002/eat.20078. [DOI] [PubMed] [Google Scholar]
- Tobin DL, Banker JD, Weisberg L, Bowers W. I know what you did last summer (and it was not CBT): a factor analytic model of international psychotherapeutic practice in the eating disorders. International Journal of Eating Disorders. 2007;40:754–757. doi: 10.1002/eat.20426. [DOI] [PubMed] [Google Scholar]
- Waller G, Stringer H, Meyer C. What cognitive behavioral techniques do therapists report using when delivering cognitive behavioral therapy for the eating disorders? Journal of Consulting and Clinical Psychology. 2012;80:171–175. doi: 10.1037/a0026559. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Fairburn CG, Mickley DE, Sysko R, Parides MK. Treatment of bulimia nervosa in a primary care setting. American Journal of Psychiatry. 2004;161:556–561. doi: 10.1176/appi.ajp.161.3.556. [DOI] [PubMed] [Google Scholar]
- Wilson GT, Grilo CM, Vitousek KM. Psychological treatments of eating disorders. American Psychologist. 2007;62:199–216. doi: 10.1037/0003-066X.62.3.199. [DOI] [PubMed] [Google Scholar]
- Wilson GT, Wilfley DE, Agras WS, Bryson SW. Psychological treatments of binge eating disorder. Archives of General Psychiatry. 2010;67:94–101. doi: 10.1001/archgenpsychiatry.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GT, Zandberg LJ. Cognitive-behavioral guided self-help for eating disorders: effectiveness and scalability. Clinical Psychology Review. 2012;32:343–357. doi: 10.1016/j.cpr.2012.03.001. [DOI] [PubMed] [Google Scholar]