SYNOPSIS
The purpose of this article is to update the otolaryngologic community on recent developments in the basic understanding of how cough, swallow, and breathing are controlled. These behaviors are coordinated to occur at specific times relative to one another to minimize the risk of aspiration. The control system that generates and coordinates these behaviors is complex and advanced computational modeling methods are useful tools to elucidate its function.
Keywords: Cough, swallow, breathing, airway, central pattern generator, brainstem
INTRODUCTION
Airway protection is the prevention and/or correction of aspiration. During swallowing, aspiration is prevented during the pharyngeal phase of swallow by closure of the vocal folds, changes in the breathing pattern, and protection of the laryngeal orifice by appropriate movement of the epiglottis. Another behavior, the expiration reflex, prevents aspiration by producing a rapidly rising expiratory airflow to eject adherent material away from the vocal folds. Other behaviors, such as laryngeal adduction and apnea, also participate in the prevention of aspiration. If aspiration occurs, cough is elicited as a defensive reflex to produce high velocity airflows that create shear forces to dislodge and eject material from the airway 1.
Most neuromuscular diseases result in impaired cough (dystussia) and/or impaired swallow function (dysphagia). Cough and swallows are controlled by complex brainstem networks which, until recently, have been studied in isolation. However, in neurologic disease, both swallow and cough function are frequently impaired. In patients with acute stroke, those with dysphagia and aspiration also have profound dystussia 2, 3. Furthermore, the risk of aspiration due to dysphagia can be predicted by several mechanical features of voluntary cough in patients with stroke and Parkinson’s disease 3, 4. These impairments of swallow and cough contribute to a high risk of aspiration 5 which “seeds” the subglottic airways with pathogen-laden material 6 resulting in a high prevalence of aspiration pneumonia. Mortality rates of aspiration pneumonia can approach 40% 5. High rates of aspiration also occur in patients following anterior cervical spinal surgery (over 40%), in elderly patients in long term care facilities, those with gastrointestinal problems, and those with other neurological disorders such as Parkinson’s Disease 5.
A specific relationship between cough and dysphagia has been recognized and is termed “silent aspiration” 5. In these patients, aspiration and/or penetration of contrast material is noted during videofluoroscopy but the aspirated dye does not provoke coughing. Patients with silent aspiration (atussia) have a 13-fold increased risk of developing pneumonia 7. By definition, this group shows the consequences of impaired cough in combination with dysphagia.
Swallow can be coordinated with breathing such that most swallows occur during expiration 8, 9, although swallowing can be observed during a brief interruption of inspiration with aspiration prevented by laryngeal adduction. This expiratory phase preference for swallow and breathing is thought to reduce the probability of aspiration as material passes through the pharynx. Until recently, the extent to which this expiratory phase preference actually protects from aspiration has not been clear. Cvejic et al 10 investigated laryngeal penetration and aspiration in patients with chronic obstructive pulmonary disease (COPD). Penetration/aspiration scores were significantly worse in COPD patients than controls and during deglutition of larger volumes (100 ml) there were fewer swallows restricted to the expiratory phase in COPD patients 10. The COPD patients had a higher prevalence of swallows at the inspiratory/expiratory phase transition than normals. On follow-up, COPD patients with penetration/aspiration during videofluoroscopy had more serious adverse outcomes. It should be noted that it took larger volumes of barium to demonstrate penetration/aspiration in these COPD patients, although all patients that had predominant swallow occurrence at the inspiration/expiration phase transition were in the penetrator/aspirator group. These findings support a hypothesis that breathing phase preference for swallow is a mechanism that becomes an important contributor to airway protection only if larger volumes are swallowed. With low bolus volumes, breathing phase preference may have relatively little influence on the risk of aspiration in dysphagic individuals.
NEUROPHYSIOLOGY OF SWALLOWING
Swallowing is composed of three phases: 1) an oral or preparative phase, 2) a pharyngeal phase, and 3) an esophageal phase 11. The pharyngeal and esophageal phases are stereotypical and can occur as isolated events or become rhythmic. Full execution of swallowing can include multiple rhythmic pharyngeal phases that precede the esophageal phase 11. Although the oral and pharyngeal phases of swallow are subject to significant modification by suprapontine mechanisms, the minimal neural circuitry necessary for their production is contained within the brainstem 11. The extent to which the esophageal phase is controlled by suprapontine mechanisms in not clear12. The pharyngeal phase of swallow is most involved in airway protection.
The function of the pharyngeal phase swallow is to move a bolus from the oral cavity through the pharynx to the esophagus. Upper airway muscle activity must be controlled and organized to close the glottis and laryngeal vestibule, move the hyolaryngeal complex superior and anterior, invert the epiglottis, and ultimately protect the subglottic airways. Failure to close the glottal opening during swallow increases the risk of penetration or aspiration. Laryngeal aspiration increases the risk that material will enter the trachea and promote aspiration pneumonia.
The pharyngeal phase of swallow is produced by the coordinated action of a variety of muscles that can be segregated by function: tongue retractors (ex. styloglossus), laryngeal elevators (ex. geniohyoid), laryngeal depressors (ex. sternothyroid), laryngeal adduction (ex. thyroartytenoid), and upper esophageal sphincter opening and closing (cricopharyngeus) 11. Motor activation of these muscles is brief (usually less than 600 ms) and ballistic-like. These muscles can be activated rhythmically, leading to repetitive swallowing 11.
NEUROPHYSIOLOGY OF COUGH AND BREATHING
The function of cough is to remove fluids, mucus, and/or foreign bodies from the respiratory tract by the generation of high velocity airflows. These airflows are generated by a complex and sequential cough motor pattern involving three phases: inspiration, compression, and expulsion 13. The inspiratory phase of cough is generated by a large burst of activity in inspiratory muscles, including the diaphragm and inspiratory intercostals 13, 14. The compressive phase of cough is generated by laryngeal adduction that is produced by ballistic-like activity in expiratory laryngeal muscles during rapidly rising expiratory thoracic and abdominal muscle activity 13. The increased intrathoracic pressure during the compression phase elicits large airflows during the expulsive phase of cough, which is driven by intense motor activation of expiratory thoracic and abdominal muscles 13.
According to current hypotheses for the neurogenesis of cough and breathing, a single network of neurons mediates both motor tasks 15, 16. The anatomical connectivity of these neurons, in combination with their intrinsic membrane properties, regulates their discharge patterns and accounts for the temporal and spatial distribution of motor drive to respiratory muscle motoneurons. The same network can produce such different behaviors by mechanisms that include alteration of the excitability of key elements, presynaptic modulation, and/or recruitment of previously silent elements. Collectively, these processes represent network reconfiguration. The term “respiratory pattern generator” describes the configuration of this network when it generates breathing and “cough pattern generator” the configuration that is responsible for cough. The neural network that makes up the swallow pattern generator may overlap somewhat with that for breathing and coughing 17, but much of it, especially the network that makes up the dorsal swallow group is considered to be separate 11.
We have proposed 18, 19 that cough is coordinated by a distributed brainstem network that includes populations of neurons (or assemblies) that cooperate to exert control over the entire network. We have described these populations of neurons as Behavioral Control Assemblies (BCAs) and also have proposed that BCAs exist for different behaviors that interact to ensure the appropriate expression of airway protective behaviors. These BCAs interact with central pattern generators (CPG) for various behaviors. A CPG contains elements for controlling the duration of action of each muscle, and regulates the temporal activation patterns between multiple muscles that contribute to a single behavior. In this context a BCA is a regulatory element that is separate from the CPG, but controls it 18–20. This hypothesis is grounded in control system theory that invokes elements known as holons 21. Holons are elements of a larger hierarchical system that exert definable control over the entire organizational apparatus. They also can be controlled by higher order holons in the control system 21. According to this hypothesis, both the cough/breathing CPG and the cough BCA can usefully be described as separate holons which constitute part of a larger regulatory system for respiratory behaviors (Figure 1). It is important to note that the evidence for BCAs is restricted to experiments on coughing. The extent to which BCAs operate to control swallowing has not yet been clearly demonstrated by experimental results. However, placing the control of swallow in context of a holarchical system may stimulate directed investigation into the existence of BCAs in the regulation of this behavior. A simplified representation for this proposed control system is shown in Fig. 1, with the BCA regulation of cough and the swallow and cough/breathing pattern generators highlighted. The figure also illustrates that the mechanisms that underlie temporal coordination of these behaviors are not currently understood.
Figure 1.
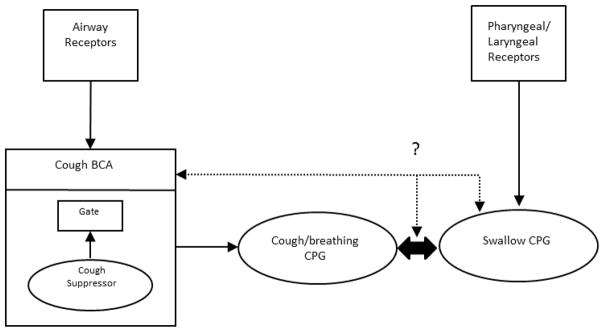
Schematic representation of proposed interaction between the cough/breathing and swallow cycle pattern generators (CPG). The cough BCA is shown with gating and cough suppressor subcomponents 26–28. The large dual arrow indicates reciprocal interactions between the cough/breathing central pattern generators. The dotted line indicates possible relationships between the cough control system and the swallow CPG that underlie temporal coordination.
CONTROL HYPOTHESIS FOR COORDINATION OF SWALLOW, BREATHING, AND COUGHING
The exact neural processes by which these behaviors are coordinated is/are not well understood. The coordinating mechanisms are a property of the brainstem circuits that generate these behaviors and their expression is manifest in what could loosely be termed as “rules” that govern how swallow, breathing, and coughing interact. The temporal relationship between swallow and coughing differs from the phase preference that is seen during breathing and is consistent with phase restriction 8. Swallowing only occurs during the period of motor quiescence between the end of the active expiratory motor burst and the onset of the next cough inspiration 8. The reason why there are differences in the relationship between swallow and cough and breathing may relate to the significant differences in mechanics between the behaviors. The large pressures and flows associated with coughing necessitate that swallowing must be prevented from occurring during the inspiratory phase of this behavior because complete laryngeal adduction would be difficult to achieve. In contrast, lower airflows during breathing are more easily interrupted by the laryngeal adduction required for swallow to occur without aspiration.
COMPUTATIONAL MODELING OF COMPLEX BRAINSTEM CIRCUITS FOR AIRWAY PROTECTION
Given the high complexity of the neural circuits governing these behaviors, we have employed computational modeling and simulation to aid in understanding their function. Computational modeling does not replace experimental investigation in animal models and humans, but it provides tools to make predictions based on current knowledge. Given that breathing and swallowing are capable of repetitive and rhythmic activation, we have hypothesized that their interaction can be explained through loose coupling between two different “oscillators”. Creating oscillatory circuits is a common modeling technique, and coupling oscillators have been used to explain the interaction of brainstem networks for breathing 22. A detailed synaptic model of the medullary network for breathing and swallow has been developed 22. This model is supported by in vitro and in vivo recordings of brainstem neurons 22. Figure 2B is a simulation from a novel network model from Figure 2A. The simulations produce trains of action potentials for each population that can be analyzed with the same tools used for the in vivo parameters which provided the experimental knowledge base for the model. In simulation, this coupled oscillator circuit was able to produce repetitive breathing with a swallow occurring during the appropriate period (expiration) of the breathing cycle. The results of this simulation can be used to predict interactions of the control systems for cough and swallow which are not currently known.
Figure 2.
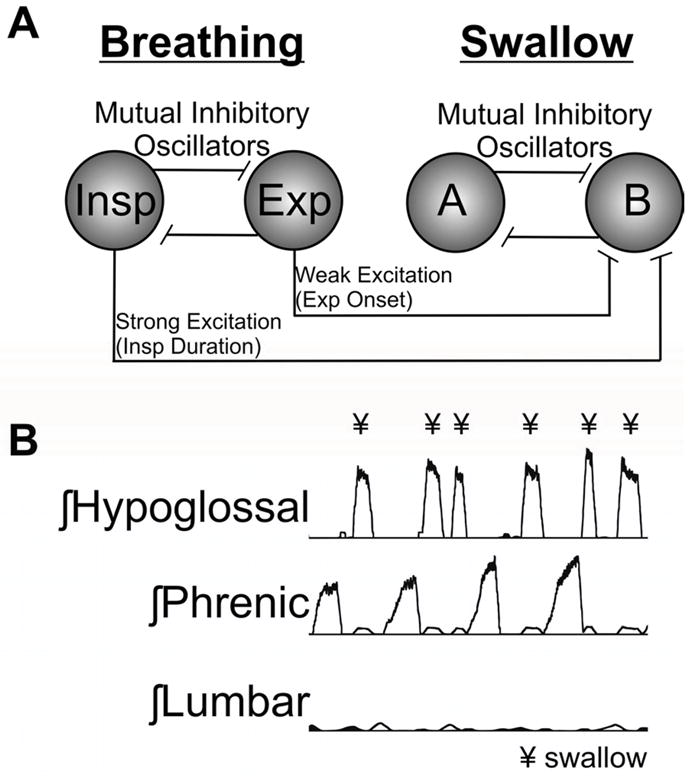
Proposed dual oscillator model for the coupling of breathing and swallow. A. Functional organizational model for the interaction between breathing and swallow. There are two neuronal populations for breathing inspiration (Insp) and expiration (Exp) and two for swallow. Population (A) controls activity for swallow production and (B) is the inter-swallow duration population. The oscillators are connected through mutual inhibition with a certain amount of accommodation which allows for oscillation. To loosely connect the two oscillators we entrained them through excitation from the breathing population to the swallow (B) population. The Insp population provided excitation throughout the inspiratory duration which suppressed swallow production during the Insp phase of breathing, and the Exp population provided excitation at the beginning of the expiratory period which should allow for swallow production at the end of the expiratory period. B. Results from a simulation from the current model producing breathing and swallow. The swallow stimulus elicited swallows (¥) at the appropriate phase of the breathing cycle.
To loosely couple the oscillators, excitatory connections between neuronal populations were chosen instead of inhibitory connections, although likely either option is appropriate. In order to inhibit the occurrence of swallow during the inspiratory phase of breathing, inspiratory populations excited the swallow B population in the model (Figure 2), which in turn suppresses the swallow A population. Note the swallow A population excites the motor neuron populations during swallow production. An additional connection was added from the expiratory populations, at the beginning of the expiratory cycle, to increase the likelihood that swallow would be produced at the end of the expiratory cycle.
The simulation shown in Figure 2B shows swallows (indicated by hypoglossal bursts) occurring during the expiratory phase of breathing. In some instances, two swallows were observed in a single simulated expiratory phase. Phrenic activity was ramp-like as has been shown in previous simulations of the breathing pattern 16, 23. There was no significant co-activation of expiratory motor activity and hypoglossal and phrenic discharge. In animal models, hypoglossal nerve or motoneuron discharge is mainly out of phase with phrenic nerve activity during fictive swallow, but in phase during fictive breathing 24, 25. Therefore, the simulation results meet several qualitative criteria used for identification of swallow in animal models.
Our preliminary simulation suggests that temporal coordination between the swallow and breathing CPGs (oscillators) can be approximated without separate intervening circuits that could be categorized as BCAs. However, production of the temporal relationship (or rule set) governing the co-expression of cough and swallow 8 may require more complex neural circuitry than we have proposed in Figure 2.
CONCLUSIONS
Airway protective behaviors, such as cough and swallow, are frequently impaired in neurologic disease and contribute to increased risk of aspiration. Emerging evidence supports the concept that impairments of multiple airway protective behaviors can result from an insult to a single unified control system in the brainstem. Our understanding of the brainstem networks that participate in cough, breathing, and swallowing has been facilitated by the use of computational modeling methods. Moving forward, simulation and prediction of the behavior of these networks and how they interact will shed significant light on the regulation of airway protection and how this regulatory system is susceptible to pathological processes.
KEY POINTS.
Airway protection is the prevention and/or correction of aspiration and a variety of behaviors, such as cough and swallow contribute to this process.
Dysphagia and dystussia (impaired cough) are frequently observed during neurologic diseases in the same patients, leading to increased probability of aspiration and a reduced ability to eject aspirated material from the airways.
Available evidence suggests that these behaviors are regulated by a common neural control system, which also controls breathing.
Investigation of this neural control system has been facilitated by computational modeling methods, which allow simulation and prediction of its behavior.
Future interrogation of this complex control system with computational modeling will promote a greater understanding of pathological processes that contribute to aspiration syndromes.
Acknowledgments
This work was supported by R01 HL103415 (DCB, PI), R01 HL109025 (PWD, PI), and K99 HL111215 (TEP, PI) from the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST:
DC Bolser, PhD and TE Pitts, PhD are partners in Sensory Integrated Solutions, LLC a startup company that will market devices for dysphagia. DC Bolser has consulted with Merck and Co. in the last calendar year. PW Davenport, PhD is a partner in Aspire Products, LLC which markets respiratory muscle training devices. KF Morris, PhD and C Gestreau, PhD have no conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Korpas J, Tomori Z. Cough and other respiratory reflexes. Basel ; New York: S. Karger; 1979. [Google Scholar]
- 2.Smith Hammond CA, Goldstein LB, Zajac DJ, et al. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56(4):502–506. doi: 10.1212/wnl.56.4.502. [DOI] [PubMed] [Google Scholar]
- 3.Smith Hammond CA, Goldstein LB, Horner RD, et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135(3):769–777. doi: 10.1378/chest.08-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitts T, Troche M, Mann G, et al. Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138(6):1426–1431. doi: 10.1378/chest.10-0342. [DOI] [PubMed] [Google Scholar]
- 5.Smith Hammond CA, Goldstein LB. Cough and aspiration of food and liquids due to oral-pharyngeal dysphagia: ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):154S–168S. doi: 10.1378/chest.129.1_suppl.154S. [DOI] [PubMed] [Google Scholar]
- 6.Scannapieco FA. Role of oral bacteria in respiratory infection. Journal of periodontology. 1999;70(7):793–802. doi: 10.1902/jop.1999.70.7.793. [DOI] [PubMed] [Google Scholar]
- 7.Daniels SK, Schroeder MF, McClain M, et al. Dysphagia in stroke: Development of a standard method to examine swallowing recovery. Journal of rehabilitation research and development. 2006;43(3):347–356. doi: 10.1682/jrrd.2005.01.0024. [DOI] [PubMed] [Google Scholar]
- 8.Pitts T, Rose MJ, Mortensen AN, et al. Coordination of cough and swallow: A meta-behavioral response to aspiration. Respiratory physiology & neurobiology. 2013 doi: 10.1016/j.resp.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin-Harris B, Brodsky MB, Price CC, et al. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: single liquid swallows. Journal of applied physiology. 2003;94(5):1735. doi: 10.1152/japplphysiol.00806.2002. [DOI] [PubMed] [Google Scholar]
- 10.Cvejic L, Harding R, Churchward T, et al. Laryngeal penetration and aspiration in individuals with stable COPD. Respirology. 2011;16(2):269–275. doi: 10.1111/j.1440-1843.2010.01875.x. [DOI] [PubMed] [Google Scholar]
- 11.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiological Review. 2001;81(2):929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 12.Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia. 2010;25(4):323–333. doi: 10.1007/s00455-010-9301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leith DE, Butler JP, Sneddon SL, et al. Cough Handbook of Physiology. The Respiratory System, V. III. Mechanics of Breathing, Part I. Bethesda: American Physiological Society; 1986. pp. 315–336. [Google Scholar]
- 14.van Lunteren E, Daniels R, Deal EC, Jr, et al. Role of costal and crural diaphragm and parasternal intercostals during coughing in cats. J Appl Physiol. 1989;66(1):135–141. doi: 10.1152/jappl.1989.66.1.135. [DOI] [PubMed] [Google Scholar]
- 15.Shannon R, Baekey DM, Morris KF, et al. Functional connectivity among ventrolateral medullary respiratory neurones and responses during fictive cough in the cat. J Physiol. 2000;525(Pt 1):207–224. doi: 10.1111/j.1469-7793.2000.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shannon R, Baekey DM, Morris KF, et al. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol. 1998;84(6):2020–2035. doi: 10.1152/jappl.1998.84.6.2020. [DOI] [PubMed] [Google Scholar]
- 17.Gestreau C, Milano S, Bianchi AL, et al. Activity of dorsal respiratory group inspiratory neurons during laryngeal-induced fictive coughing and swallowing in decerebrate cats. Experimental brain research. Experimentelle Hirnforschung Experimentation cerebrale. 1996;108(2):247–256. doi: 10.1007/BF00228098. [DOI] [PubMed] [Google Scholar]
- 18.Bolser DC, Poliacek I, Jakus J, et al. Neurogenesis of cough, other airway defensive behaviors and breathing: A holarchical system? Respiratory Physiology and Neurobiology. 2006;152(3):255–265. doi: 10.1016/j.resp.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolser DC, Pitts TE, Morris KF. The use of multiscale systems biology approaches to facilitate understanding of complex control systems for airway protection. Current opinion in pharmacology. 2011 doi: 10.1016/j.coph.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolser DC, Davenport PW. Codeine and cough: an ineffective gold standard. Current opinion in allergy and clinical immunology. 2007;7(1):32–36. doi: 10.1097/ACI.0b013e3280115145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koestler A. The Ghost in the Machine. New York: The Macmillan Company; 1967. [Google Scholar]
- 22.Lindsey BG, Rybak IA, Smith JC. Computational models and emergent properties of respiratory neural networks. Comprehensive Physiology. 2012;2(3):1619–1670. doi: 10.1002/cphy.c110016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rybak IA, O’Connor R, Ross A, et al. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J Neurophysiol. 2008;100(4):1770–1799. doi: 10.1152/jn.90416.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gestreau C, Dutschmann M, Obled S, et al. Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respiratory physiology & neurobiology. 2005;147(2–3):159–176. doi: 10.1016/j.resp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Roda F, Gestreau C, Bianchi AL. Discharge patterns of hypoglossal motoneurons during fictive breathing, coughing, and swallowing. Journal of neurophysiology. 2002;87(4):1703–1711. doi: 10.1152/jn.00347.2001. [DOI] [PubMed] [Google Scholar]
- 26.Bolser DC, Hey JA, Chapman RW. Influence of central antitussive drugs on the cough motor pattern. Journal of applied physiology. 1999;86(3):1017–1024. doi: 10.1152/jappl.1999.86.3.1017. [DOI] [PubMed] [Google Scholar]
- 27.Poliacek I, Corrie LW, Wang C, et al. Microinjection of DLH into the region of the caudal ventral respiratory column in the cat: evidence for an endogenous cough-suppressant mechanism. Journal of applied physiology. 2007;102(3):1014–1021. doi: 10.1152/japplphysiol.00616.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poliacek I, Wang C, Corrie LW, et al. Microinjection of Codeine into the Region of the Caudal Ventral Respiratory Column Suppresses Cough in Anesthetized Cats. Journal of applied physiology. 2010 doi: 10.1152/japplphysiol.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


