Abstract
Peripherally acting opioids are potentially attractive drugs for the clinical management of certain chronic pain states due to the lack of centrally mediated adverse effects. However, it remains unclear whether tolerance develops to peripheral opioid analgesic effects under neuropathic pain conditions. We subjected rats to L5 spinal nerve ligation (SNL) and examined the analgesic effects of repetitive systemic and local administration of loperamide hydrochloride, a peripherally acting opioid agonist. We found that the inhibition of mechanical hypersensitivity, an important manifestation of neuropathic pain, by systemic loperamide (1.5 mg/kg subcutaneously) decreased after repetitive drug treatment (tolerance-inducing dose: 0.75 to 6.0 mg/kg subcutaneously). Similarly, repeated intraplantar injection of loperamide (150 µg/50 µL intraplantarly) and D-Ala2-MePhe4-Glyol5 enkephalin (300 µg/50 µL), a highly selective mu-opioid receptor (MOR) agonist, also resulted in decreased inhibition of mechanical hypersensitivity. Pretreatment with naltrexone hydrochloride (5 mg/kg intraperitoneally) and MK-801 (0.2 mg/kg intraperitoneally) attenuated systemic loperamide tolerance. Western blot analysis showed that repetitive systemic administration of morphine (3 mg/kg subcutaneously), but not loperamide (3 mg/kg subcutaneously) or saline, significantly increased MOR phosphorylation in the spinal cord of SNL rats. In cultured rat dorsal root ganglion neurons, loperamide dose-dependently inhibited KCl-induced increases in [Ca2+]i. However, this drug effect significantly decreased in cells pretreated with loperamide (3 µM, 72 hours). Intriguingly, in loperamide-tolerant cells, the delta-opioid receptor antagonist naltrindole restored loperamide’s inhibition of KCl-elicited [Ca2+]i increase. Our findings indicate that animals with neuropathic pain may develop acute tolerance to the antiallodynic effects of peripherally acting opioids after repetitive systemic and local drug administration.
Keywords: Nerve injury, Neuropathic pain, Peripheral opioid receptor, Rats, Tolerance
1. Introduction
Neuropathic pain substantially reduces health-related quality of life and is often challenging to treat. Peripheral opioid receptors are being increasingly studied for their analgesic potential in the treatment of chronic pain conditions [40,44]. Our recent studies have suggested that systemic and local administration of peripherally acting opioid receptor agonists, such as loperamide hydrochloride (HCl), attenuates both mechanical and heat hypersensitivity in nerve-injured rats [6,14]. Therefore, targeting the peripheral opioid system may represent a promising new therapeutic approach for alleviating neuropathic pain [36,44].
The development of analgesic tolerance (i.e., a progressive decrease in the analgesic efficacy after repeated or prolonged drug administration) to centrally penetrating mu-opioid receptor (MOR) agonists, such as morphine, presents a substantial barrier to the treatment of chronic pain [16]. Opioid tolerance is generally considered to be primarily due to central nervous system (CNS) effects, and studies in animal models of inflammatory pain suggest that peripheral opioid analgesia is not associated with tolerance [38,43,51]. However, other reports suggest that the peripheral nervous system (PNS) is an important site of systemic morphine tolerance and that tolerance also develops to the antinociceptive effect of topically applied morphine [2,15,16,22,28]. Although some reports suggest that nerve injury facilitates the development of morphine tolerance [5,11,47], others indicate that acute morphine tolerance does not develop in rats with a mononeuropathy or in mice with herpes-associated neuropathic pain behavior [25,30]. Therefore, the development of tolerance to peripheral opioid actions, especially under neuropathic pain conditions, remains unclear.
Understanding peripheral opioid actions, including tolerance development, is important in understanding its overall contribution to opioid analgesia [37,44]. Loperamide is a MOR-preferring agonist that does not cross the blood–brain barrier after systemic administration [4,8]. Recently, we showed that systemic and local administration of loperamide can attenuate neuropathic mechanical and heat hypersensitivity [6,14,48]. Although peripherally acting opioids have potential clinical benefits owing to the minimal risk of central dose-limiting adverse effects (sedation, cognitive dysfunction) and lack of addiction and abuse potential, it remains to be determined whether repeated systemic and local administration of peripherally restricted opioids induces tolerance to their pain-inhibitory effects under neuropathic pain conditions. To establish the clinical usefulness of peripherally acting opioids for chronic pain treatment, we used loperamide as a pharmacological tool to examine whether repeated systemic and local (hind paw) drug treatments are associated with acute tolerance development in rats after an L5 spinal nerve ligation (SNL). Within the same experimental setting, we next investigated potential receptor mechanisms for loperamide analgesia tolerance in vivo. Finally, we examined MOR phosphorylation in the spinal cord and cellular adaptations in dorsal root ganglion (DRG) neurons that may contribute to the tolerance.
2. Methods
2.1. L5 spinal nerve ligation
We ligated spinal nerve L5 of adult male Sprague-Dawley rats (200 to 350 g, Harlan Bioproducts for Science, Indianapolis, IN) using a modification of the procedure described previously [6,14]. The animals were anesthetized with isoflurane (2%, Abbott Laboratories, North Chicago, IL) delivered through a nose cone. Under aseptic conditions, the skin was incised at the midline over the lumbar spine, and the L5, L6, and upper sacral vertebrae were exposed. The left transverse process of the L6 vertebra was removed, and the left L5 spinal nerve was exposed and dissected from the underlying tissue with fine forceps. The left L5 spinal nerve was then tightly ligated with a 6-0 silk suture and cut distally, with care being taken not to pull the nerve or touch the L4 spinal nerve. After hemostasis was achieved, the muscle layer was approximated with 4-0 chromic gut suture and the skin closed with metal clips. After the surgery, the rats were returned to their cages, kept warm under a heat lamp, and monitored during recovery. Skin staples were removed approximately 1 week after surgery. All procedures were approved by the Johns Hopkins University Animal Care and Use Committee as consistent with the National Institutes of Health Guide for the Use of Experimental Animals to ensure minimal animal use and discomfort.
2.2. Animal behavioral tests
The region between the foot pads in the plantar aspect of the hind paw was tested for mechanical allodynia. Animals were placed under plastic domes on a mesh floor that allowed full access to the plantar surface of the paws. Using the up-down method to quantify response to mechanical stimuli, we determined mechanical paw withdrawal thresholds (PWTs) using a series of von Frey filaments that deliver approximately logarithmic incremental forces (0.38, 0.57, 1.23, 1.83, 3.66, 5.93, 9.13, 13.1 g). The von Frey filaments were applied to the test area for 4 to 6 seconds. The 1.83-g stimulus was applied first. If a positive response occurred, the next smaller von Frey hair was used; if a negative response was observed, the next higher force was used. The test was continued until: (1) the responses to 5 stimuli were assessed after the first crossing of the withdrawal threshold, or (2) the upper or lower end of the von Frey hair set was reached before a positive or negative response had been obtained. Abrupt paw withdrawal, licking, and shaking were regarded as positive responses.
To minimize experimenter bias, the investigator who performed the behavioral tests was blinded to the drug treatment conditions. Before the behavioral testing, animals were acclimatized to the facilities for 1 week. To minimize variability of the behavioral outcome measures, we trained animals for 3 to 5 days before obtaining baseline data. All experimental conditions (animal age, room temperature, time of day for behavioral testing, drug preparation, drug injection, animal handling, etc.) were carefully controlled to maintain consistency across groups. In addition, animals were habituated to the test environment for ≥30 minutes before testing was begun on a given day.
2.3. The tolerance-inducing paradigm
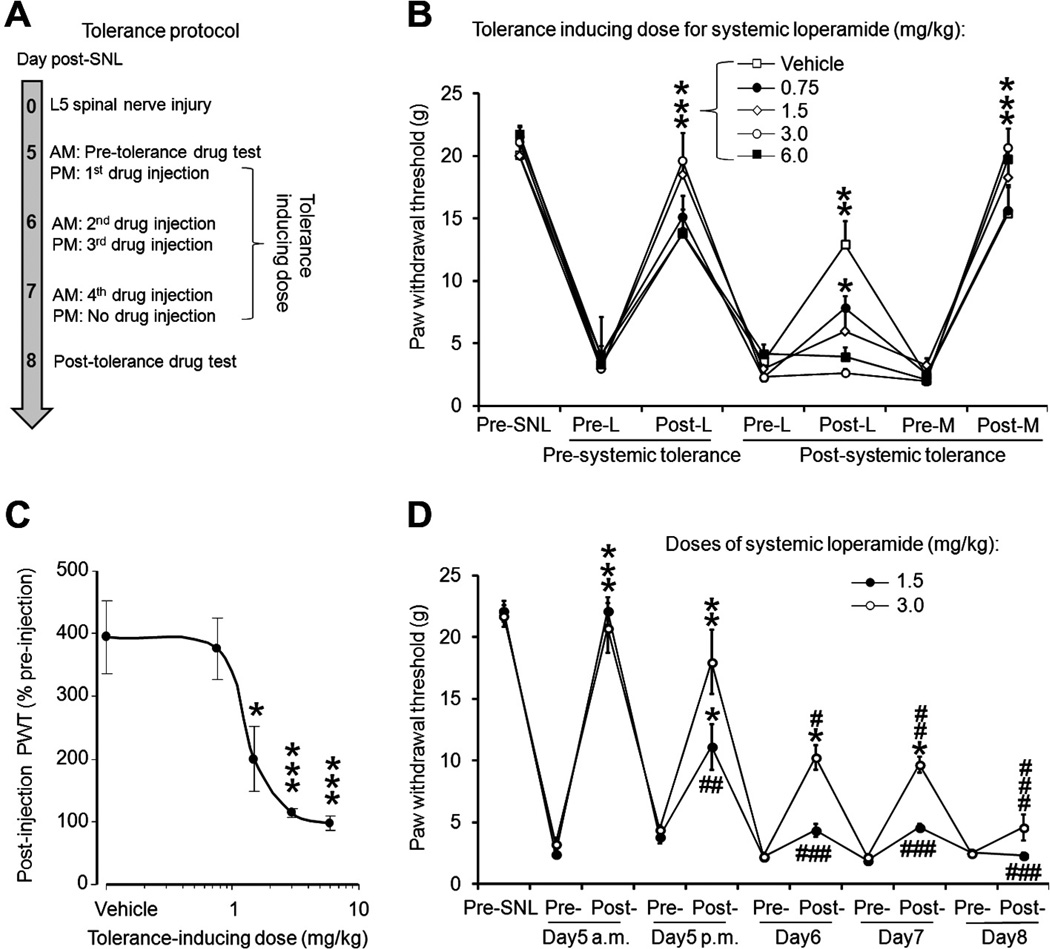
Based on previous studies and our pilot experiments (n = 6), we developed a paradigm in which repeated drug injections are used to induce acute tolerance in rats during days 5 to 8 post-SNL (n = 8 to 9 per group) (Fig. 1A and B), a time when the neuropathic mechanical and heat hypersensitivity reaches a stable maximum. The doses of systemic loperamide were twice the ED50 dose (dose estimated to produce 50% maximum possible effect [MPE]) for the pretolerance and posttolerance tests and 1 to 4 times the ED50 dose for inducing tolerance [14]. We carried out the pretolerance test in the morning on day 5 post-SNL. We first tested the rats to obtain baseline PWTs. Then we injected loperamide either systemically (1.5 mg/kg subcutaneously in the back) or locally (150 µg/50 µL intraplantarly), and then repeated the behavior tests after 30 to 45 minutes. In the afternoon of the same day we administered the first tolerance-inducing dose of loperamide (0.75, 1.5, 3.0, or 6.0 mg/kg subcutaneously in the back or 300 µg/30 µL intraplantarly) or vehicle. Tolerance-inducing doses were repeated on day 6 (morning and afternoon) and on day 7 (morning only) post-SNL. On day 8 post-SNL, we carried out the posttolerance test by first measuring the PWTs, administering loperamide at the same dose used in the pretolerance test, and then repeating the behavioral tests.
Fig. 1.
Repetitive drug administration induced acute tolerance to the antiallodynic effect of systemic loperamide in nerve-injured rats. (A) The diagram shows the tolerance-inducing protocol. (B) In rats on day 5 after an L5 spinal nerve injury (SNL), systemic administration of loperamide (L, 1.5 mg/kg subcutaneously in the back) reversed the decrease in paw withdrawal threshold (PWT) of the hind paw ipsilateral to the injured (left) side. The inhibition of mechanical hypersensitivity by systemic loperamide (1.5 mg/kg subcutaneously) decreased after repetitive systemic drug treatment at various tolerance-inducing doses (0.75 to 6.0 mg/kg subcutaneously, n = 8 to 9/group). The inhibition of mechanical hypersensitivity from a centrally penetrating opioid, morphine sulfate (M, 3.0 mg/kg subcutaneously), was not decreased in loperamide-tolerant rats. (C) After tolerance induction, the PWT measured after a 1.5-mg/kg loperamide injection was normalized to the preinjection baseline and plotted according to tolerance-inducing dose. (D) The increase in PWT induced by 1.5 mg/kg (n = 8) and 3.0 mg/kg (n = 8) systemic loperamide gradually decreased after repetitive injections. PWT was measured at 30 minutes postinjection (peak drug effect). Data are expressed as mean ± SEM. *P < .05, **P < .01, ***P < .001 versus preinjection PWT in (B) and (D), and versus vehicle in (C). # P < .05 versus postinjection PWT under pretolerance conditions.
2.4. Western blotting
Tissues from L4 and L5 spinal segments of SNL and sham-operated rats were separated and homogenized for immunoblotting. The tissues were lysed in ice-cold radioimmunoprecipitation assay buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 10% glycerol, 0.1% Triton X-100, 0.5 mg/mL bovine serum albumin (BSA)). After centrifugation, the protein concentration was determined by using a detergent-compatible protein assay with a BSA standard. Samples were separated on a 7.5% (w/v) sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and transferred onto a nitrocellulose membrane (Amersham, Pittsburgh, PA) with a Trans-Blot Transfer Cell system (Bio-Rad, Hercules, CA). Membranes were incubated with the indicated primary antibody overnight at 4°C, and immunoreactivity was detected by enhanced chemiluminescence (ECL, Amersham). Antibodies against MOR (1:2000, Neuromics, Edina, MN), phospho-MOR (p-MOR-Ser375; 1:1000, Neuromics), and actin (1:20,000, Chemicon, Temecula, CA) were used. Autoradiograms were analyzed, and the intensity of immunoreactive bands of interest was quantified (National Institutes of Health Image J 1.46r). Actin staining was used as an internal control for protein loading.
2.5. Calcium imaging
Calcium imaging with Fluo4AM was carried out in 96-well plates of DRG primary cultures. Lumbar DRGs from adult male rats were dissected and collected in ice cold Tyrode buffer (132 mM NaCl, 4.8 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5 mM dextrose, 5 mM hydroxyethyl piperazineethanesulfonic acid (HEPES)). The DRG was then digested with a solution of 1 mg/mL Dispase (Gibco 171050-041, Grand Island, NY) and 2 mg/mL Collagenase (Roche 1088831, Branford, CT) in Tyrode buffer, incubated in a 37°C shaking water bath for 45 minutes, and subsequently triturated with a fire-polished Pasteur pipet for several seconds. The neurons were then separated using a gradient Shake OptiPrep (Sigma D1556, St. Louis, MO). After centrifugation at 900g for 20 minutes, the 2 bottom layers were separated and the cells were counted. The cells were plated in a 96-well plate (Costar 3603, Corning Glassworks, Corning, NY) previously coated with poly-d-lysine (Sigma P4707) in a ratio of approximately 60,000 cells/well in Minimum Essential Medium (MEM) media (Sigma M0643) with 3 mL of 20% glucose, 10% fetal bovine serum (Invitrogen 16000-036, Grand Island, NY), 1% penicillin-streptomycin solution (Invitogen 15070-063), and 10 ng/mL of NGF (Millipore 01-125, Billerica, MA).
After 96 hours in culture, DRG neurons were washed with NaCl-based extracellular buffer (ECB) (130 mM NaCl, 2.5 mM KCl, 1 mM MgCl2, 4 mM CaCl2, 10 mM Hepes, 5 mM glucose, 2.5 mM probenecid (Molecular Probes P36400, Grand Island, NY). Cells were incubated with 1 µM Fluo4-AM (Invitrogen F14201) in ECB at 37°C for 1 hour. Cells were washed with NaCl-based extracellular buffer and incubated again for de-esterification of internal Fluo4 at 37°C for 1 hour. The plates were read on a BioTek Synergy 2 plate reader in 200 µL/well total volume. KCl (5 to 60 µM in 10 µL) was applied to the cells, and the plates were read every 3 to 6 seconds for 5 minutes on a BioTek Synergy 2 plate reader. Area under the curve (AUC) was calculated from the Ca2+ transients. AUC was normalized to the AUC of KCl-stimulated vehicle-treated cells and analyzed. A minimum of 6 wells of cells were used for each condition. All Ca2+ transients compared were analyzed on the same day and from the same plate.
A loperamide dose-response curve was generated after 96 hours of culture. Cells were loaded with Fluo4-AM as described earlier. Cells were incubated with 0.1, 1, or 10 µM loparamide or vehicle for 5 minutes at 37°C. Using the plate reader, baseline measurements were made from the wells every 4 seconds for 30 seconds. Then KCl (30 µM) or ECB was added, and the plate was read continuously every 4 seconds for 5 minutes. To induce loperamide tolerance, DRG neurons were exposed to loperamide (3 µM) for 72 hours during culture to model in vitro tolerance. DRG neuron cultures were incubated in MEM media with 3 µM loperamide for 72 hours (the media was changed every 24 hours); then fresh MEM without any drug was added and the cells were incubated for an additional 24 hours before the experiment was performed as detailed earlier.
Next we determined whether acute treatment with naltrindole, a delta-opioid receptor (DOR) antagonist, could reverse loperamide tolerance. Loperamide-tolerant DRG cells were loaded with Fluo4-AM. Individual wells are pretreated for 5 minutes with naltrindole (1 µM) or vehicle and then incubated with loperamide (3 µM, 5 minutes). KCl (30 mM) was then added, and Ca2+ transients were measured.
2.6. Drugs
Loperamide HCl, D-Ala2-MePhe4-Glyol5 enkephalin (DAMGO), naltrindole HCl, naloxone HCl (a nonselective opioid receptor antagonist that can have peripheral and central effects), naloxone methiodide (a peripherally acting opioid receptor antagonist that does not penetrate the CNS), naltrexone HCl (a long-lasting nonselective opioid receptor antagonist that can have both peripheral and central effects), MDL 105,519 (a peripherally acting N-methyl-d-aspartate [NMDA] receptor/glycine-B site antagonist that very weakly penetrates the CNS), MK-801[(+)-5-methyl-10,11-dihydroxy-5H-dibenzo(a,d)cyclohepten-5,10-imine, a non-competitive CNS-penetrating NMDA receptor antagonist with slow unbinding kinetics], and CDEX (2-hydroxypropyl-beta-cyclodextrin) were purchased from Sigma-Aldrich (St. Louis, MO). Morphine sulfate was purchased from Baxter Healthcare Corporation (Deerfield, IL). Stock solutions were freshly prepared as instructed by the manufacturer. Loperamide HCl was dissolved in 20% CDEX, made by diluting the 40% CDEX/water solution (isotonic) with saline. CDEX is a drug carrier system that can increase the water solubility of lipid-soluble drugs and reduce the rate of clearance. MDL 105,519 was dissolved in dimethyl sulfoxide initially and then further diluted to the final concentration with saline. All other drugs were dissolved initially in distilled water and then further diluted with saline (0.9%) to the final concentration.
2.7. Statistical analysis
For behavioral tests, PWT was determined by converting the pattern of positive and negative responses to the von Frey filament stimulation to a 50% threshold value with the formula provided by Dixon [9]. One-way repeated-measures ANOVA was used to compare data between different time points in each group. Two-way ANOVA was used to compare data between different groups. The Tukey honestly significant difference post-hoc test was used to compare specific behavioral data points in ANOVA. For Western blotting, relative expression levels of MOR and p-MOR-Ser375 were determined in SNL rats by first normalizing the intensity of each specific band to the intensity of the respective actin band (loading control). The ratio was then normalized to that of sham-operated rats from the same experiment. The relative levels of MOR and p-MOR-Ser375 were illustrated as fold of sham control for the purpose of comparison. For Western blotting and calcium imaging, one-way ANOVA was used to compare data between groups, and the Fisher protected least significant difference post-hoc test was used to compare specific data points. STATISTICA 6.0 software (StatSoft, Inc., Tulsa, OK) was used for analysis. Data are expressed as mean ± SEM; P < .05 was considered statistically significant.
3. Results
3.1. Nerve-injured rats developed tolerance to systemic loperamide’s inhibitory effect on mechanical hypersensitivity
On day 5 post-SNL, during the pretolerance test, systemic loperamide (1.5 mg/kg subcutaneously) significantly increased the ipsilateral PWT from the preinjection baseline after 30 minutes (i.e., time of peak drug effect). The effect largely subsided 90 to 120 minutes after administration. On day 8 post-SNL, this effect was significantly diminished in rats that had received repeated systemic injections of loperamide at various tolerance-inducing doses over the previous 3 days (n = 8 to 9/group, Fig. 1B). At this time point, loperamide (1.5 mg/kg subcutaneously) increased the PWT from preinjection level only in animals treated with vehicle or very-low-dose loperamide (0.75 mg/kg) during the tolerance-inducing period.
To examine whether repeated loperamide treatment affected central opioid analgesia, we examined the effects of morphine sulfate (3.0 mg/kg subcutaneously in the back) on PWT at 6 hours after loperamide testing on day 8 post-SNL. This dose of morphine is approximately twice the ED50 for inhibiting mechanical hypersensitivity in SNL rats [19,49]. Morphine induced a significant increase in PWT that was comparable between loperamide-treated and vehicle-treated groups. The PWT of the contralateral hind paw was not significantly changed after SNL or drug treatment.
Repetitive systemic drug treatment dose dependently leads to the development of loperamide tolerance. To compare the level of tolerance between groups treated with different tolerance-inducing doses of loperamide, we normalized the postinjection PWT on day 8 post-SNL to the preinjection baseline for each animal and plotted the values for the various tolerance-inducing doses (Fig. 1C). The inhibitory effect of loperamide (1.5 mg/kg subcutaneously) was significantly lower in animals that received tolerance-inducing doses of 1.5 mg, 3.0 mg, and 10 mg/kg loperamide than in those that received vehicle (Fig. 1C).
To further examine the rate of tolerance development, we conducted separate studies to monitor changes in the antiallodynic effect of systemic loperamide after repetitive administration at the same dose. The tolerance-inducing paradigm was the same as that described above, with the drug effect tested on day 5 (morning and afternoon) and days 6 to 8 (morning only) post-SNL (Fig. 1D). Two doses of loperamide (1.5 mg/kg, 3.0 mg/kg, n = 8/group) were tested in different groups of animals. The first injection of 1.5 mg/kg loperamide significantly inhibited mechanical allodynia. Although the second drug injection also significantly increased PWT, its effect was significantly less than that of the first injection. Beginning with the second (day 5 afternoon) and the third injection (day 6 morning) in 1.5-mg/kg and 3.0-mg/kg groups, respectively, the degree of PWT increase after drug injection was significantly less than that after the first injection, indicating a quick onset of acute tolerance.
3.2. Nerve-injured rats also developed tolerance to the inhibitory effect of locally administered loperamide on mechanical hypersensitivity
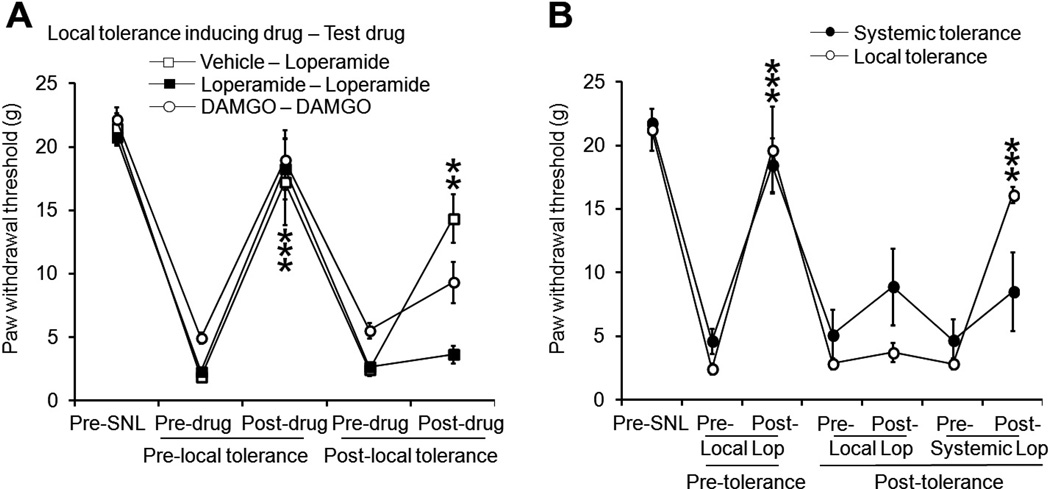
We further examined whether tolerance develops to the antiallodynic effect of locally administered loperamide in SNL rats. Using the same tolerance-inducing paradigm, we injected drugs into the plantar area of the ipsilateral hind paw. Before tolerance induction, loperamide (150 µg/50 µL, n = 7) and DAMGO (300 µg/50 µL, n = 7) significantly increased the ipsilateral PWT from the predrug baseline 30 minutes after intraplantar injection. However, these effects were mostly diminished on day 8 post-SNL after repetitive local injections of a tolerance-inducing dose of loperamide (300 µg/30 µL) or DAMGO (300 µg/30 µL) (Fig. 2A). The inhibitory effect of loperamide (150 µg/50 µL intraplantarly, n = 6) was largely maintained in rats treated with vehicle. The doses of DAMGO and loperamide, respectively, were selected based on pilot experiment and previous studies [14,27]. In the pilot study, DAMGO did not induce significant inhibition of mechanical hypersensitivity in SNL rats at a dose <50 µg/50 µL (n = 6). Animals that developed tolerance to locally administered loperamide still exhibited significant increases in ipsilateral PWT in response to systemic injection of loperamide (1.5 mg/kg subcutaneously in the back, n = 8) (Fig. 2B). However, in animals that had received repeated systemic injections of loperamide (3 mg/kg subcutaneously, n = 6, i.e., systemic tolerance), neither systemic (1.5 mg/kg subcutaneously) nor intraplantar injection of loperamide (150 µg/50 µL) could significantly increase the PWT (Fig. 2B).
Fig. 2.
Repeated local administration of loperamide into hind paw tissue also resulted in tolerance to the drug’s inhibitory effects on neuropathic mechanical hypersensitivity. (A) The antiallodynic effects of intraplantar injections of loperamide (150 µg/50 µL, n = 6) and DAMGO (300 µg/50 µL, n = 7) diminished after repetitive local drug administration. The antiallodynic effects of intraplantar loperamide (150 µg/50 µL n = 6) largely remained intact in rats treated with saline. (B) The antiallodynic effect of intraplantar loperamide (150 µg/50 µL) decreased in rats that had received repetitive systemic (3 mg/kg subcutaneously, n = 6) or repetitive local (300 µg/30 µL, intraplantarly, n = 8) injections of loperamide, compared to the effect observed before tolerance induction. However, the antiallodynic effect of systemic loperamide (1.5 mg/kg subcutaneously) was still present in rats that had become tolerant to locally administered loperamide. The paw withdrawal threshold was measured 30 minutes after injection. Data are expressed as mean ± SEM. **P < .01, ***P < .001 versus predrug paw withdrawal threshold.
3.3. Pretreatment with naltrexone HCl and MK-801 reduced systemic loperamide tolerance
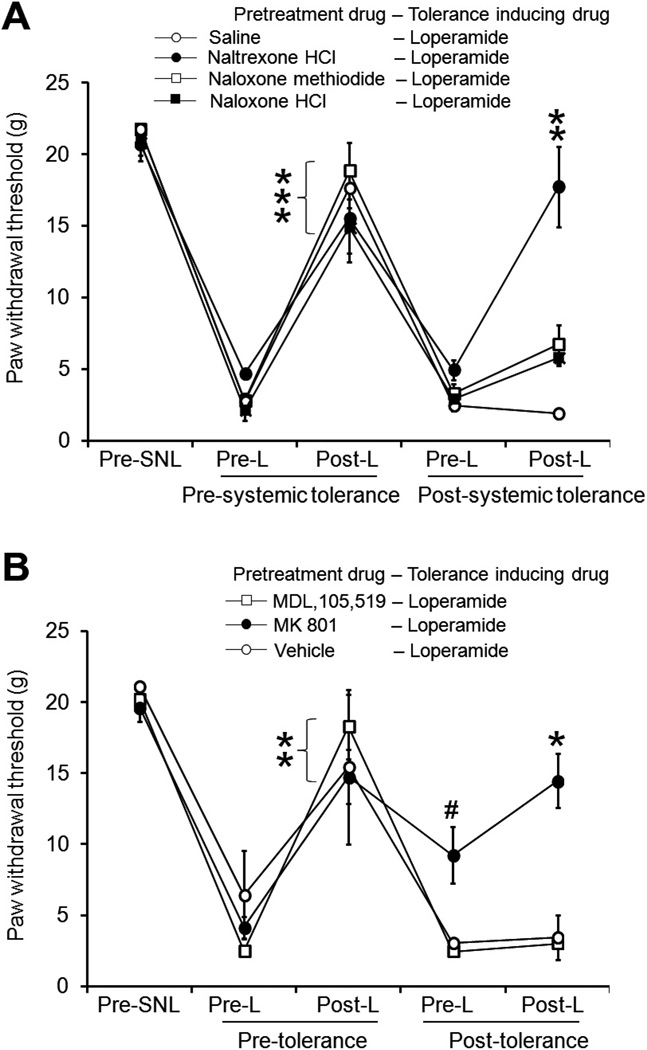
To study the receptor mechanisms that may be involved in systemic loperamide tolerance, we examined whether pretreatment with nonselective opioid receptor antagonists can limit tolerance development. In our pilot studies, pretreatment with naloxone HCl (10 mg/kg intraperitoneally), naloxone methiodide (5 mg/kg intraperitoneally, a peripherally acting opioid receptor antagonist that does not cross the blood-brain barrier), and naltrexone HCl (5 mg/kg intraperitoneally, a long-lasting opioid receptor antagonist) all blocked the antiallodynic effect of systemic (subcutaneously) 6.0 mg/kg loperamide. After undergoing pretolerance tests with 1.5 mg/kg (subcutaneously) loperamide, animals were pretreated with a systemic injection of 1 of the 3 antagonists or saline 10 minutes before receiving each tolerance-inducing dose of loperamide (6.0 mg/kg subcutaneously) during the 5- to 7-day period after SNL. Under pretolerance conditions, systemic loperamide (1.5 mg/kg subcutaneously) significantly increased the ipsilateral PWT from the preinjection level in all groups. However, the same drug dose was effective after tolerance induction only in the naltrexone-pretreated group. Surprisingly, pretreatment with naloxone HCl (10 mg/kg, intraperitoneally) or naloxone methiodide (5 mg/kg, intraperitoneally) did not block the development of tolerance (Fig. 3A).
Fig. 3.
Effects of pretreatment with opioid receptor antagonists and N-methyl-d-aspartate receptor antagonists on systemic loperamide tolerance. (A) Systemic loperamide (1.5 mg/kg subcutaneously) increased the ipsilateral paw withdrawal threshold (PWT) in spinal nerve ligation (SNL) rats under pretolerance conditions. Pretreatment with the long-lasting opioid receptor antagonist naltrexone HCl (5 mg/kg intraperitoneally), but not saline, reduced loperamide tolerance in SNL rats. However, pretreatment with naloxone methiodide (5 mg/kg intraperitoneally), an opioid receptor antagonist that does not cross the blood-brain barrier, or naloxone HCl (10 mg/kg intraperitoneally), a relatively short-acting antagonist, did not block the development of tolerance. Antagonist or saline was administered 10 minutes before each tolerance-inducing injection of loperamide (6.0 mg/kg subcutaneously). (B) Rats were administered MDL 105,519 (1 mg/kg intraperitoneally, n = 6), MK-801 (0.2 mg/kg, intraperitoneally n = 6), or saline (n = 3) 10 minutes before each tolerance-inducing injection of loperamide (6.0 mg/kg subcutaneously). After the tolerance-inducing protocol, systemic loperamide did not increase PWT in MDL 105,519- or vehicle-treated groups, but did increase PWT in the MK-801–treated group from preinjection baseline. Data are expressed as mean ± SEM. **P < .01, ***P < .001 versus preinjection PWT. #P < .05 versus preinjection PWT under pretolerance conditions.
Activation of NMDA receptors has been suggested to play an important role in morphine tolerance [42]. NMDA receptors also are present in the PNS, and a peripherally acting NMDA receptor antagonist working at a glycine-B site was shown to attenuate the development of morphine antinociceptive tolerance [7]. Therefore, we examined whether NMDA receptors also contribute to development of tolerance to the antiallodynic effect of systemic loperamide in the neuropathic pain condition by using MDL 105,519 (a peripherally acting NMDA receptor/glycine-B site antagonist) and MK-801 (a noncompetitive NMDA receptor antagonist with slow unbinding kinetics that can penetrate the CNS). In our pilot study, systemic administration of MDL 105,519 (1 mg/kg intraperitoneally) did not change PWTs in SNL rats. After the pretolerance loperamide test (1.5 mg/kg subcutaneously), animals were pretreated with a systemic injection of MDL 105,519 (1 mg/kg intraperitoneally), MK-801 (0.2 mg/kg intraperitoneally), or saline 10 minutes before each tolerance-inducing dose of loperamide (3 mg/kg subcutaneously). Before tolerance induction, systemic loperamide significantly increased the ipsilateral PWT in all groups. After tolerance induction, the drug effect was diminished in animals pretreated with MDL 105,519 or vehicle (Fig. 3B). In animals pretreated with MK-801, the preinjection PWT during the posttolerance test was significantly higher than what it had been before tolerance induction, and systemic loperamide further increased PWT from preinjection baseline (Fig. 3B). The doses of MDL 105,519 and MK-801 were chosen based on previous studies in which they both attenuated morphine tolerance [7,42]. Repetitive systemic injections of MDL 105,519 did not affect PWT in SNL rats (Fig. 3B), nor did it induce any noticeable side effect. However, MK-801 (0.2 mg/kg) induced notable impairments of cognitive function and motor performance in SNL rats at 1 to 2 hours after injection. Because the posttolerance drug test was conducted at 24 hours (day 8) after the last MK-801 treatment (day 7 morning), side effects of MK-801 may have dissipated and not significantly affected behavioral readouts at this time point. Yet it remains possible that other unknown, long-lasting side effects of MK-801 may still have been present and affected the actions of the drug and confounded data interpretation.
3.4. Intraplantar injection of naloxone methiodide and MK-801 did not block local loperamide tolerance
In additional experiments, we examined the roles of opioid receptors and NMDA receptors in the development of tolerance to locally administered loperamide in SNL rats. Under pretolerance conditions, intraplantar injection of loperamide (150 µg/50 µL) significantly increased ipsilateral PWT in all groups. Animals were then pretreated with intraplantar injections of naloxone methiodide (100 µg/20 µL, n = 6), MK-801 (10 µg/20 µL, n = 6), or saline (n = 4) 10 minutes before each tolerance-inducing dose of loperamide (300 µg/30 µL intraplantarly). In our pilot study, naloxone methiodide (100 µg/20 µL) blocked the antiallodynic effect of intraplantar loperamide (300 µg/30 µL), and intraplantar injections of MK-801 or naloxone methiodide alone did not change PWT in SNL rats. After induction of tolerance, the inhibitory effect of local loperamide diminished in all 3 groups (Fig. 4). A later systemic injection of loperamide (1.5 mg/kg subcutaneously) significantly increased PWT in local loperamide-tolerant animals.
Fig. 4.
Pretreatment with locally administered naloxone methiodide and MK-801 did not prevent the development of tolerance to intraplantar loperamide. Intraplantar injection of loperamide (150 µg/50 µL) significantly increased the ipsilateral paw withdrawal threshold (PWT) of spinal nerve ligation (SNL) rats (i.e., reversed mechanical allodynia) under pretolerance conditions. During induction of tolerance, rats were administered an intraplantar injection of MK-801 (10 µg/20 µL, n = 6), naloxone methiodide (100 µg/20 µL, n = 6), or saline (n = 4) 10 minutes before each injection of loperamide (300 µg/30 µL intraplantarly). After tolerance induction, the inhibitory effect of local loperamide (150 µg/50 µL intraplantarly) diminished in all 3 groups. However, systemic loperamide (1.5 mg/kg subcutaneously) significantly increased the PWT in local loperamide-tolerant animals. Data are expressed as mean ± SEM. **P < .01, ***P < .001 versus preinjection PWT.
3.5. Repetitive systemic administration of morphine, but not loperamide, increased MOR phosphorylation in the spinal cord of SNL rats
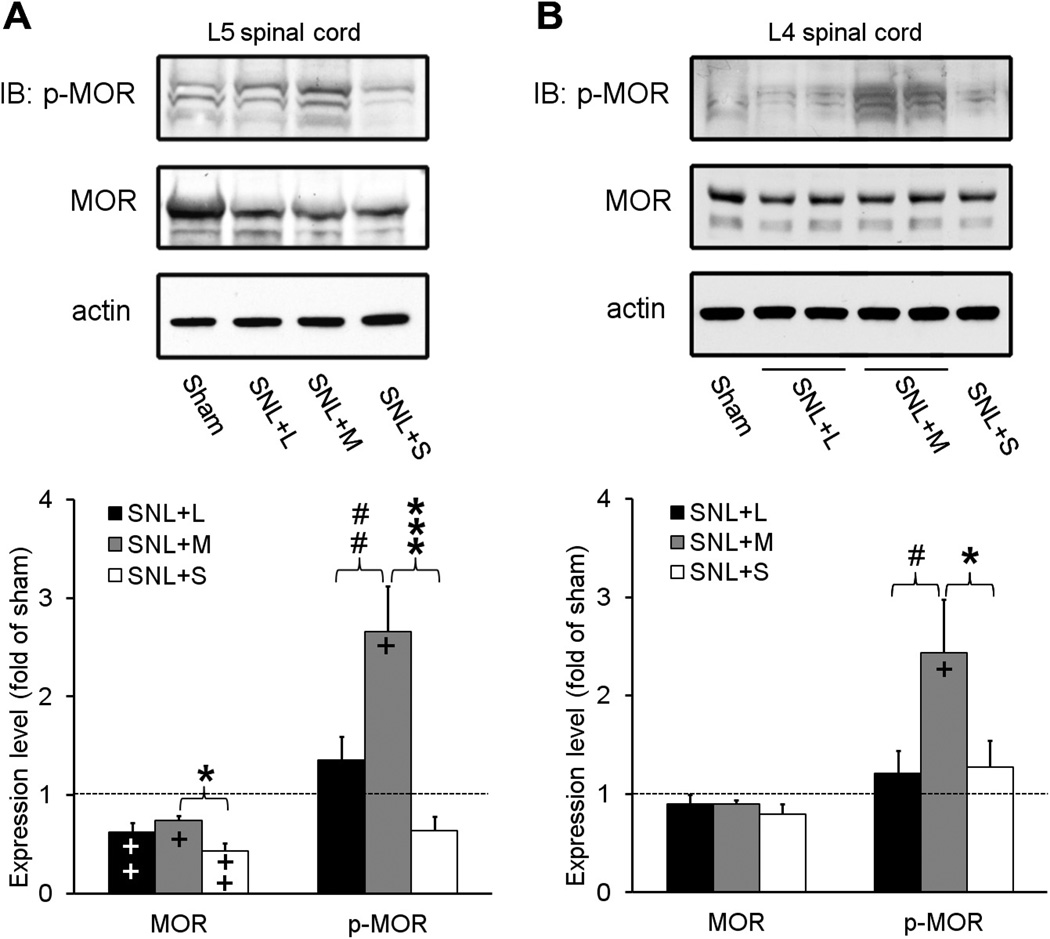
Phosphorylation at carboxyl-terminal residue 375 (Ser375) of MOR is involved in MOR desensitization and internalization and in the development of morphine tolerance [10,24,33]. Therefore, we assessed the effects of repetitive systemic injections of loperamide and morphine on spinal MOR expression and phosphorylation in SNL rats by using antibodies that selectively recognize MOR and p-MOR-Ser375. Rats received bilateral L5 SNL and were treated with repeated systemic injections of morphine sulfate (3 mg/kg subcutaneously, n = 4), loperamide (3 mg/kg subcutaneously, n = 4), or saline (n = 3) during days 5 to 8 post-SNL. The centrally penetrating morphine was used as a positive control for inducing MOR phosphorylation. To compare data between drug-treated groups within this experimental setting, we treated SNL rats with the same doses of morphine and loperamide and used the same tolerance-inducing protocol as described earlier. The 3-mg/kg dose is 3 to 4 times the ED50 for systemic morphine and loperamide to inhibit mechanical allodynia in nerve-injured rats [14,49] and is a nonsedating dose of morphine [16,23]. Sham-operated animals that did not receive drug treatment were included as injury controls (n = 3).
3.5.1. MOR
In the spinal segment that receives inputs from the injured spinal nerve (i.e., L5 segment), the levels of total MOR in morphine-, loperamide-, and saline-treated SNL groups were all significantly lower than that of the sham-operated group (Fig. 5A). The total MOR protein level in morphine-treated SNL rats was significantly higher than that in saline-treated SNL rats. There was no significant difference in the expression of MOR protein at the L4 spinal segment between groups (Fig. 5B).
Fig. 5.
Repetitive systemic administration of morphine, but not loperamide, increased MOR phosphorylation in spinal cord of nerve-injured rats. (A) Representative immunoblots (IB) show that repetitive systemic administration of morphine sulfate (M, 3 mg/kg subcutaneously, n = 3), but not loperamide (L, 3 mg/kg subcutaneously, n = 3) or saline (S, n = 3), significantly increased the level of p-MOR-Ser375 in the L5 spinal cord segment of SNL rats. The total MOR protein levels in SNL groups were all significantly lower than that in the sham-operated group. (B) Treatment with morphine, but not loperamide or saline, also induced a significant increase in MOR phosphorylation in SNL rats at the L4 spinal cord. MOR protein level at the L4 spinal segment was not significantly different between groups. Data are expressed as mean + SEM. *P < .05, ***P < .001 versus SNL+saline; +P < .05, ++P < .01 versus sham; #P < .05, ##P < .01 versus SNL+morphine.
3.5.2. Phosphorylated MOR
Immunoblotting showed that repetitive systemic morphine administration significantly increased MOR phosphorylation in both injured L5 and uninjured L4 spinal cord segments of SNL rats. Specifically, the levels of p-MOR-Ser375 in both L5 and L4 spinal segments were significantly higher in morphine-treated SNL rats than in saline-treated SNL rats or sham-operated rats (Fig. 5A and B). In contrast, repetitive systemic loperamide treatment did not induce a significant increase in p-MOR-Ser375 protein level in the L5 or L4 segment of SNL rats, as compared to either the saline-treated SNL group or the sham group. Importantly, p-MOR-Ser375 levels in both spinal segments of the loperamide-treated SNL group were significantly lower than those of morphine-treated SNL rats. The p-MOR-Ser375 level in the L5 spinal segment of the saline-treated SNL rats showed a small but statistically insignificant decrease compared to that of the sham group.
3.5.3. p-MOR-Ser375/MOR ratio
As compared with that in the sham group, the p-MOR-Ser375/MOR ratio was significantly increased in both L5 and L4 segments of morphine-treated (L5: 3.9 ± 0.6 fold, P < .01; L4: 2.7 ± 0.5 fold, P < .05), but not loperamide-treated SNL rats (L5: 1.3 ± 0.2 fold, P = .08, L4: 1.2 ± 0.2 fold, P = .51). The ratio in the L5 segment of the morphine-treated SNL group was also significantly higher than that in the saline-treated SNL group (1.7 ± 0.5 fold of sham, P < .05), and the ratio in the L4 segment of the morphine-treated SNL group was significantly higher than that of the loperamide-treated SNL group (P < .05). The p-MOR-Ser375/MOR ratios in both L5 and L4 segments of loperamide-treated SNL rats were not significantly different from those in saline-treated SNL rats.
3.6. Tolerance developed to loperamide’s inhibition of KCl-induced [Ca2+]i increase in DRG neurons in vitro
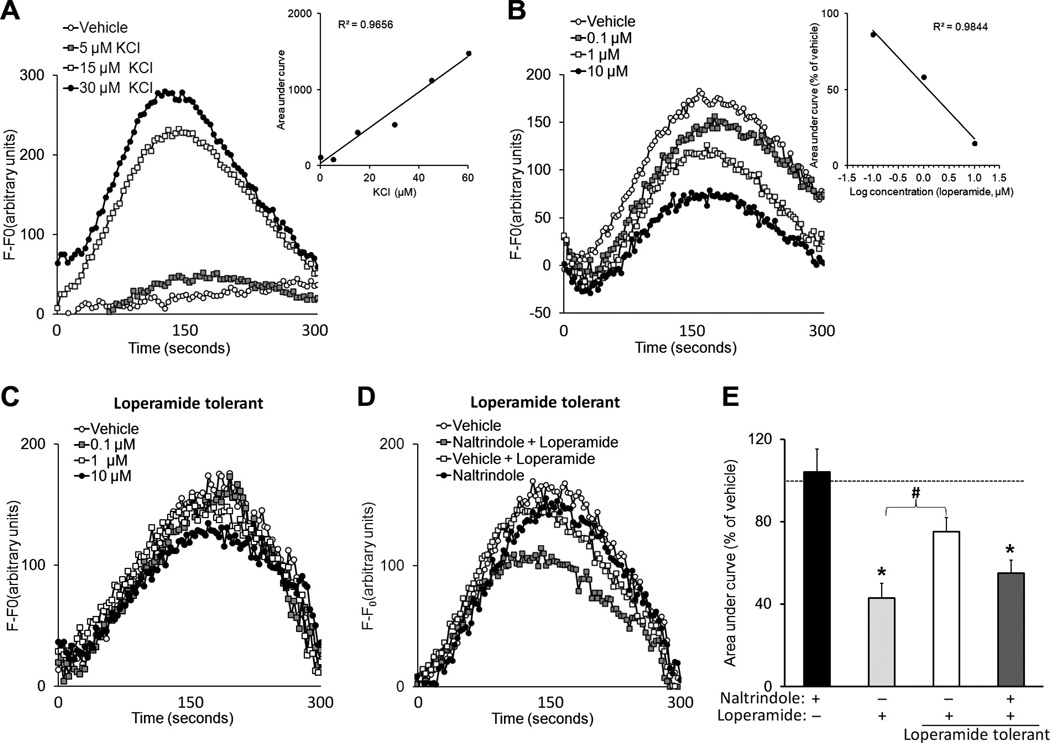
We used an in vitro approach to study the cellular adaptations that may result in loperamide tolerance. Calcium imaging was performed on DRG neurons isolated from adult rats and cultured in 96-well plates. KCl dose-dependently induced [Ca2+]i transients (Fig. 6A). Calcium-free media prevented KCl (30 µM)-induced [Ca2+]i transients. Acute bath application of loperamide (0.1 to 10 µM) dose-dependently inhibited the depolarizing KCl (30 µM)-induced [Ca2+]i in isolated DRG cells (Fig. 6B). The IC50 for inhibition was 2.65 µM. Importantly, at 24 hours after DRG cells had been exposed to loperamide (3 µM) for 72 hours, the ability of loperamide (0.1 to 10 µM) to inhibit KCl-induced [Ca2+]i transients was significantly decreased, demonstrating desensitization or tolerance (Fig. 6C). However, the DOR antagonist naltrindole (1 µM), but not vehicle, given 5 minutes before loperamide (3 µM) partially restored loperamide inhibition of KCl (30 µM)-induced [Ca2+]i in tolerant cells (Fig. 6D and E).
Fig. 6.
Tolerance developed to loperamide inhibition of KCl-induced [Ca2+]i transients in cultured DRG neurons. (A) Using a fluorescent plate reader, KCl-induced [Ca2+]i transients in adult DRG neurons were measured in a 96-well Fluo-4AM assay. Inset: The dose dependence of KCl on [Ca2+]i transient size is shown by increasing area under the curve (AUC) with increasing KCl concentration. (B) A 5-minute pretreatment with loperamide (0.1 to 10 µM) dose-dependently reduced KCl (30 µM)-induced [Ca2+]i transients. Inset: Dose-dependent loperamide inhibition on KCl-induced [Ca2+]i transients. (C) To model loperamide tolerance in vitro, cells were incubated with loperamide (3 µM) for 72 hours. At 24 hours posttreatment, loperamide (0.1 to 10 µM) inhibition of KCl-induced [Ca2+]i transients was significantly decreased across all doses, as compared to that of loperamide-naïve neurons. (D) However, a 5-minute exposure to the delta opioid receptor antagonist naltrindole (1 µM) before application of loperamide (3 µM) restored loperamide inhibition of KCl-induced [Ca2+]i transients in loperamide-tolerant cells, suggesting a reversal of tolerance. (E) Bar graph showing naltrindole alone had no effect on KCl-induced [Ca2+]i transients, and loperamide (3 µM) inhibited KCl-induced [Ca2+]i transients in nontolerant cells, but not in tolerant cells. Pretreatment with naltrindole partially restored the diminished drug effect in tolerant cells. Data are expressed as mean + SEM. *P < .05 versus naltrindole group.
4. Discussion
We characterized the tolerance that develops to the antiallodynic effect of loperamide in a preclinical model of neuropathic pain and attempted to examine potential receptor mechanisms and cellular adaptations that may contribute to loperamide tolerance. Loperamide is a MOR-preferring opioid agonist. After systemic administration, loperamide is removed quickly from the CNS by the P-glycoprotein transporter in the blood-brain barrier, and hence its actions are primarily peripheral [4,8,46]. SNL rats developed marked tachyphylaxis, or acute tolerance, to the inhibitory effect of loperamide on mechanical hypersensitivity (tactile allodynia) after receiving repetitive systemic or local drug injections. Systemic loperamide almost completely lost its antiallodynic effect after just 3 to 4 treatments. In general, the higher the tolerance-inducing dose, the greater the rate/extent of opioid tolerance development. Although drug efficacy appeared to diminish more quickly after repeated injections of loperamide at the 1.5 mg/kg dose than at the 3.0 mg/kg dose, this may not indicate a faster rate of tolerance induction by the lower dose. Under the tolerant condition, the decreased opioid analgesia can be partially compensated for by increasing/escalating the drug dose. Therefore, although tolerance may develop faster to the 3.0 mg/kg dose, 3.0 mg/kg loperamide may still induce a greater pain inhibition than the 1.5 mg/kg dose if MORs are not completely desensitized. Because loperamide is unlikely to accumulate to a significant extent in the CNS at the doses examined [4,8], our finding suggests that the PNS is also an important site for opioid tolerance development under neuropathic pain conditions. The finding that centrally penetrating morphine sulfate remained effective at reversing mechanical hypersensitivity in rats that had developed tolerance to systemic loperamide suggests that central opioid sensitivity was largely preserved when peripheral opioid effects were diminished.
Identifying the peripheral sites for systemic loperamide tolerance is important for studying the underlying mechanisms and for future development of therapeutic agents. Potential neuronal sites of action for systemic loperamide tolerance include cutaneous terminals of afferent sensory neurons, peripheral nerves, and soma of DRG neurons. The finding that locally administered loperamide also lost its antiallodynic efficacy in rats tolerant to systemic loperamide suggests that the peripheral tissue is an important site of action. However, the finding that systemic loperamide remained effective in reversing mechanical hypersensitivity in rats tolerant to locally administered loperamide indicates the existence of other sites, likely in PNS, for systemic drug action. We further characterized the dose-dependent features and demonstrated that opioid receptor-mediated mechanisms are involved in the development of systemic loperamide tolerance. We are aware that loperamide may exert several nonopioid actions, including direct inhibition of L-type calcium channels and inhibition of hyperpolarization-activated current [34,45]. However, our observation that pretreatment with naltrexone HCl blocked tolerance to systemic loperamide suggests an important role of opioid receptors in the induction of loperamide tolerance. It is unclear why pretreatment with naloxone HCl and naloxone methiodide did not block tolerance, but the major difference between these antagonists is that naltrexone has a longer duration of action. The underlying mechanism of loperamide’s antiallodynic tolerance remains to be examined, but it may involve adaptation changes at receptor and cellular levels in the PNS.
Acute cellular adaptations for morphine tolerance may involve phosphorylation and desensitization of MOR on the cell surface through a PKC-mediated process [3,17]. Our finding that repetitive systemic morphine administration increased spinal p-MOR-Ser375 in SNL rats supports this notion. However, the same tolerance-inducing protocol with loperamide did not induce a significant increase in spinal p-MOR-Ser375, suggesting that central MORs are not substrates for systemic loperamide tolerance. This notion is supported by pharmacokinetics of loperamide, which does not cross the blood-brain barrier in the dose range used in this study, and by numerous studies demonstrating the peripheral nature of loperamide actions [29,32,35]. Because the elimination half-life of loperamide (>2 hours) after subcutaneous injection is comparable to morphine (1.5 to 2 hours) [26], the lack of increased spinal p-MOR-Ser375 by repeated dosing of loperamide is not due to limited drug exposure. Although SNL may increase local blood–spinal cord barrier permeability [12], there is no evidence that loperamide gained access to the CNS. Yet, it is unclear whether loperamide does not induce MOR phosphorylation because of involvement of other mechanisms, such as differences in efficacies and properties of various opioids to induce MOR phosphorylation. Future studies may need to examine whether loperamide induces MOR phosphorylation and alters MOR trafficking in DRG neurons and at peripheral nerve terminals, actions that may influence the development of its analgesic tolerance.
Activation of central NMDA receptors has been suggested to be important to morphine tolerance [42]. NMDA receptor antagonists may attenuate morphine tolerance by inhibiting neuronal plasticity associated with tolerance in the CNS [18,20,42]. This notion is supported by our finding that the antiallodynic effect of systemic loperamide was attenuated in SNL rats pretreated with systemic MK-801, but not the peripherally acting NMDA antagonist MDL 105,519, during tolerance induction. How could tolerance to peripherally acting opioids in SNL rats involve neuronal plasticity in the CNS? Although loperamide does not penetrate the CNS to directly affect neuronal activity, it is possible that repetitive systemic drug treatments affect spinal neuronal plasticity by modulating afferent sensory neuronal activity/input, changing presynaptic neurotransmitter release, and altering receptor internalization and trafficking at central terminals. A study that examines whether pretreatment with intrathecal MK-801 blocks systemic loperamide tolerance may help to clarify the spinal site of action. However, repeated intrathecal MK-801 injections may be associated with severe CNS side effects [42]. Because MDL 105,519 did not prevent systemic loperamide tolerance, and local MK-801 did not prevent tolerance to intraplantar loperamide-induced antiallodynia, NMDA receptors in the PNS seem not to be essential for loperamide tolerance in SNL rats. However, this notion contradicts previous observations that local MK-801 treatment blocks topical morphine antinociceptive tolerance [21] and that the peripherally acting NMDA receptor/glycine-B site antagonist MRZ 2/596 attenuates systemic morphine antinociceptive tolerance in mice [7]. The inconsistencies between studies could be attributable to differences in animal species, drug treatment (e.g., dose, route, and timing of administration), tolerance-inducing paradigm, behavioral tests, and importantly, animal conditions (e.g., uninjured versus nerve-injured animals).
Inhibition of neuronal activity and pain transmission by morphine is dependent on the inhibition of calcium influx in DRG neurons. In our in vitro study, loperamide dose-dependently inhibited the depolarizing KCl-elicited calcium transient in rat DRG cells. Importantly, this inhibitory effect was significantly decreased in cells that had been pretreated with loperamide, suggesting that desensitization, or tachyphylaxis, also occurs at the cellular level. This in vitro model can be used in future studies to examine other cellular adaptations and mechanisms that result in loperamide tolerance. The finding that addition of naltrindole before the loperamide application returned the diminished drug effect in loperamide-tolerant cells is intriguing and would suggest that inhibition of DOR signaling in an opioid-tolerant state may help restore opioid analgesia. This notion is in line with previous observations that tolerance to the analgesic effects of morphine can also be decreased by pharmacologic blockade of DOR [1,31]. In addition, animals lacking DORs or the gene encoding preprotachykinin do not exhibit morphine tolerance [13,50].
Although the current study supports peripheral opioid tolerance [2,15,16,22,28], a previous finding showed that acute tolerance did not develop to the inhibitory effect of loperamide on herpetic allodynia in mice [30]. This discrepancy between these studies may be attributable to differences in the species and animal models, which could significantly affect the peripheral opioid tolerance that results from differences in neuropathic etiology and pathophysiology. It also may be related to different tolerance-inducing paradigms and behavioral outcome measures. Peripheral analgesic actions of opioids were also reported to be resistant to tolerance development under inflammatory pain conditions [38,39,41,51]. For example, no tolerance developed to peripheral morphine in spite of the plausible tonic activation of opioid receptors by immune cell–derived opioids in inflamed tissue. Loperamide remained effective in attenuating inflammatory mechanical hypersensitivity without producing tolerance in mice. Tolerance also failed to develop after repeated local administration of loperamide in the treatment of inflammatory pain [30,43]. Roles of peripheral inflammatory mediators and cytokine mechanisms in peripheral opioid tolerance under neuropathic pain conditions are worth studying in the future. It remains to be determined whether chronic use of loperamide for pain management in the clinic may be associated with tolerance.
In summary, our study suggests that repeated or prolonged use of loperamide for alleviating neuropathic mechanical hypersensitivity may lead to development of tolerance, possibly at peripheral opioid receptors. Because different MOR agonists induce different magnitudes of receptor internalization, desensitization, and tolerance processes, it remains to be tested whether analgesic tolerance also develops to other peripherally acting opioids under neuropathic pain conditions. The animal model that we used may also be useful for uncovering the mechanisms of tolerance at peripheral opioid receptors after nerve injury and for screening drugs to block tolerance development. Additional studies are needed to examine cellular adaptations involved in the genesis of peripheral opioid tolerance, such as changes in neuronal excitability and intracellular signaling transduction pathways in DRG neurons. Such studies will help to fully establish the clinical usefulness of peripherally acting opioids for a therapeutic formulation.
Acknowledgements
The authors thank Claire Levine, MS, for editing the manuscript, Matthias Ringkamp for discussing the manuscript, and Timothy V. Hartke for helping with drug preparation. This study was supported by grants from the National Institutes of Health: NS26363 (S.N.R.), NS70814 (Y.G.), DA023593 (S.M.S.), GM087369 (X.D.), and by the Blaustein Pain Research Fund.
Footnotes
The authors declare no conflict of interest.
References
- 1.Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- 2.Aley KO, Green PG, Levine JD. Opioid and adenosine peripheral antinociception are subject to tolerance and withdrawal. J Neurosci. 1995;15:8031–8038. doi: 10.1523/JNEUROSCI.15-12-08031.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey CP, Smith FL, Kelly E, Dewey WL, Henderson G. How important is protein kinase C in mu-opioid receptor desensitization and morphine tolerance? Trends Pharmacol Sci. 2006;27:558–565. doi: 10.1016/j.tips.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Baker DE. Loperamide: a pharmacological review. Rev Gastroenterol Disord. 2007;7:S11–S18. [PubMed] [Google Scholar]
- 5.Christensen D, Kayser V. The development of pain-related behaviour and opioid tolerance after neuropathy-inducing surgery and sham surgery. PAIN®. 2000;88:231–238. doi: 10.1016/S0304-3959(00)00334-1. [DOI] [PubMed] [Google Scholar]
- 6.Chung C, Carteret AF, McKelvy AD, Ringkamp M, Yang F, Hartke TV, Dong X, Raja SN, Guan Y. Analgesic properties of loperamide differ following systemic and local administration to rats after spinal nerve injury. Eur J Pain. 2012;16:1021–1032. doi: 10.1002/j.1532-2149.2012.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danysz W, Kozela E, Parsons CG, Sladek M, Bauer T, Popik P. Peripherally acting NMDA receptor/glycineB site receptor antagonists inhibit morphine tolerance. Neuropharmacology. 2005;48:360–371. doi: 10.1016/j.neuropharm.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Dehaven-Hudkins DL, Burgos LC, Cassel JA, Daubert JD, DeHaven RN, Mansson E, Nagasaka H, Yu G, Yaksh T. Loperamide (ADL 2-1294), an opioid antihyperalgesic agent with peripheral selectivity. J Pharmacol Exp Ther. 1999;289:494–502. [PubMed] [Google Scholar]
- 9.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 10.El KR, Burd AL, Erickson-Herbrandson LJ, Chang CY, Law PY, Loh HH. Phosphorylation of Ser363, Thr370, and Ser375 residues within the carboxyl tail differentially regulates mu-opioid receptor internalization. J Biol Chem. 2001;276:12774–12780. doi: 10.1074/jbc.M009571200. [DOI] [PubMed] [Google Scholar]
- 11.Foley KM. Opioids and chronic neuropathic pain. N Engl J Med. 2003;348:1279–1281. doi: 10.1056/NEJMe030014. [DOI] [PubMed] [Google Scholar]
- 12.Gordh T, Chu H, Sharma HS. Spinal nerve lesion alters blood-spinal cord barrier function and activates astrocytes in the rat. PAIN®. 2006;124:211–221. doi: 10.1016/j.pain.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Guan JS, Xu ZZ, Gao H, He SQ, Ma GQ, Sun T, Wang LH, Zhang ZN, Lena I, Kitchen I, Elde R, Zimmer A, He C, Pei G, Bao L, Zhang X. Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell. 2005;122:619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Guan Y, Johanek LM, Hartke TV, Shim B, Tao YX, Ringkamp M, Meyer RA, Raja SN. Peripherally acting mu-opioid receptor agonist attenuates neuropathic pain in rats after L5 spinal nerve injury. PAIN®. 2008;138:318–329. doi: 10.1016/j.pain.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez L, Romero A, Almela P, Garcia-Nogales P, Laorden ML, Puig MM. Tolerance to the antinociceptive effects of peripherally administered opioids. Expression of beta-arrestins. Brain Res. 2009;1248:31–39. doi: 10.1016/j.brainres.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 16.Honore P, Catheline G, Le GS, Besson JM. Chronic treatment with systemic morphine induced tolerance to the systemic and peripheral antinociceptive effects of morphine on both carrageenin induced mechanical hyperalgesia and spinal c-Fos expression in awake rats. PAIN®. 1997;71:99–108. doi: 10.1016/s0304-3959(97)03345-9. [DOI] [PubMed] [Google Scholar]
- 17.Hull LC, Llorente J, Gabra BH, Smith FL, Kelly E, Bailey C, Henderson G, Dewey WL. The effect of protein kinase C and G protein-coupled receptor kinase inhibition on tolerance induced by {micro}-opioid agonists of different efficacy. J Pharmacol Exp Ther. 2010;332:1127–1135. doi: 10.1124/jpet.109.161455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inturrisi CE. The role of N-methyl-d-aspartate (NMDA) receptors in pain and morphine tolerance. Minerva Anestesiol. 2005;71:401–403. [PubMed] [Google Scholar]
- 19.Joshi SK, Hernandez G, Mikusa JP, Zhu CZ, Zhong C, Salyers A, Wismer CT, Chandran P, Decker MW, Honore P. Comparison of antinociceptive actions of standard analgesics in attenuating capsaicin and nerve-injury-induced mechanical hypersensitivity. Neuroscience. 2006;143:587–596. doi: 10.1016/j.neuroscience.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Kolesnikov Y, Jain S, Wilson R, Pasternak GW. Lack of morphine and enkephalin tolerance in 129/SvEv mice: evidence for a NMDA receptor defect. J Pharmacol Exp Ther. 1998;284:455–459. [PubMed] [Google Scholar]
- 21.Kolesnikov Y, Pasternak GW. Topical opioids in mice: analgesia and reversal of tolerance by a topical N-methyl-d-aspartate antagonist. J Pharmacol Exp Ther. 1999;290:247–252. [PubMed] [Google Scholar]
- 22.Kolesnikov YA, Pasternak GW. Peripheral blockade of topical morphine tolerance by ketamine. Eur J Pharmacol. 1999;374:R1–R2. doi: 10.1016/s0014-2999(99)00318-0. [DOI] [PubMed] [Google Scholar]
- 23.LaBuda CJ, Little PJ. Pharmacological evaluation of the selective spinal nerve ligation model of neuropathic pain in the rat. J Neurosci Methods. 2005;144:175–181. doi: 10.1016/j.jneumeth.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 24.McPherson J, Rivero G, Baptist M, Llorente J, Al-Sabah S, Krasel C, Dewey WL, Bailey CP, Rosethorne EM, Charlton SJ, Henderson G, Kelly E. Mu-opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol Pharmacol. 2010;78:756–766. doi: 10.1124/mol.110.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neil A, Kayser V, Chen YL, Guilbaud G. Repeated low doses of morphine do not induce tolerance but increase the opioid antinociceptive effect in rats with a peripheral neuropathy. Brain Res. 1990;522:140–143. doi: 10.1016/0006-8993(90)91589-9. [DOI] [PubMed] [Google Scholar]
- 26.Nozaki-Taguchi N, Yaksh TL. Characterization of the antihyperalgesic action of a novel peripheral mu-opioid receptor agonist—loperamide. Anesthesiology. 1999;90:225–234. doi: 10.1097/00000542-199901000-00029. [DOI] [PubMed] [Google Scholar]
- 27.Obara I, Przewlocki R, Przewlocka B. Local peripheral effects of mu-opioid receptor agonists in neuropathic pain in rats. Neurosci Lett. 2004;360:85–89. doi: 10.1016/j.neulet.2004.01.056. [DOI] [PubMed] [Google Scholar]
- 28.Rashid MH, Inoue M, Toda K, Ueda H. Loss of peripheral morphine analgesia contributes to the reduced effectiveness of systemic morphine in neuropathic pain. J Pharmacol Exp Ther. 2004;309:380–387. doi: 10.1124/jpet.103.060582. [DOI] [PubMed] [Google Scholar]
- 29.Sandhu BK, Tripp JH, Candy DC, Harries JT. Loperamide: studies on its mechanism of action. Gut. 1981;22:658–662. doi: 10.1136/gut.22.8.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasaki A, Nakashima Y, Takasaki I, Andoh T, Shiraki K, Kuraishi Y. Effects of loperamide on mechanical allodynia induced by herpes simplex virus type-1 in mice. J Pharmacol Sci. 2007;104:218–224. doi: 10.1254/jphs.fp0070294. [DOI] [PubMed] [Google Scholar]
- 31.Schiller PW. Bi- or multifunctional opioid peptide drugs. Life Sci. 2010;86:598–603. doi: 10.1016/j.lfs.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schinkel AH, Wagenaar E, Mol CA, Van DL. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, Hollt V. Morphine induces terminal micro-opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J. 2004;23:3282–3289. doi: 10.1038/sj.emboj.7600334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sevostianova N, Danysz W, Bespalov AY. Analgesic effects of morphine and loperamide in the rat formalin test: interactions with NMDA receptor antagonists. Eur J Pharmacol. 2005;525:83–90. doi: 10.1016/j.ejphar.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Sklerov J, Levine B, Moore KA, Allan C, Fowler D. Tissue distribution of loperamide and N-desmethylloperamide following a fatal overdose. J Anal Toxicol. 2005;29:750–754. doi: 10.1093/jat/29.7.750. [DOI] [PubMed] [Google Scholar]
- 36.Stein C, Clark JD, Oh U, Vasko MR, Wilcox GL, Overland AC, Vanderah TW, Spencer RH. Peripheral mechanisms of pain and analgesia. Brain Res Rev. 2009;60:90–113. doi: 10.1016/j.brainresrev.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stein C, Lang LJ. Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol. 2009;9:3–8. doi: 10.1016/j.coph.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Stein C, Pfluger M, Yassouridis A, Hoelzl J, Lehrberger K, Welte C, Hassan AH. No tolerance to peripheral morphine analgesia in presence of opioid expression in inflamed synovia. J Clin Invest. 1996;98:793–799. doi: 10.1172/JCI118852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat Med. 2003;9:1003–1008. doi: 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- 40.Tegeder I, Meier S, Burian M, Schmidt H, Geisslinger G, Lotsch J. Peripheral opioid analgesia in experimental human pain models. Brain. 2003;126:1092–1102. doi: 10.1093/brain/awg115. [DOI] [PubMed] [Google Scholar]
- 41.Tokuyama S, Inoue M, Fuchigami T, Ueda H. Lack of tolerance in peripheral opioid analgesia in mice. Life Sci. 1998;62:1677–1681. doi: 10.1016/s0024-3205(98)00127-1. [DOI] [PubMed] [Google Scholar]
- 42.Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- 43.Ueda H, Inoue M. Peripheral morphine analgesia resistant to tolerance in chronic morphine-treated mice. Neurosci Lett. 1999;266:105–108. doi: 10.1016/s0304-3940(99)00285-2. [DOI] [PubMed] [Google Scholar]
- 44.Vadivelu N, Mitra S, Hines RL. Peripheral opioid receptor agonists for analgesia: a comprehensive review. J Opioid Manag. 2011;7:55–68. doi: 10.5055/jom.2011.0049. [DOI] [PubMed] [Google Scholar]
- 45.Vasilyev DV, Shan Q, Lee Y, Mayer SC, Bowlby MR, Strassle BW, Kaftan EJ, Rogers KE, Dunlop J. Direct inhibition of Ih by analgesic loperamide in rat DRG neurons. J Neurophysiol. 2007;97:3713–3721. doi: 10.1152/jn.00841.2006. [DOI] [PubMed] [Google Scholar]
- 46.Wheeler GD. ADL-2-1294 (Adolor) IDrugs. 2000;3:1373–1378. [PubMed] [Google Scholar]
- 47.Xu M, Petraschka M, McLaughlin JP, Westenbroek RE, Caron MG, Lefkowitz RJ, Czyzyk TA, Pintar JE, Terman GW, Chavkin C. Neuropathic pain activates the endogenous kappa opioid system in mouse spinal cord and induces opioid receptor tolerance. J Neurosci. 2004;24:4576–4584. doi: 10.1523/JNEUROSCI.5552-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang G, Mohammad H, Peper BD, Raja S, Wilson SP, Sweitzer SM. Enhanced peripheral analgesia using virally mediated gene transfer of the mu-opioid receptor in mice. Anesthesiology. 2008;108:305–313. doi: 10.1097/01.anes.0000299836.61785.79. [DOI] [PubMed] [Google Scholar]
- 49.Zhao C, Tall JM, Meyer RA, Raja SN. Antiallodynic effects of systemic and intrathecal morphine in the spared nerve injury model of neuropathic pain in rats. Anesthesiology. 2004;100:905–911. doi: 10.1097/00000542-200404000-00021. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 51.Zollner C, Mousa SA, Fischer O, Rittner HL, Shaqura M, Brack A, Shakibaei M, Binder W, Urban F, Stein C, Schafer M. Chronic morphine use does not induce peripheral tolerance in a rat model of inflammatory pain. J Clin Invest. 2008;118:1065–1073. doi: 10.1172/JCI25911. [DOI] [PMC free article] [PubMed] [Google Scholar]