Abstract
Genome-wide association studies (GWAS) have implicated a series of single nucleotide polymorphisms (SNPs) in Alzheimer’s disease (AD) risk. Elucidating the function of these SNPs is critical to identifying the underlying pathways and, potentially, novel therapeutic agents. SNPs within the gene ATP binding cassette A7 (ABCA7) reached significance in these studies, warranting investigation into their actions. Here, we analyzed ABCA7 expression in a set of human brain samples as a function of AD-associated SNPs and AD status. We report that the rs3764650T allele that decreases AD risk is associated with increased ABCA7 expression. However, ABCA7 expression is increased in AD individuals. We interpret our findings as suggesting a model wherein increased ABCA7 expression reduces AD risk and that the increased ABCA7 observed in AD reflects an inadequate compensatory change.
Keywords: Alzheimer’s, ABCA7, SNP, phagocytosis, microglia
1. Introduction
AD is defined by short-term memory loss progressing into dementia and neuropathology including senile plaques and neurofibrillary tangles [12, 16, 35, 42]. AD risk increases with age with the disease affecting as many as one out of three people by 85 years of age [5, 9, 11, 15, 29]. Since AD is an impending health crisis, we seek to identify the underlying mechanisms and translate these mechanisms into preventative agents.
GWAS have identified genes in pathways that modulate AD risk, including genetic variants in ABCA7 (reviewed in [3, 36]). ABCA7 is part of a large family of transporters expressed in the brain on microglia and neurons and is capable of fluxing phospholipids as well as enhancing phagocytosis of apoptotic cells [17, 22-24, 43]. As both a risk factor in AD and potential drug target, ABCA7 merits further investigation.
Here we hypothesized that rs3764650, the primary AD-associated SNP, modulates ABCA7 expression. In evaluating this hypothesis, we genotyped several SNPs and quantified ABCA7 expression in 57 human brain samples. We interpret our results as suggesting a model wherein increased ABCA7 expression reduces AD risk, possibly through enhanced microglial activation and phagocytosis.
2. Materials and methods
2.1. Ethics statement
The work described here was conducted under the approval of the University of Kentucky Institutional Review Board.
2.2. Human autopsy tissue
Human anterior cingulate samples were supplied by the University of Kentucky AD Center Neuropathology Core and have been described previously [28, 46, 47]. Evaluation of AD status was conducted by the AD Center Neuropathology and Clinical Cores using guidelines set forth by the National Institute on Aging Reagan Institute (NIA-RI) that included evaluation of neurofibrillary tangles and neuritic senile plaques [32-34]. Age at death for the cognitively intact, i.e. non-AD donors, was 82.3 ± 1.6 (mean ± SE, n = 28) while AD donors were 81.6 ± 1.1 (mean ± SE, n = 29). The post-mortem interval for non-AD and AD donors was 2.8 ± 0.2 (n = 29) hours and 3.4 ± 0.1 (n = 27), respectively. By NIA-RI neuropathology criteria, the 28 non-AD individuals included 12 with no AD likelihood, 10 with low likelihood, and six with moderate AD likelihood. Neuropathology in the 29 AD individuals was uniformly robust, i.e., definite AD by NIH-RI criteria [33].
2.3. qPCR
ABCA7 expression levels were determined by quantitative polymerase chain reaction (qPCR) (Bio-Rad, Hercules, CA). Assays contained the sense primer corresponding to sequence in exon 44, i.e., 5’-TCCTTTGGAACAGCCTTTTG-3’ and antisense primer corresponding to sequence in exon 45, i.e., 5’-CTGCCCTTGAGATGTTGC-3’. Each 20 μL reaction mixture contained 20 ng of cDNA, 1 μM of each primer, and 2x SYBR Green (Quanta Biosciences, Gaithersburg, MD) and was subjected to a qPCR profile of 40 cycles at 95°C for 15 s, 60°C for 30 s, and 72°C for 20 s. A standard curve was amplified in parallel to determine the copy number of qPCR product per sample. ABCA7 copy numbers were normalized to the geometric mean of the reference genes eukaryotic translation initiation factor 4H (EIF4H) and ribosomal protein L32 (RPL32). Assays were repeated three times.
2.4. Genotyping
DNA samples were genotyped for rs3764650, rs3752246, rs4147914, rs3752229, rs7247084, rs757232, rs2279796, rs7251792, 12710103, and rs4147930 by using Taqman FAM and VIC dye-labeled probes (Life Technologies, Foster City, CA) by qPCR (Bio-Rad, Hercules, CA).
2.5. Statistical analysis
The extent that ABCA7 mRNA levels were associated with AD-associated SNPs and/or AD-status was determined by using linear regression analysis (SPSS v.19 (IBM, Somers, NY)). A dominant allelic model was assumed, i.e. individuals homozygous for the rs3764650 minor G allele were grouped with those heterozygous for the SNP and compared with individuals homozygous for the rs3764650 major T allele. The model also included age at death and sex as covariates.
3. Results
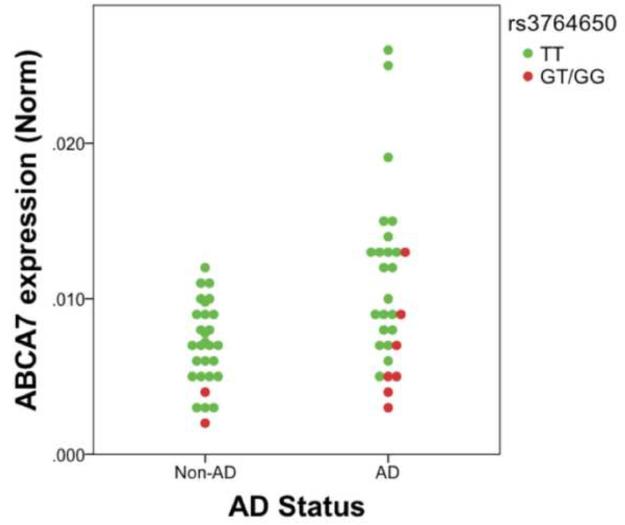
The ABCA7 SNP rs3764650 [T/G] is associated with AD risk [3]. To assess the hypothesis that ABCA7 expression is modulated by this SNP, we quantified ABCA7 copy number and then normalized these values to the geometric mean of two housekeeping genes [7, 27]. As depicted in Figure 1, ABCA7 expression appears reduced in individuals with the AD-risky G allele and increased in those with AD. To evaluate this apparent trend, we used linear modeling that included rs3764650, AD status, age, and sex. Table 1 shows that ABCA7 expression was significantly associated with rs3764650 genotype and AD status. Overall, we interpret these results as suggesting that ABCA7 expression is reduced with the minor rs3764650G allele and increased with AD.
Figure 1. ABCA7 expression as a function of rs3764650 genotype and AD status.
ABCA7 expression is decreased in individuals carrying rs3764650G and increased with. Expression was evaluated as a function of the AD-associated SNP rs3764650 in a dominant model, i.e., rs3764650 GT and GG individuals were compared against TT individuals.
Table 1.
Rs3764650 and AD status are associated with ABCA7 expression. Linear regression statistical analysis included AD status, rs3764650 genotype, age at death and sex. The adjusted R2 for the model was 0.285. Age and sex were not significantly associated with ABCA7 expression (p=.44 and p=.26, respectively).
| Standard Beta | ||
|---|---|---|
| Parameter | Coefficient | P-Value |
|
| ||
| rs3764650 | −0.375 | 3.5×10−3 |
|
| ||
| AD Status | 0.502 | 1.1×10−5 |
Several ABCA7 SNPs have been associated with ABCA7 expression in brain or monocytes by others [2, 45]. To evaluate whether these SNPs are associated with ABCA7 expression in our brain samples, we genotyped the samples for these SNPs and performed linear regression analysis with each individual SNP. Overall, the SNPs showed variable association with ABCA7 expression with rs3764650 having the lowest p value (Table 2). We hypothesized that if these other SNPs were associated with expression only through rs3764650, the linkage disequilibrium between each SNP and rs3764650 would correlate with the strength of the SNP’s association with ABCA7 expression. We found a robust correlation in this analysis (Figure 2). We interpret this positive relationship as supporting the concept that these other SNPs are associated with ABCA7 expression only through rs3764650.
Table 2.
Relationship between SNP linkage disequilibrium with rs3764650 and association with ABCA7 expression. The linkage disequilibrium between rs3764650 (r2) and each of the other SNPs was determined by using SNAP (http://www.broadinstitute.org/mpg/snap/ldsearch.php). The association between each individual SNP and ABCA7 expression was determined by linear regression analyses that included AD status, age, sex and the SNP in a dominant model. The rationale for including each SNP is noted in the comment column.
| SNP | r2 with rs3764650 |
Standard Beta Coefficient |
P-Value | Comment (reference) |
|---|---|---|---|---|
|
| ||||
| rs3764650 | 1.000 | −0.402 | 0.001 | AD GWAS [15, 31] |
|
| ||||
| rs4147914 | 0.584 | −0.296 | 0.021 | Exonic SNP |
|
| ||||
| rs3752229 | 0.541 | −0.296 | 0.028 | Associates with ABCA7 expression in brain [2] |
|
| ||||
| rs7251792 | 0.245 | 0.161 | 0.209 | Associates with ABCA7 expression in monocytes [43] |
|
| ||||
| rs3752246 | 0.230 | −0.231 | 0.066 | Non-synonymous exonic SNP [31] |
|
| ||||
| rs757232 | 0.173 | −0.170 | 0.174 | Associates with ABCA7 expression in brain [2] |
|
| ||||
| rs4147930 | 0.166 | −0.190 | 0.138 | Exonic SNP |
|
| ||||
| rs2279796 | 0.036 | 0.123 | 0.354 | Associates with ABCA7 expression in monocytes [43] |
|
| ||||
| rs7247087 | 0.007 | 0.169 | 0.171 | Associates with ABCA7 expression in brain [2] |
|
| ||||
| rs12710103 | 0.006 | 0.037 | 0.772 | Associates with ABCA7 expression in monocytes [43] |
Figure 2. SNP linkage disequilibrium with rs3764650 correlates with SNP association with ABCA7 expression.
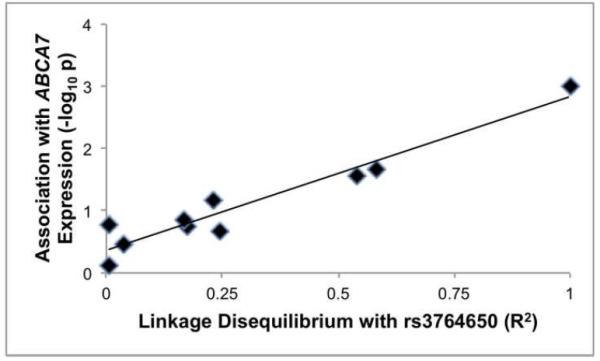
The r2 for the relationship between SNP linkage disequilibrium and association with ABCA7 expression was 0.93.
4. Discussion
Our primary findings are that ABCA7 expression is decreased in individuals that carry the AD-risky rs3764650G allele. Also, ABCA7 expression is increased with AD. The most parsimonious interpretation of our results is that the AD-risky rs3764650G allele decreases ABCA7 prior to AD onset, i.e., increased ABCA7 expression would reduce AD risk. From these results we propose that increased ABCA7 expression during AD is insufficient to block disease progression. The mechanistic underpinnings of ABCA7 action are unclear but likely involve lipid regulation or phagocytosis.
Our finding that ABCA7 expression is increased in AD is consistent with a prior report from Karch et al that ABCA7 expression was increased in individuals with increased cognitive decline [20]. Our finding that ABCA7 expression is reduced with the AD-risky rs3764650G allele has not been reported previously. Instead, Allen et al identified several other SNPs that were associated with ABCA7 expression [2]. We evaluated these SNPs and found that their association with ABCA7 expression appears to reflect their linkage disequilibrium with rs3764650. However, Karch et al examined rs3764650 directly and did not detect an association between this SNP and ABCA7 expression. Several technical and analytical differences between Karch et al and this study may underlay the differences in our findings. Technical differences include that we used SYBR-green based qPCR and normalized to the geometric mean of RPL32 and EIF4H while Karch et al used TaqMan-based qPCR and normalized relative to GAPDH. Additionally, we used a dominant model for rs3764650 while Karch et al used an additive model; we re-analyzed our data with an additive model and found a less robust significance for SNP association with ABCA7 expression (p=0.011 for additive model as opposed to p=0.0035 for dominant model). Karch et al examined 112 brain samples while our study included 57 samples; a post-hoc power analysis of the SNP association with expression here was 85.3%. A greater number of samples from non-AD individuals with the rs3764650G allele would provide greater insight on rs3764650G effects on ABCA7 expression in the absence of pathology. In summary, the normalization genes may be the most likely variable underlying our differing results, although additional unknown variables are also possible.
Two primary mechanisms may underlie the actions of ABCA7 in AD. First, ABCA7 may modulate AD risk through its ability to transfer phospholipids to apolipoproteins such as apoE, apoJ and apoA-1 [1, 43]. This is suggestive that ABCA7 may act similarly to ABCA1, which has been shown to transfer cholesterol to apoE and modulate amyloid-beta (Aβ) levels [6, 8, 19, 23, 25, 44]. For example, ABCA1 deficient mice have decreased levels of lipidated apoE and increased Aβ deposits [26]. However, a key difference between ABCA1 and ABCA7 is that apoE levels are not altered in ABCA7 deficient mice [23]. Additionally, ABCA1 deficiency causes Tangiers disease in humans and a robust phenotype in mice but ABCA7 polymorphisms do not associate with lipid homeostasis and ABCA7 deficiency has only a modest effect on lipid homeostasis in mice [10, 20, 21, 23, 31]. Overall, the actions of ABCA7 on lipid homeostasis may be modest relative to ABCA1. A second mechanism whereby ABCA7 may modulate AD risk is through microglial phagocytosis [17, 23]. Indeed, ABCA7 is a homolog of ced-7, the C. elegans gene critical for apoptotic cell engulfment [17]. Here, we note that ectopic ABCA7 expression increases microglial phagocytosis of multiple substrates, including apoptotic cells, Aβ, and synthetic substrates in vitro [17, 23, 39-41]. Consistent with this possibility, ABCA7 deficient mice have increased Aβ deposition in vivo and rs3764650G in humans has been associated with increased neuritic plaque burden [23, 37, 38]. In summary, (i) ABCA7 increases phagocytosis activity in vitro, (ii) ABCA7 deficiency increases Aβ in a murine model, and (iii) the rs3764650G allele that correlates with reduced ABCA7 expression is associated with increased neuritic plaques in human. Hence, we propose that the most likely interpretation of current results is that ABCA7 modulates AD risk through phagocytosis.
This interpretation is consistent with two recent genetic findings that have implicated microglial function in AD risk. First, TREM2 increases microglial activation; a rare, apparently inhibitory, TREM2 allele is associated with robustly increased AD risk [14, 18]. Second, CD33 reduces microglial activation; a CD33 allele that reduces functional CD33, apparently by modulating exon 2 splicing, is associated with decreased AD risk [4, 13, 30]. These results overall suggest that increased microglial activation reduces AD risk. Hence, the observation that ABCA7 expression enhances microglial activation coupled with our finding that an AD-protective ABCA7 allele is associated with increased ABCA7 expression generates a consistent overall model wherein increased microglial activation, especially phagocytosis, is AD-protective (Figure 3). TREM2 and CD33 modulate phagocytosis by signaling through Syk and a Syk phosphatase, SHP1, respectively. The signaling pathway whereby ABCA7 modulates phagocytosis is unclear but may involve the ERK signaling [17].
Figure 3. ABCA7, TREM2 and CD33 modulate microglial function to alter AD risk.

This model proposes that enhanced microglial phagocytosis due to increased ABCA7 or TREM2 function, or reduced CD33 function, leads to decreased AD risk.
In summary, our primary findings are that ABCA7 expression is decreased with the AD-risky rs3764650G allele and increased with AD. The increase in ABCA7 expression in AD could reflect that ABCA7 contributes to AD risk. However, a parsimonious model is that the ABCA7 induction in AD is in response to the disease because ABCA7 expression was also increased with the AD-protective rs3764650T allele. Hence, we interpret our data overall as suggesting a model wherein a SNP-driven, presumably long-term, increase in ABCA7 expression reduces AD risk; increased ABCA7 expression during AD is insufficient to block disease progression.
Highlights.
GWAS have identified an AD-associated SNP within ABCA7.
We investigated the extent that this AD-associated SNP modulates ABCA7 expression.
The rs3764650G allele, which increases AD risk, was associated with decreased ABCA7 expression.
ABCA7 expression was increased with AD status.
We propose a model wherein increased ABCA7 expression reduces AD risk with the increase observed in AD reflecting a poor compensatory response.
Acknowledgments
The authors acknowledge the NIH (National Institutes of Health) for funding of this work (P01-AG030128 (SE), P30-AG028383 (DWF), P20-GM103436 (DWF) as well as the University of Kentucky Alzheimer’s Center for tissue (P30-AG028383).
Abbreviations
- GWAS
Genome-wide association studies
- SNP
Single nucleotide polymorphism
- AD
Alzheimer’s disease
- ABCA7
ATP binding cassette A7
- qPCR
quantitative polymerase chain reaction
- Aβ
Amyloid-beta
- EIF4H
eukaryotic translation initiation factor 4H
- RPL32
ribosomal protein L32
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abe-Dohmae S, Ikeda Y, Matsuo M, Hayashi M, Okuhira K, Ueda K, Yokoyama S. Human ABCA7 supports apolipoprotein-mediated release of cellular cholesterol and phospholipid to generate high density lipoprotein. J Biol Chem. 279(2004):604–611. doi: 10.1074/jbc.M309888200. [DOI] [PubMed] [Google Scholar]
- 2.Allen M, Zou F, Chai HS, Younkin CS, Crook J, Pankratz VS, Carrasquillo MM, Rowley CN, Nair AA, Middha S, Maharjan S, Nguyen T, Ma L, Malphrus KG, Palusak R, Lincoln S, Bisceglio G, Georgescu C, Schultz D, Rakhshan F, Kolbert CP, Jen J, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD, Petersen RC, Graff-Radford NR, Dickson DW, Younkin SG, Ertekin-Taner N. Novel late-onset Alzheimer disease loci variants associate with brain gene expression. Neurology. 79(2012):221–228. doi: 10.1212/WNL.0b013e3182605801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertram L MM, K Mullin, D Blacker, R. Tanzi. The AlzGene Database. Alzheimer Research Forum. http://www.alzgene.org Accessed [07/15/2013]
- 4.Bradshaw EM, Chibnik LB, Keenan BT, Ottoboni L, Raj T, Tang A, Rosenkrantz LL, Imboywa S, Lee M, Von Korff A, Morris MC, Evans DA, Johnson K, Sperling RA, Schneider JA, Bennett DA, De Jager PL. CD33 Alzheimer's disease locus: altered monocyte function and amyloid biology. Nat Neurosci. 16(2013):848–850. doi: 10.1038/nn.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 88(1998):1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan SL, Kim WS, Kwok JB, Hill AF, Cappai R, Rye KA, Garner B. ATP-binding cassette transporter A7 regulates processing of amyloid precursor protein in vitro. J Neurochem. 106(2008):793–804. doi: 10.1111/j.1471-4159.2008.05433.x. [DOI] [PubMed] [Google Scholar]
- 7.Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc. 1(2006):581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- 8.Elali A, Rivest S. The role of ABCB1 and ABCA1 in beta-amyloid clearance at the neurovascular unit in Alzheimer's disease. Front Physiol. 2013;4:45. doi: 10.3389/fphys.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans DA. Estimated prevalence of Alzheimer's disease in the United States. Milbank Q. 68(1990):267–289. [PubMed] [Google Scholar]
- 10.Fasano T, Zanoni P, Rabacchi C, Pisciotta L, Favari E, Adorni MP, Deegan PB, Park A, Hlaing T, Feher MD, Jones B, Uzak AS, Kardas F, Dardis A, Sechi A, Bembi B, Minuz P, Bertolini S, Bernini F, Calandra S. Novel mutations of ABCA1 transporter in patients with Tangier disease and familial HDL deficiency. Mol Genet Metab. 107(2012):534–541. doi: 10.1016/j.ymgme.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Global prevalence of dementia: a Delphi consensus study. Lancet. 366(2005):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannakopoulos P, Kovari E, Gold G, von Gunten A, Hof PR, Bouras C. Pathological substrates of cognitive decline in Alzheimer's disease. Front Neurol Neurosci. 24(2009):20–29. doi: 10.1159/000197881. [DOI] [PubMed] [Google Scholar]
- 13.Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH, Hyman BT, Tanzi RE. Alzheimer's Disease Risk Gene CD33 Inhibits Microglial Uptake of Amyloid Beta. Neuron. 78(2013):631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J. TREM2 variants in Alzheimer's disease. N Engl J Med. 368(2013):117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 60(2003):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 16.Ivins KJ, Bui ET, Cotman CW. Beta-amyloid induces local neurite degeneration in cultured hippocampal neurons: evidence for neuritic apoptosis. Neurobiol Dis. 5(1998):365–378. doi: 10.1006/nbdi.1998.0228. [DOI] [PubMed] [Google Scholar]
- 17.Jehle AW, Gardai SJ, Li S, Linsel-Nitschke P, Morimoto K, Janssen WJ, Vandivier RW, Wang N, Greenberg S, Dale BM, Qin C, Henson PM, Tall AR. ATP-binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages. J Cell Biol. 174(2006):547–556. doi: 10.1083/jcb.200601030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 368(2013):107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karasinska JM, de Haan W, Franciosi S, Ruddle P, Fan J, Kruit JK, Stukas S, Lutjohann D, Gutmann DH, Wellington CL, Hayden MR. ABCA1 influences neuroinflammation and neuronal death. Neurobiol Dis. 54(2013):445–455. doi: 10.1016/j.nbd.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Karch CM, Jeng AT, Nowotny P, Cady J, Cruchaga C, Goate AM. Expression of novel Alzheimer's disease risk genes in control and Alzheimer's disease brains. PLoS One. 2012;7:e50976. doi: 10.1371/journal.pone.0050976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim WS, Fitzgerald ML, Kang K, Okuhira K, Bell SA, Manning JJ, Koehn SL, Lu N, Moore KJ, Freeman MW. Abca7 null mice retain normal macrophage phosphatidylcholine and cholesterol efflux activity despite alterations in adipose mass and serum cholesterol levels. J Biol Chem. 280(2005):3989–3995. doi: 10.1074/jbc.M412602200. [DOI] [PubMed] [Google Scholar]
- 22.Kim WS, Guillemin GJ, Glaros EN, Lim CK, Garner B. Quantitation of ATP-binding cassette subfamily-A transporter gene expression in primary human brain cells. Neuroreport. 17(2006):891–896. doi: 10.1097/01.wnr.0000221833.41340.cd. [DOI] [PubMed] [Google Scholar]
- 23.Kim WS, Li H, Ruberu K, Chan S, Elliott DA, Low JK, Cheng D, Karl T, Garner B. Deletion of Abca7 increases cerebral amyloid-beta accumulation in the J20 mouse model of Alzheimer's disease. J Neurosci. 33(2013):4387–4394. doi: 10.1523/JNEUROSCI.4165-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim WS, Weickert CS, Garner B. Role of ATP-binding cassette transporters in brain lipid transport and neurological disease. J Neurochem. 104(2008):1145–1166. doi: 10.1111/j.1471-4159.2007.05099.x. [DOI] [PubMed] [Google Scholar]
- 25.Koldamova R, Fitz NF, Lefterov I. The role of ATP-binding cassette transporter A1 in Alzheimer's disease and neurodegeneration. Biochim Biophys Acta. 1801(2010):824–830. doi: 10.1016/j.bbalip.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koldamova R, Staufenbiel M, Lefterov I. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J Biol Chem. 280(2005):43224–43235. doi: 10.1074/jbc.M504513200. [DOI] [PubMed] [Google Scholar]
- 27.Ling IF, Bhongsatiern J, Simpson JF, Fardo DW, Estus S. Genetics of clusterin isoform expression and Alzheimer's disease risk. PLoS One. 2012;7:e33923. doi: 10.1371/journal.pone.0033923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ling IF, Estus S. Role of SFRS13A in low-density lipoprotein receptor splicing. Hum Mutat. 31(2010):702–709. doi: 10.1002/humu.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM, Copeland JR, Dartigues JF, Jagger C, Martinez-Lage J, Soininen H, Hofman A. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54:S4–9. [PubMed] [Google Scholar]
- 30.Malik M, Simpson JF, Parikh I, Wilfred BR, Fardo DW, Nelson PT, Estus S. CD33 Alzheimer's Risk-Altering Polymorphism, CD33 Expression, and Exon 2 Splicing. J Neurosci. 33(2013):13320–13325. doi: 10.1523/JNEUROSCI.1224-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNeish J, Aiello RJ, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe KL, Roach ML, Royer LJ, de Wet J, Broccardo C, Chimini G, Francone OL. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Natl Acad Sci U S A. 97(2000):4245–4250. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson PT, Abner EL, Scheff SW, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Patel E, Markesbery WR. Alzheimer's-type neuropathology in the precuneus is not increased relative to other areas of neocortex across a range of cognitive impairment. Neurosci Lett. 450(2009):336–339. doi: 10.1016/j.neulet.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 68(2009):1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson PT, Kukull WA, Frosch MP. Thinking outside the box: Alzheimer-type neuropathology that does not map directly onto current consensus recommendations. J Neuropathol Exp Neurol. 69(2010):449–454. doi: 10.1097/NEN.0b013e3181d8db07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perl DP. Neuropathology of Alzheimer's disease. Mt Sinai J Med. 77(2010):32–42. doi: 10.1002/msj.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schellenberg GD, Montine TJ. The genetics and neuropathology of Alzheimer's disease. Acta Neuropathol. 124(2012):305–323. doi: 10.1007/s00401-012-0996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shulman JM, Chen K, Keenan BT, Chibnik LB, Fleisher A, Thiyyagura P, Roontiva A, McCabe C, Patsopoulos NA, Corneveaux JJ, Yu L, Huentelman MJ, Evans DA, Schneider JA, Reiman EM, De Jager PL, Bennett DA. Genetic susceptibility for Alzheimer disease neuritic plaque pathology. JAMA Neurol. 70(2013):1150–1157. doi: 10.1001/jamaneurol.2013.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shulman JM, Chen K, Keenan BT, Chibnik LB, Fleisher A, Thiyyagura P, Roontiva A, McCabe C, Patsopoulos NA, Corneveaux JJ, Yu L, Huentelman MJ, Evans DA, Schneider JA, Reiman EM, De Jager PL, Bennett DA. Genetic Susceptibility for Alzheimer Disease Neuritic Plaque Pathology. JAMA Neurol. 2013:1–7. doi: 10.1001/jamaneurol.2013.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka N, Abe-Dohmae S, Iwamoto N, Fitzgerald ML, Yokoyama S. Helical apolipoproteins of high-density lipoprotein enhance phagocytosis by stabilizing ATP-binding cassette transporter A7. J Lipid Res. 51(2010):2591–2599. doi: 10.1194/jlr.M006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka N, Abe-Dohmae S, Iwamoto N, Fitzgerald ML, Yokoyama S. HMG-CoA reductase inhibitors enhance phagocytosis by upregulating ATP-binding cassette transporter A7. Atherosclerosis. 217(2011):407–414. doi: 10.1016/j.atherosclerosis.2011.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka N, Abe-Dohmae S, Iwamoto N, Yokoyama S. Roles of ATP-binding cassette transporter A7 in cholesterol homeostasis and host defense system. J Atheroscler Thromb. 18(2011):274–281. doi: 10.5551/jat.6726. [DOI] [PubMed] [Google Scholar]
- 42.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 30(1991):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 43.Wang N, Lan D, Gerbod-Giannone M, Linsel-Nitschke P, Jehle AW, Chen W, Martinez LO, Tall AR. ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. J Biol Chem. 278(2003):42906–42912. doi: 10.1074/jbc.M307831200. [DOI] [PubMed] [Google Scholar]
- 44.Wildsmith KR, Holley M, Savage JC, Skerrett R, Landreth GE. Evidence for impaired amyloid beta clearance in Alzheimer's disease. Alzheimers Res Ther. 2013;5:33. doi: 10.1186/alzrt187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeller T, Wild P, Szymczak S, Rotival M, Schillert A, Castagne R, Maouche S, Germain M, Lackner K, Rossmann H, Eleftheriadis M, Sinning CR, Schnabel RB, Lubos E, Mennerich D, Rust W, Perret C, Proust C, Nicaud V, Loscalzo J, Hubner N, Tregouet D, Munzel T, Ziegler A, Tiret L, Blankenberg S, Cambien F. Genetics and beyond--the transcriptome of human monocytes and disease susceptibility. PLoS One. 2010;5:e10693. doi: 10.1371/journal.pone.0010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu H, Tucker HM, Grear KE, Simpson JF, Manning AK, Cupples LA, Estus S. A common polymorphism decreases low-density lipoprotein receptor exon 12 splicing efficiency and associates with increased cholesterol. Hum Mol Genet. 16(2007):1765–1772. doi: 10.1093/hmg/ddm124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zou F, Gopalraj RK, Lok J, Zhu H, Ling IF, Simpson JF, Tucker HM, Kelly JF, Younkin SG, Dickson DW, Petersen RC, Graff-Radford NR, Bennett DA, Crook JE, Younkin SG, Estus S. Sex-dependent association of a common low-density lipoprotein receptor polymorphism with RNA splicing efficiency in the brain and Alzheimer's disease. Hum Mol Genet. 17(2008):929–935. doi: 10.1093/hmg/ddm365. [DOI] [PMC free article] [PubMed] [Google Scholar]