Highlights
-
•
Mathematical model to predict the outcome of broiler chicken with and without vaccination after exposure to MDV.
-
•
Chance of MDV outbreak within a barn increases with the virulence of an MDV strain.
-
•
Vaccination significantly reduces the chance of an MDV outbreak.
-
•
Mortality due to MDV is an insufficient metric to assess the prevalence of MDV.
Keywords: Cohort dynamics, Poultry, Pathogens, Vaccination, Virulence
Abstract
Marek's disease virus (MDV), a poultry pathogen, has been increasing in virulence since the mid twentieth century. Since multiple vaccines have been developed and widely implemented, losses due to MDV have decreased. However, vaccine failure has occurred in the past and vaccine breakthroughs remain a problem. Failure of disease control with current vaccines would have significant economic and welfare consequences. Nevertheless, the epidemiology of the disease during a farm outbreak is not well understood. Here we present a mathematical model to predict the effectiveness of vaccines to reduce the outbreak probability and disease burden within a barn. We find that the chance of an outbreak within a barn increases with the virulence of an MDV strain, and is significantly reduced when the flock is vaccinated, especially when there the contaminant strain is of low virulence. With low quantities of contaminated dust, there is nearly a 100% effectiveness of vaccines to reduce MDV outbreaks. However, the vaccine effectiveness drops to zero with an increased amount of contamination with a middle virulence MDV strain. We predict that the larger the barn, and the more virulent the MDV strain is, the more virus is produced by the time the flock is slaughtered. With the low-to-moderate virulence of the strains studied here, the number of deaths due to MDV is very low compared to all-cause mortality regardless of the vaccination status of the birds. However, the cumulative MD incidence can reach 100% for unvaccinated cohorts, and 35% for vaccinated cohorts. These results suggest that death due to MDV is an insufficient metric to assess the prevalence of MDV broiler barns regardless of vaccine status, such that active surveillance is required to successfully assess the probability of MDV outbreaks, and to limit transmission of MDV between successive cohorts of broiler chickens.
Introduction
Marek's disease virus (MDV) is an airborne poultry pathogen primarily affecting chickens and causing losses to the industry of $1–2 billion annually (Morrow and Fehler, 2004). MDV causes a wide range of clinical signs depending on the virulence of the strain and the susceptibility of the host (Anderson et al., 1971; Witter, 1998; Payne, 2004; Nair and Kung, 2004; Nair, 2005). In order of increasing severity, clinical signs of Marek's disease (MD) include chronic polyneuritis, immunosuppression, lymphomas in visceral organs and other tissues, transient paralysis and acute brain oedema (Nair, 2005).
MDV has been increasing in virulence since the middle of the twentieth century (Witter, 1998; Morrow and Fehler, 2004) and live vaccines have been widely used to control MD since their development in 1970 (Powell and Lombardini, 1987; Bublot and Sharma, 2004). A feature of increased virulence of MDV in the USA has been the failure of successive vaccines (Witter, 1998) and recent outbreaks in both unvaccinated and vaccinated birds caused by more virulent strains of MDV have prompted fears that the current vaccines may be rendered ineffective with the emergence and spread of more virulent strains (Nair, 2005).
Despite these concerns, the epidemiological dynamics of MDV within a farm are not well understood, and the within-barn effectiveness of vaccines has not been systematically established. While there have been MDV outbreaks in many countries worldwide, data on the endemicity of MD within barns is not widely available due to the fact that hygiene and infection data are not made public by poultry companies and that MDV is not a notifiable disease (Morrow and Fehler, 2004). Only a limited number of field studies have evaluated either within-flock MD prevalence or mortality (Biggs et al., 1972, 1973; Jackson et al., 1976; Heier et al., 1999; Karpathy et al., 2003) or quantified within-barn viral burden (Walkden-Brown et al., 2013). Indeed, only three of these studies have collected data within the past 35 years. A recent Australian study reported that on average 26% of unvaccinated barns and 16% of vaccinated barns tested PCR-positive in dust samples for MDV-1, the pathogenic strain of MDV, during 2005–2011 (Walkden-Brown et al., 2013). However, these prevalence estimates varied between 3 and 55% depending on the region and year in which the sample was collected. Moreover, while there are known to be many strains of MDV-1, only one may be present within each barn at any one time, suggesting that without subsequent pathotyping experiments, it is difficult to understand the effect that MDV virulence has on MD outbreak dynamics within a barn. Moreover, the effect of farm conditions on outbreak potential and disease prevalence has never been systematically evaluated. Together, these limitations mean the effectiveness of vaccines and the impact of strain virulence on outbreak potential and disease prevalence have not been evaluated in the field. Similarly, we know of only a limited number of modeling studies evaluating on-farm MDV epidemiology, all using a deterministic approach (Gao et al. 2004, Gao et al. 2005a, Gao et al. 2005b). Moreover, while there is much literature on epidemiological systems in the role of pathogen persistence and virulence selection in a variety of systems (e.g. Anderson and May, 1982; Dieckmann, 2002; Read and Mackinnon, 2008), the dynamics within a temporally explicit cohort structure have not been well studied (with the notable exception of a modeling study of coccidiosis in broiler chickens Klinkenberg and Heesterbeek (2007)). For these reasons, there is a dearth of information about how vaccination and strain virulence may alter the probability of an MD outbreak if barn contamination occurs.
In a previous mathematical modeling study we have estimated the epidemiological implications for birds infected with an MDV strain under a range of strain virulence–vaccination combinations (Atkins et al., 2011). While this parameter estimation was important to understand the epidemiological implications of virulence within a single bird, these parameters on their own could not describe the barn-level dynamics of an outbreak. Understanding the effectiveness of vaccines within a barn can help poultry farms quantify the risk of MDV outbreak and better evaluate optimal control strategies. Furthermore, there are concerns that vaccinated birds can become infected with MDV, albeit at a reduced rate compared to unvaccinated birds, a viral reservoir will be maintained on which selection can act (Morrow and Fehler, 2004). Evaluating the predicted prevalence in an MDV outbreak as a function of strain virulence can elucidate selection pressure on MDV within a barn.
To aid the control of MD, here we provide a novel quantitative stochastic assessment of the epidemiological dynamics of MDV within a broiler cohort. Using a mathematical modeling approach, we predict the effectiveness of vaccines to control MD outbreaks and reduce MDV prevalence after an outbreak within a barn. We evaluate this vaccine effectiveness for different strain virulences under different barn conditions. We also extend these results to understand the relative impact of barn hygiene on the persistence of MDV from one cohort to another.
Methods
We developed an individual-based stochastic model of MDV infection within a flock of chickens. The model simulates a single cohort of susceptible broiler birds in a barn during an outbreak of MDV initiated by infected dust. This infected dust either enters the cohort barn at the same time as the cohort of birds, or is present in the barn as a result of insufficient cleaning after the previous flock of chickens has been removed.
We parameterized the individual-based model of MDV transmission dynamics with previously estimated posterior distributions for parameters governing the survival of MDV-infected birds and the timing and rate of virus shedding (Atkins et al., 2011) using data from laboratory experiments (Renz, 2008). We used these distributions to parameterize the characteristics of each individual bird (please refer to Table 2 for distributions of parameters). For each bird in the cohort, we sampled its viral shedding parameters, and its day of death given it becomes infected with MDV from these distributions. This Monte Carlo sampling approach provided a way to quantify the variability in disease progression observed in experimental data.
Table 2.
Sampled parameters for individual based model. All individual birds have infection characteristics sampled from these distributions. The single number refers to the median of the fitted posterior distribution estimated previously, and the range in brackets corresponds to the respective 95% credible interval of the posterior distribution. The two distributions provided for each parameter are from the two posterior distributions estimated from two replicate experiments using the same MDV virulence and vaccination status. The individual infection characteristics for each bird are sampled from either replicate distribution with equal probability. RND is a uniform random variable on the interval [0,1].
| Parameter | Symbol | Value/s |
|---|---|---|
| Primary viral shedding rate (logVCN/mg dust) | ||
| .5, no vaccine | 4.87 (3.79–5.25), 3.37 (2.79–4.05) | |
| , no vaccine | 3.86 (3.30–4.56), 3.28 (2.71–3.95) | |
| , no vaccine | 3.20 (2.64–3.90), 2.89 (2.34–3.59) | |
| , HVT | 3.79 (3.34–4.20), 2.44 (1.93–3.02) | |
| , HVT | 4.48 (4.19–4.92), 4.56 (4.13–4.91) | |
| , HVT | 2.70 (2.16–3.42), 4.15 (3.58–4.82) | |
| Secondary viral shedding rate (VCN/mg dust) | ||
| , no vaccine | 6.99 (6.72–7.21), 6.89 (6.72–7.07) | |
| , no vaccine | 7.34 (7.17–7.51), 7.58 (7.41–7.76) | |
| , no vaccine | 7.69 (7.51–7.85), 7.52 (7.32–7.72) | |
| , HVT | 6.83 (6.61–7.04), 6.35 (6.18–6.52) | |
| , HVT | 7.49 (7.28–7.70), 7.53 (7.31–7.75) | |
| , HVT | 7.50 (7.32–7.67), 7.29 (7.11–7.46) | |
| Primary delay until viral shedding (days) | ||
| , no vaccine | 6 (2–6), 4 (0–6) | |
| , no vaccine | 4 (0–6), 4 (0–6) | |
| , no vaccine | 3 (0–6), 3 (0–6) | |
| , HVT | 5 (0–6), 2 (0–6) | |
| , HVT | 6 (4–6), 6 (2–6) | |
| , HVT | 3 (0–6), 3 (0–6) | |
| Secondary delay until viral shedding (days) | ||
| , no vaccine | 10 (7–14), 9 (6–13) | |
| , no vaccine | 9 (6–13), 9 (6–13) | |
| , no vaccine | 9 (6–13), 9 (5–13) | |
| , HVT | 12 (8–17), 10 (7–13) | |
| , HVT | 11 (8–14), 12 (8–16) | |
| , HVT | 10 (6–13), 9 (6–13) | |
| Weibull scale intercept | β0 | 4.54 (4.23–4.87) |
| Weibull scale coefficient | ||
| β1 | −0.53 (−1.01–0.06) | |
| HVT | β2 | 0.44 (0.24–0.66) |
| Weibull scale parameter | ||
| no vaccine | ||
| HVT | ||
| Time until death due to MDV | ||
We estimated the probability of an outbreak with an initial quantity of infected dust for three relatively low-pathogenic strains for which we have parameter estimates. There are no longitudinal studies assessing the per-cohort MDV outbreak probability, so these model predictions serve as the first published estimates of MDV within-barn outbreak risk. Our model allows us to predict the infection prevalence and the quantity of virus left at the end of such an outbreak. Without much field data on prevalence caused by an MDV outbreak, the model provides information on the ramifications of an MDV outbreak within a flock. The model also predicts the extent to which MDV-associated mortality can be detected within a flock. For many flocks where MDV has not been detected and DNA samples have not been extracted, a model is able to predict the extent to which infection may still be occurring without detection. Due to the limited amount of field data for MDV load, infection prevalence, and mortality within flocks, we do not undertake any model calibration or validation. However, we offer discussion of the results and future data collection requirements towards the end of the paper.
Host infection
Infectious birds shed virus via excreted feather follicle epithelial cells, that collect as dust within the barn environment. MDV is transmitted only by inhalation of this infectious dust (Calnek et al., 1970). The daily probability of infection depends only on the concentration of virus-infected dust in the air and a bird's vaccination status (Table 1, estimated from data reported in Atkins et al. (2013)).
Table 1.
Parameters used in individual based simulation of on-farm epidemiology of MDV. Personal communication refers to Nick Sparks (NS). is the quantity of virus in the atmosphere (VCN) on day k for an outbreak of virulence in a cohort with vaccination status j (Fig. 1).
| Parameter | Symbol | Value/s | Reference |
|---|---|---|---|
| Daily dust production per bird (mg) | d(t, Tc) | 368 exp(− P(Tc)/t1.64) + 10.8 | Atkins et al. (2013) |
| Daily transmission probability per bird per VCN/m3 | |||
| Unvaccinated | αsham | 4.93 × 10−8 | From data in Atkins et al. (2011) |
| HVT | αHVT | 8.26 × 10−10 | From data in Atkins et al. (2011) |
| Total flock mortality (%) | μtotal | 3.6–6.8 | Sheppard (2004) |
| Daily non-MDV death probability | μ | Atkins et al. (2013) | |
| Age at introduction (days) | e | 2 | Sheppard (2004) |
| Height of barn (m) | h | 2.5 | NS pers. comm |
| Finishing weight (kg) | 2.5 | NS pers. comm | |
| Maximum dust threshold (mg/m3) | E | 7.15 | Takai et al. (1998) |
| Initial dust (mg) | D0 | 0–560 | Unknown |
| Vaccination status | j | Sham, HVT | Bublot and Sharma (2004) |
| Probability MDV infection on day k | p | Atkins et al. (2013) | |
| Growth scaling constant | P(Tc) | Atkins et al. (2013) | |
| Weibull shape parameter | r | 4.18 (3.38–4.99) | Atkins et al. (2011) |
| Stocking density (kg/m2) | sd | 5, 20, 35 | NS pers. comm |
| Initial cohort size (birds) | S0 | 500, 5000, 30000 | NS pers. comm |
| Cohort duration (days) | Tc | 30, 60 | Sheppard (2004) |
| Dust reduction at end of cohort (%) | ϵ | 0–100 | Unknown |
| Virus virulence score (%) | 16.5, 36, 46 | Atkins et al. (2011) | |
| Transformed virulence score | Atkins et al. (2011) | ||
| Barn volume (m3) | V(S0, sd) | S0wh/sd | Atkins et al. (2013) |
| Virus extinction threshold (VCN/m3) | ze | 10−9, 10−5 | Unknown |
Once infection occurs, we assume there is a delay, (days) until the bird sheds virus at a constant primary rate, (viral copy number (VCN)/mg dust). We then assume the bird sheds virus at a constant secondary rate, (VCN/mg dust) after a further delay, (days). The bird continues to shed at this secondary rate until it is removed from the barn. The subscript j refers to either sham (assumed to be identical to unvaccinated), or HVT (vaccinated), and corresponds to the virulence of the infecting MDV strain (see Methods section ‘Virus Strain’). We have previously estimated the distributions of these two delays and the two shedding rates (for more information, refer to Tables 2 and 3 in Atkins et al. (2011)). Each bird has its own primary and secondary delays/shedding rates sampled from these parameter distributions. Viral shedding is assumed to occur as infectious dust into the barn atmosphere.
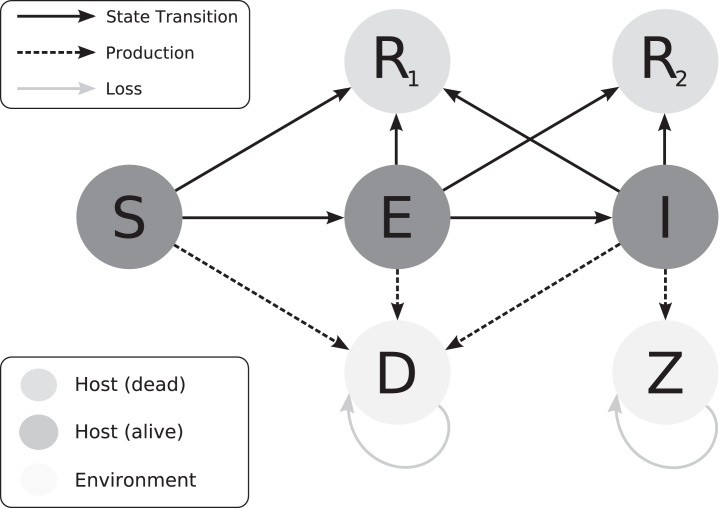
If infected, each bird's lifespan is dependent on the virulence of the infecting virus and its own vaccination status. The daily probability of death due to MDV is determined by the parameters of a Weibull survival analysis: the shape parameter (r), and the scale parameter (β), the latter of which is dependent on both the virulence of the infected virus and the bird's vaccination status. Parameter values are given in Table 2. More details on the estimation of these parameters and their values are given in Atkins et al. (2011). There is also an underlying daily mortality probability, μ, that acts to remove chickens from the population for reasons other than MDV. The bird may then die as a result of MDV infection only if it has not yet died of other causes or been slaughtered at the end of the cohort duration. A schematic of the chicken states and its environment is shown in Fig. 1.
Fig. 1.
Schematic of the state transitions of the host individuals in one cohort. Susceptible (S), infected-uninfectious (E), infected-infectious (I), removed-other cause (R1), removed-MDV related (R2) and production/loss of dust (D) and virus (Z).
Broiler population
We model a broiler population as a single cohort of S0 floor-reared broiler chickens. Consistent with broiler barns, we assume there is no immigration, but there is host mortality, both non-MDV- and MDV-related. The removal of dead birds is assumed to be done daily. At the end of day Tc, the remaining chicken population is removed for slaughter. Each bird sheds an amount of feather dander per day, d(t, Tc) = 326 exp(− P(Tc)/t1.64) + 10.8 where P is a decreasing function of the duration of the cohort that measures how quickly birds reach their finishing weight (Islam and Walkden-Brown, 2007; Atkins et al., 2013). Therefore, a bird raised in a 30-day cohort reaches its finishing weight twice as quickly than a bird raised in a 60-day cohort. These assumptions can therefore give rise to possible non-linear relationships between cohort duration and the quantity of virus and thus infection prevalence within the barns.
Barn housing
At the start of a cohort, we assume that the barn is seeded with an amount of infectious dust D0 (mg). We have previously estimated the average virus concentration in dust for a virus strain of virulence and host vaccination status, j (measured in VCN/mg) (Atkins et al., 2011) as . Therefore the quantity of virus at the start of the cohort, , is calculated as (VCN). Due to legal occupational exposure limits (OELs), dust concentrations are maintained between 2 and 10 mg/m3 in broiler barns (based on Northern European estimates at 28 days into the cohort) (Wathes, 1994, 1998; Takai et al., 1998). Therefore, we assume that ventilation of the barn is assumed to keep the concentration of dust in the air less than E, 7.15 mg/m3. We assume that the virus is well-mixed in the dust, and that the dust is well-mixed in the barn atmosphere.
If the virus concentration in the air drops below a critical point, ze (VCN/m3), below which no virus transmission can occur, the virus is assumed to be effectively extinct. Jurajda and Klimes (1970) showed that 44-day old virus had a similar infection potential as recently shed virus, and Carrozza et al. (1973) demonstrated that even infected dust 205 days old was still able to infect birds to a high degree. Therefore, we assume there is no virus decay rate for the relatively short period of each cohort duration. Nevertheless, the initial concentration of virus will drop due to ventilation in the event that no virus is shed into the atmosphere.
We evaluated MDV outbreak outcomes for different barn sizes: small – 500 birds at 5 kg/m2 (6253), medium – 5000 birds at 20 kg/m2 (1563 m3), and large – 30,000 birds at 35 kg/m2 (5357 m3). For comparison we also present results for a barn of 5000 birds at 5 kg/m2 (6250 m3) to evaluate the effect of increasing bird number (by comparison with 500 birds at 5 kg/m2) or stocking density (by comparison with 5000 birds at 20 kg/m2).
Virus strain
MDV isolates are characterized by a virulence score, similar to the pathotype devised by Witter (1997) and Witter et al. (2005). The virulence score is the percentage of cases exhibiting gross MD lesions 56 days following infection of birds vaccinated (calculated as an average for birds vaccinated with either HVT (herpesvirus of turkeys) or Bivalent (HVT and a non-pathogenic MDV serotype 2) vaccine). The three MDV isolates (04CRE, MPF57 and 02LAR) for which we have estimates for shedding rate and shedding delay and mortality rates have virulence scores of 16.5, 36, and 46 respectively (Atkins et al., 2011).
Model parameterization and simulation
The individual based model captures the variation in bird MD pathogenesis previously estimated. The daily infection probability (per VCN/m3 air), viral shedding parameters, MDV-related survival times are each assigned values for each individual bird, together with the gradient of the infection rate with virus concentration. The model was run 2500 times. Parameters are given in Tables 1 and 2. The model was written in C++ and compiled in Xcode 2.6.3.
Results
The standard deviations of the results are shown in all figures.
The probability of an outbreak
The probability of an MDV outbreak in a cohort was defined as the probability that at least one individual bird becomes infected with the initial contaminated dust. The expected probability of an outbreak can be calculated analytically as,
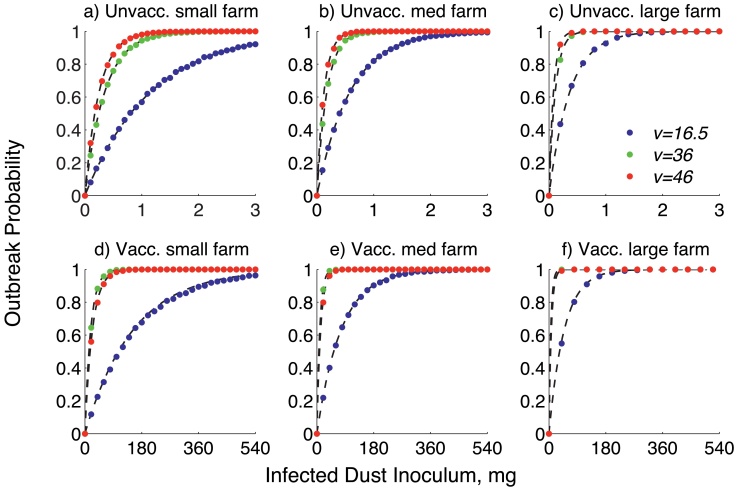
where is the average probability of a single bird being infected on day k given vaccination status, j, atmospheric quantity of virus, (VCN), and barn volume, V(S0, sd) (m3) (Fig. 2). Changing the extinction threshold for virus between 10−9 and 10−5 VCN/m3 does not change the probability of an outbreak. Note that any contaminated dust contains a quantity of virus, Z, the size of which depends on the virulence of the strain, , and the vaccination status of the birds in the cohort, j, because viral concentration in the dust depends on both and j.
Fig. 2.
Probability of an outbreak of MDV within a cohort of birds with varying initial dust, and different strains (blue, red, and green dots). Farms are small (500 birds at 5 kg/m2), medium (5000 birds at 20 kg/m2) or large (30,000 birds at 35 kg/m2). Birds are either all unvaccinated or vaccinated. The probability that there is any virus left (colored dots) is equivalent to the probability that at least one bird is infected (analytic results given by black dashed lines). Note the different x-axis domains on the plots d, e, and f. (a) Unvacc. small farm, (b) unvacc. medium farm, (c) unvacc. large fram, (d) vacc. small farm, (e) vacc. medium farm and (f) vacc. large farm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
The probability of an MDV outbreak was found to be equivalent to a positive amount of virus at the end of the cohort (Fig. 2). To the first order approximation, the number of birds in the barn does not affect the chance of an outbreak, as the probability of infection per bird is very small (results not shown).
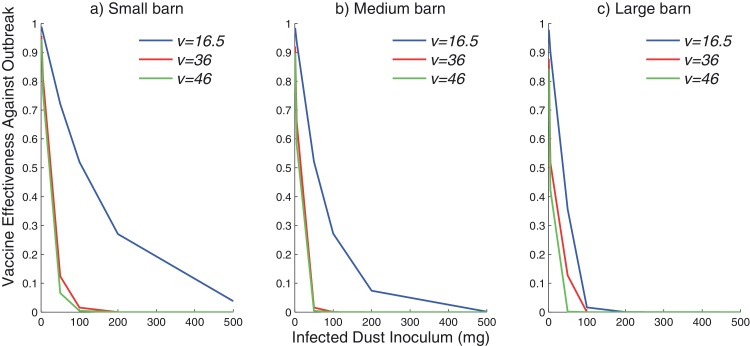
Vaccination can be effective at reducing the chance of an outbreak in a flock of birds, especially when the outbreak strain is of low virulence (Fig. 3). As the stocking density increases, vaccination becomes less effective at controlling the probability of an outbreak. For large industrial barns with a high stocking density of birds, vaccination does not offer any protection against outbreaks of all strains analyzed here when infected dust over 100mg is introduced (Fig. 3c).
Fig. 3.
Vaccine effectiveness for reducing an outbreak of MDV within a cohort of broiler birds with varying initial dust inocula and different strain virulence scores (). Farms are (a) small (500 birds at 5 kg/m2), (b) medium (5000 birds at 20 kg/m2), and (c) large (30,000 birds at 35 kg/m2). (a) Small barn, (b) medium barn and (c) large barn.
Cumulative virus
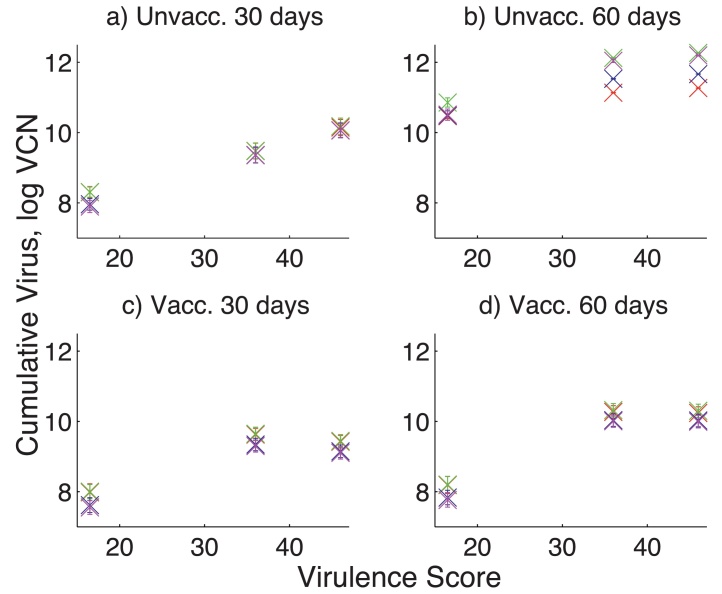
When an outbreak does occur, vaccination reduces the amount of virus left at the end of the cohort duration (Fig. 4). The total amount of virus predicted to be left at the end of an outbreak increases with both virulence of the strain and cohort duration (Fig. 4). The largest barn by volume (housing 5000 birds at 5 kg/m2), all else being equal, gives the largest end quantity of virus in all cases. Vaccinated cohorts infected with two of the most virulent strains cause similar virus densities at the end, due to their similar secondary shedding rates (Atkins et al., 2011).
Fig. 4.
Quantity of virus in the barn at the end of the cohort duration, given an outbreak of MDV has occurred. The amounts are calculated for different strain virulence, duration of the cohort (30 days or 60 days) and unvaccinated or vaccinated birds. Different colors on the figures show different sizes of farm: 500 birds at 5 kg/m2 (red), 5000 birds at 5 kg/m2 (green), 5000 birds at 20 kg/m2 (blue), 30,000 birds at 35 kg/m2 (magenta). (a) Unvacc. 30 days, (b) Unvacc. 60 days, (c) vacc. 30 days and (d) vacc. 60 days. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
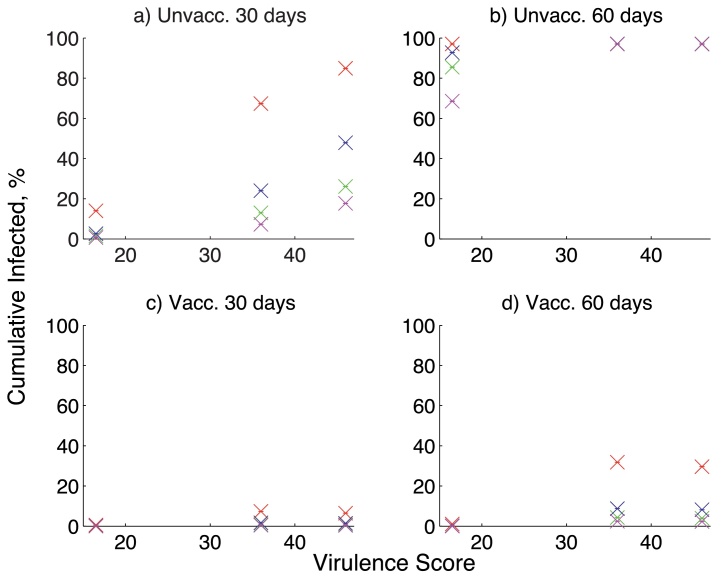
Cumulative infected individuals
For unvaccinated cohorts, more virulent strains infect a greater proportion of birds (Fig. 5). There is a considerable difference in the percentage of individuals infected after 30 days, but after 60 days, most, if not all, individuals are infected. This is in contrast to a vaccinated cohort, which only becomes 35% infected, even after 60 days and with the most virulent strain. Thus, vaccination is highly effective at reducing the infection prevalence by the end of the cohort duration (Table 3).
Fig. 5.
Cumulative percentage of infected birds by the end of the cohort duration, given an outbreak of MDV has occurred. The percentages are calculated for different strain virulence, duration of the cohort (30 days or 60 days) and unvaccinated or vaccinated birds. Different colors on the figures show different sizes of farm: 500 birds at 5 kg/m2 (red), 5000 birds at 5 kg/m2 (green), 5000 birds at 20 kg/m2 (blue), 30,000 birds at 35 kg/m2 (magenta). (a) Unvacc. 30 days, (b) unvacc. 60 days, (c) vacc. 30 days and (d) vacc. 60 days. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Table 3.
The effectiveness of vaccine introduction into a barn of broiler birds (% reduction compared to unvaccinated birds). Both measures of effectiveness are calculated at the end of a 60-day cohort for a vaccinated flock compared with an unvaccinated flock. Results are conditional on an outbreak occurring and are given for difference stocking densities (sd), flock size (N), and virulence scores () of the outbreak virus. Numbers in brackets are the 95% confidence intervals.
| Stocking density, sd | 5 | 5 | 20 | 35 |
| Cohort size, N | 500 | 5000 | 5000 | 30,000 |
| Effectiveness (%) against: | ||||
| Infection prevalence | ||||
| 98.9 (98.9,98.9) | 99.9 (99.9,99.9) | 99.9 (99.9,99.9) | 99.8 (99.8,99.8) | |
| 67.2 (67.2,67.3) | 97.5 (97.5,97.5) | 95.6 (95.6,95.6) | 91.0 (91.0,91.0) | |
| 69.5 (69.4,69.5) | 97.6 (97.6,97.6) | 95.9 (95.9,95.9) | 91.6 (91.6,91.6) | |
| Cumulative mortality | ||||
| 9.20 (−∞,100) | −55.0 (−∞,100) | 86.4 (−38.4,100) | −52.0 (−∞,100) | |
| 41.7 (−225,100) | 74.0 (31.2,94.0) | 36.2 (−230,100) | 73.2 (5.82,97.7) | |
| 97.0 (90.3,100) | 98.9 (97.8,99.8) | 98.6 (96.5,100) | 98.7 (97.0,100) | |
Cumulative removed individuals
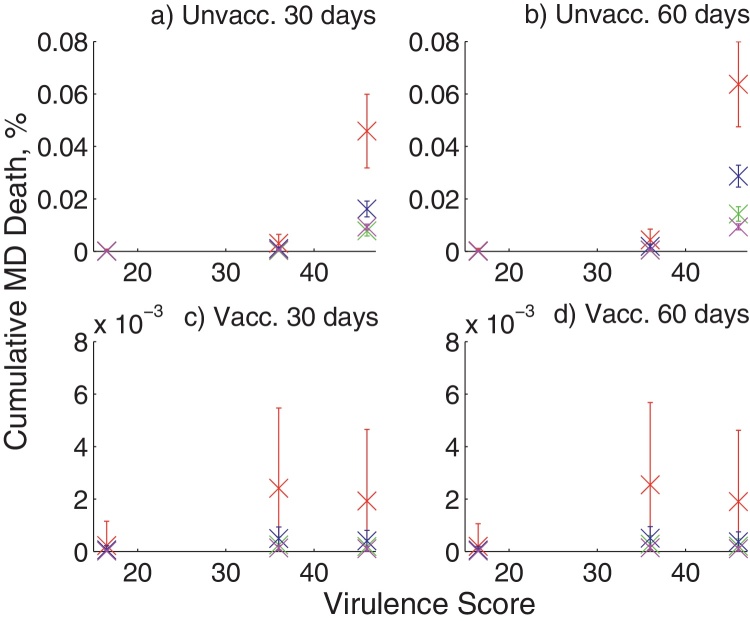
Vaccination is highly effective at reducing cumulative mortality (Table 3), although the percentage of deaths due to MDV is predicted to be very low for the wide range of scenarios we evaluated. In all cases the percentage of removed individuals due to MDV was less than 0.08% for unvaccinated cohorts, and below 0.006% for vaccinated cohorts (Fig. 6).
Fig. 6.
Cumulative percentage of birds that have been removed as a result of MDV by the end of the cohort duration, given an outbreak of MDV has occurred. The percentages are calculated for different strain virulence, duration of the cohort (30 days or 60 days) and unvaccinated or vaccinated birds. Different colors on the figures show different sizes of farm: 500 birds at 5 kg/m2 (red), 5000 birds at 5 kg/m2 (green), 5000 birds at 20 kg/m2 (blue), 30,000 birds at 35 kg/m2 (magenta). (a) Unvacc. 30 days, (b) unvacc. 60 days, (c) vacc. 30 days and (d) vacc. 60 days. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
The results describing the percentage of removed individuals who have died through MDV-induced disease match those found relating to the total infected individuals. Namely, increasing virulence and stocking density, in general, increase the percentage of removed individuals, whereas introducing vaccination and increasing population size reduces the fraction of removed individuals. Increasing cohort duration in a vaccinated population will increase the total removed individuals, which is not true in the unvaccinated population since the population can be saturated with infection.
Prevention of further outbreaks
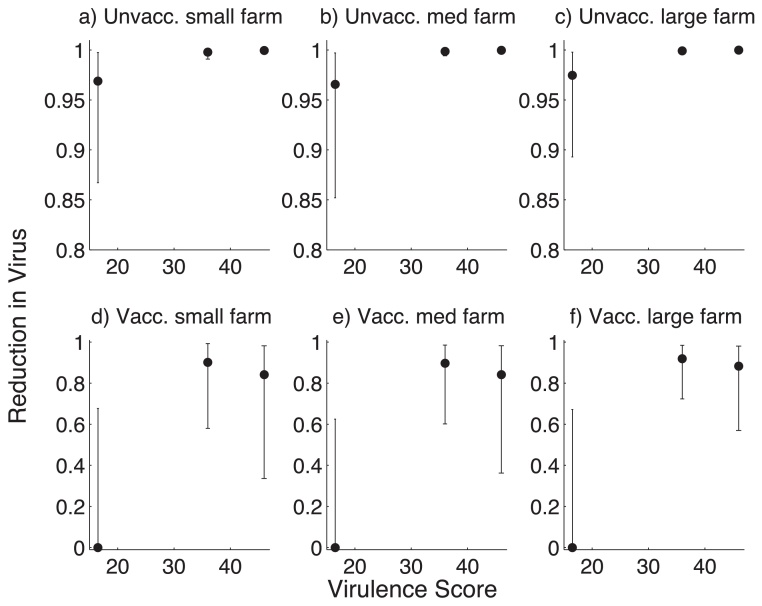
In many countries, barns are cleaned out, disinfected, and the litter replaced between cohorts. We predicted the reduction in virus after the first cohort required to decrease the probability of outbreak in the next cohort to 5, 50, and 95% (Fig. 7). We found that the reduction in virus required was less for vaccinated cohorts than for unvaccinated cohorts. For example, for moderately virulent strains in all cases, for a 50% or less probability of an outbreak in the next cohort, the reduction in virus had to be greater than 80% for vaccinated cohorts, whereas for unvaccinated cohorts, this value had to be greater than 99%.
Fig. 7.
Reduction of infected dust between cohorts that is required to reduce the probability of an outbreak in the next cohort to 50%, 95%, 5% (dots, upper and lower confidence intervals, respectively). Farms are small (5000 birds at 5 kg/m2), medium (20,000 birds at 20 kg/m2) or large (30,000 birds at 35 kg/m2). Birds are either all unvaccinated or vaccinated. Cohort duration is 30 days. (a) Unvacc. small farm, (b) unvacc. medium farm, (c) unvacc. large fram, (d) vacc. small farm, (e) vacc. medium farm and (f) vacc. large farm.
Discussion
We built an individual-based mathematical model to simulate a cohort of broiler chickens exposed to MDV infected dust to evaluate the effectiveness of vaccines to prevent MDV outbreaks and the effectiveness of vaccines against the impact of such an outbreak. We used previously estimated epidemiological parameters to capture the probabilistic nature of an outbreak occurring. These epidemiological parameters quantified the infection probability for birds, their viral shedding capabilities, and their disease-related mortality probability. We evaluated the probability of a barn MDV outbreak, the level of virus after an MDV outbreak, and the cumulative incidence of disease and mortality within the barn. We calculated the probability of an MDV outbreak as a function of the initial amount of virus in the barn. Therefore, the effect of strain virulence, of barn stocking density, of number of birds in the barn, and of vaccination status can all be evaluated by comparing the probability that a single bird gets infected with a given virus concentration in the atmosphere. The probability of an outbreak given a quantity of infected dust was dependent on the virulence of the strain. This is unsurprising because the probability of infection depends only on the concentration of virus material in the air, and this increases with virulence (i.e., the more virulent strains have larger quantities of virus per quantity of infected dust, and hence a greater initial concentration in the air). Assuming that the number of individual birds remains the same, the probability of an outbreak increases with the stocking density of birds. This is true because the initial virus concentration will be higher in the high stocking density environment, and hence the probability that the initial infected dust infects at least one bird is greater. Conversely, if the number of individuals doubles, the chances for new infections doubles, however, the volume of the barn also doubles, which means the virus concentration halves and thus the probability of an outbreak remains the same (density-independent transmission). Therefore an increase in both the stocking density and the number of individuals to current industrial conditions is also predicted to increase the chance of an outbreak if the barn was exposed to any MDV.
Dust within the barn is made up of several constituents such as feather dander and dust from feed, faeces, and litter materials. Our model only considers dust from feather dander, as it has been previously quantified (Islam et al., 2007; Atkins et al., 2011). Therefore, the atmospheric viral concentration calculated in the model is an upper estimate of the true viral concentration. A recent study showed that the viral concentration in sampled dust from an unvaccinated broiler cohort increased until at least 53 days of age, when it reached a concentration on the order of 4.6–4.9 logs (Walkden-Brown et al., 2013). Our results suggest that at 60 days during an unvaccinated cohort, the total virus amount varied between 10.5 and 12.2 logs, depending on the barn size and the virulence score. This corresponds to a viral concentration (VCN/mg dust) at the end of the cohort between 5.5 and 7.2 logs. Therefore, our estimates are slightly higher than those measured, consistent with our upper bound estimate of viral concentration in the dust. The discrepancy could also be due to other reasons, such as variation in host, MDV strain virulence, or barn dust concentration.
There is scant information on MDV prevalence and severity around the world, and even fewer data providing on-farm estimates. Three studies show wide range of MD incidence and mortality: first, Biggs et al. (1972) report mortality due to MD in unvaccinated broiler breeders. Biggs et al. (1972) estimate 0.6–23.4% of chickens die of MD in a production house between 8 and 20 weeks. The total mortality in the rearing house in the first 8 weeks of age was less than 2% in all pens, giving an upper limit to the losses attributable to MDV; second, Heier et al. (1999) report an estimated upper limit for MD-associated mortality for vaccinated layer flocks between 16 and 68 weeks as 5–8.2%; and third, Karpathy et al. (2003) report clinical signs and mortality from breeders from a large field vaccine trial of 21 flocks. Karpathy et al. (2003) report for the vaccinated flocks in which MD was detected, 2.1–51% of birds showed signs of MD lesions, with an estimated 0.11–4.82% dying of MDV infection within the 40 weeks of the study. Therefore, mortality due to MD in field studies is higher than those predicted by this study. For example, for an unvaccinated 60-day cohort, less than 0.08% of the flock die with an outbreak of medium virulence MDV. It is difficult to compare our results directly to field data due to potential differences in the method of housing, the duration of the cohort and when the data were collected, the vaccination status of the birds, the pathogenicity of the outbreak MDV strain, and the type of chicken and chicken line. It is known that all of these aforementioned factors can alter the mortality expected during an outbreak of MD. In particular, we have previously estimated that a bird infected with a strain of virulence score 50% can expect to live for an average of 80 days before it dies as a result of MDV infection (Atkins et al., 2011). Therefore, most of the MDV-related death would occur after the cohort duration of 30–60 days. Indeed, broiler birds do not usually live for long enough to die from MDV infection, unless the infecting strain is hyper-pathogenic, which we have not analyzed in this study (Witter, 1997).
Direct mortality due to MDV is not the only source of commercial loss as a result of MDV outbreaks; immunosuppression resulting in increased concurrent disease and reductions in weight gain are both important indirect effects associated with MDV infection (Islam et al., 2001, 2002; Baigent et al., 2006). As we have not included these indirect contributions of MDV infection, our model predictions regarding the overall impact of an MDV outbreak will be conservative. To assess the feasibility of cleaning barns to control within–barn MDV, it would be necessary to calibrate our model with longitudinal data assessing the persistence of MDV between cohorts. Ideally, we would require information on how the barn size, stocking density, vaccination status, and cleaning effort impacted the probability of outbreak persistence between subsequent cohorts. These data would help to identify the recurrence rate of MDV infection and to identify whether the most probable cause of MDV outbreaks are within–barn persistence or constant reintroduction. As there is very little known about the prevalence of MDV on farms around the world, this modeling work sheds lights on the extent to which farms may be affected by MDV. For industrial broiler farms, there are typically about 30,000 birds living together in one barn at a stocking density of 35 kg/m2 for between 30 and 60 days. Within this environment, if an outbreak occurs and there is no vaccination, we would expect only 0.01% cumulative mortality due to MDV (about three birds), which would be undetectable against the normal background cumulative mortality of around 5%. However, our model predicts an MDV prevalence before slaughter of between 25 and 100% for a medium virulence strain (02LAR, ), which would be persisting at extraordinary high concentrations within the barn. Therefore, despite there being no visible signs of Marek's disease, it would still require a 99.999% reduction in the MDV persisting at the end of the cohort to reduce the chance of an MDV occurring in the next generation to 5%. If the flock were vaccinated, there would likely be no deaths due to MDV, but up to 4% of the flock would be infected, and require over a 95% reduction in MDV within the barn to reduce the chance of a further outbreak in the next cohort. It is also worth noting that the virulence of the strains are mild to medium pathogenicity, and recent isolates can cause much higher rates of morbidity and a much quicker death (Witter, 2001). Despite the high effectiveness of vaccines to reduce MDV outbreaks and infection prevalence, vaccination allows the maintenance of high viral concentrations within a barn. These results suggest to industry that the death toll from a cohort may not be the best indicator for MDV prevalence in the flock and reliance of observing mortality may allow MDV to circulate freely in the broiler population. This undetected virus within environments will allow undetected spread from barn to barn, and onwards to other farms. Therefore, to limit MDV outbreaks within a cohort and to reduce the chance of farm persistence of MDV, these results suggest it may be necessary to combine a cohort vaccination with excellent hygiene. These model predictions are consistent with recent studies suggesting that mortality alone is a poor measure of virus load within a barn and that surveillance methods based on dust monitoring are likely to provide a better indicator of MDV outbreaks (Walkden-Brown et al., 2013).
This analysis provides the first quantitative assessment of the within-barn vaccine effectiveness and the impact of MDV exposure for a broiler barn. By better understanding the on-farm epidemiological dynamics of an increasingly virulent disease, improved methods for surveillance, prevention, and virulence management can be developed.
Acknowledgements
We thank Nick Sparks, Sue Baigent and Venu Nair for helpful discussion, David Kennedy for critical reading of the manuscript, and Don Klinkenberg for an incisive review. The work was funded by a BBSRC CASE (Pfizer) award.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Katherine E. Atkins, Email: Katherine.Atkins@yale.edu.
Andrew F. Read, Email: a.read@psu.edu.
Stephen W. Walkden-Brown, Email: swalkden@une.edu.au.
Nicholas J. Savill, Email: Nick.Savill@ed.ac.uk.
Mark E.J. Woolhouse, Email: Mark.Woohouse@ed.ac.uk.
References
- Anderson R.M., May R.M. Directly transmitted diseases: control by vaccination. Science. 1982;215:1053–1060. doi: 10.1126/science.7063839. [DOI] [PubMed] [Google Scholar]
- Anderson D.P., Eidson C.S., Richey D.J. Age susceptibility of chickens to Marek's disease. Am. J. Vet. Res. 1971;32:935–938. [PubMed] [Google Scholar]
- Atkins K.E., Read A.F., Savill N.J., Renz K.G., Walken-Brown S.W., Woolhouse M.E. Modelling Marek's Disease Virus (MDV) infection: parameter estimates for mortality rate and infectiousness. BMC Vet. Res. 2011;7:70. doi: 10.1186/1746-6148-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins K.E., Read A.F., Savill N.J., Renz K.G., Islam A.F.M.F., Walkden-Brown S.W., Woolhouse M.E.J. Vaccination and reduced cohort duration can drive virulence evolution: Mareks disease virus and industrialized agriculture. Evolution. 2013;67:851–860. doi: 10.1111/j.1558-5646.2012.01803.x. [DOI] [PubMed] [Google Scholar]
- Baigent S.J., Smith L.P., Nair V.K., Currie R.J.W. Vaccinal control of Marek's disease: current challenges, and future strategies to maximize protection. Vet. Immunol. Immunopathol. 2006;112:78–86. doi: 10.1016/j.vetimm.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Biggs P., Powell D., Churchill A., Chubb R. The epizootiology of Marek's disease: I. Incidence of antibody, Viraemia and Marek's disease in six flocks. Avian Pathol. 1972;1:5–25. doi: 10.1080/03079457208418048. [DOI] [PubMed] [Google Scholar]
- Biggs P., Jackson C., Powell D. The epizootiology of Marek's disease. 2. The effect of supply flock, rearing, house and production house on the incidence of Marek's disease. Avian Pathol. 1973;2:127–134. doi: 10.1080/03079457309353790. [DOI] [PubMed] [Google Scholar]
- Bublot M., Sharma J. Vaccination against Marek's disease. In: Davison F., Nair V., editors. Marek's Disease: An Evolving Problem. Elsevier Academic Press; London, UK: 2004. [Google Scholar]
- Calnek B.W., Adldinger H.K., Kahn D.E. Feather follicle epithelium: a source of enveloped and infectious cell-free herpesvirus from Marek's disease. Avian Dis. 1970;14:219–233. [PubMed] [Google Scholar]
- Carrozza J.H., Fredrickson T.N., Prince R.P., Luginbuhl R.E. Role of desquamated epithelial cells in transmission of Marek's disease. Avian Dis. 1973;17:767–781. [PubMed] [Google Scholar]
- Dieckmann U. Adaptive dynamics of pathogen-host interactions. In: Dieckmann U., Metz J.A.J., Sabelis M.W., Sigmund K., editors. Adaptive Dynamics Of Infectious Diseases: In Pursuit of Virulence Management. Cambridge University Press; New York, USA: 2002. [Google Scholar]
- Gao Z., Walkden-Brown S., Islam A., Groves P., Underwood G., Sergeant E. Proc. Aust. Poult. Sci. Symp. 16, volume 145–148. 2004. A model for Marek's disease transmission in broiler chickens; pp. 145–148. [Google Scholar]
- Gao Z., Walkden-Brown S., Islam A., Groves P., Underwood G. Proc. Aust. Poult. Sci. Symp. 17, volume 17. 2005. Effects of HVT vaccination-challenge interval and external contamination level on Marek's disease transmission among broiler chickens – a mathematical model based assessment; pp. 113–117. [Google Scholar]
- Gao Z., Walkden-Brown S., Islam A., Groves P., Underwood A. A mathematical model of Mareks disease epidemiology in broiler chickens. In: Nair V., Davison T., editors. Recent Advances in Mareks Disease Research: Proceedings of the 7th International Mareks Disease Symposium; Oxford; 2005. pp. 77–82. [Google Scholar]
- Heier B.T., Jarp J., Kaldhusdal M.I., Schaller G., Forus I.B. A longitudinal field study of mortality and Marek's disease in Norwegian and imported white leghorns. Prev. Vet. Med. 1999;40:207–219. doi: 10.1016/s0167-5877(99)00031-8. [DOI] [PubMed] [Google Scholar]
- Islam A., Walkden-Brown S.W. Quantitative profiling of the shedding rate of the three Marek's disease virus (MDV) serotypes reveals that challenge with virulent MDV markedly increases shedding of vaccinal viruses. J. Gen. Virol. 2007;88:2121–2128. doi: 10.1099/vir.0.82969-0. [DOI] [PubMed] [Google Scholar]
- Islam A.F.M.F., Walkden-Brown S.W., Burgess S.C., Groves P.J. Marek's disease in broiler chickens: effect of route of infection and herpesvirus of turkey-vaccination status on detection of virus from blood or spleen by polymerase chain reaction, and on weights of birds, bursa and spleen. Avian Pathol. 2001;30 doi: 10.1080/03079450120092116. [DOI] [PubMed] [Google Scholar]
- Islam A.F.M.F., Wong C.W., Walkden-Brown S.W., Colditz I.G., Arzey K.E., Groves P.J. Immunosuppressive effects of Marek's disease virus (MDV) and herpesvirus of turkeys (HVT) in broiler chickens and the protective effect of HVT vaccination against MDV challenge. Avian Pathol. 2002;31:449–461. doi: 10.1080/0307945021000005824. [DOI] [PubMed] [Google Scholar]
- Islam A.F.M.F., Walkden-Brown S.W., Groves P.J., Underwood G.J. Effects of vaccine dose, virus challenge dose and interval from vaccination to challenge on protection of broiler chickens against Marek's disease virus challenge. Aust. Vet. J. 2007;85:348–355. doi: 10.1111/j.1751-0813.2007.00195.x. [DOI] [PubMed] [Google Scholar]
- Jackson C., Biggs P., Bell R., Lancaster F., Milne B. The epizootiology of Marek's disease 3. The interrelationship of virus pathogenicity, antibody and the incidence of Marek's disease. Avian Pathol. 1976;5:105–123. doi: 10.1080/03079457608418176. [DOI] [PubMed] [Google Scholar]
- Jurajda V., Klimes B. Presence and survival of {M}arek's disease agent in dust. Avian Dis. 1970;14:188–190. [PubMed] [Google Scholar]
- Karpathy R., Firth G., Tannock G. Field evaluations of safety and efficacy of an Australian Mareks disease vaccine. Aust. Vet. J. 2003;81:222–225. doi: 10.1111/j.1751-0813.2003.tb11475.x. [DOI] [PubMed] [Google Scholar]
- Klinkenberg D., Heesterbeek J.A.P. A model for the dynamics of a protozoan parasite within and between successive host populations. Parasitology. 2007;134:949–958. doi: 10.1017/S0031182007002429. [DOI] [PubMed] [Google Scholar]
- Morrow C., Fehler F. Marek's disease: a worldwide problem. In: Davison F., Nair V., editors. Marek's Disease: An Evolving Problem. Elsevier Academic Press; London, UK: 2004. [Google Scholar]
- Nair V., Kung H.-J. In: Marek's Disease: An Evolving Problem. Davison F., Nair V., editors. Elsevier Academic Press; London, UK: 2000. [Google Scholar]
- Nair V. Evolution of Marek's disease – a paradigm for incessant race between the pathogen and the host. Vet. J. 2005;170:175–183. doi: 10.1016/j.tvjl.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Payne L.N. Pathological responses to infection. In: Davison F., Nair V., editors. Marek's Disease: An Evolving Problem. Elsevier Academic Press; London, UK: 2004. [Google Scholar]
- Powell P.C., Lombardini F. Vaccination against Marek's disease – past, present and future. Zootecnica Int. 1987 [Google Scholar]
- Read A., Mackinnon M. Oxford University Press; 2008. Pathogen Evolution in a Vaccinated World. [Google Scholar]
- Renz K. University of New England; Australia: 2008. In vitro and in vivo characterisation of selected Australian isolates of Marek's disease virus, Ph.D. Thesis. [Google Scholar]
- Sheppard, A., 2004. The Structure and Economics of Broiler Production in England, Defra Commissioned: Special Studies in Agricultural Economics, 65.
- Takai H., Pedersen S., Johnsen J.O., Metz J.H.M., Koerkamp P.W.G.G., Uenk G.H., Phillips V.R., Holden M.R., Sneath R.W., Short J.L., White R.P., Hartung J., Seedorf J., Schröder M., Linkert K.H., Wathes C.M. Concentrations and emissions of airborne dust in Livestock buildings in Northern Europe. J. Agric. Eng. Res. 1998;70:59–77. [Google Scholar]
- Walkden-Brown S., Islam A., Groves P., Rubite A., Sharpe S., Burgess S. Development, application and results of routine monitoring of Mareks disease virus in broiler house dust using real-time quantitative PCR. Avian Dis. 2013;57(2 Suppl):544–554. doi: 10.1637/10380-92112-REG.1. [DOI] [PubMed] [Google Scholar]
- Wathes C.M. Air and surface hygiene. In: Wathes C.R.C.D., editor. Livestock Housing. CAB International; Wallingford: 1994. pp. 123–148. [Google Scholar]
- Wathes C.M. Aerial emissions from poultry production. World Poultry Sci. J. 1998;54:241–251. [Google Scholar]
- Witter R.L., Calnek B.W., Buscaglia C., Gimeno I.M., Schat K.A. Classification of Marek's disease viruses according to pathotype: philosophy and methodology. Avian Pathol. 2005;34:75–90. doi: 10.1080/03079450500059255. [DOI] [PubMed] [Google Scholar]
- Witter R.L. Increased virulence of Marek's disease virus field isolates. Avian Dis. 1997;41:149–163. [PubMed] [Google Scholar]
- Witter R.L. The changing landscape of Marek's disease. Avian Pathol. 1998;27:S46–S53. [Google Scholar]
- Witter R.L. Protective efficacy of Marek's disease vaccines. Curr. Top. Microbiol. 2001;255:58–91. doi: 10.1007/978-3-642-56863-3_3. [DOI] [PubMed] [Google Scholar]