Abstract
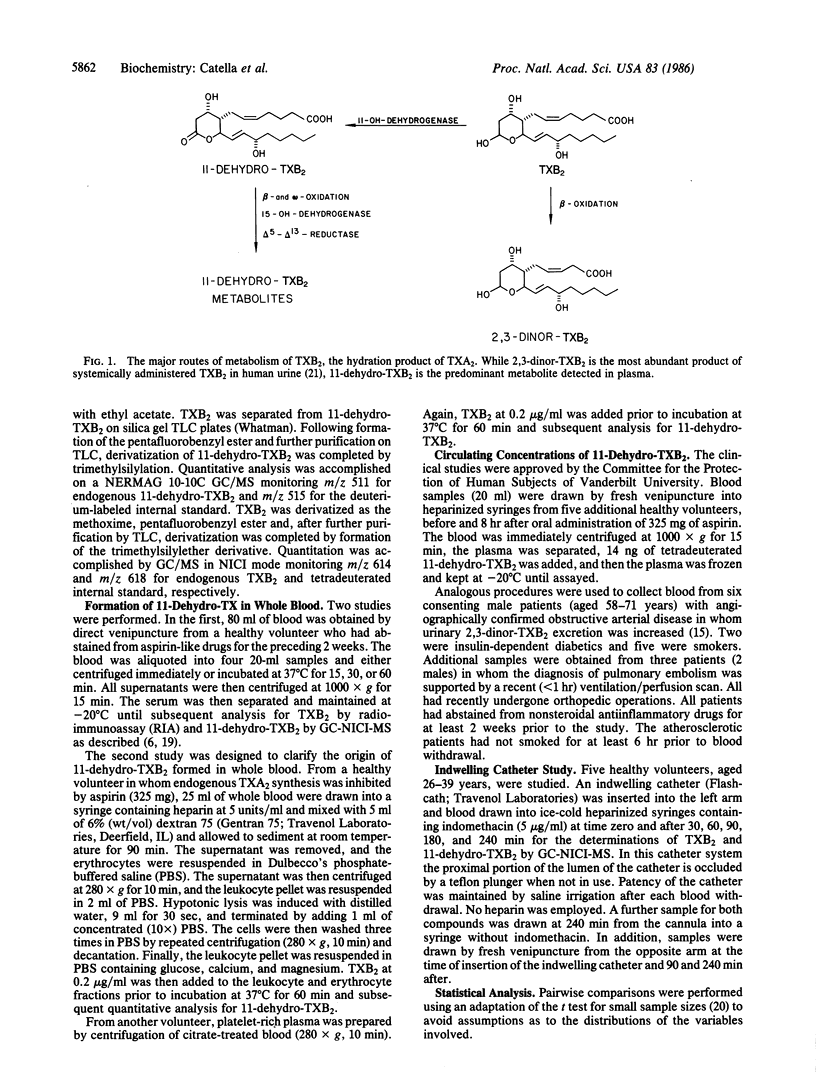
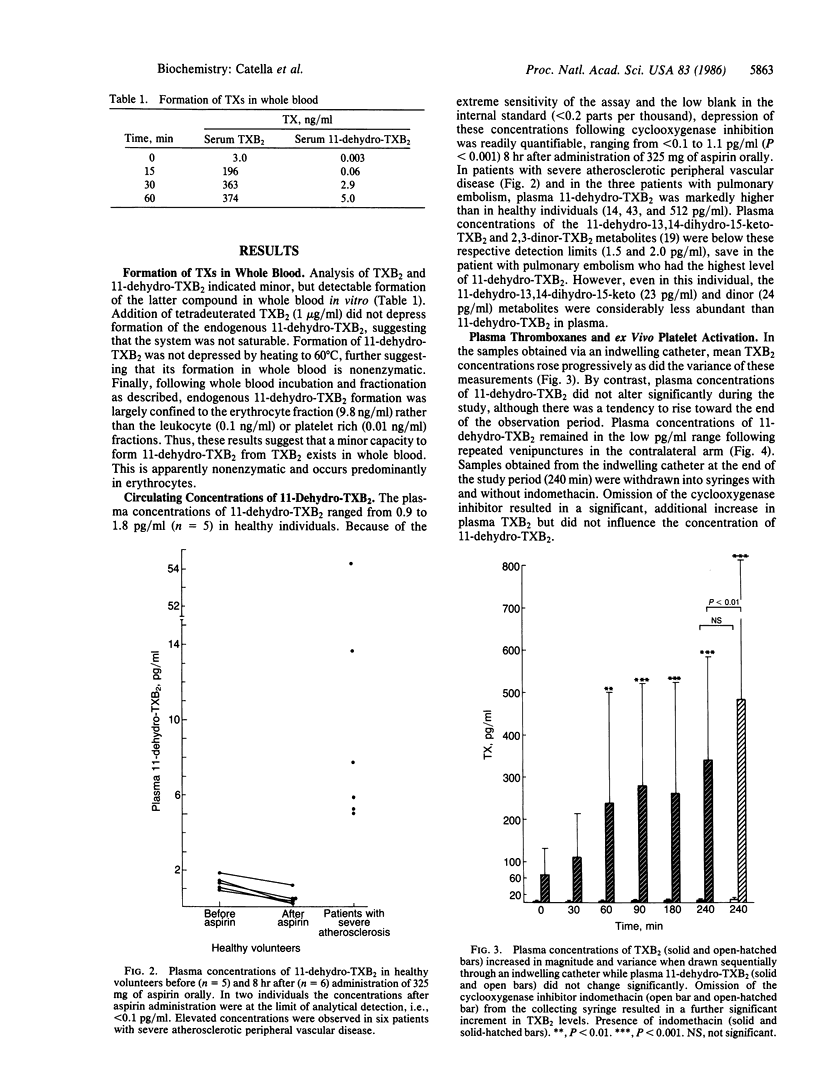
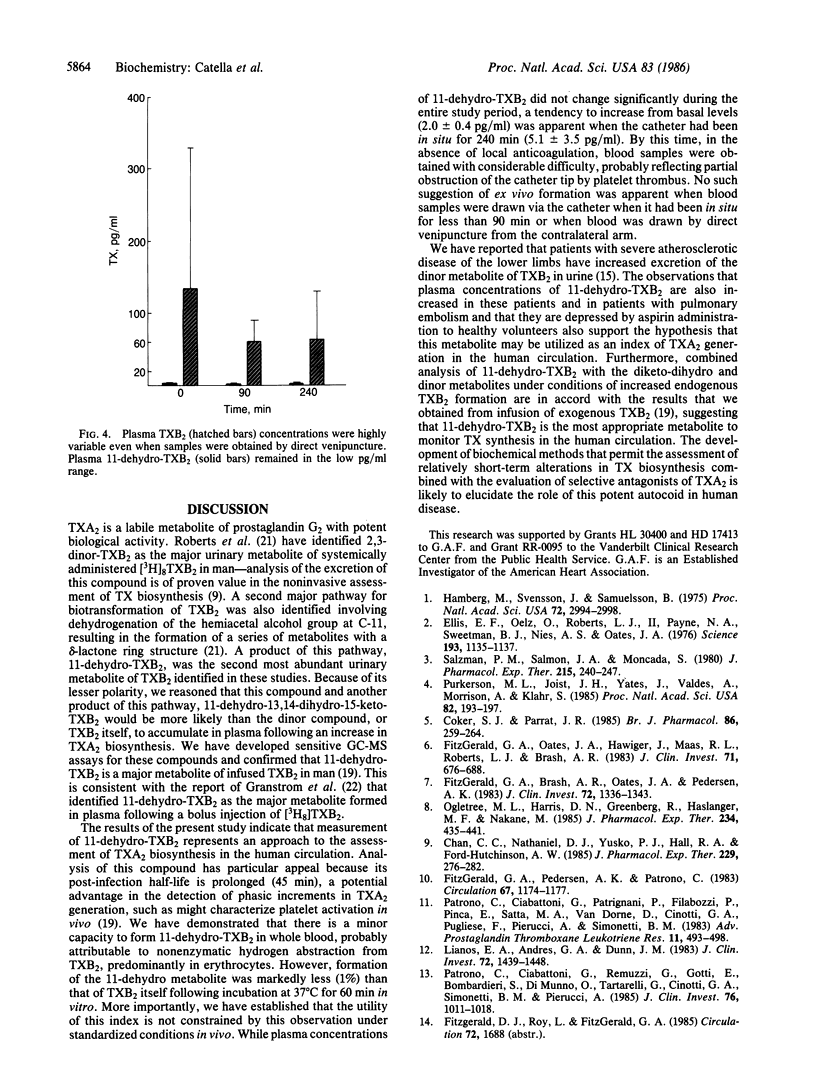
In human plasma, 11-dehydrothromboxane (TX) B2 is a major long lived metabolite (t1/2 45 min) formed from infused TXB2, the hydration product of biologically active TXA2. Plasma concentrations of TXB2 itself are readily confounded by ex vivo platelet activation and, theoretically, an enzymatic derivative of this compound, not subject to formation in whole blood, would more accurately reflect TXA2 formation in vivo. To address this hypothesis, we developed a sensitive assay for both 11-dehydro-TXB2 and TXB2, using capillary gas chromatography/negative-ion chemical ionization mass spectrometry. We established that whole blood possesses a minor capacity to form 11-dehydro-TXB2, attributable to nonenzymatic formation in erythrocytes. However, the nonenzymatic formation of 11-dehydro-TXB2 was not a practical limitation to its use as an index of TX biosynthesis. Blood was drawn from healthy volunteers (i) via an indwelling catheter at the time of insertion and at 30, 60, 90, 180, and 240 min thereafter and (ii) via separate venipunctures at 0 time and at 90 and 240 min thereafter. Plasma TXB2 drawn via the catheter at baseline (66 +/- 63 pg/ml) was substantially greater than the maximal estimate of endogenous TXB2 (1-2 pg/ml) in plasma [Patrono, C., Ciabattoni, G., Pugliese, F., Perruci, A., Blair, I. A. & FitzGerald, G. A. (1986) J. Clin. Invest. 77, 590-594] and increased in magnitude and variance over time (339 +/- 247 pg/ml at 240 min). By contrast, 11-dehydro-TXB2 did not change significantly in the sequential catheter samples or in the samples drawn by separate venipuncture. Basal plasma concentrations in volunteers were depressed by pretreatment with 325 mg of aspirin. Furthermore, the range of concentrations in patients with severe atherosclerosis in whom urinary 2,3-dinor-TXB2 was increased was significantly higher (5-50 pg/ml, P less than 0.01) than in healthy subjects (0.9-1.8 pg/ml). Concentrations of 11-dehydro-TXB2 were increased in patients who had recently suffered a pulmonary embolism to a greater extent than either the 11-dehydro-13,14-dihydro-15-keto-TXB2 or the 2,3-dinor-TXB2 metabolites in plasma. These results indicate that plasma TXB2 is readily confounded by platelet activation ex vivo. Measurement of enzymatic metabolites of TXB2 minimizes this problem. The 11-dehydro metabolite is the most appropriate analytic target to detect phasic release of TXA2 in the human circulation, such as might occur in human syndromes of platelet activation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan C. C., Nathaniel D. J., Yusko P. J., Hall R. A., Ford-Hutchinson A. W. Inhibition of prostanoid-mediated platelet aggregation in vivo and in vitro by 3-hydroxymethyl-dibenzo(b,f)thiepin 5,5-dioxide (L-640,035). J Pharmacol Exp Ther. 1984 Apr;229(1):276–282. [PubMed] [Google Scholar]
- Coker S. J., Parratt J. R. AH23848, a thromboxane antagonist, suppresses ischaemia and reperfusion-induced arrhythmias in anaesthetized greyhounds. Br J Pharmacol. 1985 Sep;86(1):259–264. doi: 10.1111/j.1476-5381.1985.tb09457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis E. F., Oelz O., Roberts L. J., 2nd, Payne N. A., Sweetman B. J., Nies A. S., Oates J. A. Coronary arterial smooth muscle contraction by a substance released from platelets: evidence that it is thromboxane A2. Science. 1976 Sep 17;193(4258):1135–1137. doi: 10.1126/science.959827. [DOI] [PubMed] [Google Scholar]
- FitzGerald G. A., Brash A. R., Oates J. A., Pedersen A. K. Endogenous prostacyclin biosynthesis and platelet function during selective inhibition of thromboxane synthase in man. J Clin Invest. 1983 Oct;72(4):1336–1343. doi: 10.1172/JCI111089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald G. A., Oates J. A., Hawiger J., Maas R. L., Roberts L. J., 2nd, Lawson J. A., Brash A. R. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest. 1983 Mar;71(3):676–688. doi: 10.1172/JCI110814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald G. A., Pedersen A. K., Patrono C. Analysis of prostacyclin and thromboxane biosynthesis in cardiovascular disease. Circulation. 1983 Jun;67(6):1174–1177. doi: 10.1161/01.cir.67.6.1174. [DOI] [PubMed] [Google Scholar]
- Granström E., Westlund P., Kumlin M., Nordenström A. Monitoring thromboxane production in vivo: metabolic and analytical aspects. Adv Prostaglandin Thromboxane Leukot Res. 1985;15:67–70. [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson J. A., Patrono C., Ciabattoni G., Fitzgerald G. A. Long-lived enzymatic metabolites of thromboxane B2 in the human circulation. Anal Biochem. 1986 May 15;155(1):198–205. doi: 10.1016/0003-2697(86)90247-2. [DOI] [PubMed] [Google Scholar]
- Lianos E. A., Andres G. A., Dunn M. J. Glomerular prostaglandin and thromboxane synthesis in rat nephrotoxic serum nephritis. Effects on renal hemodynamics. J Clin Invest. 1983 Oct;72(4):1439–1448. doi: 10.1172/JCI111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogletree M. L., Harris D. N., Greenberg R., Haslanger M. F., Nakane M. Pharmacological actions of SQ 29,548, a novel selective thromboxane antagonist. J Pharmacol Exp Ther. 1985 Aug;234(2):435–441. [PubMed] [Google Scholar]
- Patrono C., Ciabattoni G., Patrignani P., Filabozzi P., Pinca E., Satta M. A., van Dorne D., Cinotti G. A., Pugliese F., Pierucci A. Evidence for a renal origin of urinary thromboxane B2 in health and disease. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:493–498. [PubMed] [Google Scholar]
- Patrono C., Ciabattoni G., Pugliese F., Pierucci A., Blair I. A., FitzGerald G. A. Estimated rate of thromboxane secretion into the circulation of normal humans. J Clin Invest. 1986 Feb;77(2):590–594. doi: 10.1172/JCI112341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrono C., Ciabattoni G., Remuzzi G., Gotti E., Bombardieri S., Di Munno O., Tartarelli G., Cinotti G. A., Simonetti B. M., Pierucci A. Functional significance of renal prostacyclin and thromboxane A2 production in patients with systemic lupus erythematosus. J Clin Invest. 1985 Sep;76(3):1011–1018. doi: 10.1172/JCI112053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkerson M. L., Joist J. H., Yates J., Valdes A., Morrison A., Klahr S. Inhibition of thromboxane synthesis ameliorates the progressive kidney disease of rats with subtotal renal ablation. Proc Natl Acad Sci U S A. 1985 Jan;82(1):193–197. doi: 10.1073/pnas.82.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly I. A., Doran J. B., Smith B., FitzGerald G. A. Increased thromboxane biosynthesis in a human preparation of platelet activation: biochemical and functional consequences of selective inhibition of thromboxane synthase. Circulation. 1986 Jun;73(6):1300–1309. doi: 10.1161/01.cir.73.6.1300. [DOI] [PubMed] [Google Scholar]
- Roberts L. J., 2nd, Sweetman B. J., Oates J. A. Metabolism of thromboxane B2 in man. Identification of twenty urinary metabolites. J Biol Chem. 1981 Aug 25;256(16):8384–8393. [PubMed] [Google Scholar]
- Salzman P. M., Salmon J. A., Moncada S. Prostacyclin and thromboxane A2 synthesis by rabbit pulmonary artery. J Pharmacol Exp Ther. 1980 Oct;215(1):240–247. [PubMed] [Google Scholar]
- Taylor B. M., Sun F. F. Tissue distribution and biliary excretion of prostacyclin metabolites in the rat. J Pharmacol Exp Ther. 1980 Jul;214(1):24–30. [PubMed] [Google Scholar]



