Abstract
Background
Diffusion-weighted imaging (DWI) along with the calculation of apparent diffusion coefficient (ADC), is a novel, non-invasive, and reliable technique of choice for accurate assessment and for the treatment planning of different types of brain tumors. It is more advantageous in the distinction and differentiation of benign from malignant meningiomas on the basis of ADC values.
Purpose
To investigate the utility of DW magnetic resonance imaging (MRI), and to compare the apparent diffusion coefficient (ADC) obtained at two b-values for an authentic and preoperative characterization of meningiomas.
Material and Methods
Twenty-six patients with clinically diagnosed or histologically verified meningioma (18 benign and 8 malignant) underwent imaging including DWI at 1.5 T. DW images were obtained at b = 1000 s/mm2 and b = 2000 s/mm2, ADC maps were generated at both the b-values. Signal intensities (SIs) and ADCs for solid tumorous tissues, contralateral normal tissues, and peritumoral edema were calculated and normalized ADC (NADC) ratio were determined for tumorous tissues. SI scores, ADC maps, and ADC values were analyzed visually and quantitatively, and were compared at both the b-values.
Results
DW images at b = 2000 s/mm2 were more conspicuity (either hyperintense or hypointense) with improved contrast. The mean ADC of malignant meningiomas (0.64 ± 0.05 and 0.42 ± 0.03) was significantly lower (P < 0.05) as compared with benign meningiomas (1.04 ± 0.12 and 0.80 ± 0.07) at both the b-values. Mean NADC ratio in the malignant type was also significantly lower (P < 0.05) than the benign type at both the b-values. Mean ADC values for peritumoral edema do not differ between benign and malignant meningiomas.
Conclusion
1.5-T DWI using high b-values improved our ability to differentiate benign from malignant meningiomas. DWI may play an important role in the preoperative radiological evaluation and the recognition of these types for proper surgical treatment.
Keywords: Diffusion-weighted imaging (DWI), apparent diffusion coefficient (ADC), meningioma, malignant, benign
Introduction
Meningiomas are promptly diagnosed by magnetic resonance imaging (MRI), constituting 14–20% of almost all intracranial tumors. In spite of the fact that they are usually benign, up to 10% of these tumors are atypical or malignant (1). Typical benign meningiomas are easily diagnosed, but their distinction from more malignant by using conventional MRI is still a difficulty (2). Heterogeneous appearance and enhancement, edema around the lesion, and irregular cerebral surface are not consistent and unique neuroimaging features for diagnosing malignant meningiomas (3 –6). To define a diagnostic method is desirable for accurate differentiation between benign and malignant meningiomas before surgery, to aid in surgical and treatment planning. This distinction is not reliably accomplished, when assessing the imaging features of meningiomas on conventional MR images (7 –9).
Diffusion-weighted imaging (DWI) along with the calculation of apparent diffusion coefficient (ADC), is a novel, non-invasive, and reliable technique of choice for the preoperative assessment and for the treatment planning of different types of brain tumors (10 –13). Quantitatively, the signal attenuation depends on ADC exponentially. ADCs calculated most frequently from the mono-exponential model Sb/S0 = exp (– b × ADC) (S0, signal intensity with diffusion gradients turned off, b = 0; Sb, signal intensity with diffusion gradients turned on; S(b), described as a decaying exponential: S(b) αe- b × A DC).
The objectives of our study were to evaluate the benefits of DW MRI method, using greater b-values (b = 2000 s/mm2) and to investigate whether it is more advantageous in the distinction and differentiation of benign from malignant meningiomas on the basis of ADC values. A preoperative authentic characterization of disease would be of supreme importance for treatment planning.
Material and Methods
Twenty-six patients with meningiomas (age range, 32–82 years; mean age, 42.9 years; 6 men, 20 women) were enrolled in this study. Patients who underwent MR exams between April 2012 and March 2013 were included in the study. Eighteen patients underwent surgery and biopsy, and the diagnosis was confirmed by a histopathologic examination. All other patients were clinically diagnosed on the basis of MRI.
MR techniques and imaging data
All patients were examined using Philips new generation Medical Imaging system, (Achieva Intera 1.5 T “A” series; Philips Medical Systems, Best, The Netherlands), equipped with an 8-channel head coil with SENSE factor. The images were analyzed using DICOM software (Philips Dicom Viewer R1.1V1L1-SP01; Philips Medical Systems, Best, The Netherlands) and at the Precision work station (670 series; software version, R5.1V1L1SP1). All patients underwent DW MRI at b = 1000 s/mm2 and 2000 s/mm2. In addition, we obtained unenhanced axial and sagittal T2-weighted images (T2W) and unenhanced axial and sagittal T1-weighted (T1W) images.
DW images were obtained using a single-shot echo-planar spin echo technique, following the parameters in Table 1 with diffusion gradients in three directions.
Table 1.
MRI pulse parameters.
| Parameters | T1W | T2W | DW |
|---|---|---|---|
| Pulse sequence | FSE | TSE | SS-EP-SE |
| Repetition time (ms) | 422 | 4500 | 2575/3153 |
| Echo time (ms) | 11 | 100 | 89 |
| b-values (s/mm2) | - | - | 0, 1000, 2000 |
| Slice thickness/interslice gap (mm) | 5/1.5 | 5/1.5 | 5/1.5 |
| Field of view (mm) | 220 | 220 | 220 |
Data calculation
DW images were visually inspected and classified as hyperintense, isointense, or hypointense compared with normal white matter. ADC maps were generated automatically for both the b-values (1000 s/mm2, 2000 s/mm2) with a pixel-by-pixel analysis, using advanced workstation software (Philips Medical Imaging System) after DWI data acquisition. ADC values were recorded from the ADC maps for solid portion of the tumor, for the contralateral normal area, and for edema (these regions were sampled avoiding the cystic and necrotic areas), by drawing the circular region of interest (ROI) manually, on all axial ADC maps, using the manufacturer’s software. The ROIs measurements varied from 20 to 40 mm2. For the tumor giving variable SI, highest intensity ROI was selected and its SI was noted. In some cases more than one ROI were drawn and an average ADC value was recorded. ADC of the tumor were divide by the ADC of the normal white matter to find the normalized ADC (NADC) ratios, considering NADCs < 1.00 represent relatively restricted diffusion.
Statistical analysis
Statistical analysis was performed using the SPSS (Statistical Package for the Social Sciences) software package, version 17 (SPSS Inc., Chicago, IL, USA).
Results
Conventional MRI scans (T1W, T2W) revealed heterogeneous signal intensities. Solid tumorous tissues exhibited usual high intensity (hyperintense or bright) on T2W images and less intensity (hypointense or dark) on T1W images. On DW MRI, the SI of 18 benign meningiomas was variable (Fig. 1) with 12 hypointense or four isointense or two hyperintense at b = 1000 s/mm2 and 15 hypointense or three isointense at b = 2000 s/mm2.
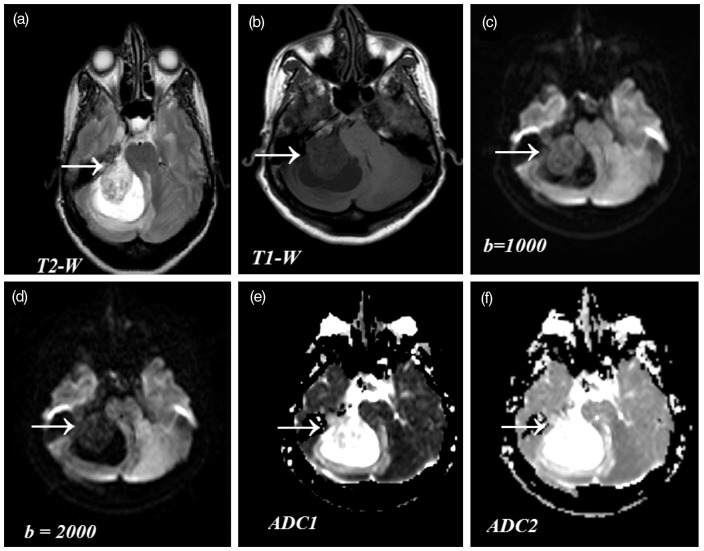
Fig. 1.
Comparison of SIs in conventional MR images and DW images. Findings of meningioma in a 47-year-old woman (biopsy proven case). (a) Axial T2W image (4500/100 ms) showing mixed intensity (from iso to high) with hyperintense peritumoral edema. (b) Axial T1W image (422/11 ms) showing hypointense lesion. (c) DW axial image (2575/89 ms, b = 1000), showing solid portion of the tumor as isointense to mildly hypointense. (d) DW axial image (3153/89 ms b = 2000) showing markedly hypointensity with increased diffusion. (e) ADC1 map at b = 1000 and (f) ADC2 map at b = 2000, show more conspicuous intensities differentiating tumor and edema.
Of eight malignant meningiomas, two were hypointense, one was isointense, three were hyperintense, and two had mixed hyperintense and isointense signal intensity at b = 1000 s/mm2, and two were hypointense, two were isointense, and four were hyperintense at b = 2000 s/mm2.
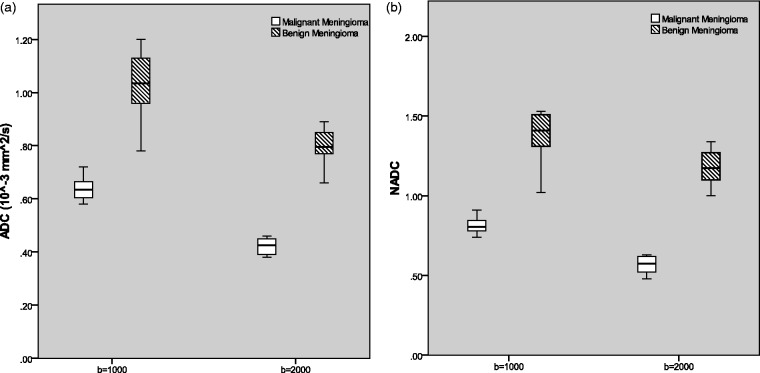
Table 2 shows the calculated mean values SD and ranges at both b-values. Absolute ADC in benign meningiomas ranged from (0.66 to 1.20 × 10–3 mm2/s), whereas the NADC ratio ranged from (1.0 to 1.53 × 10–3 mm2/s). In malignant meningiomas, absolute ADC ranged from (0.38 to 0.72 × 10–3 mm2/s), and NADC ranged from (0.52 to 0.91 × 10–3 mm2/s). All of the malignant meningiomas had absolute ADC and NADC ratios < 1. The variation of the absolute ADC values, NADC values and data ranges are displayed in Fig. 2 for malignant and benign groups at both b-values.
Table 2.
Mean ADC (10–3 mm2/s) and NADC values.
| Mean ADCs (10−3 mm2/s) and NADCs | At b = 1000 s/mm2 Mean ± SD | Range | At b = 2000 s/mm2 Mean ± SD | Range |
|---|---|---|---|---|
| ADC of malignant meningioma | 0.64 ± 0.05 | 0.58–0.72 | 0.42 ± 0.03 | 0.38–0.46 |
| ADC of benign meningioma | 1.04 ± 0.12 | 0.78–1.20 | 0.80 ± 0.07 | 0.66–0.89 |
| NADC of malignant meningioma | 0.81 ± 0.06 | 0.74–0.91 | 0.57 ± 0.06 | 0.52–0.63 |
| NADC of benign meningioma | 1.39 ± 0.15 | 1.02–1.53 | 1.18 ± 0.11 | 1.0–1.3 |
Fig. 2.
Box and whisker plot showing a comparison of ADC values between benign and malignant type at both the b-values. (a) Absolute ADC values of tumorous tissues in benign and malignant meningiomas showing some overlap between two types. (b) Comparison of NADC ratios between benign and malignant meningiomas at b = 1000 and 2000 s/mm2. The horizontal line is the median, vertical lines represent data ranges; two halves of the rectangular boxes represent the upper and lower quartiles.
Mean ADC of malignant meningiomas was found significantly lower (P < 0.05) than the benign meningioma (0.64 ± 0.05 vs. 1.04 ± 0.12 and 0.42 ± 0.03 vs. 0.80 ± 0.07) at both b-values. The mean NADC ratio in the malignant group was also significantly lower (P < 0.05) than in the benign group (0.81 ± 0.06 vs. 1.39 ± 0.15 and 0.57 ± 0.06 vs. 1.18 ± 0.11) at both b-values. Although absolute ADC values showed considerable overlap, there was no NADC ratio overlap between groups. ADC and NADC ratios in malignant meningiomas were significantly lower than in benign tumors.
Mean ADCs of tumorous tissues were found to be reduced from 21% to 38%, when they were compared with the ADCs of normal contralateral area, at both b-values. The overall accuracy of conventional MRI to predict patients having malignant meningioma was 73.2%, with a sensitivity of 67.0%, specificity of 83.2%, positive predictive value (PPV) of 82.0%, and negative predictive value (NPV) of 68.3%.
Discussion
The technique of high b-value diffusion imaging was found useful in distinguishing areas of predominantly peritumoral edema from the areas of predominantly non-enhancing tumor. Moreover, DW images at b = 2000 s/mm2 were more sensitive than images at b = 1000 s/mm2 on visual and on quantitative analysis for predicting localized tumor.
For the normal contralateral area, mean ADC values were found 0.77 ± 0.087 at b = 1000 s/mm2 and 0.67 ± 0.06 at b = 2000 s/mm2.
For peritumoral edema, mean ADC values at b = 1000 s/mm2 were significantly different than mean ADC at b = 2000 s/mm2 (P = 0.017). Meningioma showed a wide range of ADCs from 0.58 to 1.02 × 10–3 mm2/s at b = 1000 s/mm2 and from 0.38 to 0.89 × 10–3 at b = 2000 s/mm2. Mean ADC values for both benign and malignant type at b = 1000 s/mm2 were significantly lower than mean ADC at b = 2000 s/mm2. The basic mechanism behind this is based on the fact that free water molecule diffusivity is restricted by increased cellularity of tumors, causing a relative reduction in the ADC values. ADC is altered by the translational movement of hydrogen spins in the extracellular space because of the swelling or increased cellularity.
The delineation of meningioma on DW images and ADC maps was inferior to that on T2W turbo spin-echo and unenhanced T1W spin-echo images.
DWI allows us to assess the tumor cellularity non-invasively because cellular and subcellular elements significantly impede the mobility of water molecules, thus these densely cellular regions exhibit low ADCs. As increased cellularity in the tumorous tissues restrict the normal water movement and reduced the ADC value than normal and vice versa. The presence of edema exhibits an increased diffusion coefficient; in addition ADC values can differentiate cytotoxic edema from vasogenic edema.
Appearance of meningiomas on the DW images was variable. This study reliably predicts that abnormally increased ADC values of meningiomas would have histopathological findings, e.g. all benign meningiomas have the highest ADCs. One of the benign type meningiomas, rich in intracellular fluid, has the most elevated ADC value (2.47 × 10–3 mm2/s) and was found to be microcystic. Another produces prominent edema out of proportion to lesion size, which may be associated to the marked pericytic proliferations often present in this tumor type. After analyzing the hypointensity of meningiomas on DWI, we hypothesize that all the meningiomas which appeared hypointense on DW images with increased ADCs contained increased amounts of fluid within them. This fluid may produce a suitable environment for the free movement of hydrogen spins or less restriction to molecular diffusion. ADCs of the meningiomas were found to be correlated inversely with the grade of meningiomas on the basis of histological results, and at high b-value, a low ADC might imply a high-grade meningioma. Decrease in ADC values with the increase in b-values can be explained by bi-exponential signal decay. Human brain model describes two components of diffusion, fast and slow, so the SI is governed by slow diffusion at high b-values, and by fast diffusion at comparatively low b values (14 –16). Our study used a monoexponential model for water diffusion decay and the mean ADC values of tumors at a b-value of 1000 s/mm2 were higher than those at a b-value of 2000 s/mm2. The reason for this decrease is unknown but one can speculate that the fraction for the component of slow diffusion is larger in brain tumors and edema than in normal contralateral tissues of brain.
In conclusion, the technique of high b-value DWI was found useful in distinguishing abnormalities located in the white matter. ADCs of tumors provided additional information. DW images at b = 2000 s/mm2 showed contrast features reflecting the relative ADCs accurately and were more clear and of high quality than the images at b = 1000 s/mm2. It increases the sensitivity and specificity. Dependence of ADC on b-value must be included in the interpretation of quantitative measurements. Interpretation of DW images obtained with b-values > 1000 s/mm2 must consider an increasing contribution of diffusion weighting and diminished T2 effects.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Hakyemez B, Yildirim N, Gokalp G, et al. The contribution of diffusion weighted MR imaging to distinguishing typical from atypical meningiomas. Neuroradiology 2006; 48: 513–520 [DOI] [PubMed] [Google Scholar]
- 2. Carpeggiani P, Crisi G, Trevisan C. MRI of intracranial meningiomas: correlations with histology and physical consistency. Neuroradiology 1993; 35: 532–536 [DOI] [PubMed] [Google Scholar]
- 3. Jaaskelainen J, Haltia M, Laasonen E, et al. The growth rate of intracranial meningiomas and its relation to histology: an analysis of 43 patients. Surg Neurol 1985; 24: 165–172 [DOI] [PubMed] [Google Scholar]
- 4. Mahmood A, Caccamo DV, Tomecek FJ, et al. Atypical and malignant meningiomas: a clinicopathological review. Neurosurgery 1993; 33: 955–963 [DOI] [PubMed] [Google Scholar]
- 5. Zee CS, Chin T, Segall HD, et al. Magnetic resonance imaging of meningiomas. Semin Ultrasound CT MR 1992; 13: 154–169 [PubMed] [Google Scholar]
- 6. Modha A, Gutin PH. Diagnosis and treatment of atypical and anaplastic meningiomas: a review. Neurosurgery 2005; 57: 538–550 [DOI] [PubMed] [Google Scholar]
- 7. Verheggen R, Finkenstaedt M, Bockermann V, et al. Atypical and malignant meningiomas: evaluation of different radiological criteria based on CT and MRI. Acta Neurochir (Wien) 1996; 65: 66–69 [DOI] [PubMed] [Google Scholar]
- 8. Yamaguchi N, Kawase T, Sagoh M, et al. Prediction of consistency of meningiomas with preoperative magnetic resonance imaging. Surg Neurol 1997; 48: 579–583 [DOI] [PubMed] [Google Scholar]
- 9. Goebell E, Paustenbach S, Vaeterlein O, et al. Low-grade and anaplastic gliomas: differences in architecture evaluated with diffusion-tensor MR imaging. Radiology 2006; 239: 217–222 [DOI] [PubMed] [Google Scholar]
- 10. Kwee TC, Galban CJ, Tsien C, et al. Intravoxel water diffusion heterogeneity imaging of human high-grade gliomas. NMRBiomed 2010; 2: 179–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higano S, Yun X, Kumabe T, et al. Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology 2006; 241: 839–846 [DOI] [PubMed] [Google Scholar]
- 12. Murakami R, Hirai T, Sugahara T, et al. Grading astrocytic tumors by using apparent diffusion coefficient parameters: superiority of a one- versus two-parameter pilot method. Radiology 2009; 251: 838–845 [DOI] [PubMed] [Google Scholar]
- 13. Filippi CG, Edgar MA, Ulug AM, et al. Appearance of meningiomas on diffusion-weighted images: correlating diffusion constants with histopathologic findings. Am J Neuroradiol 2001; 22: 65–72 [PMC free article] [PubMed] [Google Scholar]
- 14. Kawahara Y, Nakada M, Hayashi Y, et al. Prediction of high-grade meningioma by preoperative MRI assessment. J Neurooncol 2012; 108: 147–152 [DOI] [PubMed] [Google Scholar]
- 15. Hakyemez B, Yildirim N, Gokalp G, et al. The contribution of diffusion-weighted MR imaging to distinguishing typical from atypical meningiomas. Neuroradiology 2006; 48: 513–520 [DOI] [PubMed] [Google Scholar]
- 16. Alvarez-Linera J, Benito-León J, Escribano J, et al. Predicting the histopathological grade of cerebral gliomas using high b value MR DW imaging at 3-tesla. J Neuroimaging 2008; 18: 276–281 [DOI] [PubMed] [Google Scholar]