Summary
Neurologists have a new toolbox of options for neurorehabilitation of disabling brain disorders such as stroke and traumatic brain injury. An emerging intellectual paradigm for neurologic recovery that includes neural regeneration, repair, and dynamic reorganization of functional neural systems, as well as increasing awareness of behavioral principles that may support best return to function and freedom, brought forward treatments based on experience-dependent learning, neurophysiologic stimulation, and a combination of these concepts. In this article, we summarize five rehabilitative approaches to watch: constraint therapy for motor and language recovery, synergy of motor-language rehabilitation, prism adaptation training and other virtual feedback approaches, and noninvasive magnetic and electrical brain stimulation.
Neurorehabilitation interventions have exploded since the year 2000, in parallel with a shift in the paradigm of neurologic care. In the mid-20th century, we turned away from the assumption that the effect of a brain injury such as a stroke on function, activity, and participation is permanent and became increasingly aware of the brain's regenerative potential, as well as dynamic brain reorganization, months and even many years later. Neurorehabilitation scientists pushed for translational research to define the permissive conditions under which optimal brain change and recovery occurs,1 apparently requiring controlled, intensive stimulation of impaired brain networks.2
Here, we summarize 5 treatments to rehabilitate motor and cognitive recovery based on behavioral or noninvasive physiologic stimulation (using magnetic fields or electricity). They have been explored primarily in stroke rehabilitation but are also potentially useful after brain trauma and in other neurologic conditions (e.g., spinal cord injury, multiple sclerosis).
Constraint-induced movement therapy
Constraint-induced movement therapy (CIMT) for upper extremity paresis may be a prototypical example of translational neurorehabilitation.3,e1 Intensive, experience-based, repetitive motor training of a paretic limb was first used in stroke survivors in the 1980s, based on the observation of “learned nonuse” in monkeys with a deafferented limb but intact motor capability. Competent, symmetric movements were restored by immobilizing the unaffected forelimb. Thus, during CIMT administration, stroke survivors may wear a mitt on the unaffected hand during most waking hours to reinforce paretic arm use, and perform task-specific, repetitive movement shaping during 2 weeks of long daily sessions (3–6 hours). A multisite CIMT study in chronic stroke, the EXCITE trial,e2 yields good results at time points 3–9 months and 15–21 months poststroke—later than most patients are eligible for conventional therapy. The participants were relatively mildly affected stroke survivors who had some ability to extend the wrist, thumb, or fingers and were able to stand unassisted for at least 2 minutes. Although CIMT has frequently been compared with less intensive usual and standard care in studies supporting its effectiveness (including the EXCITE study4), the treatment is theoretically appealing: it may provide massed practice of functional movement in a paretic limb; the intensive practice, rather than the constraint, appears to support CIMT treatment effect on brain reorganization.1 CIMT may also improve motor function in other neurologic disorders (cerebral palsye3,e4). Since the main cost of treatment is therapist training, it is low risk and feasible for many clinical environments, but unfortunately third-party payers may not accept a daily treatment plan, even with 2 weeks' duration. Also, studies indicate patients must have some preserved movement to improve. A “transfer package” therapeutic contract may aid with patient commitment and engagement to obtain best treatment results.e5
Weight-supported treadmill training
Weight-supported treadmill walking, an intensive, experience-dependent functional movement training, is sometimes conceptually grouped with CIMT. Patients wear a supportive harness for this labor-intensive therapy, usually requiring a technician stationed at each leg to assist in leg advancement at a minimal walking speed. A third therapist/technician may be needed on the treadmill with the patient, to assist in trunk movements and balance. Although a recent study, the LEAPS trial, comparing treadmill-based training to home-based exercise showed no definite benefit of this approach on gait speed, walking ability, and balance control,5 some researchers still urge that we investigate whether stroke survivors, vulnerable to the ill effects of a sedentary lifestyle, may benefit from upright aerobic training with respect to general health (insulin resistance), bone mass, or psychological well-being.6
Constraint-induced language therapy
Therapeutic effects of motor training on language recovery
Taking a motor rehabilitation approach to a cognitive function such as language might seem odd. Traditionally, training gestures or movements in a communication program would be viewed as a way to avoid working on speech intensively—a “compensatory” rather than “experience-based” approach. However, it is possible that some treatments might support a function indirectly, an effect called “vicariation.” “Vicariative” interventions may activate a neural system closely interacting with the stroke-impaired network so that both neural systems are functionally active during the treatment. We recently summarized reports7 that training stroke survivors with aphasia to use the left, nonparetic hand to make communicative and noncommunicative gestures improved their oral language. Left brain stimulation and arm training after left brain stroke also resulted in language improvement.8 One explanation may be that movement training stimulates interrelated left brain networks and supports both language and purposive arm/hand use.
Another similarity between motor training and aphasia therapy is that a constraint approach has been applied to both hemiparesis and aphasia. Since intensive, experience-dependent CIMT may induce large improvements in chronic paralysis, researchers hoped intensive oral communication would promote speech output and language recovery. An initial study (17 stroke survivors) administered 30 hours of “constraint-induced language therapy” (CILT) over 2 weeks; no physical “constraint” was applied, but physical barriers limited the ability to communicate nonverbally. Therapists continuously employed shaping techniques to stimulate verbal interaction (e.g., a variation on the “Go Fish” card game). CILT improved formal language performance and daily life communication activities compared with a control therapy. Further studies confirmed benefits in both acute and chronic aphasia and also suggested that lay people can be trained to perform CILT.9
Prism adaptation training for spatial neglect
Pathologically asymmetric reporting, response, or orienting to contralesional stimuli, causing functional disability (spatial neglect), affects >50% of acute stroke survivors, adversely affects recovery, and is associated with higher in-hospital and posthospital care expense. Spatial neglect treatments primarily addressed visual dysfunction, even though neuroscientists tell us that, distinct from visual-spatial errors, people with spatial neglect may make disabling, body-based, motor-exploratory spatial errors. Studies using a visual approach probably recruited subjects and assessed outcomes in ways that stack the deck against detecting improvements in spatial motor function. This translational block between neuroscience and clinical research may have confused clinicians. This may explain why neglect treatments reported to result in functional benefit are still not widely used.10,11
Compared with commonly used visual therapies, prism adaptation training (PAT) is simple and more amenable to short, frequent in-hospital rehabilitation sessions.11 Although dose-response studies do not yet support a specific prism type for PAT, the 20-diopter, 12.4° right-displacing wedge prism lenses are usually worn for 10 short sessions of intensive motor training (figure 1).
Figure 1. Rehabilitation of spatial neglect by prism adaptation training: Wedge prism goggles.
Optical prism goggles used for prism adaptation therapy (photo credit: Joan Banks for the Kessler Foundation, with permission).
Patients repeatedly point to targets or perform continuous manual tasks while their view of their own arm movements is partially blocked. During training, participants initially err rightward, in the direction of optical displacement, but as they perform repeated movements (usually ∼ 50 trials), many begin to point accurately. Training sessions are brief (15–30 minutes); prisms are worn only during training, leaving the rest of the day free for other activities or rehabilitation. After training, with the lenses removed, participants typically demonstrate immediate aftereffects. Movements err in the opposite direction (leftward). In stroke survivors with neglect, aftereffects may persist longer than in healthy controls, and improved leftward spatial motor “aiming” may generalize to improved daily life function.
Dramatic improvements after PAT have been reported in some patients, who started self-ambulating in a wheelchair for navigation or gained new ability to self-dress after receiving PAT. PAT may have an efficacy advantage because it employs motor learning principles, a potentially optimal approach to support motor functional rehabilitation. It is experience-dependent, or activity-dependent, rather than strategic; it is a procedure, rather than a set of verbal instructions or conscious goals to be remembered; it provides multiple opportunities for learning and direct experience of error.
Other virtual feedback
We do not know whether distorting visual feedback by wearing optical prisms, intense movement practice, or some other factor determines PAT efficacy after stroke. However, optical displacement while wearing wedge prisms induces a “virtual” mismatch between perception and action, prompting functional, automatic self-corrections. Similarly, “virtual reality” platforms allowing immersive, novel visual-motor experience have been used to rehabilitate hemiparesis, spatial dysfunction, memory and learning, organizational skills, and psychiatric problems such as anxiety disorders.12,13 Right now, there are very few studies supporting any virtual reality rehabilitation approaches. However, virtual reality treatment may bypass a top-down set of didactic, verbal instructions common in therapy. Neuropsychological deficits after stroke interfere with the ability to understand conditional instructions (“when you are in your room and not doing anything, squeeze this ball to exercise your hand”) and self-implement therapy activities.14 Virtual, immersive activities may retain their effectiveness even when cognitive problems impair the ability to access abstract ideas or concepts or anticipate outcomes. By directly activating complex brain networks and prompting the patient to move and react, virtual reality interaction could reduce the executive requirements of task scheduling and execution and increase treatment adherence and engagement.
Transcranial magnetic stimulation
Transcranial magnetic stimulation (TMS) is a noninvasive method to stimulate the human brain; TMS has a role in evaluation of neurophysiology and diagnosis of many neurologic conditions. It is also emerging as a neuromodulating modality of great potential in debilitating psychiatric and neurologic disorders, although further studies are needed to support these preliminary findings.
A brief, strong magnetic field created by an electric current circulating within a coil on the scalp penetrates the skull and induces electrical current that can depolarize neurons and axons15 (figure 2). Since TMS was first introduced in 1985, it has been used for clinical neurophysiology, intraoperative monitoring, and therapeutic purposes in a wide spectrum of neurologic and psychiatric conditions.16 As a diagnostic tool, TMS is primarily used for evaluation of cortical motor areas and motor pathways. Standard TMS methods of evaluation include assessing the motor threshold of the motor evoked potential (MEP; lowest stimulation intensity able to evoke an MEP of minimal size) and central motor conduction time. Absent or low MEP suggests a loss of neurons or axons, and prolonged central conduction time may reflect demyelination of central motor pathway or loss of large fibers. However, clinical use of these methods is limited due to their complexity.
Figure 2. Transcranial magnetic stimulation of the brain using neuronavigation.
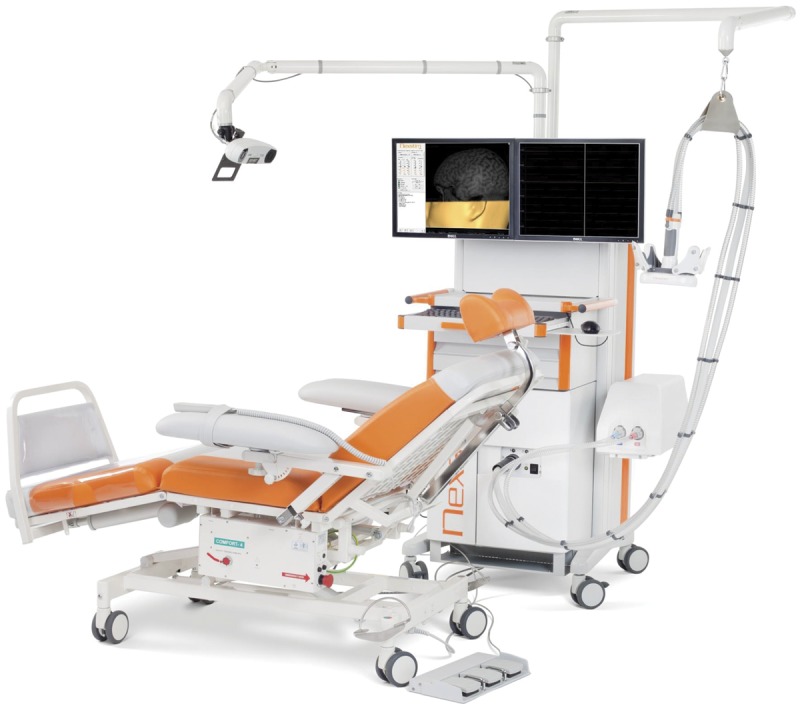
Transcranial magnetic stimulation setup for measuring the motor evoked potential using neuronavigation guidance (photo credit: Nextstim, with permission).
Research demonstrated that the excitatory or inhibitory effect of TMS on cortical excitability may persist when trains of repetitive TMS (rTMS) are delivered over cortical areas. The effect of rTMS on cortical excitability may depend on the frequency of stimulation: downregulation may follow low-frequency rTMS (e.g., 1 Hz) and excitation, high-frequency rTMS (e.g., 10 Hz). There are some promising results using rTMS as an add-on therapy in a number of neurologic and psychiatric disorders characterized by dysfunction of distinct brain networks, including stroke, tinnitus, chronic pain, and posttraumatic stress disorders. However, in United States, the only US Food and Drug Administration–approved indication of rTMS application is single-drug resistant unipolar depression. Further clinical trials are needed to support most claims of therapeutic utility of rTMS in many conditions.16
A recent publication summarized safety precautions, ethical considerations, and application guidelines from a consensus conference for application of TMS in research and clinical settings.17 Although medical experience with the technique has in many ways been satisfactory, absolute contraindications for TMS use include the presence of metallic hardware in close contact to the discharging coil (such as cochlear implants, implanted brain electrodes, medical pumps). TMS use is also generally avoided in persons with a history of epilepsy, tumoral or infectious lesions of the brain, sleep deprivation, alcoholism, pregnancy, or severe heart disease. In general, an acceptable safety profile of TMS is well supported by the literature, although it is essential for TMS applicants to be familiar with potential side effects for safe, well-tolerated application (headache, syncope, seizure, hearing loss, magnetic induction of a metal or paramagnetic object, etc.).
Transcranial direct current stimulation
Almost 200 years after low-voltage direct electrical current was first used transcranially to treat mental disorders, standard parameters to stimulate the human motor cortex with transcranial direct current stimulation (tDCS) became available.18 Since then, a growing body of evidence suggests tDCS may exert therapeutic effects on a variety of clinical disorders, including improvement of motor dysfunction, chronic pain, memory impairment, working memory deficits, additive craving, major depression, speech production in aphasia, and spatial neglect.e6-–e17 An excellent safety profile and low cost of equipment (a few hundred to a few thousand dollars) make tDCS extremely appealing as a primary or adjunctive neurorehabilitation treatment. However, tDCS is not yet appropriate for clinical application. Electrode montage, stimulation duration, therapy duration, and interval between sessions may all significantly determine tDCS effects; optimal parameters need to be clinician ready.
Modern tDCS is a safe, easy-to-use, low-cost, and noninvasive brain stimulation technique.18 In fact, tDCS probably does not “stimulate” neurons. Rather, it modifies ongoing neuronal activities through weak direct currents up to 2 milliamperes (2 mA) for 10 to 30 minutes per session, typically delivered by saline-soaked, sponge-surrounded, rubber electrodes of 5 x 5 or 5 x 7 cm2 (see figure 3 for an example of a setup for tDCS stimulation). Smaller electrodes with higher spatial resolution are also under development. Anodal tDCS promotes tonic depolarization of the resting membrane potentials in the underlying cortical brain regions, inducing excitatory effects; cathodal tDCS increases the likelihood of hyper- or repolarization, leading to inhibitory effects. Applying this basic principle to rehabilitation research, researchers use tDCS on specific locations of the scalp in order to improve pathologic symptoms. However, to date, most of the reported effects were observed results after a single stimulation session. Such effects may be too short-lived to have effect on long-term, daily function. Therefore, some researchers use multiple spaced, therapy-like sessions (e.g., 5 daily sessions over a week) to increase the duration of the effect and to explore generalization of the stimulation to long-lasting rehabilitative effects in daily life. However, how to optimize dosing with respect to frequency and intensity is not yet known.
Figure 3. Transcranial direct current stimulation: Equipment setup for treatment administration.
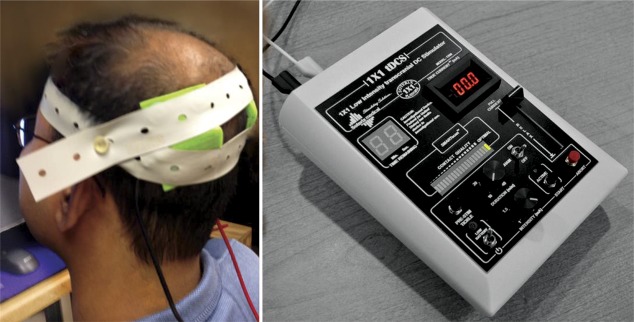
Transcranial direct current stimulation (tDCS): electrode (inside sponge) placement on the head for bilateral parietal stimulation (left side of figure; photo credit: Kessler Foundation, with permission) and an example of tDCS stimulator controls (right side of figure; photo credit: Soterix Medical, with permission).
Researchers are also examining the combination of tDCS with established therapies. Studies suggested combining tDCS with several standardized treatments improved outcomes: CIMT, transcutaneous electrical nerve stimulation, vision restoration therapy for hemianopia, speech-language therapy for aphasia, and aerobic exercise for appetite reduction and weight loss. e18–e22
Individual differences in brain shape, size, and volume, especially when the brain or skull has been injured, may significantly alter tDCS effects.19 Not only the mechanism of tDCS but also how it interacts with the brain mechanisms of the target disorder, impairment, or dysfunction need to be better understood. If stimulating the brain modulates the relative dominance of interacting brain systems, unexpected effects may occur, such as modulation of neuroimmunologic networks,20 which need to be carefully considered.
DISCUSSION
Limited medical resources make it imperative that neurorehabilitation develop better ways to improve neurologic disabilities in shorter periods of time. This means focusing on experience-dependent learning but also employing biological techniques to induce a permissive state for instantiating new optimal, functional brain activation patterns. Neurorehabilitation also needs to take into account the special challenges of research in its domain: difficulty blinding experimenters who are administering active vs control treatments; need to assess trajectories of recovery that require collecting multiple data points and using special analytic approaches that take intercorrelation within subjects into accounte23; and vulnerability of older stroke survivors with significant deficits to health care disparities that affect distribution of medical research resources as well as resources for medical care.
Clinicians reading rehabilitation research studies must weigh what they read in light of the study's care in including representative subjects from the population being studied, balancing treatment and control groups by appropriate subtypes of a condition (e.g., different aphasia syndromes), choosing control conditions (active vs usual and standard care), translating what is known about the mechanisms of a disorder and its treatment, and using rigorous statistical approaches (especially when negative studies may be the result of misestimation of variancee23). Studies evaluating sequential and simultaneous treatment combinations, and treatments designed to improve more than one domain of function, may help us advance the field. There is also “personalized medicine,” which may refer either to an individual's genotypic profile or to a neuropsychological deficit pattern limiting his or her recovery.14 The 5 behavioral and noninvasive brain stimulation techniques we described above may well be hotly discussed as neurology decides how to balance the cost of rehabilitation with treatment benefits to individuals, their families and loved ones, and society.
Neurorehabilitation: Five new things.
Constraint-induced movement therapy and other intensive, experience-dependent learning may improve rehabilitation outcomes in people with hemiparesis from stroke and other brain disorders.
Constraint-induced language therapy and other methods to stimulate speech and motor output may improve rehabilitation outcomes in aphasia.
Prism adaptation therapy and therapies using virtual feedback and implicitly integrating 3D motor and perceptual function may improve function in spatial neglect.
Transcranial magnetic stimulation may induce a permissive brain state therapeutic for depression and promoting better motor and cognitive recovery.
Transcranial direct current stimulation may promote better mood and motor and cognitive rehabilitation outcomes and has an appealing risk/cost profile for feasible future implementation.
Supplementary Material
Footnotes
Supplemental data: neurology.org/cp
STUDY FUNDING
Manuscript preparation was funded by the Kessler Foundation (AMB, MO-P, PC), the NIH (R01NS 055808 and K24HD062647: PI Barrett), and the Department of Education (NIDRR grant H133G120203). These contents do not necessarily represent the policy of the Department of Education, and you should not assume endorsement by the Federal Government.
DISCLOSURES
A. M. Barrett wrote three chapters for e-text WebMD/emedicine.com and receives research support from Pfizer/Eisai, O'Brien Technologies, the NIH (NINDS, NICHD, NCMRR), Kessler Foundation, the Healthcare Foundation of New Jersey, and the Wallerstein Foundation for Geriatric Improvement. M. Oh-Park reports no disclosures. P. Chen receives research support from the NIH (NIDRR, NINDS , NICHD). N. Ifejika receives research support from the NIH/NINDS. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.

REFERENCES
- 1.Barrett AM, Rothi LJG. Treatment innovation in the rehabilitation of stroke: removing limits on recovery. J Rehabil Res Dev 2006;43:vii–ix. [DOI] [PubMed] [Google Scholar]
- 2.Cramer SC, Duncan PL, Barrett AM. Final report of the stroke Progress review group, recovery and rehabilitation [Online]. Available at: www.ninds.nih.gov/strokeprg. Accessed December 1, 2012.
- 3.Huang WC, Chen YJ, Chien CL, Kashima H, Lin KC. Constraint-induced movement therapy as a paradigm of translational research in neurorehabilitation: reviews and prospects. Am J Transl Res 2011;3:48–60. [PMC free article] [PubMed] [Google Scholar]
- 4.Corbetta D, Sirtori V, Moja L, Gatti R. Constraint-induced movement therapy in stroke patients: systematic review and meta-analysis. Eur J Phys Rehabil Med 2010;46:537–544. [PubMed] [Google Scholar]
- 5.Duncan PW, Sullivan KJ, Behrman A, et al. ; LEAPS Investigative Team. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med 2011;364: 2026–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hicks AL, Ginis KAM. Treadmill training after spinal cord injury: it's not just about the walking. J Rehabil Res Dev 2008;45:241–248. [DOI] [PubMed] [Google Scholar]
- 7.Ifejika-Jones NL, Barrett AM. Rehabilitation—emerging technologies, innovative therapies and future objectives. Neurotherapeutics 2011;8:452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesse S, Werner C, Schonhardt EM, Bardeleben A, Jenrich W, Kirker SG. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: a pilot study. Restor Neurol Neurosci 2007;25(1):9–15. [PubMed] [Google Scholar]
- 9.Berthier ML, Pulvermüller F. Neuroscience insights improve neurorehabilitation of poststroke aphasia. Nat Rev Neurol 2011;7:86–97. [DOI] [PubMed] [Google Scholar]
- 10.Barrett AM, Buxbaum LJ, Coslett HB, et al. Cognitive rehabilitation interventions for neglect and related disorders: moving from bench to bedside in stroke patients. J Cogn Neurosci 2006;18:1223–1236. [DOI] [PubMed] [Google Scholar]
- 11.Barrett AM, Goedert KM, Basso JC. Prism adaptation for spatial neglect after stroke: translational practice gaps. Nat Rev Neurol 2012;8:567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corey JB, Bradly A, Biocca FA. Virtual reality in neuroscience research and therapy. Nat Rev Neurosci 2011;12:752–762. [DOI] [PubMed] [Google Scholar]
- 13.Laver K, George S, Thomas S, Deutsch JE, Crotty M. Cochrane review: virtual reality for stroke rehabilitation. Eur J Phys Rehabil Med 2012;48:523–530. [PubMed] [Google Scholar]
- 14.Barrett AM. Rose-colored answers: neuropsychological deficits and patient-reported outcomes after stroke. Behav Neurol 2010;22(1-2):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallett M. Transcranial magnetic stimulation and the human brain. Nature 2000;406:147–150. [DOI] [PubMed] [Google Scholar]
- 16.Rossini PM, Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology 2007;68:484–488. [DOI] [PubMed] [Google Scholar]
- 17.Rossi S, Hallett M, Rossini PM, Pascual-Leone A; Safety of TMSCG. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009;120:2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000;527:633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bikson M, Rahman A, Datta A. Computational models of transcranial direct current stimulation. Clin EEG Neurosci 2012;43:176–183. [DOI] [PubMed] [Google Scholar]
- 20.Frisina PG, Kutlik AM, Barrett AM. Left brain injury associated with more hospital-acquired infections during inpatient rehabilitation. Arch Phys Med Rehabil 2013;94:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



