Abstract
Background
Adjusting to symptom flares, treatment regimens, and side effects places youth with inflammatory bowel disease (IBD) at increased risk for emotional and behavioral problems and adverse disease outcomes. Implementation of psychosocial screening into clinical practice remains a challenge. This study examines the clinical utility of health-related quality of life (HRQOL) screening in predicting disease outcome and healthcare utilization.
Methods
One-hundred twelve youth 7-18 years diagnosed with IBD and their parents. Youth completed standardized measures of HRQOL and depression. Parents completed a proxy-report of HRQOL. Pediatric gastroenterologists provided the Physician Global Assessment. Families were recruited from a pediatric gastroenterology clinic. Retrospective chart reviews examined disease outcome and healthcare utilization 12-months following baseline measurement.
Results
Linear regressions, controlling for demographic and disease parameters, revealed that baseline measurement of youth and parent proxy-reported HRQOL predicted the number of IBD-related hospital admissions, gastroenterology clinic visits, Emergency Department visits, psychology clinic visits, telephone contacts, and pain management referrals over the next 12 months. Disease outcome was not significant.
Conclusions
Lower HRQOL was predictive of increased healthcare utilization among youth with IBD. Regular HRQOL screening may be the impetus to providing better case management and allocating resources based on ongoing care needs and costs. Proactive interventions focused on patients with poor HRQOL may be an efficient approach to saving on healthcare costs and resource utilization.
Keywords: inflammatory bowel disease, health-related quality of life, disease outcomes, healthcare utilization
Inflammatory bowel disease (IBD) is a chronic idiopathic disease characterized by an unpredictable course that includes intermittent periods of symptom exacerbation and remission. In addition to coping with the uncertainties of their disease, youth with IBD are responsible for managing a complex and potentially burdensome treatment regimen that is sometimes associated with negative side effects.1,2 Collectively, these factors place youth with IBD at increased risk for emotional and behavioral problems,3,4 which in turn have been associated with medication nonadherence in adolescents5 and poor disease outcomes, including increased relapses and disease activity, in adults with IBD.6,7 Thus, identifying patients at risk for experiencing psychosocial difficulties is critical in preventing adverse outcomes; yet the implementation of screening measures into standard clinical practice remains a challenge.
One measure of psychosocial adjustment to chronic illness that has traditionally been conceptualized as an important outcome in adult and pediatric health is health related quality of life (HRQOL), defined as a patient's current perception of functioning across several areas. In addition to its responsiveness to treatment intervention8 and association with disease severity9,10 and barriers to care,11 to name a few, HRQOL has demonstrated its clinical utility in several adult chronic illness populations. For instance, HRQOL predicted mortality and hospitalizations independent of several demographic (e.g., age, gender, socioeconomic status) and comorbid factors (e.g., medication dose, time on dialysis, and body mass index) in a large sample of adults with end-stage renal disease.12 In adults with myocardial infarction, lower physical HRQOL was related to poorer treatment adherence, using both cross-sectional and prospective approaches, even after controlling for depression.13 In one study of pediatric patients on managed care health plans, parent proxy-reported HRQOL predicted healthcare costs over 6, 12, and 24 months.14 Although youth perspective on how their illness impacts daily functioning and HRQOL is the preferred method, parent proxy-reported HRQOL is a vital and valid estimate of youth HRQOL when the child is either unable to participate in the evaluation or the validity of his responses is in question.15
In addition to its predictive utility which has seldom been examined, HRQOL is an advantageous clinical tool because a large proportion of youth with chronic illness present with sub-clinical levels of depression but significant functional impairment in important life domains.16,17 This is particularly concerning given that poor HRQOL is related to increased school/work absenteeism, lower standardized achievement and intelligence testing scores, and work productivity loss.18-21 Without HRQOL screening, a subgroup of patients may be overlooked and not receive the necessary resources or referrals for additional services (e.g., psychological, education specialists, social services). Taken together, these findings support the need for screening youth HRQOL during clinic visits in order to identify those at risk for further psychosocial difficulties and poor disease outcomes.
To our knowledge, few studies have examined the relationship between HRQOL and clinical outcomes,12,13,22 and only one included a pediatric sample14; however, that study did not evaluate youth-reported HRQOL and included costs accrued as the sole measure of healthcare utilization. The current study advances the existing literature by examining the clinical utility of youth and parent proxy-reported HRQOL in predicting disease severity and healthcare utilization in a sample of youth with IBD. Specifically, lower HRQOL was hypothesized to predict higher physician-rated disease severity at baseline and increased healthcare utilization, including more IBD-related hospital admissions, Emergency Department (ED) visits, phone calls to the provider, psychological services, GI clinic visits, and referrals for pain management over the next 12 months.
Materials and Methods
Participants and Procedure
Participants included 112 youth, ages 7-18 years (M = 14.45, SD = 2.92), with a confirmed diagnosis of IBD (73% Crohn's disease, 24% ulcerative colitis, and 3% indeterminant colitis) who were receiving medical care at a pediatric IBD specialty clinic in the Midwest region of the United States between March 2010 and July 2012.
All participants who met the aforementioned inclusion criteria were asked to complete psychological screeners as part of their standard clinical care during a scheduled medical visit with a pediatric gastroenterologist. Patients were given the option to decline participation without it impacting their medical care. Retrospective chart review was completed to collect data on demographic variables (e.g., age, sex, and type of insurance), patient-reported general well-being and physician-reported global assessment of disease severity, and healthcare utilization (e.g., IBD-related phone calls, psychological services, and ED visits). A waiver of consent was granted as the study involved only retrospective deidentified data collection. The study was approved by the hospital's Institutional Review Board.
Measures
Pediatric Quality of Life Inventory™, Version 4.0 (PedsQL 4.0)
The PedsQL 4.023 is a well-validated 23-item self-report measure of perceived functioning across several areas including physical, emotional, social, and school functioning. Using a 5-point Likert scale ranging from “Never” to “Always”, youth are asked to rate the frequency with which they have experienced problems in each domain in the past month. Items are reverse scored and linearly transformed (possible range: 0 – 100), with higher scores indicating better HRQOL. The parent proxy-report version is similar to the youth-report but asks parents to rate how much of a problem each item has been for their child or adolescent in the last month. The PedsQL 4.0 yields a Total Scale Score and two Summary Scores: Physical Health (8 items; same as Physical functioning subscale) and Psychosocial Health (15 items; mean score of three subscales). Additional subscales include Emotional, Social, and School functioning (5 items each). The measure is easy to administer and takes approximately five minutes to complete.24 Internal consistency reliability for the current sample was excellent for child-, teen-, (α = .87 and .94, respectively) and parent proxy-reported Total HRQOL (α = .93 and .97, respectively).
Children's Depression Inventory: Short Version (CDI:S)
The CDI:S25 is a brief 10-item self-report screening measure of cognitive, emotional, and behavioral signs of depression in youth between 7-17 years. For each item, youth rate the degree to which they have experienced depressive symptoms in the past two weeks. Items are summed, and T-scores based on combined age (7-12 and 13-17) and gender are calculated. T-scores of 65 or higher are considered to be within the clinical range. The CDI:S is a reliable and valid screener of depressive symptoms in children and adolescents.25 Internal consistency reliability for the current sample was good (α = .76). In the current study, youth between 7-16 years completed the CDI:S.
Beck Depression Inventory-Second Edition (BDI-II)
The BDI-II26 is a widely used 21-item self-report measure of depressive symptoms in adolescents and adults ages 13-80 years. Respondents rate the severity of each item on a 4-point Likert-type scale ranging from 0 to 3. Items are summed, with higher scores indicating greater depressive symptomatology. Total scores of at least 19 are considered to be within the clinical range. The BDI-II has demonstrated excellent psychometric properties,26 and for the current sample, internal consistency reliability was excellent (α = .91). Youth 17 years and older enrolled in the current study completed the BDI-II.
Physician Global Assessment of Disease Severity
During GI clinic visits, one of eight physicians provided an assessment of each IBD patients' disease severity using the Physician Global Assessment (PGA) score as part of standard clinical practice.27 Disease severity was categorized as quiescent/remission, mild, moderate, or severe based on the presence of specific factors during the last week, including abdominal pain, diarrhea, bloody stools, fatigue, a fistula, weight loss, abdominal mass or tenderness, toxic appearance, and laboratory tests.27,28 PGA ratings have been shown to be highly correlated with other validated measures of disease severity (e.g., PCDAI, r = .80, p = .001).29,30,31
Healthcare Utilization
Healthcare utilization was assessed by the following: the number of GI clinic visits, IBD-related hospital admissions, IBD-related ED visits (e.g., fracture not included), psychological visits, IBD-related phone calls; and whether the patient was referred for pain management. Measures of healthcare utilization were gathered from chart review, and all outcomes were tracked for 12 months following baseline measurement.
Statistical Analyses
Preliminary statistical analyses included bivariate Pearson correlation coefficients to examine the relationship between demographic and disease parameters (e.g., sex, age, illness duration, IBD subtype), physician-reported disease severity (i.e., PGA), and youth and parent proxy-reported HRQOL. Covariates were chosen based on statistically significant correlations between youth- and parent proxy-reported HRQOL and clinical endpoints (e.g., disease severity via PGA ratings, healthcare utilization variables). Although there was not a significant relationship between PGA and youth (r = .08, p = .39) or parent-proxy reported HRQOL (r = .13, p = .17), the PGA rating was selected as covariate to determine whether HRQOL adds validity above and beyond disease symptoms in predicting healthcare utilization.
In order to test the primary hypotheses that HRQOL would predict disease outcomes at baseline and healthcare utilization for 12 months following, separate linear regression analyses were conducted. Specifically, the PedsQL Total Score and the same five covariates including illness duration, IBD subtype, type of insurance (private versus public), severity of depressive symptoms (clinical versus nonclinical), and PGA rating were entered as predictors, with PGA and measures of healthcare utilization entered separately as outcome variables in regression equation. Of note, referrals for pain management services were categorical in nature (yes/no), while all other outcome measures were continuous variables (frequency of visits/phone calls). Depressive symptoms were dichotomized as clinical or nonclinical, with CDI:S T-scores of 65 or higher (1.5 SD above the mean) and BDI-II total scores of at least 19 (moderate depressive symptoms) falling in the clinical range. All analyses were conducted using SPSS Version 20 software.32
Results
Descriptive Analyses
Data on demographic and disease-related factors are presented in Table 1. Descriptive data on youth and parent proxy-reported HRQOL scores and health care utilization are presented in Tables 2 and 3.
Table 1. Patient demographic and disease-related descriptive data.
| Percentage | |
|---|---|
| Sex (% male) | 56 |
| Ethnicity (% white, not Hispanic origin) | 98.2 |
| Insurance (% private) | 94.6 |
| IBD diagnosis | |
| Crohn's disease | 73.2 |
| Ulcerative colitis | 24.1 |
| Indeterminant colitis | 2.7 |
| Illness duration | |
| Diagnosed at visit | 1.8 |
| Less than 6 months | 13.6 |
| 6 months – 1 year | 12.7 |
| 1 year – 5 years | 29.1 |
| Greater than 5 years | 6.4 |
| Unknown | 36.4 |
| Physician-rated disease activity | |
| Quiescent | 58.1 |
| Mild | 21.9 |
| Moderate | 19.0 |
| Severe | 1.0 |
| Reason for GI clinic visit | |
| Follow-up visit | 83.1 |
| Sick visit | 9.3 |
| New patient visit | 5.1 |
| 2nd opinion visit | 2.5 |
IBD, inflammatory bowel disease; GI, gastroenterology
Table 2. Youth and parent proxy-reported health-related quality of life scores.
| Mean | Standard Deviation | Observed Range | |
|---|---|---|---|
| Youth-report | |||
| Total score | 78.01 | 16.56 | 29.35 – 100 |
| Physical health | 77.85 | 20.41 | 09.38 – 100 |
| Psychosocial healtha | 78.88 | 15.00 | 38.33 - 100 |
| Emotional functioning | 77.55 | 18.33 | 25.00 - 100 |
| Social functioning | 89.37 | 12.65 | 35.00 – 100 |
| School functioning | 71.33 | 19.24 | 20.00 - 100 |
| Parent proxy-report | |||
| Total score | 74.64 | 18.20 | 26.09 - 100 |
| Physical health | 75.43 | 22.06 | 09.38 - 100 |
| Psychosocial healtha | 74.21 | 18.05 | 26.67 - 100 |
| Emotional functioning | 69.26 | 21.47 | 25.00 – 100 |
| Social functioning | 84.40 | 17.06 | 35.00 – 100 |
| School functioning | 70.28 | 22.13 | 00.00 - 100 |
Psychosocial health is the mean score of emotional, social, and school functioning
Table 3. Health care utilization 12-months post baseline measurement.
| Mean | Standard Deviation | Range | |
|---|---|---|---|
| IBD-related hospital admissions | 0.50 | 1.13 | 0 – 5 |
| IBD-related ED visits | 0.52 | 1.24 | 0 - 10 |
| GI clinic visits | 3.30 | 2.50 | 0 - 15 |
| IBD-related telephone calls | 3.41 | 4.37 | 0 - 23 |
| Psychological visits | 1.91 | 4.34 | 0 - 22 |
IBD, inflammatory bowel disease; ED, emergency department; GI, gastroenterology
Regression Analyses
Physician Global Assessment of Disease Severity
To test the hypothesis that youth and parent proxy-reported HRQOL are significant predictors of disease severity, separate linear regression analyses were conducted for each reporter. Youth or parent-proxy reported PedsQL Total Score and four covariates (e.g., illness duration, IBD subtype, type of insurance, and severity of depressive symptoms) were entered simultaneously as the predictor variables, and PGA (e.g., quiescent, mild, moderate, or chronically severe) served as the dependent variable. Results revealed that the overall model was not significant (p > .05), such that neither youth nor parent proxy-reported HRQOL predicted disease severity during baseline measurement. In addition, a one-way ANOVA revealed no significant difference in PGA distribution based on the physician completing the rating of disease severity (F(2,102) = .52, p = .60). It should be noted that analyses examined potential differences among three GI physicians given that they provided the majority (93%) of PGA ratings for the current sample.
Healthcare Utilization
In order to test the hypothesis that youth and parent proxy-reported HRQOL at baseline are significant predictors of healthcare utilization over the next 12 months, separate linear regressions were conducted with youth or parent proxy-reported PedsQL Total Score and five demographic and disease parameters (i.e., covariates) entered simultaneously as predictors and each individual measure of healthcare utilization entered as the dependent variable.
Hospital Admissions
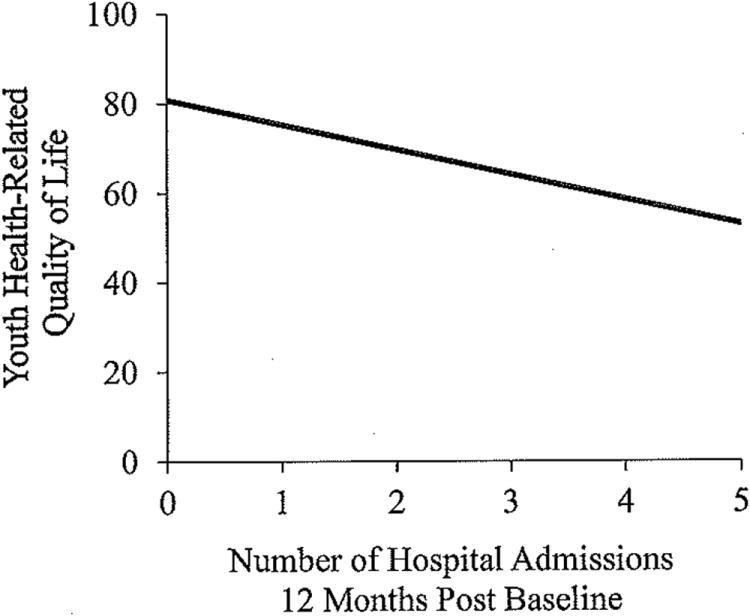
For youth-reported HRQOL, the overall regression model evaluating the number of IBD-related hospital admissions for 12 months following baseline measurement was significant, F(6,103) = 3.51, p = .003, and explained 18% (R2 = .18) of the variance in the number of hospital admissions (Figure 1). The regression model was also significant for parent proxy-reported HRQOL (F(6,99) = 2.62, p = .02, R2 = .15).
Figure 1. Youth health-related quality of life and the number of IBD-related hospital admissions 12-months post baseline measurement.
Emergency Department Visits
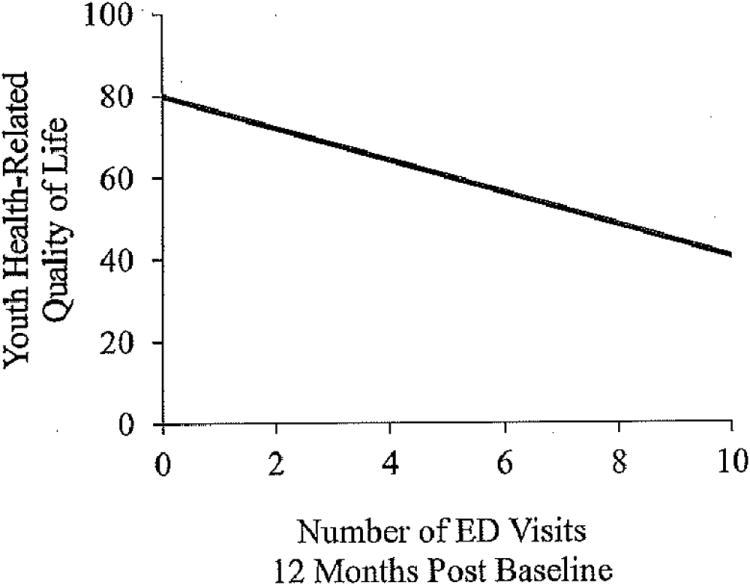
Results also revealed that youth and parent proxy-reported HRQOL were significant predictors of the number of IBD-related ED visits even after controlling for demographic and disease parameters (F(6,103) = 4.19, p = .001, R2 = .21, Figure 2 and F(6,99) = 3.27, p = .006, R2 = .17).
Figure 2. Youth health-related quality of life and the number of emergency department visits 12-months post baseline measurement.
Psychological Services
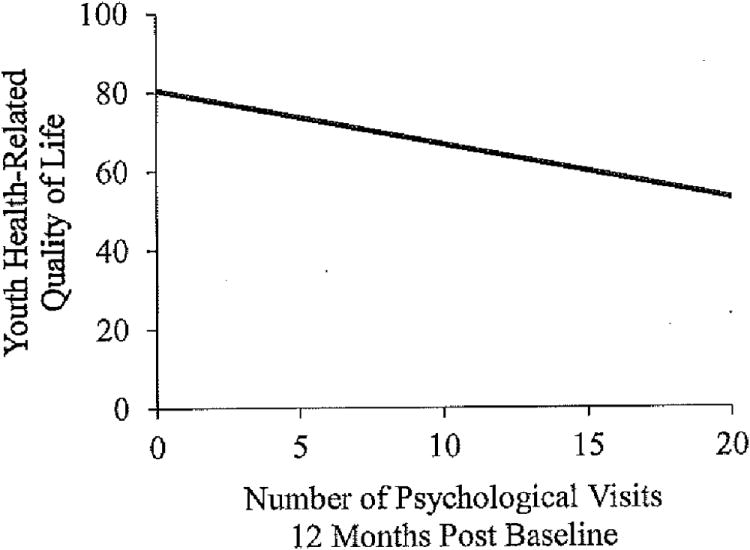
The overall regression model for the number of psychological visits 12 months following baseline measurement was significant for youth (F(6,103) = 6.04, p < .001, R2 = .27) and parent proxy-reported HRQOL (F(6,99) = 6.36, p < .001, R2 = .29), with poorer HRQOL at baseline predicting an increased number of psychology visits over the next 12 months (Figure 3).
Figure 3. Youth health-related quality of life and the number of psychological visits 12-months post baseline measurement.
Telephone Contacts
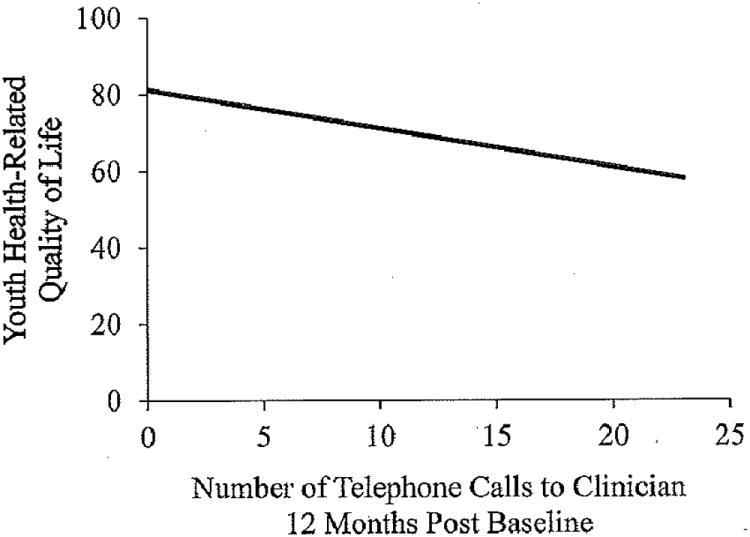
After controlling for demographic and disease parameters, youth and parent proxy-reported HRQOL were significant predictors of the number of IBD-related telephone calls (F(6,103) = 2.67, p = .02, R2 = .14 and F(6,99) = 3.21, p = .007, R2 = .17), such that poorer HRQOL at baseline was associated with more phone calls to clinicians for the next 12 months (Figure 4).
Figure 4. Youth health-related quality of life and the number of IBD-related telephone calls 12-months post baseline measurement.
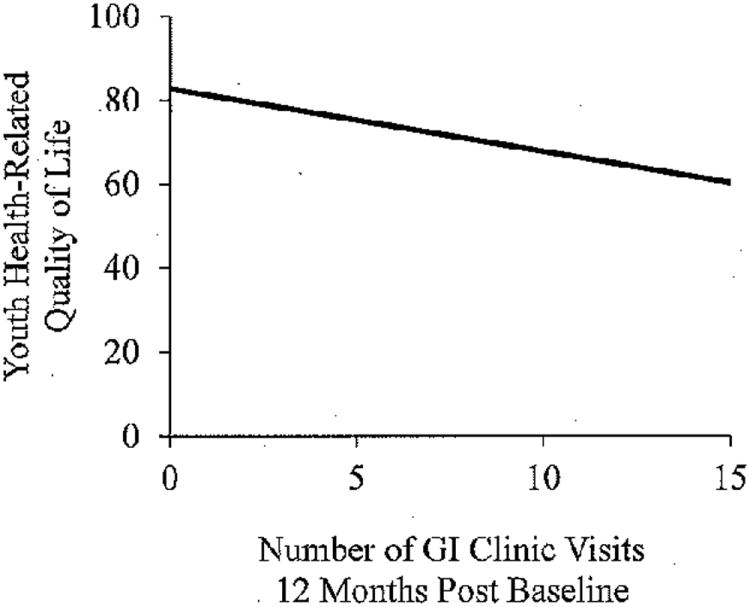
GI Clinic Visits
Youth and parent proxy-reported HRQOL were significant predictors of the number of GI clinic visits even after controlling for five demographic and disease parameters (F(6,103) = 3.99, p = .001, R2 = .20, Figure 5 and F(6,99) = 3.74, p = .002, R2 = .19).
Figure 5. Youth health-related quality of life and the number of GI clinic visits 12-months post baseline measurement.
Pain Management
The regression model evaluating whether patients were referred for pain management (yes or no) was significant for youth (F(6,103) = 3.47, p = .004, R2 = .18) and parent proxy-reported HRQOL (F(6,99) = 2.43, p = .03, R2 = .14). Compared to youth with higher HRQOL, those with lower scores (i.e., poorer HRQOL) at baseline were more likely to be referred for pain management services over the next 12 months after completing baseline measures.
Discussion
This study is the first to examine the clinical utility of both youth and parent proxy-reported HRQOL screening for predicting disease outcome and healthcare utilization in a pediatric IBD clinic setting. Consistent with our hypotheses, HRQOL predicted healthcare utilization, such that youth and parents who reported lower HRQOL at baseline, on average, had more IBD-related hospital admissions, psychology clinic visits, ED visits, telephone contacts to the medical clinician, and referrals for pain management over the following 12 months. Contrary to our first hypothesis, youth and parent proxy-reported perceptions of HRQOL were not associated with disease severity (i.e., PGA) at the time of baseline measurement. One possible explanation for this finding is that the majority of patients in our sample had either quiescent or mild disease severity (58% and 22%, respectively). As such, there was limited variability in moderate to severe disease, which is more commonly related to poor HRQOL among youth with chronic illness.33
In general, findings are consistent with the adult chronic illness literature demonstrating that poor HRQOL greatly impacts a patient's healthcare resource use.12,14 Perhaps more importantly, the current study addresses gaps in the literature by focusing on a pediatric clinical sample, demonstrating the clinical utility of HRQOL as a predictive measure, using a universal rather than referral-based screening approach, and examining multiple sources of healthcare utilization. Indeed, HRQOL predicted several markers of healthcare utilization even after controlling for five socioeconomic and disease parameters (e.g., IBD subtype, illness duration, private versus public insurance, physician-reported disease severity, and level of depressive symptoms). Notably, only 5% of the same reported depressive symptoms in the clinical range while concurrently reporting significant impairment in areas of social, academic, emotional, and/or physical functioning. Such discrepancies highlight the need to screen for HRQOL and functional impairment that may otherwise go undetected with traditional measures of depressive symptoms and ultimately lead to increased use of healthcare resources and higher healthcare costs.
These findings should be interpreted within the context of some limitations. Demographic and disease characteristics of the current sample (e.g., primarily Caucasian, predominantly quiescent disease status, and majority of families with private insurance) may limit generalizability of the findings to the general pediatric IBD population. However, the demographics of the current sample are similar to those of published reports in the pediatric IBD literature.3,4,34 In addition, although the PedsQL 4.0 is well-validated and frequently used, it is a generic measure of HRQOL that was designed for use across a wide range of clinical conditions and may be less sensitive than disease-specific measures.23 Nevertheless, the PedsQL 4.0 has been frequently used in pediatric IBD research and allows for comparison with normative data and across disease groups.35-40 There is the potential for underreporting of healthcare utilization given that outcome measures for the current study were limited to the participating hospital and could not account for additional visits to the family's pediatrician, urgent care clinics, or other health providers. Similarly, if patients report poor HRQOL during a GI clinic visit and are referred for additional services (i.e., psychological or pain management), there is the potential for bias and underreporting during subsequent visits. On the other hand, underreporting in HRQOL or psychopathology would further support the current findings and the need for regular screening. Lastly, given the clinical nature of the current study reliability ratings of PGA scores were not conducted. Although the study design precluded ratings to be done by a separate physician for all of the patients, training was provided to physicians in terms of assessing PGA and there are descriptions of each value to guide rating assignment.
The current findings have important clinical implications. HRQOL should be monitored and screening measures should be incorporated into standard clinical practice, with particular attention given to patients coming for second opinions (PedsQL M = 53.62) and sick visits (PedsQL M = 63.04) as they appear to have lower HRQOL compared to new patients (PedsQL M = 78.44) and patients scheduled for regular follow-up visits (PedsQL M = 80.64). In comparison, these overall HRQOL scores are lower than those observed in healthy children (M = 83.00).24 Ideally, however, HRQOL screening would be done with all patients in order to identify those at risk for adverse outcomes including excessive and sometimes unnecessary use of health resources. Regularly inquiring about youth perceptions of his/her well-being can assist healthcare providers in clinical decision-making (e.g., medication changes) and whether interventions need to be tailored to the specific needs of the patient or if the patient would benefit from a referral to a mental health specialist.41,42 Specifically, pediatric psychologists are trained in evidence-based approaches (e.g., Cognitive Behavioral Therapy, Behavioral Family Systems Therapy) for adjustment to chronic illness and could assist the patient and family in developing coping strategies to help manage negative feelings or thoughts and problem-solving around barriers to improved HRQOL (e.g., perceived illness intrusiveness, overprotective parenting).43,44 Our clinical observations suggest that introducing the need for psychological intervention in the context of HRQOL data is less intimidating to the patient and parents as it does not seem to be as stigmatizing as assessing for symptoms of depression or anxiety.
For children who are unable (e.g., too ill or fatigued) or unwilling to participate in the assessment process, findings of the current study suggest that parent proxy-report can provide a valid estimation of the child's HRQOL. This is consistent with studies demonstrating that even moderate concordance between youth and parent reports of HRQOL using the PedsQL substantiates the ideal use of multiple informants and allows for proxies when estimations are needed.33,40 Moreover, a HRQOL screener should be selected that measures the perspectives of both the child and parent given that caregiver perceptions of their child's functioning often influence subsequent healthcare utilization.45 Although there are numerous generic measures of HRQOL, the PedsQL 4.023 is a highly-used and psychometrically sound measure of HRQOL across several domains, allows for youth and parent proxy-report, and is easy to administer and score without disrupting clinic flow. In the adult literature, HRQOL screeners have been easily completed, scored, and printed while patients were in the waiting room.46 Additionally, implementing HRQOL screening does not necessarily lengthen consultation time between the physician and patient; anecdotally, some physicians in an outpatient oncology clinic reported that HRQOL summaries even increased their efficiency during clinic visits.46 Computer-assisted HRQOL measurement may also help reduce perceived barriers in the administration and scoring of standardized screening tools and help make clinic visits more efficient.47 For example, physicians could be provided with a brief computer-generated summary of HRQOL results including areas of concern at the time of service and recommendations for targeted intervention and/or referral.41
Finally, these results suggest that early identification and proactive interventions focused on patients with poor HRQOL may demonstrate through further research to be an efficient approach to improving clinical outcomes and saving on healthcare costs and resources. Although a percentage of healthcare costs are inevitable, identifying high-risk children (i.e., combination of poor health status and poor HRQOL) as early as their initial clinic visit may be the impetus to providing better case management and allocating resources based on ongoing care needs and costs. For instance, one evidence-based intervention found that providing at-risk asthma patients with individualized orientation programs on illness management and maintaining regular phone contact with an outreach nurse resulted in significant reductions in ED and hospital admissions (79% and 86%, respectively) and saved approximately $87,000 in costs.48 Thus, implementing HRQOL screening into pediatric clinical practice has the potential to be advantageous on several levels, namely improving patient functional and health status and saving on healthcare utilization and costs over time.
References
- 1.Ingerski LM, Baldassano RN, Denson LA, Hommel KA. Barriers to oral medication adherence for adolescents with inflammatory bowel disease. J Pediatr Psychol. 2010;35(6):683–691. doi: 10.1093/jpepsy/jsp085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenley RN, Stephens M, Doughty A, Raboin T, Kugathasan S. Barriers to adherence among adolescents with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16(1):36–41. doi: 10.1002/ibd.20988. [DOI] [PubMed] [Google Scholar]
- 3.Greenley RN, Hommel KA, Nebel J, et al. A meta-analytic review of the psychosocial adjustment of youth with inflammatory bowel disease. J Pediatr Psychol. 2010 Sep;35(8):857–869. doi: 10.1093/jpepsy/jsp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackner LM, Sisson DP, Crandall WV. Review: Psychosocial issues in pediatric inflammatory bowel disease. J Pediatr Psychol. 2004 Jun;29(4):243–257. doi: 10.1093/jpepsy/jsh027. [DOI] [PubMed] [Google Scholar]
- 5.Gray WN, Denson LA, Baldassano RN, Hommel KA. Treatment adherence in adolescents with inflammatory bowel disease: The collective impact of barriers to adherence and anxiety/depressive symptoms. Journal of Pediatric Pscyhology. 2012;37(3):282–291. doi: 10.1093/jpepsy/jsr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mardini HE, Kip KE, Wilson JW. Crohn's disease: a two-year prospective study of the association between psychological distress and disease activity. Dig Dis Sci. 2004 Mar;49(3):492–497. doi: 10.1023/b:ddas.0000020509.23162.cc. [DOI] [PubMed] [Google Scholar]
- 7.Mittermaier C, Dejacto C, Waldhoer T, et al. Impact of depressive mood on relapse in patients with inflammatory bowel disease: A prospective 18-month follow-up study. Psychosom Med. 2004;66(1):79–84. doi: 10.1097/01.psy.0000106907.24881.f2. [DOI] [PubMed] [Google Scholar]
- 8.Varni JW, Seid M, Smith Knight T, Burwinkle T, Brown J, Szer IS. The PedsQL in pediatric rheumatology: reliability, validity, and responsiveness of the Pediatric Quality of Life Inventory Generic Core Scales and Rheumatology Module. Arthritis Rheum. 2002 Mar;46(3):714–725. doi: 10.1002/art.10095. [DOI] [PubMed] [Google Scholar]
- 9.Gerson AC, Wentz A, Abraham AG, et al. Health-related quality of life of children with mild to moderate chronic kidney disease. Pediatrics. 2010 Feb;125(2):e349–357. doi: 10.1542/peds.2009-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varni JW, Seid M, Knight TS, Uzark K, Szer IS. The PedsQL 4.0 Generic Core Scales: sensitivity, responsiveness, and impact on clinical decision-making. J Behav Med. 2002 Apr;25(2):175–193. doi: 10.1023/a:1014836921812. [DOI] [PubMed] [Google Scholar]
- 11.Seid M, Sobo EJ, Gelhard LR, Varni JW. Parents' reports of barriers to care for children with special health care needs: development and validation of the barriers to care questionnaire. Ambul Pediatr. 2004 Jul-Aug;4(4):323–331. doi: 10.1367/A03-198R.1. [DOI] [PubMed] [Google Scholar]
- 12.Mapes DL, Lopes AA, Satayathum S, et al. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2003 Jul;64(1):339–349. doi: 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 13.Fogel J, Fauerbach JA, Ziegelstein RC, Bush DE. Quality of life in physical health domains predicts adherence among myocardial infarction patients even after adjusting for depressive symptoms. J Psychosom Res. 2004 Jan;56(1):75–82. doi: 10.1016/S0022-3999(03)00563-4. [DOI] [PubMed] [Google Scholar]
- 14.Seid M, Varni JW, Segall D, Kurtin PS. Health-related quality of life as a predictor of pediatric healthcare costs: a two-year prospective cohort analysis. Health Qual Life Outcomes. 2004;2:48. doi: 10.1186/1477-7525-2-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpentieri SC, Meyer EA, Delaney BL, et al. Psychosocial and behavioral functioning among pediatric brain tumor survivors. J Neurooncol. 2003 Jul;63(3):279–287. doi: 10.1023/a:1024203323830. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Tejera G, Canino G, Ramirez R, et al. Examining minor and major depression in adolescents. J Child Psychol Psyc. 2005 Aug;46(8):888–899. doi: 10.1111/j.1469-7610.2005.00370.x. [DOI] [PubMed] [Google Scholar]
- 17.Brent DA, Birmaher B, Kolko D, Baugher M, Bridge J. Subsyndromal depression in adolescents after a brief psychotherapy trial: course and outcome. J Affect Disord. 2001 Mar;63(1-3):51–58. doi: 10.1016/s0165-0327(00)00189-0. [DOI] [PubMed] [Google Scholar]
- 18.Cortina S, McGraw KJ, deAlarcon A, Ahrens A, Rothernberg ME, Drotar D. Psychological functioning of children and adolescents with eosinophil-associated gastrointestinal disorders. Children's Health Care Oct. 2010;39(4):266–278. doi: 10.1080/02739615.2010.515927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varni JW, Burwinkle TM, Seid M. The PedsQL (TM) 4.0 as a school population health measure: Feasibility, reliability, and validity. Qual Life Res. 2006 Mar;15(2):203–215. doi: 10.1007/s11136-005-1388-z. [DOI] [PubMed] [Google Scholar]
- 20.Bastiaansen D, Koot HM, Bongers IL, Varni JW, Verhulst FC. Measuring quality of life in children referred for psychiatric problems: psychometric properties of the PedsQL 4.0 generic core scales. Qual Life Res. 2004 Mar;13(2):489–495. doi: 10.1023/B:QURE.0000018483.01526.ab. [DOI] [PubMed] [Google Scholar]
- 21.Bolge SC, Doan JF, Kannan H, Baran RW. Association of insomnia with quality of life, work productivity, and activity impairment. Qual Life Res. 2009 May;18(4):415–422. doi: 10.1007/s11136-009-9462-6. [DOI] [PubMed] [Google Scholar]
- 22.Montazeri A, Milroy R, Hole D, McEwen J, Gillis CR. Quality of life in lung cancer patients - As an important prognostic factor. Lung Cancer. 2001 Feb-Mar;31(2-3):233–240. doi: 10.1016/s0169-5002(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 23.Varni JW, Seid M, Rode CA. The PedsQL: Measurement model for the pediatric quality of life inventory. Med Care. 1999 Feb;37(2):126–139. doi: 10.1097/00005650-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001 Aug;39(8):800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Kovacs M. Children's Depression Inventory. North Tonawanda, NY: Multi-Health Systems; 1992. [Google Scholar]
- 26.Beck A, Steer R, Brown G. Manual for Beck Depression Inventory II (BDI-II) San Antonio: Psychology Corporation; 1996. [Google Scholar]
- 27.Crandall W, Kappelman MD, Colletti RB, et al. ImproveCareNow: The Development of a Pediatric Inflammatory Bowel Disease Improvement Network. Inflamm Bowel Dis. 2011 Jan;17(1):450–457. doi: 10.1002/ibd.21394. [DOI] [PubMed] [Google Scholar]
- 28.Stjernman H, Tysk C, Almer S, Strom M, Hjortswang H. Factors Predicting the Outcome of Disease Activity Assessment in Crohn's Disease. Inflamm Bowel Dis. 2009 Dec;15(12):1859–1866. doi: 10.1002/ibd.20975. [DOI] [PubMed] [Google Scholar]
- 29.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn's Disease Activity Index. J Pediatr Gastroenterol Nutr. 1991;12(4):439–447. [PubMed] [Google Scholar]
- 30.Hyams JS, Markowitz J, Otley A, et al. Evaluation of the Pediatric Crohn Disease Activity Index: A prospective multicenter experience. J Pediatr Gastroenterol Nutr. 2005;41(4):416–421. doi: 10.1097/01.mpg.0000183350.46795.42. [DOI] [PubMed] [Google Scholar]
- 31.Turner D, Hyams J, Markowitz J, et al. Appraisal of the pediatric ulcerative colitis activity index (PUCAI) Inflamm Bowel Dis. 2009 Aug;15(8):1218–1223. doi: 10.1002/ibd.20867. [DOI] [PubMed] [Google Scholar]
- 32.SPSS [computer program] Version 17. Chicago, IL: 2009. [Google Scholar]
- 33.Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:43. doi: 10.1186/1477-7525-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hommel KA, Baldassano RN. Brief report: Barriers to treatment adherence in pediatric inflammatory bowel disease. J Pediatr Psychol. 2010;35(9):1005–1010. doi: 10.1093/jpepsy/jsp126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingerski LM, Modi AC, Hood KK, et al. Health-related quality of life across pediatric chronic conditions. J Pediatr. 2010 Apr;156(4):639–644. doi: 10.1016/j.jpeds.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perrin JM, Kuhlthau K, Chughtai A, et al. Measuring quality of life in pediatric patients with inflammatory bowel disease: psychometric and clinical characteristics. J Pediatr Gastroenterol Nutr. 2008 Feb;46(2):164–171. doi: 10.1097/MPG.0b013e31812f7f4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcus SB, Strople JA, Neighbors K, et al. Fatigue and health-related quality of life in pediatric inflammatory bowel disease. Clinical Gastroenterology and Hepatology. 2009;7(5):554–561. doi: 10.1016/j.cgh.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 38.Patrick DL, Deyo RA. Generic and disease-specific measures in assessing health status and quality of life. Med Care. 1989 Mar;27(3 Suppl):S217–232. doi: 10.1097/00005650-198903001-00018. [DOI] [PubMed] [Google Scholar]
- 39.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993 Apr 15;118(8):622–629. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 40.Kunz JH, Hommel KA, Greenley RN. Health-related quality of life of youth with inflammatory bowel disease: A comparison with published data using the PedsQL 4.0 generic core scales. Inflamm Bowel Dis. 2010 Jun;16(6):939–946. doi: 10.1002/ibd.21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varni JW, Burwinkle TM, Lane MM. Health-related quality of life measurement in pediatric clinical practice: an appraisal and precept for future research and application. Health Qual Life Outcomes. 2005;3:34. doi: 10.1186/1477-7525-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner AK, Ehrenberg BL, Tran TA, Bungay KM, Cynn DJ, Rogers WH. Patient-based health status measurement in clinical practice: a study of its impact on epilepsy patients' care. Qual Life Res. 1997 May;6(4):329–341. doi: 10.1023/a:1018479209369. [DOI] [PubMed] [Google Scholar]
- 43.Szigethy E, Kenney E, Carpenter J, et al. Cognitive-behavioral therapy for adolescents with inflammatory bowel disease and subsyndromal depression. J Am Acad Child Adolesc Psychiatry. 2007;46(10):1290–1298. doi: 10.1097/chi.0b013e3180f6341f. [DOI] [PubMed] [Google Scholar]
- 44.Wysocki T, Harris MA, Buckloh LM, et al. Effects of behavioral family systems therapy for diabetes on adolescents' family relationships, treatment adherence, and metabolic control. J Pediatr Psychol. 2006 Oct;31(9):928–938. doi: 10.1093/jpepsy/jsj098. [DOI] [PubMed] [Google Scholar]
- 45.Janicke DM, Finney JW, Riley AW. Children's health care use: A prospective investigation of factors related to care-seeking. Med Caree. 2001 Sep;39(9):990–1001. doi: 10.1097/00005650-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Detmar SB, Aaronson NK. Quality of life assessment in daily clinical oncology practice: a feasibility study. Eur J Cancer. 1998 Jul;34(8):1181–1186. doi: 10.1016/s0959-8049(98)00018-5. [DOI] [PubMed] [Google Scholar]
- 47.Carlson LE, Speca M, Hagen N, Taenzer P. Computerized quality-of-life screening in a cancer pain clinic. J Palliat Care Spring. 2001;17(1):46–52. [PubMed] [Google Scholar]
- 48.Greineder DK, Loane KC, Parks P. Reduction in resource utilization by an asthma outreach program. Arch Pediatr Adolesc Med. 1995 Apr;149(4):415–420. doi: 10.1001/archpedi.1995.02170160069010. [DOI] [PubMed] [Google Scholar]


