G-quadruplexes (G4s) are DNA or RNA four-stranded supramolecular architectures that may form in G-rich regions. G4s have been found in biologically significant regions of the genome such as telomeres, gene promoters, and in sequences associated with human disease.[1] Due to their critical roles in key biological processes, G4s have been the object of intense studies for their potential as therapeutic targets.[2] To date, a broad range of compounds has been identified as G4 ligands[3] both in vitro and in vivo with encouraging results in clinical trials.[2a] Among them, numerous tri- and tetra-substituted naphthalene diimides (NDIs) have shown high affinity for telomeric G4s and good antiproliferative activity.[4] In this context, we recently began the development of hybrid ligand-alkylating NDIs that possess a binding core tethered to an electrophile precursor, such as a quinone methide (QM, see Scheme 1),[5] which can interact covalently with G4 structures.[4d-f] Covalent G4 targeting was also explored using Pt(II)-terpyridine complexes.[6] The strategy highlighted in Scheme 1 affords the possibility of triggering the alkylating activity under well-defined environmental conditions (e.g., light or mild digestion at 40°C), which would help minimize typical off-target reactivity before the site of attack is reached.[7] Unfortunately, the QMs tested to date have yielded rather reversible DNA adducts, which eluded structural characterization.[4e, f] In the attempt to overcome such a drawback we have developed NDIs tethering the reversible ligands 1-4 (Scheme 1), to both a QM precursor (5 and 6) and an intrinsically reactive oxirane (7). The reversible binding of 1-4 and the alkylating properties of 5-7 were evaluated against a model G4 structure corresponding to the human telomere. The superior reactivity and selectivity of the alkylating oxirane 7 were unambiguously and unprecedentedly assessed by different mass spectrometric approaches.
Scheme 1.
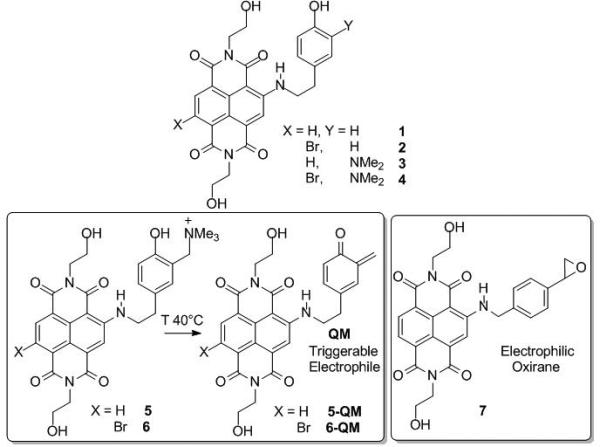
NDIs as reversible (1-4) and alkylating G4 binders (5-7).
The NDIs 1-6 were synthesized by a nucleophilic aromatic substitution (SNAr), on the dibromo-NDI 8,[8] in the presence of 4-(2-aminoethyl) phenol and 4-ethenylbenzenemethanamine for 1-6 and 7, respectively (ESI, Scheme 1S). The desired side chains and the alkylating moieties were introduced only subsequently to the SNAr, in order to preserve their structural integrity. In this way, the multi-step synthesis for 1-4 involved a SNAr and a Mannich reaction, followed by an exhaustive methylation of the amines 3 and 4, yielding the quaternary ammonium salts (5, 6). The oxirane 7 was obtained as a racemic mixture by SNAr using 4-vinylbenzylamine and subsequent epoxidation with dimethyldioxirane (DMD) or meta-chloroperbenzoic acid (MCPBA, yield > 90%).
The activity of reversible ligands 3 and 4 was initially assayed by mixing them with a labelled oligonucleotide that reproduced the human telomeric DNA sequence (F21T, Figure 1S) and by using FRET to measure the melting of the structure.[9] Addition of 1.0 μM NDIs to a 0.25 μM solution of F21T in the presence of K+ induced melting temperature increases (ΔTm) of 10 ± 1.0°C and 11 ± 0.5°C for 3 and 4, respectively. In contrast, no Tm variations were observed upon incubation with labelled double-stranded DNAs. Possible structural perturbations were also monitored by circular dichroism (CD).[10] In this case, minor spectral variations were detected when the NDIs were added to a fully-folded hTel, which displayed the typical G4's fingerprint with a maximum at 290 nm. When the compounds were added before folding, an additional maximum was noted at 260 nm (Figure 2S). This behavior indicates that the NDIs may partially direct the folding towards a parallel-like conformation, as described for other NDIs.[4e, f] The continuous variation method (Job Plot)[11] was employed to evaluate the 1:1 binding stoichiometry (Figure 2S).
The alkylating properties of 5 and 6 were assayed by incubating them with selected substrates at 40°C and by monitoring adduct formation by denaturing polyacrylamide gel electrophoresis (PAGE). Reactivity was tested against the folded hTel and ss- and ds-scrambled hTel (Figure 1S). Adduct formation was observed at a molar ratio of 0.8:1 NDI:hTel, and yields increased to ~10% adduct at a ratio of 12:1 (Figure 1, A and B). In contrast, the same amount of ss substrate failed to produce detectable adducts even when treated with up to 50:1 NDI:ss (Figure 1B). Similarly, the ds substrate produced only a modest ~2% adduct when the analogue concentrations were increased to 200:1 NDI:ds (Figures 1B and 3S). Under the same conditions, the oxirane 7 displayed greater reactivity than the corresponding QM activatable counterparts (Figure 1C). In this case, up to ~16% adduct yield was obtained at 12:1 NDI:hTel (Figure 1D). Conversely, alkylation of ss and ds substrates was modest (<2%, when treated with up to 200:1 NDI:substrate). The negligible reactivity manifested by substrates that do not fold into G4 suggests a mechanism involving specific substrate-ligand recognition before the actual alkylation can take place. Substrates that are unable of sustaining NDI binding appear incapable of undergoing significant alkylation, even though they still contain putative reactive sites of A and G.
Figure 1.
Alkylation of 6 and 7 analyzed by PAGE. A) P32-labelled hTel alone (lane 1) and digested with 6 at 40°C for 24 h (lanes 2-9) or at 4°C for 24 h (lane 10). C) Compound 7 with P32-labelled hTel, ss- and ds-scrambled hTel. Quantification of adduct bands obtained reacting compound 6 B) and 7 D) with hTel, ss- and ds-scrambled hTel
These data indicate a selective alkylation of the G4 structure over unstructured or double-helix DNA. The possible reversible character of the alkylated G4 product was explored as a function of temperature and salt concentration. The adduct was stable after 10 min incubation at 95°C and in up to 1.0 M KCl solution (Figure 4S). By comparison, these conditions were found sufficient to partially revert adducts formed by the activatable QM compound 6 (Figure 5S). The higher efficiency in the modification of hTel by 7 in comparison to 6 (see Figure 1A vs Figure 1C) and the increased stability of the resulting 7-hTel (Figure 4S) in comparison to 6-hTel (Figure 5S), prompted us to further study the nature of the former. Therefore, the structure of the 7-hTel adduct was investigated by electrospray ionization mass spectrometry (ESI-MS), which was performed on both unreacted and alkylated species after isolation by PAGE. The observed mass of 7464.33 Da matched very closely the mass of 7464.34 Da calculated for a 1:1 adduct by adding the masses of the initial G4 construct and 7 (Figure 6S). These data suggested that the putative adduct was produced by opening of the oxirane induced by hTel. Submitted to tandem mass spectrometry (MS/MS), the alkylated product provided typical fragment ions that matched the sequence of hTel. As typically observed for covalent adducts of nucleic acids,[13] the fragments included a characteristic mass shift corresponding to analogue 7 (i.e., 501 Da incremental mass), which enabled to locate its putative position at A1, A7, A13, and A19 (Figure 2). The detection of a fragment corresponding to the adduct 7 bound to an A nucleobase (i.e., m/z 637.2, Figure 7SA) was consistent with the mechanism of DNA fragmentation, which is prompted by initial base loss.[13] A similar alkylation selectivity has been reported for Pt(II)-terpyridines which coordinate exclusively the adenine nucleobases present in the G4 loops.[6] Considering the well-known styrene oxide reactivity towards all four deoxyribonucleosides dG>dC>dA>>dT[14] it was surprising to observe that A exhibited greater susceptibility than G. In contrast, the alkylated adduct of 7 with G nucleobase was detected when the substrate considered was the ss construct with scrambled sequence. Indeed, a fragment corresponding to alkylated G was recognizable in the MS/MS spectrum of the ss'oxirane adduct (Figure 7SB). At the same time, no product of A alkylation could be detected, thus suggesting that adduct formation was profoundly affected by the structural context.
Figure 2.
MS/MS spectrum of 7–hTel adduct obtained in negative ion mode. Characteristic ion series (black font) are labelled according to standard nomenclature.[12] [M-5H]5- marks the precursor ion at m/z 1492.46. Ions labelled in green included a 501 Da mass shift from the corresponding un-modified fragment, in agreement with the presence of one unit of 7. All fragments are summarized on the hTel sequence to enable a direct comparison of ion series with (marked with an asterisk symbol) and without (unmarked) adduct.
In conclusion, we have developed two types of G4 alkylating agents. Both alkylation efficiency and selectivity were enhanced in the oxirane derivative. While QM adducts proved to be insufficiently stable for isolation and characterization, the stability of oxirane adducts enabled MS analysis. The results showed a highly selective alkylation of A vs G in h-Tel, which is consistent with a lower G accessibility within the G4 structure. The presence of As in the loop regions of most oncogene promoters that form G4s15 suggests that NDI-oxirane conjugates may covalently target different cancer-related G4 structures based on its affinity for G-quartets and selective alkylation of As.
Supplementary Material
Acknowledgements
Financial support from MIUR, Rome (FIRB-IdeasRBID082ATK, and PRIN 2009MFRKZ8), University of Pavia, University of Padua, and the National Institutes of Health (GM064328-12to DF) is gratefully acknowledged.
Footnotes
Supporting information for this article is available on the WWW under http://www.chemeurj.org/ or from the author.
References
- 1.a Huppert JL, Balasubramanian S. Nucleic Acids Res. 2005;33:2908–2916. doi: 10.1093/nar/gki609. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Huppert JL, Balasubramanian S. Nucleic Acids Res. 2007;35:406–413. doi: 10.1093/nar/gkl1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a Balasubramanian S, Hurley LH, Neidle S. Nat. Rev. Drug. Discov. 2011;10:261–275. doi: 10.1038/nrd3428. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Paeschke K, Simonsson T, Postberg J, Rhodes D, Lipps HJ. Nat Struct. Mol. Biol. 2005;12:847–854. doi: 10.1038/nsmb982. [DOI] [PubMed] [Google Scholar]
- 3.a De Cian A, Grellier P, Mouray E, Depoix D, Bertrand H, Monchaud D, Teulade-Fichou MP, Mergny JL, Alberti P. Chembiochem. 2008;9:2730–2739. doi: 10.1002/cbic.200800330. [DOI] [PubMed] [Google Scholar]; b Petenzi M, Verga D, Largy E, Hamon F, Doria F, Teulade-Fichou M-P, Guédin A, Mergny J-L, Mella M, Freccero M. Chem. Eur. J. 2012;18:14487–14496. doi: 10.1002/chem.201202097. [DOI] [PubMed] [Google Scholar]
- 4.a Collie GW, Promontorio R, Hampel SM, Micco M, Neidle S, Parkinson GN. J. Am. Chem. Soc. 2012;134:2723–2731. doi: 10.1021/ja2102423. [DOI] [PubMed] [Google Scholar]; b Gunaratnam M, de la Fuente M, Hampel SM, Todd AK, Reszka AP, Schätzlein A, Neidle S. Bioorg. Med. Chem. 2011;23:7151–7157. doi: 10.1016/j.bmc.2011.09.055. [DOI] [PubMed] [Google Scholar]; c Hampel SM, Sidibe A, Gunaratnam M, Riou JF, Neidle S. Bioorg. Med. Chem. Lett. 2008;20:6459–6463. doi: 10.1016/j.bmcl.2010.09.066. [DOI] [PubMed] [Google Scholar]; d Di Antonio M, Doria F, Richter SN, Bertipaglia C, Mella M, Sissi C, Palumbo M, Freccero M. J. Am. Chem. Soc. 2009;131:13132–13141. doi: 10.1021/ja904876q. [DOI] [PubMed] [Google Scholar]; e Nadai M, Doria F, Di Antonio M, Sattin G, Germani L, Percivalle C, Palumbo M, Richter SN, Freccero M. Biochimie. 2011;93:1328–1340. doi: 10.1016/j.biochi.2011.06.015. [DOI] [PubMed] [Google Scholar]; f Doria F, Nadai M, Folini M, Di Antonio M, Germani L, Percivalle C, Sissi C, Zaffaroni N, Alcaro S, Artese A, Richter SN, Freccero M. Org. Biomol. Chem. 2012;10:2798–2806. doi: 10.1039/c2ob06816h. [DOI] [PubMed] [Google Scholar]
- 5.Rokita SE. Quinone Methides. Wiley-VCH; Hoboken: 2009. [Google Scholar]
- 6.Bertrand H, Bombard S, Monchaud D, Talbot E, Guedin A, Mergny JL, Grunert R, Bednarski PJ, Teulade-Fichou MP. Org. Biomol. Chem. 2009;7:2864–2871. doi: 10.1039/b904599f. [DOI] [PubMed] [Google Scholar]
- 7.a Doria F, Percivalle C, Freccero M. J. Org. Chem. 2012;77:3615–3619. doi: 10.1021/jo300115f. [DOI] [PubMed] [Google Scholar]; b Verga D, Nadai M, Doria F, Percivalle C, Di Antonio M, Palumbo M, Richter SN, Freccero M. J. Am. Chem. Soc. 2010;132:14625–14637. doi: 10.1021/ja1063857. [DOI] [PubMed] [Google Scholar]
- 8.Doria F, di Antonio M, Benotti M, Verga D, Freccero M. J. Org. Chem. 2009;74:8616–8625. doi: 10.1021/jo9017342. [DOI] [PubMed] [Google Scholar]
- 9.De Cian A, Guittat L, Kaiser M, Sacca B, Amrane S, Bourdoncle A, Alberti P, Teulade-Fichou MP, Lacroix L, Mergny JL. Methods. 2007;42:183–195. doi: 10.1016/j.ymeth.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Karsisiotis AI, Hessari NM, Novellino E, Spada GP, Randazzo A, Webba da Silva M. Angew. Chem. Int. Ed. 2011;50:10645–10648. doi: 10.1002/anie.201105193. [DOI] [PubMed] [Google Scholar]
- 11.Huang CY. Methods Enzymol. 1982;87:509–525. doi: 10.1016/s0076-6879(82)87029-8. [DOI] [PubMed] [Google Scholar]
- 12.McLuckey SA, Habibi-Goudarzi S. J. Am. Chem. Soc. 1993;115:12085–12095. [Google Scholar]
- 13.a Nordhoff E, Kirpekar F, Roepstorff P. Mass Spectrom. Rev. 1996;15:67–138. doi: 10.1002/(SICI)1098-2787(1996)15:2<67::AID-MAS1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]; b Kellersberger KA, Yu E, Kruppa GH, Young MM, Fabris D. Anal. Chem. 2004;76:2438–2445. doi: 10.1021/ac0355045. [DOI] [PubMed] [Google Scholar]
- 14.Savela K, Hesso A, Hemminki K. Chem. Biol. Interact. 1986;60:235–246. doi: 10.1016/0009-2797(86)90055-4. [DOI] [PubMed] [Google Scholar]
- 15.Brooks TA, Kendrick S, Hurley L. Febs J. 2010;277:3459–3469. doi: 10.1111/j.1742-4658.2010.07759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




