Abstract
The net effect of mu opioid receptor agonists on intracranial self-stimulation (ICSS) in rats reflects an integration of rate-increasing and rate-decreasing effects. Prior opioid exposure is associated with tolerance to rate-decreasing effects and augmented expression of abuse-related rate-increasing effects. This finding was replicated here with morphine. Subsequent studies then tested the hypothesis that opioid agonist-induced rate-decreasing effects require activation of a larger relative fraction of mu receptors, and hence are more vulnerable to tolerance-associated reductions in receptor density, than rate-increasing effects. Two sets of experiments were conducted to test this hypothesis. First, morphine effects on ICSS were examined after pretreatment with the irreversible mu antagonist β-funaltrexamine (β-FNA) to reduce density of available mu receptors. Second, effects were examined for a range of mu opioids that varied in relative efficacy at mu receptors. The hypothesis predicted that (a) morphine after β-FNA treatment or (b) low-efficacy mu agonists would mimic effects of morphine tolerance to produce reduced expression of rate-decreasing effects and enhanced expression of rate-increasing effects. Neither of these predictions was supported. These results suggest that mu agonist-induced facilitation and depression of ICSS may be mediated by distinct populations of mu receptors that respond differently to regimens of opioid exposure.
Keywords: Morphine, opioid, efficacy, intracranial self-stimulation, drug abuse, rats
Introduction
Mu opioid receptor agonists constitute one class of abused drugs (Hughes and Rieche, 1995; Cicero et al., 2007), and intracranial self-stimulation (ICSS) is one preclinical experimental procedure that has been used to examine abuse-related effects of mu agonists and other drugs (Kornetsky and Esposito, 1979; Wise, 1998). In ICSS, an operant response such as lever press is maintained by delivery of electrical stimulation to a brain region such as the medial forebrain bundle. Drug-induced increases in low-rates of ICSS are often interpreted as a rewarding effect associated with abuse potential (Esposito et al., 1980; Cryan et al., 2003; Altarifi and Negus, 2011). Evaluation of factors that determine the expression of drug-induced rate-increasing effects in ICSS may improve the usefulness of this procedure as an experimental tool for assessing the abuse potential of opioids and other drugs.
We reported previously that morphine effects on ICSS in rats were determined by factors that included dose, time after morphine treatment, and extent of prior morphine exposure (Altarifi and Negus, 2011). Abuse-related rate-increasing effects of morphine were most apparent early after administration of low morphine doses, later in the time course after higher morphine doses, or after repeated morphine exposure. However, morphine also produced rate-decreasing effects that appeared to oppose and limit the expression of rate-increasing effects. These rate-decreasing effects of morphine were most apparent early in the time course after high morphine doses, and repeated morphine treatment produced tolerance to these rate-decreasing effects and a corresponding increase in the expression of rate-increasing effects. These results agree with other studies in suggesting that effects of morphine and other opioids on ICSS reflect an integration of both rate-increasing and rate-decreasing effects, and that opioid exposure can produce tolerance to rate-decreasing effects and increased expression of abuse-related rate-increasing effects (Lorens and Mitchell, 1973; Koob et al., 1975; Carlezon and Wise, 1993; Easterling and Holtzman, 1997).
One possible explanation of these findings is that mu agonist-induced facilitation of ICSS requires lower levels of receptor occupation and activation (i.e. has lower efficacy requirements and higher receptor reserve) than mu agonist-induced depression of ICSS. According to this hypothesis, lower morphine doses would produce sufficient receptor occupancy and activation to facilitate ICSS, whereas higher doses would produce higher levels of receptor occupancy sufficient to recruit opposing rate-decreasing effects. Repeated morphine could selectively attenuate rate-decreasing effects and enhance expression of rate-increasing effects by desensitizing and/or downregulating some fraction of mu receptors to reduce the functional receptor density and attenuate the ability of morphine to produce rate-decreasing effects dependent on high levels of receptor occupancy (Martini and Whistler, 2007). The present study tested this hypothesis in two ways. First, morphine effects on ICSS were examined in different groups of rats after pretreatment with different doses of the irreversible mu opioid receptor antagonist β-funaltrexamine (β-FNA) (Ward et al., 1982; Walker et al., 1999; Bardo et al., 2003). Because irreversible antagonists decrease the density of available receptors, the hypothesis predicted that β-FNA would be more potent to antagonize rate-decreasing than rate-increasing effects, and that it might be possible to titrate β-FNA dose to selectively attenuate morphine’s rate-decreasing effects and enhance expression of rate-increasing effects. Second, effects on ICSS were examined for a range of other mu agonists that varied in their relative efficacy at mu receptors. The drugs tested were methadone, fentanyl, hydrocodone, buprenorphine, nalbuphine and naltrexone (listed in order from highest to lowest efficacy as determined by in vitro functional assays of agonist-stimulated GTPγS binding) (Selley et al., 1997; Selley et al., 1998; Thompson et al., 2004). The hypothesis predicted that higher-efficacy mu agonists would have sufficient efficacy to produce both morphine-like rate-increasing and rate-decreasing effects, but that lower efficacy mu agonists might lack sufficient efficacy to produce rate-decreasing effects and might therefore produce enhanced rate-increasing effects.
Methods
Subjects and ICSS electrode implantation
Male Sprague-Dawley rats (Harlan, Fredrick, MD, USA) weighing 310–350 g at the time of surgery were used for these studies. Rats were individually housed and maintained on a 12-h light/dark cycle with lights on from 6:00 a.m. to 6:00 p.m. Rats had free access to food and water except during testing. Animal maintenance and research were in compliance with National Institutes of Health guidelines on care and use of animal subjects in research, and all animal use protocols were approved by the Virginia Commonwealth University Institutional Care and Use Committee.
Rats were anesthetized with isoflurane gas (2.5–3% in oxygen; Webster Veterinary, Phoenix, AZ) for the implantation of stainless steel electrodes. The cathode of each bipolar electrode (Plastics One, Roanoke, VA) was 0.25 mm in diameter and covered with polyamide insulation except at the flattened tip. The anode was 0.124 mm in diameter and uninsulated. The cathode was implanted in the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior and 1.7 mm lateral from bregma, and 8.8 mm below the skull). The anode was wrapped around one of three skull screws to serve as the ground, and the skull screws and electrode assembly were secured with orthodontic resin. Animals were allowed to recover for at least 7 days prior to commencing ICSS training.
Experimental procedure
Apparatus and training
Experiments were conducted in sound attenuating chambers that contained modular acrylic test chambers (29.2 × 30.5 × 24.1) equipped with a response lever (4.5 cm wide, extended 2.0 cm through the center of one wall, 3 cm off the floor), stimulus lights (three lights colored red, yellow and green positioned 7.6 cm directly above the lever), a 2-W white house light, and an ICSS stimulator (Med Associates, St. Albans, VT). Electrodes were connected to the stimulator via bipolar cables and a commutator (Model SL2C, Plastics One, Roanoke, VA). A computer and software (Med Associates, St. Albans, VT) controlled the stimulator, programming parameters and data collection.
Rats were trained under a continuous reinforcement schedule of brain stimulation using procedures similar to those described previously (Pereira Do Carmo et al., 2009a,b; Negus et al., 2010; Altarifi and Negus, 2011). Each lever press resulted in the delivery of a 0.5-s train of square wave cathodal pulses (0.1-ms pulse duration), and stimulation was accompanied by illumination of the stimulus lights above the lever. Responses during the 0.5-s stimulation period did not result in additional stimulation. During the initial phase of training, sessions lasted 30 to 60 min, the frequency of stimulation was held constant at 158 Hz, and the stimulation intensity was adjusted to the lowest value that would sustain at least 30 stimulations per minute. Once this criterion was met, frequency manipulations were introduced during sessions that consisted of sequential 10 min components. During each component, a descending series of 10 current frequencies (158-56 Hz in 0.05 log increments) was presented, with a 60-s trial at each frequency. A frequency trial began with a 5-s time out followed by a 5-s “priming” phase during which animals received 5 non-contingent stimulations with a 0.5-s interval between each stimulation. This non-contingent stimulation was followed by a 50-s “response” phase during which responding produced electrical stimulation under a continuous reinforcement schedule. Training continued with three to twelve sequential components per day, and the current intensity was adjusted until rats reliably responded during the first three to four frequency trials of all components for at least three consecutive days. This intensity (range: 100–290 µA) was held constant for the remainder of the study. In addition, rats were habituated to saline injections until these injections had no effect on ICSS frequency-rate curves. Once training and habituation to saline injections were completed, testing was initiated using the experimental designs described below.
Effects of repeated morphine and of β-FNA on cumulative morphine dose-effect curves
The first study was designed to compare effects of repeated morphine with effects of the irreversible mu opioid receptor antagonist β-FNA on changes in ICSS produced by cumulative morphine dosing. Four groups of rats (N=5 per group) received 0 (no pretreatment), 0.32, 1.0 or 3.2 mg/kg β-FNA 24hr before determination of a cumulative morphine dose-effect curve. Cumulative morphine test sessions began with three consecutive "control" ICSS components. The first control component was considered to be an acclimation component, and data from this component were discarded. Data from the second and third control components were used to calculate control parameters of frequency-rate curves for that session (see Data Analysis). Immediately after completion of these control ICSS components, cumulative morphine doses (0.32–10 mg/kg) were administered at 50 min intervals such that each sequential dose increased the total cumulative dose by 0.5 log units. Thirty min after each dose, ICSS was evaluated during two consecutive ICSS test components (10 min each, 20 min total). The group receiving no β-FNA pretreatment was also used to evaluate effects of repeated morphine treatment during a 14-day regimen of repeated morphine dosing and testing that began on the day after determination of the initial cumulative morphine dose-effect curve. On Days 1–6, sessions consisted of three consecutive control ICSS components followed 30 min later by two test components, and 3.2 mg/kg morphine was administered immediately after completion of the control components. On Day 7, the cumulative morphine dose-effect curve was redetermined as described above. On Days 8–13, sessions again consisted of three consecutive control components followed 30 min later by two test components, and the daily morphine dose was increased to 10 mg/kg. On Day 14, the cumulative morphine dose-effect curve was redetermined a second time.
Effects of opioid agonists with varying efficacies at the mu receptor
Methadone (0.032–5.6 mg/kg), fentanyl (0.001–0.1 mg/kg), hydrocodone (0.1–10 mg/kg), morphine (0.32–10 mg/kg), buprenorphine (0.001–0.1 mg/kg), nalbuphine (0.032–10 mg/kg), naltrexone (0.1 mg/kg) (in order from highest to lowest efficacy at mu opioid receptors) and vehicle (saline) were tested in one group of animals (n = 5). Morphine was tested first, and results were similar to those reported previously (Altarifi and Negus, 2011) and to results with cumulative morphine described above, and are not discussed further. The remaining drugs were tested in order of buprenorphine, methadone, hydrocodone, fentanyl, nalbuphine and naltrexone. For each drug except naltrexone, testing was conducted in two phases. During the first phase, dose was manipulated from doses that produced no effect on ICSS to doses that either (a) significantly decreased ICSS or (b) were at least 10 times greater than the lowest dose to significantly facilitate ICSS. Test sessions consisted of three consecutive control components followed immediately by drug injection and then 30 min later by two consecutive test components. During the second phase, the time course of the dose producing peak facilitation of ICSS was determined. Test sessions consisted of three consecutive control components followed immediately by drug injection and then by consecutive pairs of test components that began 10, 30, 100, 180 and 300 min after injection. Naltrexone was tested at a single dose (0.1 mg/kg) alone and as a 20 min pretreatment to 1.0 mg/kg methadone. One rat died prior to studies with naltrexone, so these studies were conducted with only four rats.
Four other experiments were conducted in three other groups of rats to expand on results obtained in the experiment described above. First, the time course of a higher buprenorphine dose (1.0 mg/kg) was determined in a group of six rats that had a history of intermittent nalbuphine injections (i.e. opioid testing no more than twice per week). Second, another group of four rats that had a history of intermittent methadone and loperamide injections received a high morphine dose (10 mg/kg) with or without pretreatment of nalbuphine (1.0 mg/kg). Nalbuphine (or saline) was injected 10 min prior to morphine injection, and ICSS started 20 min after morphine administration. The remaining two experiments examined effects of the highest efficacy mu agonist methadone (0.032–3.2 mg/kg) and the lowest efficacy mu agonist nalbuphine (0.032–10 mg/kg) in separate groups of opioid naïve rats. Table 1 summarizes all the groups used in this study.
Table 1.
Summary table showing the pharmacological history and number of rats used in each group. “Intermittent” indicates that the drug was administered no more often than twice per week.
| Figure | Test Drug (s) | Pharmacological History | Group size |
|---|---|---|---|
| 1 | Morphine | Daily morphine (0, 3.2, 10 mg/kg/day) |
5 |
| 2 (a,b) | Morphine | 0.32 mg/kg β-FNA | 5 |
| 2 (c,d) | Morphine | 1.0 mg/kg β-FNA | 5 |
| 2 (e,f) | Morphine | 3.2 mg/kg β-FNA | 5 |
| 3, 4, 5 | Buprenorphine, Methadone, Hydrocodone, Fentanyl, Nalbuphine, Naltrexone (in order of testing) |
Intermittent morphine (0.1–10 mg/kg) |
5 |
| 6 | Buprenorphine | Intermittent nalbuphine (0.1–1.0 mg/kg) |
6 |
| 7 | Nalbuphine, morphine | Intermittent methadone (0.032–3.2 mg/kg), loperamide (0.32–10 mg/kg) |
4 |
| 8 (a,b) | Methadone | Naïve | 6 |
| 8 (c,d) | Nalbuphine | Naïve | 6 |
In all experiments with different efficacy opioids, sessions were typically conducted on Tuesdays and Fridays and were separated by at least three days. This intermittent dosing regimen was intended to minimize development of tolerance to drug effects on ICSS. Training sessions consisting of three components were conducted on Mondays, Wednesdays, Thursdays, and occasionally, on Saturdays. In some cases, data from these training sessions were used to assess ICSS performance 24 h after injection in the time-course studies. In these cases, data from the second and third components of the training session were used as the "24 h time point" for data analysis.
Data Analysis
The primary dependent variable was the reinforcement rate in stimulations/trial during each frequency trial. To normalize these raw data, reinforcement rates from each trial were converted to Percent Maximum Control Rate (%MCR) for that rat on that day. The maximum control rate was determined during control components of each test session and was defined as the mean of the maximal rates observed in any frequency trial during the second and third control components. Thus, %MCR for each trial was calculated as (Reinforcement Rate During a Frequency Trial ÷ Maximum Control Rate) × 100. Normalized data from the frequency trials of each pair of consecutive test components were then averaged across rats for display and for statistical analysis using two-way ANOVA, with drug dose or time as one factor and ICSS frequency as the other factor. A significant ANOVA was followed by a Holm-Sidak post hoc test, and the criterion for significance was set at p < 0.05.
To provide an additional summary of ICSS performance, the total number of stimulations per component was calculated as the average of the total stimulations delivered across all 10 frequency trials of each component. Test data were expressed as a percentage of the total stimulations earned during the “control” components (% Control Stimulations). Thus, % Control stimulations for each test was calculated as (Mean Total Stimulations during Test Components ÷ Mean Total Stimulations during Control Components) × 100.
To determine the effects of treatments on maximum control rates and on total stimulations during control components, data were analyzed as appropriate with paired t-tests or with one-way repeated measures ANOVA followed by the Newman-Keuls post hoc test. In each case, the criterion for significance was set at p<0.05.
Drugs
Morphine sulfate, beta-Funaltrexamine HCl, buprenorphine HCl, methadone HCl, hydrocodone bitartrate, naltrexone HCl and fentanyl HCl were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). Nalbuphine HCl was provided by Dr. Kenner Rice (Chemical Biology Branch, National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD). All drugs were dissolved in saline and delivered s.c. in a volume of 1 ml/kg body weight.
Results
Effects of repeated morphine and of β-FNA on cumulative morphine dose-effect curves
Table 2 and figure 1 show control ICSS parameters and effects of cumulative morphine before repeated morphine and after repeated daily treatment with 3.2 and 10 mg/kg morphine in the group receiving 0 β-FNA pretreatment. Under control conditions before cumulative morphine, little responding was maintained by the lower frequencies of stimulation (56–89 Hz), whereas reinforcement rates increased at the intermediate and higher intensities (100–158 Hz). Prior to repeated morphine, testing with cumulative morphine produced dose-dependent changes in ICSS (figure 1a,b). The lowest cumulative dose of 0.32 mg/kg morphine had no effect on ICSS. Treatment with 1.0 mg/kg morphine increased ICSS at the intermediate frequency of 100 Hz. Cumulative 3.2 mg/kg morphine produced a biphasic effect, increasing ICSS at intermediate frequencies (89 and 100 Hz) but decreasing ICSS at the highest frequency (158 Hz). Cumulative 10 mg/kg morphine exclusively produced rate-decreasing effects at intermediate to high frequencies (100–158 Hz) and nearly eliminated ICSS.
Table 2.
Control MCR and Total Stimulations obtained during experiments to determine the effects of repeated morphine on cumulative morphine dose-effect curves.
| MCR ± SE | Total Stimulations ± SE | |
|---|---|---|
| Before repeated morphine | 56.0 ± 6.5 | 221.9 ± 33.3 |
| After Repeated 3.2 mg/kg/day | 54.0 ± 6.1 | 176.1 ± 26.0 |
| After Repeated 10.0 mg/kg/day | 51.6 ± 6.7 | 155.5 ±28.9* |
Asterisk indicates significant difference from data obtained before repeated morphine (p < 0.05) as indicated by one-way repeated measures ANOVA with Newman-Keuls post-hoc test.
Fig. 1.
Effects of repeated morphine on cumulative morphine dose-effect curves. Cumulative morphine dose effect curves were determined before repeated daily morphine administration (a,b), following six days of repeated daily administration of 3.2 mg/kg morphine (c,d), and six days of repeated daily administration of 10 mg/kg morphine (e,f). The left column of panels (a, c, and e) displays ICSS frequency-rate curves. Horizontal axes: frequency of electrical brain stimulation in hertz (log scale). Vertical axes: ICSS rate expressed as percent maximum control rate (%MCR). Data obtained for 0.32 mg/kg morphine, which had no effect under any conditions tested, are omitted from the graph for clarity but were included in statistical analysis. Filled symbols indicate frequencies at which morphine ICSS rates were greater than those obtained during control components, as determined by the Holm-Sidak post-hoc test following a significant two-way ANOVA. The right column of panels (b, d, and f) displays the total number of stimulations per test component expressed as a percentage of total control stimulations. Horizontal axes: dose of morphine. Vertical axes: percent control stimulations per test component. Upward and downward arrows indicate the presence and valence of effects of morphine as determined by analyses of frequency-rate data. Thus, upward arrows indicate significant facilitation of ICSS at ≥1 frequency of the frequency-rate curve, whereas downward arrows indicate significant depression of ICSS at ≥1 frequency of the frequency-rate curve. ANOVA results were as follows: Naive: Significant main effect of frequency [F(9,36)=60.1; P<0.001], significant main effect of dose [F(4,16)=46.3; P<0.001], and significant dose X frequency interaction [F(36,144)=13.2; P<0.001]; Repeated 3.2 mg/kg/day: Significant main effect of frequency [F(9,36)=50.6; P<0.001], significant main effect of dose [F (4,16)=60.7; P<0.001], and significant dose X frequency interaction [F(36,144)=9.4; P<0.001]; Repeated 10.0 mg/kg/day: Significant main effect of frequency [F(9,36)=30.8; P<0.001], no significant main effect of dose [F(4,16)=1.8; NS], and significant dose X frequency interaction [F(36,144)=4.5; P<0.001]. All points show mean ± SEM for five rats.
Repeated 3.2 mg/kg/day morphine did not significantly alter control ICSS parameters determined 24hr after the last repeated morphine dose and before testing with cumulative morphine (table 2). The effects of cumulative morphine after repeated daily treatment with 3.2 mg/kg/day are shown in figure 1c,d. The lowest cumulative dose of morphine, 0.32 mg/kg, again had no effect on ICSS. Cumulative 1.0 mg/kg morphine exclusively produced rate-increasing effects, but did so at more frequencies (89–112 Hz) than during the test conducted prior to repeated morphine. Likewise, cumulative 3.2 mg/kg morphine exclusively produced rate-increasing effects (89–112 Hz) during this test, which is in contrast to the biphasic effects of this dose prior to repeated morphine. Responding was again largely eliminated by cumulative 10 mg/kg morphine.
Repeated 10 mg/kg/day morphine decreased control ICSS determined 24hr after the last repeated morphine dose, as indicated by a decrease in the total control number of stimulations per component (table 2). Effects of cumulative morphine are shown in figure 1e,f. Cumulative 0.32 mg/kg was again without effect. As was the case following repeated administration of 3.2 mg/kg/day morphine, only rate-increasing effects were produced by cumulative 1.0 mg/kg (126 Hz) and 3.2 mg/kg (100–126 Hz) morphine. Cumulative 10 mg/kg morphine again produced only rate-decreasing effects, but these effects were attenuated such that decreases were only observed at the two highest frequencies (141–158 Hz). There was also a trend toward rate-increasing effects at the lower frequencies (71–100 Hz) that did not reach statistical significance. Overall, repeated morphine had three general effects: it decreased control ICSS prior to cumulative morphine, enhanced expression of cumulative morphine-induced facilitation of ICSS, and reduced expression of cumulative morphine-induced rate-decreasing effects.
Table 3 and figure 2 show that β-FNA pretreatment did not mimic effects of repeated morphine. No dose of β-FNA altered control parameters of ICSS (table 3), and β-FNA reduced both rate-increasing and rate-decreasing effects of cumulative morphine. β-FNA was most potent in antagonizing rate-increasing effects of morphine, and morphine failed to produce significant rate-increasing effects at any frequency after any β-FNA dose. The low dose of 0.32 mg/kg β-FNA failed to antagonize the rate-decreasing effects of cumulative 3.2 or 10 mg/kg morphine (figure 2a,b), but higher doses of 1.0–3.2 mg/kg β-FNA dose-dependently eliminated the rate-decreasing effects of cumulative 3.2 and 10 mg/kg morphine (figure 2c,d and e,f).
Table 3.
Control MCR and Total Stimulations obtained during experiments to determine the effects of β-FNA on cumulative morphine dose-effect curves.
| MCR± SE | Total Stimulations ± SE | |||
|---|---|---|---|---|
| Pre-β-FNA | Post-β-FNA | Pre-β-FNA | Post-β-FNA | |
| 0.32 β-FNA | 69.2 ± 3.1 | 66.8 ± 3.7 | 347.1 ± 34.2 | 295.3 ± 49.3 |
| 1.0 β-FNA | 49.4 ± 4.5 | 50.1 ± 4.5 | 223.5 ± 22.7 | 207.3 ± 30.7 |
| 3.2 β-FNA | 60.2 ± 4.1 | 59.0 ± 4.3 | 235.8 ± 57.8 | 225.4 ± 68.0 |
Fig. 2.
Effects of β-FNA on cumulative morphine dose-effect curves. Cumulative morphine dose effect curves were determined 24 hours after administration of 0.32 mg/kg (a,b), 1.0 mg/kg (c,d), or 3.2 mg/kg β-FNA (e,f). ANOVA results were as follows: 0.32 mg/kg β-FNA: Significant main effect of frequency [F(9,36)=20.7; P<0.001], significant main effect of dose [F(4,16)=36.1; P<0.001], and significant dose X frequency interaction [F(36,144)=7.9; P<0.001]; 1.0 mg/kg β-FNA: Significant main effect of frequency [F(9,36)=55.0; P<0.001], significant main effect of dose [F(4,16)=5.9; P<0.01], and significant dose X frequency interaction [F(36,144)=5.9; P<0.001]; 3.2mg/kg β-FNA : Significant main effect of frequency [F(9,36)=33.7; P<0.001], no significant main effect of dose [F(4,16)=1.2; NS], and no significant dose X frequency interaction [F(36,144)=1.1; NS]. For description of axes and symbols, please refer to figure 1. All points show mean ± SEM for five rats.
Effects of opioid agonists with varying efficacies at the mu receptor
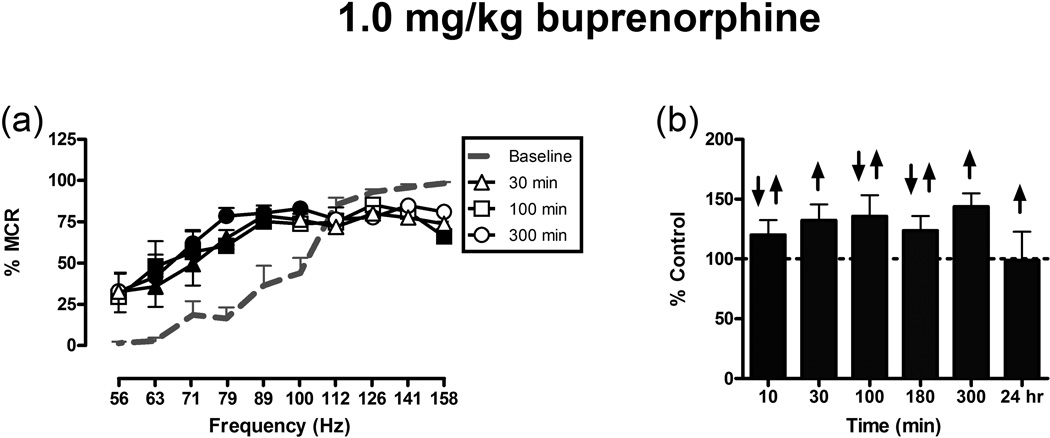
Control parameters of ICSS did not significantly vary during sequential testing with mu opioid receptor ligands in a group of 5 rats, and overall mean control parameters of this group are shown in table 4. The high-efficacy mu agonists methadone, fentanyl and hydrocodone produced qualitatively similar dose-dependent effects on ICSS manifested as exclusive facilitation of ICSS at relatively low doses and emergence of rate-decreasing effects at doses 10-fold higher than the lowest doses to produce facilitation (figure 3). The lower efficacy mu agonists buprenorphine and nalbuphine also produced exclusive facilitation of ICSS at relatively low doses; however, in contrast to the higher efficacy mu agonists, buprenorphine and nalbuphine continued to produce exclusive facilitation of ICSS at doses up to 10-fold (buprenorphine) and 30-fold (nalbuphine) higher than the lowest doses to produce facilitation (figure 4a,b,c,d). The opioid antagonist naltrexone did not alter ICSS at a dose sufficient to antagonize methadone-induced facilitation of ICSS (figure 4e,f). The mu agonists differed in their potencies to facilitate ICSS (fentanyl = buprenorphine > methadone = nalbuphine > hydrocodone), and figure 5 shows that they also differed in their time courses to facilitate ICSS. Methadone, fentanyl, hydrocodone and nalbuphine all produced peak facilitation of ICSS at the earliest time tested (10 min), whereas buprenorphine had a slower onset of action (peak facilitation at 100 min). Speed of offset was fastest for fentanyl (off after 30 min), followed by hydrocodone and nalbuphine (off at 100 min), methadone (off at 180 min) and buprenorphine (off at 24hr). Finally, offset of ICSS facilitation was followed by transient but significant decreases in ICSS for all drugs except buprenorphine. Treatment with vehicle (saline) did not produce significant changes in ICSS at any time point.
Table 4.
Control MCR and Total Stimulations obtained during experiments to determine the effects of µ-opioid receptor ligands.
| MCR ± SE | Total Stimulations ± SE | |
|---|---|---|
| Mu ligands | 54.8 ± 12.6 | 291.7 ± 82.6 |
| 1.0 Buprenorphine | 65.7 ± 11.2 | 311.4 ± 57.7 |
| Methadone (naïve) | 57.1 ± 6.1 | 221.5 ± 48.4 |
| Nalbuphine (naïve) | 57.3 ± 10.6 | 207.8 ± 91.6 |
Fig. 3.
Effects of the high-efficacy µ opioid receptor ligands methadone (a,b), fentanyl (c,d), or hydrocodone (e,f) on ICSS in opioid-experienced rats. ANOVA results were as follows: Methadone: Significant main effect of frequency [F(9,36)=71.2; P<0.001], significant main effect of dose [F(5,20)=3.8; P=0.014], and significant dose X frequency interaction [F(45,180)=3.8; P<0.001]; Fentanyl: Significant main effect of frequency [F(9,36)=39.7; P<0.001], significant main effect of dose [F(5,20)=9.8; P<0.001], and significant dose X frequency interaction [F(45,180)=5.4; P<0.001]; Hydrocodone: Significant main effect of frequency [F(9,36)=75.1; P<0.001], significant main effect of dose [F(5,20)=19.0; P<0.001], and significant dose X frequency interaction [F(45,180)=3.9; P<0.001]. For description of axes and symbols, please refer to figure 1.
Fig. 4.
Effects of low-efficacyµ-opioid receptor ligands buprenorphine (a,b) and nalbuphine (c,d) and the opioid antagonist naltrexone ± methadone (e,f) on ICSS in opioid-experienced rats. ANOVA results were as follows: Buprenorphine: Significant main effect of frequency [F(9,36)=62.8; P<0.001], no significant main effect of dose [F(5,20)=0.8; P=0.543], and significant dose X frequency interaction [F(45,180)=2.8; P<0.001]; Nalbuphine: Significant main effect of frequency [F(9,36)=27.1; P<0.001], significant main effect of dose [F(5,20)=10.8; P<0.001], and significant dose X frequency interaction [F(45,180)=1.8; P=0.005]; Naltrexone: Significant main effect of frequency [F(9,27)=27.4; P<0.001], significant main effect of treatment [F(3,9)=7.1; P=0.009], and significant treatment X frequency interaction [F(27,81)=3.5; P<0.001]. For description of axes and symbols, please refer to figure 1.
Fig. 5.
Time courses of µ-opioid receptor agonist effects on ICSS. Time course of the dose producing peak facilitation of ICSS for each drug as shown in figures 3 and 4 was determined. Horizontal axes: time elapsed after injection of test drug. Vertical axes: percent control stimulations per test component. ANOVA results were as follows: Methadone: Significant main effect of frequency [F(9,36)=92.8; P<0.001], significant main effect of time [F(6,24)=15.1; P<0.001], and significant time X frequency interaction [F(45,216)=2.2; P<0.001]; Fentanyl: Significant main effect of frequency [F(9,27)=37.5; P<0.001], significant main effect of time [F(6,18)=13.3; P<0.001], and no significant time X frequency interaction [F(54,162)=1.3; P=0.122]; Hydrocodone: Significant main effect of frequency [F(9,36)=57.5; P<0.001], significant main effect of time [F(6,24)=36.3; P<0.001], and significant time X frequency interaction [F(45,216)=3.1; P<0.001]; Buprenorphine: Significant main effect of frequency [F(9,27)=28.6; P<0.001], significant main effect of time [F(6,18)=7.6; P<0.001], and no significant time X frequency interaction [F(54,162)=1.0; P=0.545]; Nalbuphine: Significant main effect of frequency [F(9,27)=36.5; P<0.001], significant main effect of time [F(5,15)=7.8; P<0.001], and no significant time X frequency interaction [F(45,135)=1.2; P=0.181]; Vehicle: Significant main effect of frequency [F(9,27)=36.7; P<0.001], no significant main effect of time [F(6,18)=1.6; P=0.215], and no significant time X frequency interaction [F(54,162)=1.2; P=0.250]. For description of symbols, please refer to figure 1.
Four other experiments were conducted in four other groups of rats, and control ICSS parameters for each group are shown in table 4. Figure 6 shows the time course of effects produced in a group of opioid-experienced rats by 1.0 mg/kg buprenorphine, a dose 100-fold higher than the lowest dose to facilitate ICSS in the experiment above. The main effect was facilitation of low ICSS rates maintained by low frequencies of brain stimulation (63–100 Hz). Rate decreasing effects were observed at some time points at high frequencies (e.g. 158 Hz at 100 min), but even this high dose of buprenorphine failed to reduce ICSS to the degree observed with high doses of higher efficacy mu agonists (figure 3). As expected, this high dose of buprenorphine had a faster rate of onset and longer duration of action than observed with the lower buprenorphine dose shown in figure 5. Next, another group of opioid-experienced rats received morphine alone (10 mg/kg), nalbuphine alone (1.0 mg/kg), or a combination of both drugs (figure 7). In this group of rats, morphine alone decreased ICSS, nalbuphine alone had no effect on ICSS, and nalbuphine pretreatment completely blocked the rate-decreasing effects of morphine. Figure 8 shows effects of methadone and nalbuphine in two separate groups of opioid-naïve rats. Relative to their effects in the initial group of opioid-experienced rats shown in figure 3–figure 4, methadone and nalbuphine produced weaker ICSS facilitation, and methadone produced more potent ICSS depression. For example, 1.0 mg/kg methadone facilitated low rates of ICSS maintained by low frequencies of brain stimulation in opioid-experienced rats (figure 3) but only depressed high rates of ICSS maintained by high frequencies of brain stimulation in opioid-naïve rats (figure 8). Similarly, nalbuphine produced greater magnitudes of ICSS facilitation across a broader range of frequencies in opioid experienced rats (figure 4) than in opioid-naïve rats (facilitation only at 89 Hz after 0.32 and 10 mg/kg, figure 8). Notably, nalbuphine did not significantly decrease ICSS at any dose in opioid-naïve rats, but this absence of rate-decreasing effects was not associated with enhanced expression of rate-increasing effects relative to methadone.
Fig. 6.
Time course of effects produced by 1.0 mg/kg buprenorphine on ICSS. The left panel (a) displays ICSS frequency-rate curves. Horizontal axes: frequency of electrical brain stimulation in hertz (log scale). Data from 10, 180 min, and 24 h are not shown in frequency-rate graph (a) for clarity, but they were included in statistical analysis. The right panel (b) displays the total number of stimulations per test component expressed as a percentage of total control stimulations. There was significant main effect of frequency [F(9,45)=28.6; P<0.001], no significant main effect of time [F(6,30)=1.6; P=0.179], and significant time X frequency interaction [F(54,270)=3.4; P<0.001]. For description of axes and symbols, please refer to figure 1.
Fig. 7.
Effects of morphine alone, nalbuphine alone, or morphine plus nalbuphine on ICSS. Horizontal axes: dose of test drug. There was significant main effect of frequency[F(9,27)=24.3; P<0.001], significant main effect of treatment [F(3,9)=54.5.6; P<0.001], and significant treatment X frequency interaction [F(27,81)=7.1; P<0.001]. For description of axes and symbols, please refer to figure 1.
Fig. 8.
Effects of the high-efficacy µ agonist methadone (a,b) and the low-efficacy nalbuphine (c,d) on ICSS in separate groups of opioid-naïve rats. ANOVA results were as follows: Methadone: Significant main effect of frequency [F(9,45)=33.8; P<0.001], significant main effect of dose [F(5,25)=18.5; P<0.001], and significant dose X frequency interaction [F(45,225)=8.2; P<0.001]; Nalbuphine: Significant main effect of frequency [F(9,45)=30.0; P<0.001], no significant main effect of dose [F(6,30)=2.2; P=0.074], and significant dose X frequency interaction [F(54,270)=2.2; P<0.001]. For description of axes and symbols, please refer to figure 1.
Discussion
Mu opioid receptor agonists such as morphine can either facilitate or depress intracranial self-stimulation (ICSS), and prior opioid exposure can increase expression of abuse-related ICSS facilitation. This study tested the hypothesis that rate-decreasing effects require activation of a larger relative fraction of mu receptors, and hence are more vulnerable to tolerance-associated reductions in receptor density, than rate-increasing effects. Contrary to this hypothesis, the irreversible mu antagonist β-FNA failed to display higher potency to block rate-decreasing vs. rate-increasing effects of morphine, and no dose of β-FNA produced a tolerance-like enhancement in expression of ICSS facilitation. Similarly, in opioid-naïve rats, a reduction in efficacy of mu agonists was associated with decreased expression of rate-decreasing effects but not a tolerance-like enhancement of ICSS facilitation. Taken together, these results suggest that opioid exposure increases expression of abuse-related ICSS facilitation by mechanisms other than global reductions in mu opioid receptor density.
Effects of repeated morphine
In the present series of experiments, studies with cumulative morphine replicated three key phenomena observed in previous studies with individual morphine doses (Kornetsky and Esposito, 1979; Carlezon and Wise, 1993; Easterling and Holtzman, 1997; Craft et al., 2001; Jha et al., 2004; Altarifi and Negus, 2011). First, morphine produced both rate-increasing and rate-decreasing effects. Second, rate-increasing effects predominated at lower morphine doses, and rate-decreasing effects emerged and predominated at higher morphine doses. Thus, the dose-effect curve for morphine effects on overall rates of ICSS displayed an inverted-U shape. Third, repeated daily morphine treatment enhanced expression of morphine’s rate-increasing effects while producing tolerance to rate-decreasing effects. This study tested the hypothesis that opioid agonist-induced rate-decreasing effects require activation of a larger relative fraction of mu receptors, and hence are more vulnerable to tolerance-associated reductions in receptor density, than rate-increasing effects.
Antagonism of morphine by β-FNA
As one approach to test this hypothesis, the effects of cumulative morphine were redetermined in different groups of rats after pretreatment with the mu-selective antagonist β-FNA. By virtue of its irreversible binding to mu receptors, β-FNA has the effect of reducing functional mu receptor density (Martin et al., 1993), and β-FNA and other irreversible antagonists have been widely used to examine both the relative efficacies of different mu agonists (Comer et al., 1992; Walker et al., 1998; Walker et al., 1999; Negus et al., 2003) and the efficacy requirements of different effects produced by a given mu agonist (Zernig et al., 1994; Walker et al., 1995; Walker et al., 1999). For example, mu agonists can produce thermal antinociception against both low- and high-intensity noxious stimuli, and irreversible antagonists generally produce greater or more potent antagonism of antinociception at higher intensities (Zernig et al., 1994; Walker et al., 1995), consistent with other evidence to suggest higher efficacy requirements for mu agonist antinociception at higher noxious stimulus intensities (Morgan et al., 1999). As another example, equivalent doses of β-FNA produced greater antagonism of morphine antinociception than of morphine-induced depression of food-maintained operant responding in Long-Evans rats, and these results were interpreted to suggest higher efficacy requirements for antinociception, the effect more vulnerable to the irreversible antagonist (Walker et al., 1999).
In the present study, the hypothesis of higher efficacy requirements for rate-decreasing than rate-increasing effects of mu agonists predicted that β-FNA would have higher potency to antagonize rate-decreasing effects and that β-FNA dose could be titrated to selectively attenuate morphine’s rate-decreasing effects and enhance expression of rate-increasing effects. However, there was no evidence that β-FNA was more potent to block depression than facilitation of ICSS. If anything, the reverse was observed, with the lowest β-FNA dose (0.32 mg/kg) blocking expression of significant facilitation while having little effect on morphine-induced depression of ICSS. Moreover, although β-FNA dose-dependently blocked morphine rate-decreasing effects, no dose of β-FNA produced a reciprocal enhanced expression of rate-increasing effects. Overall then, these results with β-FNA do not support the hypothesis that morphine-induced depression of ICSS has higher efficacy requirements at mu receptors than morphine-induced facilitation of ICSS.
Effects of mu agonists that vary in efficacy
Drugs with varying efficacies at mu receptors enable a second approach to dissociate the efficacy requirements of different effects produced by mu agonists. For example, low-efficacy mu agonists such as nalbuphine and buprenorphine often produce antinociception against low- but not high-intensity noxious thermal stimuli, whereas higher efficacy mu agonists such as morphine, fentanyl and methadone are more likely to produce antinociception against both low- and high-intensity noxious stimuli (Morgan et al., 1999; Negus and Mello, 1999; Cook et al., 2000). Such data provide one source of evidence to suggest that antinociception against low-intensity noxious stimuli has lower efficacy requirements than antinociception against high-intensity noxious stimuli.
In the present study in opioid-experienced rats, the low-efficacy mu agonists nalbuphine and buprenorphine facilitated ICSS across a broad range of doses but produced little or no depression of ICSS, whereas higher efficacy mu agonists produced biphasic facilitation and depression of ICSS. Moreover, nalbuphine pretreatment antagonized the rate-decreasing effects of morphine, although nalbuphine alone failed to facilitate ICSS in this group. These findings are superficially consistent with lower efficacy requirements for facilitation than depression of ICSS and discrepant with implications of β-FNA studies described above. However, initial results with different efficacy mu agonists (Figures 3–5) were collected in opioid-experienced rats, albeit using a twice-per-week testing regimen intended to minimize opioid tolerance. Given both (a) the known potential for opioid exposure to attenuate ICSS-depressing effects (Altarifi and Negus, 2011; cumulative morphine data in Figure 1 above), and (b) the discrepancy with β-FNA experiments, a follow-up study was conducted with nalbuphine and methadone in opioid-naïve rats. Methadone was more potent in depressing ICSS and both drugs were less effective in facilitating ICSS in opioid-naïve than opioid-experienced rats. The differences in methadone effects between opioid-naïve and opioid-experienced rats suggests that even intermittent opioid exposure associated with twice-per-week testing was sufficient to produce tolerance to ICSS depression similar to that observed with more intensive daily regimens of morphine treatment (Altarifi and Negus, 2011; cumulative morphine data in Figure 1 above). Moreover, these results suggest that opioid exposure also augments expression of rate-increasing effects produced by low-efficacy mu agonists like nalbuphine that do not reliably facilitate ICSS in naïve rats. Indeed, nalbuphine was tested in three different groups of rats in this study, and facilitation of ICSS was greatest in the group with the greatest extent of opioid exposure prior to nalbuphine testing. Overall, the limited ability of nalbuphine to facilitate ICSS in rats with little or no history of opioid exposure fails to support the hypothesis that opioid-induced facilitation of ICSS has lower efficacy requirements at mu receptors than opioid-induced depression of ICSS.
Effects of opioid exposure vs. irreversible antagonism
One premise for the experimental design of this study was that both opioid exposure and β-FNA might produce qualitatively similar effects on mu agonist-induced changes in ICSS by decreasing the functional density of mu opioid receptors. However, the differing effects of these treatments suggest that they produced effects on distinct receptor populations mediating mu agonist-induced facilitation and depression of ICSS. Consistent with this possibility, previous studies have reported different effects of treatment with chronic morphine or β-FNA on the anatomical distribution of mu agonist-stimulated GTPγS binding in rat brain (Sim et al., 1996; Martin et al., 1997). Thus, a regimen of chronic morphine selectively decreased this measure of functional mu opioid receptors in brain stem areas including periaqueductal gray but not in forebrain areas including the striatum and nucleus accumbens. Conversely, β-FNA produced a different anatomical profile of effects that included decreases in functional mu receptors in both brain stem and forebrain areas, with some of the greatest reductions observed in striatum and nucleus accumbens. The anatomical differences in effects of chronic morphine and β-FNA on mu receptor function may be relevant to ICSS, because a previous brain mapping study reported that morphine produced primarily depression of ICSS after local injection in brain stem areas proximal to periaqueductal gray and primarily facilitation of ICSS after intracerebral injection in forebrain areas proximal to striatum (Broekkamp et al., 1976). Taken together, these studies suggest that brain stem mu receptors mediating ICSS depression might be inactivated by either chronic morphine or β-FNA, whereas forebrain mu receptors mediating ICSS facilitation might be inactivated by β-FNA but not by chronic morphine.
A second pharmacological factor that might contribute to differential effects of opioid exposure vs. β-FNA is the contribution of non-mu opioid receptors to opioid agonist effects. For example, both kappa and delta opioid receptor agonists depress ICSS (Todtenkopf et al., 2004; Do Carmo et al., 2009; Negus et al., 2010), raising the theoretical possibility that delta or kappa receptor activation might contribute to the rate-decreasing effects of opioid agonists like morphine that are selective for mu receptors but retain lower affinity for delta and kappa receptors (Emmerson et al., 1994; Raynor et al., 1994; Toll et al., 1998). At least two findings in this study argue against this possibility. First, mu agonists with greater mu-selectivity than morphine [e.g. fentanyl; (Emmerson et al., 1994; Raynor et al., 1994; Toll et al., 1998)] produced a morphine-like biphasic effect on ICSS, suggesting that mu receptor activation is sufficient to produce both facilitation and depression of ICSS. Second, both morphine-induced facilitation and depression of ICSS were fully antagonized by the mu-selective antagonist β-FNA, suggesting that mu receptors are necessary for both morphine effects.
A third factor that may contribute to different effects of chronic opioid exposure vs. β-FNA is that the former may produce opioid dependence in addition to tolerance. In ICSS, opioid dependence may be expressed as depression of ICSS during periods of opioid withdrawal (Schaefer and Michael, 1983; Altarifi and Negus, 2011). In the present study, withdrawal-associated depression of ICSS was apparent both after dissipation of rate-increasing effects produced by acute treatment with most opioid agonists (see figure 5) or after repeated treatment with morphine (see table 2). Accordingly, the increased expression of rate-increasing effects produced by mu agonists in opioid-experienced subjects may be due in part to a reversal of withdrawal-associated depression in baseline ICSS.
A final nonpharmacological consideration is that behavioral tolerance may also have contributed to differential effects of opioid exposure and β-FNA treatment. The opportunity to engage in behavior in the presence or absence of a drug can modify the development of tolerance. For example, in an assay of food-maintained schedule-controlled behavior, tolerance to the rate-decreasing effects of morphine develops more rapidly when operant behavior occurs in the presence of morphine than when the behavior and drug administration occur in separate contexts (Sannerud and Young, 1986). Furthermore, drug effects that disrupt the ability to satisfy reinforcement contingencies are more susceptible to the development of tolerance than are drug effects that enhance or do not disrupt rates of reinforcement (Schuster et al., 1966). In the present study, administration of opioid agonists always occurred in a context in which reinforcement of the operant also occurred. Thus, in opioid-experienced rats, the interaction of a history of mu agonist exposure and the opportunity to engage in ICSS may contribute to the development of tolerance to rate-decreasing effects and enhanced expression of rate-increasing effects. Continued evaluation of the role of pharmacological and non-pharmacological factors in the effects of acute and repeated opioid agonist administration in ICSS may improve the usefulness of this procedure as an experimental tool for assessing the abuse potential of opioids and other drugs.
Acknowledgments
This research was supported by NIH grants R01-NS070715, T32-DA007027 and Jordan University of Science and Technology.
Footnotes
Conflicts of Interest: None declared.
References
- Altarifi AA, Negus SS. Some determinants of morphine effects on intracranial self-stimulation in rats: Dose, pretreatment time, repeated treatment, and rate dependence. Behav Pharmacol. 2011;22:663–673. doi: 10.1097/FBP.0b013e32834aff54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Gehrke BJ, Shortridge BE, Rauhut AS. Effects of beta-funaltrexamine and naloxonazine on single-trial morphine-conditioned place preference and locomotor activity. Pharmacol Biochem Behav. 2003;74:617–622. doi: 10.1016/s0091-3057(02)01049-3. [DOI] [PubMed] [Google Scholar]
- Broekkamp CL, Van Den Bogaard JH, Heijnen HJ, Rops RH, Cools AR, Van Rossum JM. Separation of inhibiting and stimulating effects of morphine on self-stimulation behaviour by intracerebral microinjections. Eur J Pharmacol. 1976;36:443–446. doi: 10.1016/0014-2999(76)90099-6. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Morphine-induced potentiation of brain stimulation reward is enhanced by mk-801. Brain Res. 1993;620:339–342. doi: 10.1016/0006-8993(93)90177-o. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Dart RC, Inciardi JA, Woody GE, Schnoll S, Munoz A. The development of a comprehensive risk-management program for prescription opioid analgesics: Researched abuse, diversion and addiction-related surveillance (radars) Pain Med. 2007;8:157–170. doi: 10.1111/j.1526-4637.2006.00259.x. [DOI] [PubMed] [Google Scholar]
- Comer SD, Burke TF, Lewis JW, Woods JH. Clocinnamox: A novel, systemically-active, irreversible opioid antagonist. J Pharmacol Exp Ther. 1992;262:1051–1056. [PubMed] [Google Scholar]
- Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ. Sex-related differences in the antinociceptive effects of opioids: Importance of rat genotype, nociceptive stimulus intensity, and efficacy at the mu opioid receptor. Psychopharmacology (Berl) 2000;150:430–442. doi: 10.1007/s002130000453. [DOI] [PubMed] [Google Scholar]
- Craft RM, Stoffel EC, Stratmann JA. Effects of chronic morphine treatment on responding for intracranial stimulation in female versus male rats. Exp Clin Psychopharmacol. 2001;9:198–208. doi: 10.1037//1064-1297.9.2.198. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl) 2003;168:347–358. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Do Carmo GP, Folk JE, Rice KC, Chartoff E, Carlezon WA, Jr, Negus SS. The selective non-peptidic delta opioid agonist snc80 does not facilitate intracranial self-stimulation in rats. Eur J Pharmacol. 2009;604:58–65. doi: 10.1016/j.ejphar.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterling KW, Holtzman SG. Intracranial self-stimulation in rats: Sensitization to an opioid antagonist following acute or chronic treatment with mu opioid agonists. J Pharmacol Exp Ther. 1997;281:188–199. [PubMed] [Google Scholar]
- Emmerson PJ, Liu MR, Woods JH, Medzihradsky F. Binding affinity and selectivity of opioids at mu, delta and kappa receptors in monkey brain membranes. J Pharmacol Exp Ther. 1994;271:1630–1637. [PubMed] [Google Scholar]
- Esposito RU, Perry W, Kornetsky C. Effects of d-amphetamine and naloxone on brain stimulation reward. Psychopharmacology (Berl) 1980;69:187–191. doi: 10.1007/BF00427648. [DOI] [PubMed] [Google Scholar]
- Hughes PH, Rieche O. Heroin epidemics revisited. Epidemiol Rev. 1995;17:66–73. doi: 10.1093/oxfordjournals.epirev.a036186. [DOI] [PubMed] [Google Scholar]
- Jha SH, Knapp CM, Kornetsky C. Effects of morphine on brain-stimulation reward thresholds in young and aged rats. Pharmacol Biochem Behav. 2004;79:483–490. doi: 10.1016/j.pbb.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Koob GF, Spector NH, Meyerhoff JL. Effects of heroin on lever pressing for intracranial self-stimulation, food and water in the rat. Psychopharmacologia. 1975;42:231–234. doi: 10.1007/BF00421261. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: Effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Lorens SA, Mitchell CL. Influence of morphine on lateral hypothalamic self-stimulation in the rat. Psychopharmacologia. 1973;32:271–277. doi: 10.1007/BF00422149. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Dworkin SI, Smith JE. Effects of intracerebroventricular administration of beta-funaltrexamine on [3h]damgo binding to rat brain sections. J Pharmacol Exp Ther. 1993;267:506–514. [PubMed] [Google Scholar]
- Martin TJ, Sim LJ, Selley DE, Demontis MG, Childers SR. Effects of intracerebroventricular administration of beta-funaltrexamine on damgo-stimulated [35s]gtp-gamma-s binding in rat brain sections. Synapse. 1997;27:177–182. doi: 10.1002/(SICI)1098-2396(199711)27:3<177::AID-SYN3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Martini L, Whistler JL. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol. 2007;17:556–564. doi: 10.1016/j.conb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Morgan D, Cook CD, Smith MA, Picker MJ. An examination of the interactions between the antinociceptive effects of morphine and various mu-opioids: The role of intrinsic efficacy and stimulus intensity. AnesthAnalg. 1999;88:407–413. doi: 10.1097/00000539-199902000-00035. [DOI] [PubMed] [Google Scholar]
- Negus SS, Brandt MR, Gatch MB, Mello NK. Effects of heroin and its metabolites on schedule-controlled responding and thermal nociception in rhesus monkeys: Sensitivity to antagonism by quadazocine, naltrindole and beta-funaltrexamine. Drug Alcohol Depend. 2003;70:17–27. doi: 10.1016/s0376-8716(02)00331-9. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Opioid antinociception in ovariectomized monkeys: Comparison with antinociception in males and effects of estradiol replacement. J Pharmacol Exp Ther. 1999;290:1132–1140. [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC. Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 2010;210:149–159. doi: 10.1007/s00213-009-1770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: Further studies on pain-depressed behavior. Pain. 2009;144:170–177. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol. 1994;45:330–334. [PubMed] [Google Scholar]
- Sannerud CA, Young AM. Modification of morphine tolerance by behavioral variables. J Pharmacol Exp Ther. 1986;237:75–81. [PubMed] [Google Scholar]
- Schaefer GJ, Michael RP. Morphine withdrawal produces differential effects on the rate of lever-pressing for brain self-stimulation in the hypothalamus and midbrain in rats. Pharmacol Biochem Behav. 1983;18:571–577. doi: 10.1016/0091-3057(83)90283-6. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Dockens WS, Woods JH. Behavioral variables affecting the development of amphetamine tolerance. Psychopharmacologia. 1966;9:170–182. doi: 10.1007/BF00404721. [DOI] [PubMed] [Google Scholar]
- Selley DE, Liu Q, Childers SR. Signal transduction correlates of mu opioid agonist intrinsic efficacy: Receptor-stimulated [35s]gtp gamma s binding in mmor-cho cells and rat thalamus. J Pharmacol Exp Ther. 1998;285:496–505. [PubMed] [Google Scholar]
- Selley DE, Sim LJ, Xiao R, Liu Q, Childers SR. Mu-opioid receptor-stimulated guanosine-5-o-(gamma-thio)-triphosphate binding in rat thalamus and cultured cell lines: Signal transduction mechanisms underlying agonist efficacy. Mol Pharmacol. 1997;51:87–96. doi: 10.1124/mol.51.1.87. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Dworkin SI, Childers SR. Effects of chronic morphine administration on mu opioid receptor-stimulated [35s]gtpgammas autoradiography in rat brain. J Neurosci. 1996;16:2684–2692. doi: 10.1523/JNEUROSCI.16-08-02684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CM, Wojno H, Greiner E, May EL, Rice KC, Selley DE. Activation of g-proteins by morphine and codeine congeners: Insights to the relevance of o- and n-demethylated metabolites atmu- and delta-opioid receptors. J Pharmacol Exp Ther. 2004;308:547–554. doi: 10.1124/jpet.103.058602. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, et al. Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr. 1998;178:440–466. [PubMed] [Google Scholar]
- Walker EA, Tiano MJ, Benyas SI, Dykstra LA, Picker MJ. Naltrexone and beta-funaltrexamine antagonism of the antinociceptive and response rate-decreasing effects of morphine, dezocine, and d-propoxyphene. Psychopharmacology(Berl) 1999;144:45–53. doi: 10.1007/s002130050975. [DOI] [PubMed] [Google Scholar]
- Walker EA, Zernig G, Woods JH. Buprenorphine antagonism of mu opioids in the rhesus monkey tail-withdrawal procedure. J Pharmacol Exp Ther. 1995;273:1345–1352. [PubMed] [Google Scholar]
- Walker EA, Zernig G, Young AM. In vivo apparent affinity and efficacy estimates for mu opiates in a rat tail-withdrawal assay. Psychopharmacology(Berl) 1998;136:15–23. doi: 10.1007/s002130050534. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Portoghese PS, Takemori AE. Pharmacological characterization in vivo of the novel opiate, beta-funaltrexamine. J Pharmacol Exp Ther. 1982;220:494–498. [PubMed] [Google Scholar]
- Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- Zernig G, Butelman ER, Lewis JW, Walker EA, Woods JH. In vivo determination of mu opioid receptor turnover in rhesus monkeys after irreversible blockade with clocinnamox. J Pharmacol Exp Ther. 1994;269:57–65. [PubMed] [Google Scholar]