Abstract
Vangl2, a core component of the Planar Cell Polarity pathway, is necessary for the caudal migration of Facial Branchiomotor (FBM) neurons in the vertebrate hindbrain. Studies in zebrafish suggest that vangl2 functions largely non-cell autonomously to regulate FBM neuron migration out of rhombomere 4 (r4), but the cell-type within which it acts is not known. Here, we demonstrate that vangl2 functions largely in floor plate cells to regulate caudal neuronal migration. Furthermore, FBM neurons fail to migrate caudally in the mouse Gli2 mutant that lacks the floor plate, suggesting an evolutionarily conserved role for this cell type in neuronal migration. Although hindbrain floor plate cilia are disorganized in vangl2 mutant embryos, cilia appear to be dispensable for neuronal migration. Notably, Vangl2 is enriched in the basolateral, but not apical, membranes of floor plate cells. Taken together, our data suggest strongly that Vangl2 regulates FBM neuron migration by acting in floor plate cells, independently of cilia function.
Keywords: Hindbrain, Facial Branchiomotor Neuron, Floor plate cells, Van gogh-like 2, Neuronal migration, Cilia
INTRODUCTION
During nervous system development, newborn neurons migrate away from the germinal zone to their final locations where they generate functional neural networks. Neuronal migration disorders in humans can lead to severe physiological and cognitive problems (Copp and Harding, 1999; Baraban, 2007). Cortical neurons in the forebrain migrate extensively along radial and tangential pathways (Nadarajah and Parnavelas, 2002; Marin and Rubenstein, 2003). Similarly, facial branchiomotor (FBM) neurons, a subset of cranial motor neurons, migrate along tangential and radial pathways from their birthplace in rhombomere 4 (r4) of the hindbrain to caudal rhombomeres (r6/r7) in most vertebrates (Chandrasekhar, 2004; Gilland and Baker, 2005).
Studies in zebrafish and mice have identified several molecules necessary for the migration of FBM neurons, including components of the Wnt/Planar Cell Polarity (Wnt/PCP) pathway. Wnt/PCP signaling regulates convergence and extension cell movements during gastrulation (Roszko et al., 2009) and establishes the polarity of neural progenitors (Ciruna et al., 2006). Inactivation of core Wnt/PCP components such as Van gogh-like 2 (Vangl2) (Bingham et al., 2002; Jessen et al., 2002; Vivancos et al., 2009; Glasco et al., 2012), Prickle1a (Pk1a) (Carreira-Barbosa et al., 2003), Prickle1b (Pk1b) (Rohrschneider et al., 2007), Scribble1 (Scrib) (Wada et al., 2005) and Frizzled3a (Fzd3a) and Celsrs 1–3 (Wada et al., 2006; Qu et al., 2010) abolishes FBM neuron migration in zebrafish and mouse. Interestingly, inactivation of Wnt/PCP components like Wnt11, Wnt5a, Glypican4 and Dishevelled have little effect on FBM neuron migration (Bingham et al., 2002; Jessen et al., 2002; Vivancos et al., 2009; Glasco et al., 2012), suggesting redundant or non-essential roles in this process. Furthermore, mosaic analyses suggest that vangl2, fzd3a, celsr2 and scrib largely function non-cell autonomously during FBM neuron migration (Jessen et al., 2002; Wada et al., 2005; Wada et al., 2006). These observations suggest that some core PCP genes may regulate FBM neuron migration through novel cellular mechanisms. These mechanisms must also be compatible with the behavior of “pioneer” and “follower” FBM neurons in zebrafish (Wanner and Prince, 2013).
Wada et al (2006) proposed that zebrafish fzd3a and celsr2 function in neuroepithelial cells adjacent to the FBM neurons, and regulate their caudal migration by preventing their integration into the neuroepithelium in rhombomere 4. In contrast, pk1b functions within the FBM neurons to regulate their polarity and midline-directed protrusions during their migration (Mapp et al., 2010; Mapp et al., 2011). Although vangl2 largely functions non-autonomously for neuronal migration (Jessen et al., 2002), the cell-type within which it acts is not known. Walsh et al. (2011) also suggested an FBM neuron-autonomous role for vangl2 during migration. Our analyses employing genetic mosaics, an inducible transgene, and mutants suggest strongly that vangl2 functions primarily in floor plate cells to regulate FBM neuron migration. Our data also indicate that floor plate cilia are not required for migration.
MATERIALS AND METHODS
Animals
Zebrafish were maintained following standard protocols and IACUC guidelines as described previously (Westerfield, 1995; Sittaramane et al., 2009). Embryos were developed at 28.5°C and staged by hours post fertilization (hpf) (Kimmel et al., 1995). Tg(isl1:gfp) and Tg(zcrest:mRFP) fish (Higashijima et al., 2000; Mapp et al., 2010), were used to analyze FBM neuron migration. SAGFF187A/Tg(Fp:Gal4FF; UAS:GFP) was generated by the gene trap method using the SAGFF Tol2 construct (Asakawa et al., 2008), and expressed Gal4FF and GFP in the floor plate from 16–48 hpf. Tg(vangl2:GFP-Vangl2) and Tg(UAS:Myc-vangl2) were generated using the Tol2 Gateway cloning kit (X.P. and A.C., unpublished data; Tol2 kit provided by Kristen Kwan and Chi-Bin Chien, University of Utah). The following mutant lines were used: trilobite (tritc240a; (Hammerschmidt et al., 1996)), detour (dtrts269 and dtrte370a; (Brand et al., 1996)), iguana (iguts294e; (Brand et al., 1996)), and oval (ovltz288b; (Tsujikawa and Malicki, 2004; Huang and Schier, 2009)).
Mouse Gli2+/− mice were maintained according to IACUC guidelines at UMDNJ. Embryos were staged and processed as described previously (Matise et al., 1998; Glasco et al., 2012).
Morpholino and mRNA Injections
The following morpholinos were obtained from Gene Tools and injected at the indicated doses: sox32 MO ((Sakaguchi et al., 2001); 4–8 ng/embryo), no tail MO ((Nasevicius and Ekker, 2000); 4 ng/embryo) and vangl2 MO ((Jessen et al., 2002); 4 ng/embryo). The following mRNAs were used: TARAM-Ad/TAR RNA ((Thisse and Thisse, 1999); 50 pg/embryo) and mRFP RNA (100 pg/embryo).
Mesoderm transplantations
Donor cells were targeted to the host mesoderm by injecting TARAM-Ad/TAR RNA into donor embryos. TARAM-Ad/TAR is a constitutively active activin type I receptor that cell autonomously induces mesendodermal fates, and TAR-expressing cells preferentially form mesoderm at low doses (50 pg RNA/embryo) (Thisse and Thisse, 1999) (Fig. S4). Donor embryos were injected at the one-cell stage with a mixture of 2% rhodamine dextran, 3% lysine-fixable biotin dextran (Molecular Probes), and TAR RNA. At the late blastula stage, cells were transplanted to the margin of 50% epiboly stage hosts. Host embryos were screened at 24 hpf for those containing donor-derived cells in the cranial mesoderm. In some experiments, the donor cells also contained vangl2 MO, resulting in the knockdown of vangl2 expression in donor-derived mesodermal cells, a strategy employed previously for knocking down BMP function in the endoderm (Holzschuh et al., 2005).
Floor plate transplantations
Donor cells were targeted to the floor plate by injecting no tail (ntl) MO into donor embryos, since ntl-deficient cells preferentially contribute to the floor plate (Halpern et al., 1993). Donor embryos were injected with ntl MO (4–6 ng/embryo) and 2% rhodamine dextran, and late blastula stage cells were transplanted to the margin of Tg(isl1:gfp) hosts (3 hpf). Host embryos (Fig. S5) containing donor-derived cells in the hindbrain floor plate were selected for further analysis. We verified that ntl morphant donor cells did not differentiate into motor neurons by transplanting Tg(isl1:gfp), ntl MO cells into wildtype non-transgenic host embryos. In three independent experiments (80 embryos), we obtained 38 host embryos with donor-derived cells in the hindbrain floor plate. Importantly, no GFP-expressing FBM neurons were found in any of these embryos, indicating that ntl MO donor cells are highly unlikely to differentiate into FBM neurons.
Quantification of FBM neuron migration phenotypes
Phenotypes were scored by examining the distribution of FBM neurons in rhombomeres 4–7. “Normal migration” indicates >90% (qualitative estimate) of neurons migrated out of r4, (e.g., Fig. 2A, B). “Abrogation/abrogated” in the transplant experiments indicates >20% of neurons failed to migrate out of r4 on one or both sides of the host wildtype hindbrain (e.g., Fig. 3A, C). Control vangl2-deficient embryos exhibit complete migration block since FBM neurons never exited r4 (e.g., Fig. 6D and 3F). “Rescued migration” in transplant experiments indicates at least 50% of neurons migrated out of r4 in vangl2 mutant embryos, (e.g., Figs. 2F, 3D and 3F). Rhombomere assignments (particularly in Fig. 4) were made by dividing the anterior-posterior axis of the hindbrain from r2 to r7 into six roughly equal parts. The anterior limit of r2 was defined by the presence of trigeminal motor neurons (in most embryos), and the caudal limit of r7 was defined by the presence of vagal motor neurons (in wildtype and vangl2-deficient embryos). When visible, the characteristic genu and/or exit point of the facial motor axons reliably marked r4. In detour mutant host embryos, r2 and r4 were readily identified, and the dimensions of r4-r6 were borrowed from wildtype siblings because rhombomere patterning is not affected in these mutants (Chandrasekhar et al., 1999).
Figure 2. Ventral neural tube expression of vangl2, but not the endoderm, is required for FBM neuron migration.
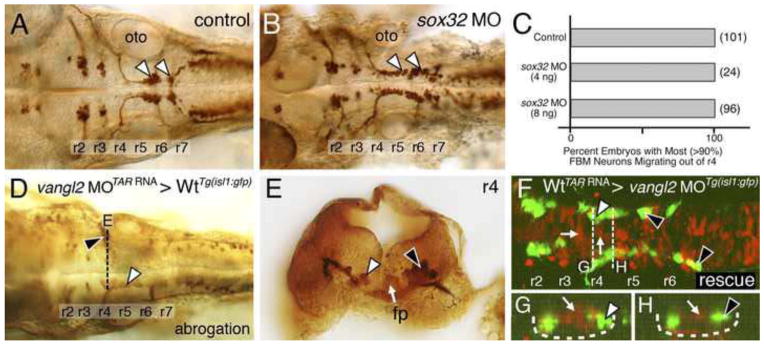
(A, B) Dorsal views of 36 hpf Tg(isl1:GFP) hindbrains stained for FBM neurons (arrowheads, GFP antibody). In sox32 morphant embryos, which lack endoderm, FBM neurons largely migrate normally out of r4, as in wildtype embryos. (C) Quantification of the FBM neuron migration phenotype in sox32 morphant embryos. Number of embryos in parentheses. (D–H) Dorsal views of 36 hpf Tg(isl1:GFP) host hindbrains (D, F), with the level of r4 cross-sections (E, G, H) indicated by broken lines. In D and E, motor neurons and donor-derived cells are both labeled brown due to streptavidin-conjugated alkaline phosphatase reactivity used to detect anti-GFP antibody binding (motor neurons) and biotinylated rhodamine dextran (vangl2 MO donor cells). In F–H, the endogenous fluorescence from GFP and rhodamine were used to identify the motor neurons and donor-derived cells, respectively. Inadvertant targeting of vangl2 morphant cells to the ventral neural tube (white arrow, E) in a wildtype host (D) results in failure of host FBM neurons to migrate (black arrowhead in D, E) on one side, with normal migration on the other (white arrowheads). Conversely, inadvertant targeting of wildtype cells (rhodamine dextran tracer, red) to the ventral neural tube (white arrows, F–H), including the floor plate (not visible due to very weak fluorescence), in a vangl2 morphant host (F) rescues the migration defect of host FBM neurons (black arrowheads, F–H). fp, floor plate; oto, otic vesicle.
Figure 3. Vangl2 functions in floor plate cells in r4 to regulate FBM neuron migration.
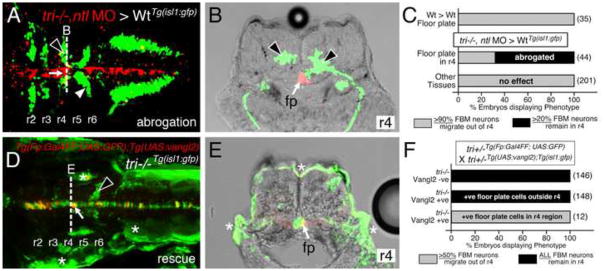
Panels A, D show dorsal views of 36 hpf (A) and 48 hpf (D) Tg(isl1:GFP) host hindbrains, and panels B, E show cross-sections at the levels indicated by the broken lines. Vangl2 mutants are indicated as tri−/− throughout. (A, B) Presence of vangl2 mutant donor cells in the floor plate (fp) in r4 (arrows) of a wildtype host can abrogate (partially block) the migration of some host FBM neurons (black arrowheads) out of r4. A large number of host neurons (white arrowhead) migrate caudally. (C) Quantification of vangl2 mutant > Wt transplantation data. Number of embryos in parentheses. (D, E) Floor plate-specific expression of vangl2 (arrows) in a vangl2 mutant completely rescues the migration of host FBM neurons (arrowhead). Gal4+ve floor plate cells are green, Myc-Vangl2+ve cells are red or yellow. Asterisks indicate Gal4FF-induced GFP expression in otic vesicle, trunk muscles, and skin. Many GFP-expressing cells are Myc-Vangl2-ve (and vice versa) probably due to independent and variable silencing of the UAS:GFP and UAS:Myc-vangl2 transgenes integrated at different sites. (F) Quantification of Gal4-UAS rescue data. Number of embryos in parentheses. fp, floor plate.
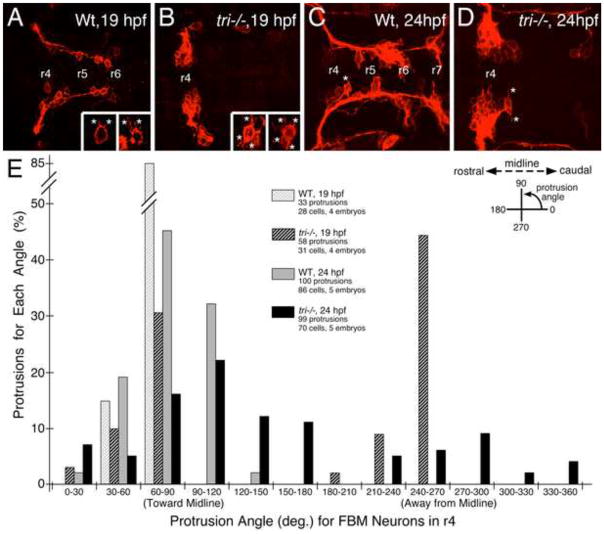
Figure 6. Loss of orientated FBM neuron protrusions in vangl2 mutants.
(A–D) Dorsal views of Tg(zCREST:mRFP) hindbrains processed for RFP immunostaining. In wildtype embryos (A, C and insets), protrusions (asterisks) generated by FBM neurons in r4 are oriented toward the midline. In vangl2 mutant (tri−/−) embryos (B, D and insets), the protrusions of FBM neurons in r4 are oriented in various directions, with a large number directed away from the midline. (E) Quantification of the orientation of protrusions of FBM neurons in r4 of wildtype and vangl2 mutant embryos. Whereas the orientation angles for all wildtype protrusions at 19 and 24 hpf are limited to the quadrants directed toward the midline, a large number of protrusions of mutant neurons are directed 180 degrees away from the midline, especially at 19 hpf (significant difference from wildtype at p<0.001; Watson-Williams F-test).
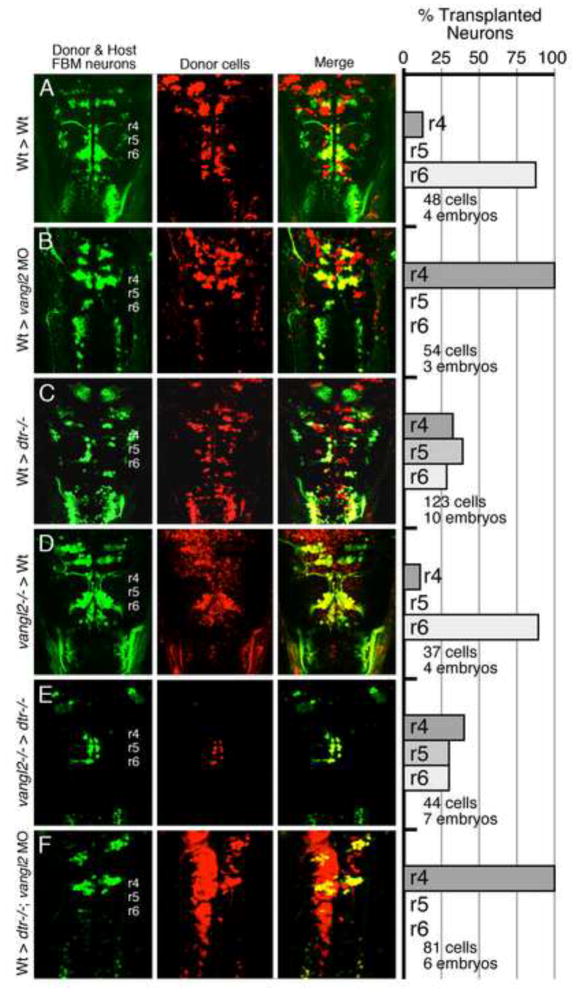
Figure 4. Vangl2 functions outside FBM neurons to regulate their migration.
(A–F) Dorsal views of the hindbrain in 48 hpf host embryos with anterior to the top. Both the donor and host embryos are Tg(isl1:GFP). Donor derived cells contain the lineage tracer rhodamine dextran (red), hence the donor-derived motor neurons are yellow, while the host motor neurons are green. Quantification of non-migrated FBM neurons in r4 and migrated FBM neurons in r5 and r6 is shown in the histograms. Different transplant conditions are indicated on the far left, and written as Donor > Host. Vangl2−/− refers to trilobite mutants. Wildtype, donor-derived motor neurons (yellow cells) in r6 of vangl2 morphant (B) and dtr−/−; vangl2 morphant (F) host embryos are not scored as FBM neurons because they do not have anteriorly directed axons exiting in r4, and are likely glossopharyngeal motor neurons. (A–D) depict control experiments. (E) Many vangl2 mutant FBM neurons migrate out of r4 in a vangl2+/+ environment, even in the absence of host wildtype FBM neurons. (F) Conversely, wildtype FBM neurons never migrate out of r4 in a vangl2-deficient environment, even in the absence of host mutant FBM neurons (compare to B).
Genotyping of rescued embryos
Rescued vangl2 mutant host embryos (short trunks with thin somites) could usually be definitively identified by examining the distribution of FBM neurons (e.g., Fig. 3D), and were confirmed to be vangl2−/− by genotyping. We amplified a 449 bp genomic region spanning the point mutation in the trilobite (tritc240a) allele (Jessen et al., 2002) (Forward: 5′-GCCTGGATGGTCACAGATTT-3′ and Reverse: 5′-CCGGAAGTTTATCAGTATGGGAAACAC-3′), and identified mutants by sequencing.
Immunohistochemistry and in situ hybridization
Immunohistochemistry was performed as described previously (Bingham et al., 2002; Sittaramane et al., 2009) using the following antibodies: acetylated α-tubulin (Sigma, 1:500), GFP (Invitrogen, 1:1000 for DAB reaction and 1:2000 for Alexafluor), rhodamine dextran (Invitrogen, 1:500), RFP and Myc (Cell Signaling Technology, 1:250), and Alexafluor-conjugated secondary antibodies (Invitrogen, 1:500). Mesoderm transplant embryos were processed for anti-GFP immunostaining and biotin-streptavidin chemistry to simultaneously visualize donor-derived mesodermal cells and GFP-expressing host motor neurons. Floor plate transplant embryos were processed for anti-GFP and anti-rhodamine immunostaining to visualize donor-derived floor plate cells and host FBM neurons.
Synthesis of digoxygenin labeled probes and wholemount in situ hybridization were performed as described previously (Vanderlaan et al., 2005). For cross-sections, embryos were embedded in OCT (EMS), and processed (30 μm thickness) on a Leica CM1900 cryostat. Fluorescent imaging was performed on a Zeiss LSM 510 confocal microscope. Mouse in situs and NeuroVue labeling were performed as described previously (Qu et al., 2010; Glasco et al., 2012). Images were processed in Adobe Photoshop to adjust brightness and contrast only.
Analysis of motor neuron protrusions
Optical sections were obtained on Zeiss LSM 510 and 710 confocal microscopes. Cell protrusions and their angles relative to the midline were measured in LSM Image browser or Zen Black software (Zeiss, Inc.). For comparing angular distributions between wild type and mutant embryos, protrusions were placed in two groups (0°–180° and 180°–360°) for each genotype. The differences in distributions were tested for significance using Watson-Williams F-test (JMP software, SAS Institute Inc.).
RESULTS
Vangl2 expression in non-neural tissues is dispensable for FBM neuron migration
Van gogh-like 2 (vangl2) is expressed broadly in the hindbrain and mesendoderm between 18–24 hpf, spanning the period of FBM neuron migration (Figs. 1 and S1). We showed previously that vangl2 function largely functions non-cell autonomously for FBM neuron migration (Jessen et al., 2002). We hypothesized that neuroepithelial tissues co-expressing vangl2 and prickle1a (pk1a) would be good candidates for vangl2 site of action since 1) pk1a is expressed along the lateral margins of the hindbrain, except in rhombomeres 4–6 (Carreira-Barbosa et al., 2003; Veeman et al., 2003), 2) vangl2 and pk1a interact genetically during for FBM neuron migration (Carreira-Barbosa et al., 2003), and 3) Drosophila Van gogh and Prickle interact physically (Bastock et al., 2003; Jenny et al., 2003). Surprisingly, cross-sections revealed that pk1a is mostly expressed outside the neural tube, in endodermal and mesodermal tissues (Fig. S2). Since vangl2 is also expressed in these tissues, in close proximity to the migrating FBM neurons, we tested whether vangl2 functions in the mesoderm or endoderm to regulate FBM neuron migration.
Figure 1. FBM neurons contact vangl2-expressing floor plate cells in rhombomere 4.
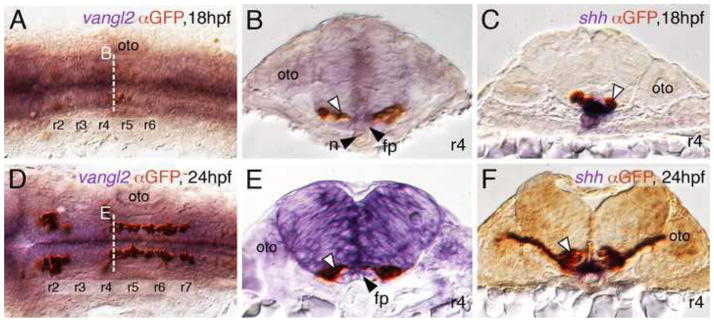
(A, D) Dorsal views of Tg(islet1:GFP) hindbrains processed for vangl2 in situs (purple) and anti-GFP immunostaining to label FBM neurons (brown). The level of the cross-sections is indicated by the broken lines. (B, E) vangl2 is expressed broadly in the endoderm, mesoderm and within the neural tube, including the floor plate (fp, black arrowhead). Some FBM neurons (white arrowhead) are immediately adjacent to the floor plate cells. (C, F) FBM neuron cell bodies and processes (white arrowhead) contact floor plate cells marked by shh expression (purple). n, notochord; oto, otocyst.
To test whether endoderm played a role, we generated embryos lacking endoderm using morpholinos (MO) against casanova (cas), which encodes Sox32, which is essential for endoderm specification (Kikuchi et al., 2001; Sakaguchi et al., 2001). In cas/sox32 MO-injected embryos, endodermal cells at the hindbrain level were absent, while mesoderm was not affected (Fig. S3). Nevertheless, caudal migration of FBM neurons was largely unaffected (Fig. 2A–C), with >90% (qualitative estimate) of motor neurons exiting r4 in 36 hpf morphant embryos, demonstrating that the endoderm (and expression of vangl2 and pk1a within) is not required for initiating caudal migration. However, while the FBM neurons were organized in two distinct clusters in r6 and r7 in control embryos, the neurons were more evenly distributed between r5–r7 in morphant embryos, suggesting that the endoderm may influence subsequent phases of caudal migration.
Next, we tested whether vangl2 expression in the mesoderm played a role in FBM neuron migration. Cells from embryos co-injected with vangl2 MO and TAR RNA were transplanted into Tg(isl1:GFP) hosts to generate mosaic embryos containing vangl2-deficient mesoderm (see Materials and Methods). Host wildtype FBM neurons migrated normally even when donor-derived vangl2 morphant mesodermal cells were located adjacent to the motor neurons in r4 (Fig. S4; 219/243 embryos), suggesting that mesodermal expression of vangl2 is not required for FBM neuron migration. Conversely, the presence of donor-derived wildtype mesodermal cells at the r4 level in vangl2 mutant hosts was unable to induce (rescue) the migration of host mutant FBM neurons (137/167 embryos; data not shown). Intriguingly, the migration of host wildtype neurons was abrogated (partially blocked) (Fig. 2D, E; 24/243 embryos) only when the donor-derived, vangl2 MO + TAR RNA-injected cells formed ventral neuroepithelial cells, including the floor plate. Similarly, the migration of host vangl2-morphant (or vangl2−/−) neurons was partially rescued (Fig. 2F–H; 30/167 embryos) only when the donor-derived, TAR RNA-injected wildtype cells formed ventral neuroepithelial cells, including the floor plate and possibly FBM neurons. This unanticipated location of TAR RNA-injected donor cells in the ventral neural tube and their ability to influence the migration of host FBM neurons suggested a role for vangl2 in floor plate cells or other ventral neuroepithelial cells in regulating FBM neuron migration.
Vangl2 can function in floor plate cells to regulate FBM neuron migration
To directly test a role for vangl2 in floor plate cells, we used cell transplantation to target vangl2-deficient cells to the floor plate in Tg(isl1:GFP) host embryos. Donor cells were targeted to the floor plate by injecting no tail (ntl) MO into donor embryos, since ntl-deficient cells preferentially contribute to the floor plate (Halpern et al., 1993) (see Materials and Methods for details). In control experiments, donor-derived wildtype cells differentiating into floor plate in wildtype hosts did not affect the migration of host FBM neurons (Fig. 3C; 35 embryos). However, when vangl2 mutant donor cells contributed to the floor plate in the r4 region of 36 hpf wildtype hosts, a large number of host neurons failed to migrate out of r4 in a majority of the embryos (Figs. 3A–C and S6; 30/44 embryos), consistent with a requirement for vangl2 function in floor plate cells for neuronal migration. The blocking ability of vangl2 mutant cells was variable since host neurons migrated normally in several embryos containing donor floor plate cells in r4 (Fig. 3C; 14/44 embryos). Importantly, vangl2 mutant donor cells were able to block the migration of wildtype FBM neurons only if they contributed to the floor plate in the r4 region of the host embryos, since donor-derived cells in other locations in the hindbrain, including the floor plate in other rhombomeres failed to block migration even partially (Fig. 3C; 201 embryos).
Next, we used the Gal4-UAS system to ask whether floor plate-specific expression of a vangl2 transgene could rescue FBM neuron migration in vangl2 mutant embryos. We employed a Tol2 gene trap line SAGFF187A (henceforth called Tg(Fp:Gal4FF); Tg(UAS:GFP)), which expresses Gal4FF and GFP (Asakawa et al., 2008) in the floor plate from 16–48 hpf, to drive expression of a UAS:Myc-vangl2 transgene (Fig. S7; X.P. and A.C., unpublished data). Vangl2 mutant (tri−/−) embryos obtained from a cross of Tg(Fp:Gal4FF); Tg(UAS:GFP); tri+/− and Tg(UAS:Myc-vangl2); Tg(islet1:gfp); tri+/− fish were processed for anti-MYC (Vangl2 expression) and anti-GFP (FBM neurons and floor plate) immunostaining at 48 hpf and scored for the presence of caudally migrating FBM neurons outside r4. Consistent with a role for vangl2 in the floor plate, >50% of vangl2 mutant FBM neurons migrated out of r4 in mutant embryos containing at least 1–2 Myc-Vangl2 expressing floor plate cells in the r4 region (12/12 embryos; Figs. 3D–F and S8; Table S1). Migration of mutant neurons was not rescued either in embryos not expressing Myc-Vangl2 (146/146) or in embryos containing Myc-Vangl2 expressing floor plate cells outside the r4 region (148/148; Fig. 3F). Taken together, the floor plate transplant and transgene experiments suggest strongly that vangl2 functions primarily in floor plate cells to regulate FBM neuron migration.
It is also possible that vangl2 functions in part in other cell types such as the FBM neurons themselves to regulate migration. Indeed, the inability of transplanted wildtype FBM neurons to migrate in vangl2-deficient hosts (Fig. 4B) (Jessen et al., 2002) may reflect a partial requirement for vangl2 in the FBM neurons, since non-migrating host motor neurons may inhibit the migration of donor-derived wildtype neurons through vangl2-mediated cell interactions. Recently, Walsh et al. (2011) performed elegant transplant experiments that indicated that vangl2 function is required in the environment (non cell-autonomous role) and in FBM neurons (cell autonomous role) for migration. Specifically, the authors suggest that vangl2 acts in FBM neurons, mediating interactions between motor neurons needed to migrate out of r4 (collective migration mode). To definitively resolve this issue, one must eliminate the influence of host FBM neurons on the behavior of donor-derived motor neurons. Therefore, we performed similar transplant experiments in detour mutant (gli1−/−) host embryos that generate few or no branchiomotor neurons (Chandrasekhar et al., 1999; Vanderlaan et al., 2005).
In control experiments, ~85% of wildtype donor-derived FBM neurons migrated out of r4 in wildtype host embryos (Fig. 4A). Next, as expected, all wildtype FBM neurons failed to migrate out of r4 in vangl2 morphant embryos (Fig. 4B), and ~85% of vangl2 mutant FBM neurons migrated out of r4 in wildtype hosts (Fig. 4D), similar to previous results (Jessen et al., 2002; Walsh et al., 2011), and consistent with a non-autonomous role for vangl2. About 70% of wildtype FBM neurons migrated out of r4 in detour (dtr) mutant (but vangl2+/+) hosts (Fig. 4C). Importantly, ~60% of vangl2 mutant FBM neurons migrated out of r4 in dtr mutant hosts (Fig. 4E), which is comparable in efficiency to the ability of wildtype FBM neurons to migrate in dtr mutants (Fig. 4C), indicating that vangl2−/− neurons do not require the presence of vangl2+/+ neurons to migrate caudally (one interpretation of Fig. 4D). Further discounting a motor neuron-autonomous role for vangl2, donor-derived wildtype FBM neurons completely failed to migrate out of r4 in vangl2 morphant, dtr mutants (Fig. 4F), identical to the results in vangl2 morphant hosts (Fig. 4B), and ruling out an influence of host motor neurons on the behavior of donor wildtype FBM neurons in experiment 4B. Lastly, since the migration of wildtype FBM neurons was completely blocked by inactivating vangl2 in an environment lacking FBM neurons (compare Figs. 4C and F), we conclude that vangl2 regulates FBM neuron migration out of r4 by acting largely outside motor neurons, likely in neuroepithelial cells such as the floor plate.
FBM neurons fail to migrate caudally in the mouse Gli2 mutant, which fails to induce the floor plate
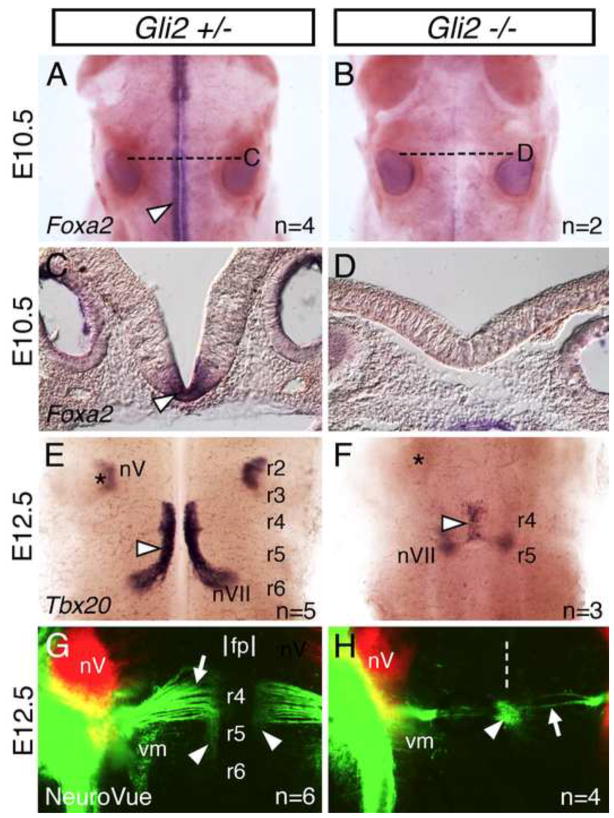
To test whether a potential role for the floor plate in FBM neuron migration was evolutionarily conserved, we analyzed Gli2 mutant mice, which fail to induce the floor plate but still generate motor neurons (Matise et al., 1998). Gli2 mutant hindbrains did not express the floor plate marker Foxa2 (Fig. 5A–D), as shown previously in the spinal cord (Matise et al., 1998). In situ hybridization with Tbx20, a branchiomotor neuron marker (Song et al., 2006), showed that in wildtype E12.5 siblings, FBM neurons formed a continuous longitudinal stream of caudally migrating cells spanning r4 to r6, with radial migration within r6 to form the facial motor nucleus (nVII) (Figs. 5E and S9). In Gli2 mutants, FBM neurons were greatly reduced in number (Fig. 5F). Importantly, however, they failed to migrate caudally out of r4, but still migrated radially within r4 (Figs. 5F and S9). Consistent with this, the nVII nucleus at E14.5, detected by Tbx20 and Phox2b in situs (Glasco et al., 2012), was located rostrally in the r4 region of Gli2 mutants, rather than in r6, as in wildtype siblings (Fig. S9). The neuronal populations were fused across the midline at E12.5 due to the absence of the floor plate, as shown previously for spinal motor neurons (Matise et al., 1998). To confirm that the Tbx20-expressing cells in mutants were FBM neurons, we performed retrograde labeling with NeuroVue dyes (Fritzsch et al., 2005). As expected, the cell bodies of the nV and nVII nuclei were located in r2 and r4–r6, respectively, in wildtype embryos (Fig. 5G). In Gli2 mutants, a small number of back-filled FBM neurons were located within r4 at the midline, with axons extending laterally (Fig. 5H), indicating the failure of caudal migration.
Figure 5. FBM neurons fail to migrate caudally in mouse Gli2 mutants, which lack the floor plate.
(A, B, E–H) Dorsal views of hindbrains with anterior to the top. (C, D) Cross-sections (dorsal up) at the levels indicated by broken lines (A, B). (A–D) Foxa2 in situs. In a Gli2+/− embryo (A, C), Foxa2 is expressed along the midline, corresponding to the floor plate (arrowheads), at all axial levels. In a Gli2−/− embryo (B, D), Foxa2 expression is missing, indicating the absence of a floor plate. (E–H) Tbx20 in situs (E, F) or NeuroVue injection at the cranial nerve V (red) and VII (green) exit points (G, H). In a Gli2+/− embryo (E), FBM neurons migrate caudally from r4 into r6 (arrowhead). In a Gli2−/− embryo (F), FBM neurons fail to migrate out of r4, and are found in a single cluster (arrowhead) fused across the midline. Asterisks in E, F indicate location of the trigeminal motor nucleus (nV) in r2. In a Gli2+/− embryo (G), many retrogradely-labeled FBM neurons (arrowheads) are located in r5, and the motor axons (arrow) exhibit a characteristic fan shape (genu) indicative of caudal migration. The approximate location of the floor plate (fp) is indicated. In a Gli2−/− embryo (H), FBM neurons (arrowhead) are found in a single cluster at the midline (broken line), and their axons (arrow) lack the genu reflecting the failure to migrate. The locations of the trigeminal motor (nV) and visceromotor (vm) neurons in r2 and r5, respectively, are similar between wildtype and mutant embryos.
Potential role for Vangl2-dependent floor plate polarity, but not for floor plate cilia function, in FBM neuron migration
We showed previously that FBM neurons in vangl2-deficient embryos generate protrusions and change shapes at the same rate as neurons in wildtype embryos, but fail to migrate out of r4 because they are unable to maintain caudally directed protrusions (Jessen et al., 2002; Sittaramane et al., 2009). Since our data suggested strongly that vangl2 functions in floor plate cells in r4 to regulate caudal migration, we wondered whether FBM neurons located in r4 exhibit previously undetected protrusive behaviors. Therefore we quantified protrusions of FBM neurons located in r4 in fixed wildtype and vangl2 mutant Tg(zCREST:mRFP) embryos at 19 and 24 hpf (Fig. 6). Protrusions of wildtype neurons were directed largely toward the midline at both ages, as shown previously (Mapp et al., 2010). Imaging of Tg(Fp:Gal4FF); Tg(UAS:GFP); Tg(zCREST:mRFP) embryos to simultaneously examine floor plate cells (GFP+ve) and motor neurons (mRFP+ve) revealed that some midline-directed protrusions contacted floor plate cells (Fig. S10). Importantly, protrusions on vangl2 mutant neurons arose from all around the cell periphery (Fig. 6B, E, D), with a majority of protrusions directed away from the midline at 19 hpf (Fig. 6E; significant difference from wildtype at p<0.001). These data suggest that vangl2 acting non-autonomously, likely in floor plate cells, affects the ability of FBM neurons to generate or preferentially maintain midline-directed protrusions.
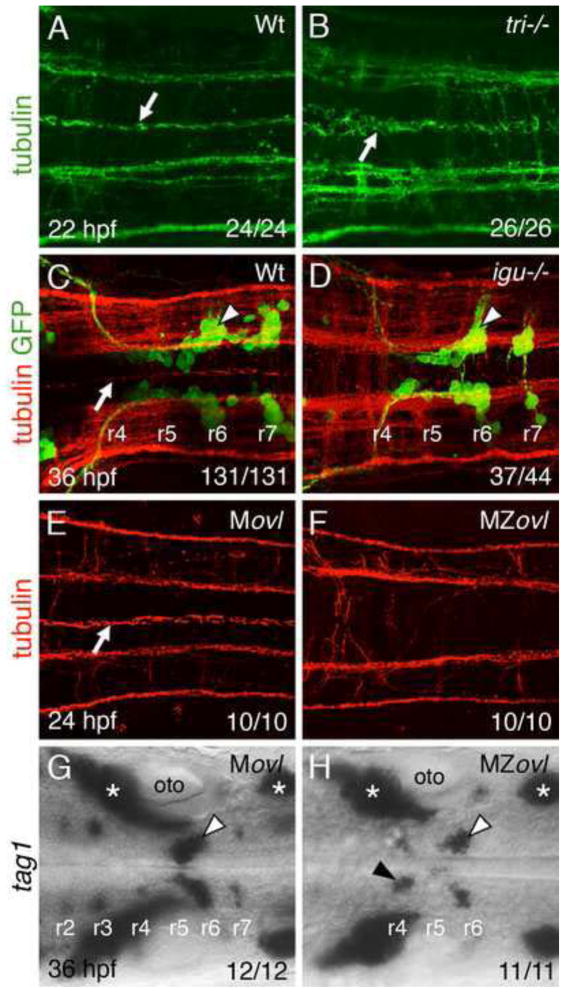
Vangl2 may function in, and influence the polarity of, floor plate cells to regulate FBM neuron migration. Indeed, Borovina et al. (2010) showed that floor plate polarity, defined by the position of the centrosome generating the primary cilium and by the orientation of the cilium, is disrupted in the spinal cord floor plate of MZvangl2 mutants. We confirmed that the organization of floor plate cilia was also disrupted in the hindbrain region of vangl2 mutants (Fig. 7A, B). To test whether floor plate cilia are necessary for FBM neuron migration, analogous to the role of ependymal cilia in olfactory neuroblast migration (Sawamoto et al., 2006), we first examined iguana (igu) mutants, which form very few floor plate cilia (Kim et al., 2010). In wildtype siblings, floor plate cilia were longitudinally oriented, and a majority of FBM neurons had migrated out of r4 (Fig. 7C). In igu mutants, although floor plate cilia were absent (37/44 embryos; Fig. 7D) or greatly reduced (7/44) in number, most FBM neurons migrated normally out of r4, suggesting that cilia are not required for migration. Since floor plate cilia eventually form in iguana mutants by 48 hpf (Kim et al., 2010), we sought to rule out any involvement of cilia by analyzing maternal zygotic mutants of the intraflagellar transport protein Ift88/Polaris/Oval (MZovl), which lack all cilia (Huang and Schier, 2009). We verified that floor plate cilia were absent in the hindbrain region of MZovl mutants (Fig. 7E, F). Tag1 in situs revealed that inspite of the complete absence of cilia, a majority of FBM neurons migrated out of r4 in MZovl mutants (Fig. 7H). The numbers of trigeminal (r2, r3) and facial (r4–r7) branchiomotor neurons cells were reduced in MZovl mutants, likely reflecting dampened hedgehog signaling (Huang and Schier, 2009). A small number of tag1-expressing neurons consistently remained in the r4 region of MZovl embryos, reflecting either a partial dependence of caudal migration on cilia function or subtle defects in PCP signaling.
Figure 7. Floor plate cilia are largely dispensable for FBM neuron migration.
(A–H) Dorsal views of hindbrains with anterior to the left. The ratio of the number of embryos exhibiting a phenotype to the total number of embryos examined is indicated in each panel. (A, B) Anti-tubulin immunostaining (green). In a wildtype hindbrain (A), floor plate cilia (arrow) are uniformly orientated along the anterior-posterior axis, whereas in a vangl2 mutant (tri−/−) mutant (B), the floor plate cilia (arrow) are disorganized and misorientated. (C, D) Tg(isl1:GFP) embryos processed for anti-tubulin (red) and anti-GFP (green) immunostaining. In a wildtype sibling (C), floor plate cilia (arrow) are uniformly orientated, and FBM neurons (arrowhead) migrate out of r4. In an iguana−/− embryo (D), although floor plate cilia are absent, FBM neurons (arrowhead) migrate normally out of r4. (E, F) While floor plate cilia (white arrow) are present and uniformly orientated in the Movl hindbrain (E), they are absent in the MZovl hindbrain (F). Prominent longitudinal axon tracts appear unaffected in MZovl embryos. (G, H) Tag1 in situs to label branchiomotor and sensory neurons. FBM neurons (white arrowheads) migrate out of r4 in both Movl and MZovl embryos, although a small number of neurons (black arrowhead) consistently remained in r4 in MZovl embryos. Asterisks indicate the location of sensory ganglia, which develop normally in both genotypes.
Vangl2 is enriched in basolateral membranes of floor plate cells
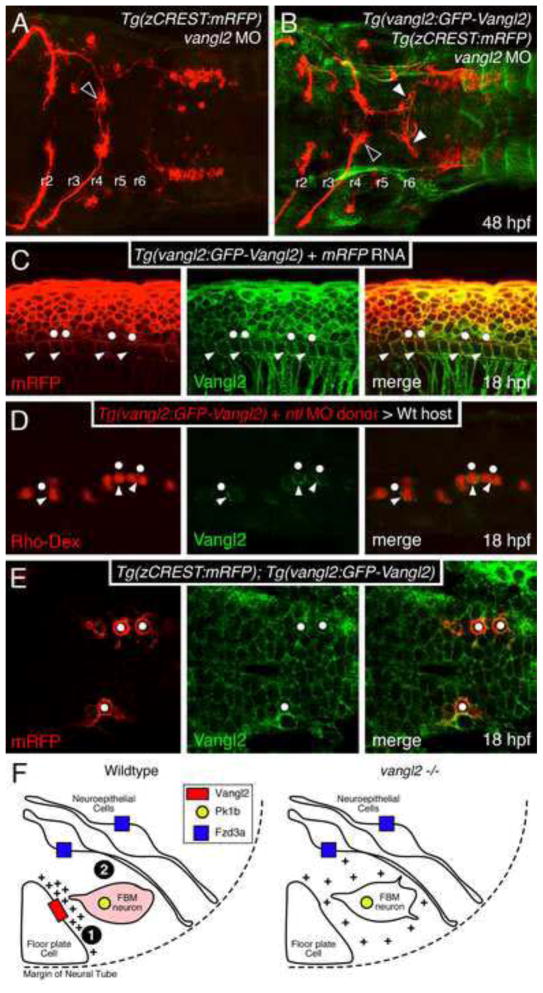
Our data suggest that vangl2 functions in floor plate cells to regulate FBM neuron migration independently of floor plate cilia function. To explore this issue further, we asked whether Vang2 itself exhibited asymmetric localization in floor plate cells. We generated a transgenic line driving an N-terminally fused GFP-Vangl2 protein from a 5 kb vangl2 promoter element (X.P. and A.C., unpublished data). The GFP-Vangl2 transgene was expressed in a similar pattern to the endogenous gene, but also in some ectopic tissues such as the somites (Fig. 8B). Importantly, the vangl2 transgene was able to efficiently rescue FBM neuron migration in vangl2 morphants (Fig. 8A, B; 16/16 embryos), indicating that the fusion protein is expressed at appropriate levels and retains the biochemical activity of the endogenous protein. To examine localization in floor plate cells, we analyzed Tg(vangl2:GFP-Vangl2) embryos injected with RNA encoding membrane-RFP. In >90% of floor plate cells, GFP-Vangl2 was enriched in the anterior, posterior, and ventral membranes compared to the apical membrane (Fig. 8C; 131/141 cells, 6 embryos). To determine whether GFP-Vangl2 showed preferential accumulation in the anterior or posterior membranes, we tried to generate isolated transgene-expressing floor plate cells using the ntl MO to generate floor plate mosaics. We found again that GFP-Vangl2 was enriched in all floor plate cell membranes except the apical (Fig. 8D; 21/31 cells, 3 embryos), but did not observe consistent differences between the anterior and posterior membranes. We also could not detect preferential enrichment of GFP-Vangl2 on anterior or posterior membranes of migrating FBM neurons (Fig. 8E; 60 cells, 4 embryos). Together, these data suggest that differential enrichment of GFP-Vangl2 on floor plate membranes reflects an aspect of floor plate polarity that may influence FBM neuron migration.
Figure 8. Vangl2 is enriched in basolateral membranes of floor plate cells.
Anterior is to the left in panels A–E. (A, B) Dorsal views of hindbrains processed for RFP and GFP immunostaining. In a control vangl2 morphant embryo (A), FBM neurons fail to migrate out of r4 (black arrowhead). In a Tg(vangl2:GFP-Vangl2) vangl2 morphant (B), many FBM neurons migrate out of r4 (white arrowheads). (C) Lateral view of an embryo processed for immunostaining to detect mRFP and GFP-Vangl2. Whereas mRFP is present on basolateral (arrowheads) and apical (dots) surfaces of floor plate cells, the apical surfaces are mostly devoid of GFP-Vangl2. (D) Lateral view of a host embryo containing donor-derived cells targeted to the floor plate, and processed for Rhodamine and GFP immunostaining. Donor-derived floor plate cells (Rho-Dex) exhibit basolateral enrichment of GFP-Vangl2 (arrowheads) with weaker signal at the apical surface (dots). (E) Dorsal view of the hindbrain showing uniform distribution of GFP-Vangl2 on the surface (mRFP) of FBM neurons (dots). (F) Model for regulation of FBM neuron migration by floor plate-derived Vangl2. In the wildtype rhombomere 4 hemi cross-section, Vangl2 on basolateral membranes of floor plate cells sequester a floor plate-derived orientating cue (+). Orientated protrusions on FBM neurons and associated signaling make them competent (primed; denoted by pink shading) to respond to other environmental cues (Step 1). These primed FBM neurons may respond to cell adhesive and/or cell repulsive cues in a Pk1b- and Fzd3a-dependent manner (Step 2), get excluded from the neuroepithelium in r4, and migrate caudally, as proposed previously (Wada et al., 2006). In rhombomere 4 of vangl2 mutants, the floor plate-derived cue does not get sequestered at the basolateral membranes of floor plate cells, and the FBM neurons do not become competent (Step 1 fails). The neurons are unable to respond to adhesive/repulsive cues on neuroepithelial cells, resulting in their integration into the neuroepithelium in r4 and consequent failure to migrate caudally. The depiction of Vangl2 and Fzd3a on floor plate and neuroepithelial cells, respectively, indicates their functional requirement for neuronal migration, and not their expression pattern, which is ubiquitous in the neural tube.
DISCUSSION
Vangl2 is a core component of the PCP signaling pathway, and is essential for directional movement of gastrulating cells and FBM neurons (Jessen et al., 2002; Roszko et al., 2009). Consistent with its broad expression pattern, previous studies have suggested cell autonomous and non-cell autonomous roles for vangl2 in regulating FBM neuron migration (Jessen et al., 2002; Walsh et al., 2011). Here, we provide multiple lines of evidence that suggest strongly that vangl2 functions primarily in floor plate cells to regulate migration of FBM neurons out of rhombomere 4. Importantly, we also find that floor plate cilia function is not required for neuronal migration.
Site of action of PCP genes for FBM neuron migration
Several broadly expressed PCP genes (vangl2, celsr2, fzd3a, scrib) appear to function non cell-autonomously for FBM neuron migration (Jessen et al., 2002; Wada et al., 2005; Wada et al., 2006). Wada and colleagues performed mosaic analyses to propose that fzd3a (and possibly celsr2 and scrib) functions in neuroepithelial cells surrounding the FBM neurons to inhibit their radial migration (and integration into the neuroepithelium) within r4, consequently enabling their caudal migration into r6. One caveat of these mosaic analyses is that the donor-derived FBM neurons of one genotype (e.g., scrib mutant) were surrounded by host FBM neurons of the second genotype (e.g., wildtype), leaving open the possibility that the migratory behaviors of the donor-derived neurons were being dictated by interactions with host motor neurons (motor neuron-autonomous) and not by interactions with neuroepithelial cells in the environment (non cell-autonomous). This issue was addressed recently (Walsh et al., 2011) by examining the behaviors of donor-derived vangl2- and scrib-deficient FBM neurons in vangl2+ve and scrib+ve host embryos, respectively, where the host FBM neurons failed to migrate due to pk1b knockdown. Whereas vangl2-deficient FBM neurons frequently migrated out of r4 in wildtype hosts, consistent with a non-autonomous role for vangl2, vangl2-deficient motor neurons failed to migrate out of r4 in pk1b morphant hosts, indicating that the migratory behavior of the host motor neurons was influencing the migratory ability of the donor-derived FBM neurons (motor neuron autonomous effect). Two mechanisms are consistent with this motor neuron autonomous effect (Walsh et al., 2011). Firstly, migratory FBM neurons in r4 may interact and influence each other’s decision to migrate out of r4. Importantly, these interactions would not be dependent on the function of core PCP genes (collective migration, independent of PCP function). Secondly, PCP proteins may also function within FBM neurons to facilitate their migration (PCP-dependent migration), but this function would be masked by the collective mode of migration. Our mosaic analysis with detour mutants to resolve this issue suggests strongly that vangl2 does not function in FBM neurons for migration.
Vangl2 functions in floor plate cells to regulate FBM neuron migration
Since the collective mode of FBM neuron migration (Walsh et al., 2011) can confound mosaic analysis of vangl2 function, we performed chimeric analysis in detour mutant (gli1−/−) host embryos, which fail to induce branchiomotor neurons (Chandrasekhar et al., 1999; Vanderlaan et al., 2005). This allowed us to examine the migratory behavior of donor-derived FBM neurons in the absence of host-derived branchiomotor neurons, thereby removing the contribution of collective migration to mosaic analysis phenotypes (Fig. 4). Vangl2 mutant FBM neurons migrated out of r4 in detour mutant hosts in a similar fashion to wildtype neurons, indicating that vangl2 function in FBM neurons is dispensable for migration. Importantly, wildtype FBM neurons failed to migrate out of r4 in vangl2 morphant, detour mutant hosts, similar to their behavior in vangl2 morphant hosts, demonstrating an exclusive requirement for vangl2 function outside of motor neurons. Interestingly, FBM neuron migration out of r4 was less efficient in detour mutant hosts compared to wildtype hosts (Fig. 4A, C–E), suggesting that the dtr mutant hindbrain may be less conducive to migration. Consistent with this, expression of chemokine genes cxcl12a and cxcr7b, which play roles in FBM neuron migration (Cubedo et al., 2009), was altered in putative detour mutant hindbrains (Fig. S11).
While the detour transplant experiments argue against a motor neuron-autonomous role for vangl2 in FBM neurons, the no tail transplant and UAS:Myc-vangl2 transgene experiments strongly favor a non cell-autonomous role for vangl2 in floor plate cells for regulating migration (Fig. 3). In the transplant studies, the moderate efficiency of abrogating wildtype neuron migration (Fig. 3A) may be attributed to variability in the position and number of donor-derived floor plate cells in host embryos. Similarly, while expression of the UAS:Myc-vangl2 transgene in floor plate cells in the r4 region rescued neuronal migration in every vangl2 mutant (Fig. 3F), rescue efficiency ranged from ~50 to >90%. This variable efficiency is likely due to the mosaic expression of the transgene in floor plate cells resulting from methylation-mediated transcriptional silencing of the 10XUAS element in the UAS:Myc-vangl2 transgene (Goll et al., 2009; Akitake et al., 2011). Nevertheless, taken together, the transplantation and transgenic data suggest strongly that vangl2 functions primarily in floor plate cells for FBM neuron migration. However, we cannot definitively rule out that vangl2 may also function in the neuroepithelial cells surrounding the FBM neurons to regulate their migration (Walsh et al., 2011), as proposed for fzd3a (Wada et al., 2006).
Our observation that caudal migration of FBM neurons is lost in mouse Gli2 mutants, which fail to induce the floor plate (Matise et al., 1998), lends further support to the idea that floor plate cells play an evolutionarily conserved role in this migration. However, since other ventral cells, in addition to the floor plate, are also missing in Gli2 mutants, the migration phenotype cannot be attributed exclusively to floor plate-associated defects. Nevertheless, because Vangl2 is expressed in the mouse hindbrain floor plate (Glasco et al., 2012), and Vangl2 mutant mice exhibit defective floor plate development (Greene et al., 1998; Murdoch et al., 2001) and defective FBM neuron migration (Glasco et al., 2012), we propose that Vangl2 functions in the floor plate to regulate FBM neuron migration in mice, as in zebrafish. Demonstration of a specific role for Vangl2 in the mouse floor plate will require analyses of conditional knockouts using the Vangl2 flox allele (Song et al., 2010). However, because most FBM neurons in mice are positioned several cell diameters away from the floor plate (Qu et al., 2010), it is unlikely that floor plate cells (and Vangl2 function within) will directly regulate caudal migration of all FBM neurons, and other cell types including ventral neuroepithelial cells adjacent to the floor plate and motor neurons may also play a role.
Role of floor plate polarity and cilia function in FBM neuron migration
Given the importance of the floor plate for FBM neuron migration, it will be critical to identify the aspects of floor plate structure and function that are necessary for migration. One attractive possibility is that motile floor plate cilia (Yu et al., 2008; Cruz et al., 2010) produce directed fluid flow in the fourth ventricle that could influence FBM neuron migration by generating gradients of floor plate-secreted molecules within the neural tube. Indeed, Sawamoto et al. (2006) demonstrated that fluid flow caused by ependymal cilia generates a gradient of the chemorepulsive Slit proteins in the subventricular zone, and that cilia function and Slits were necessary for the directed migration of olfactory neuroblasts in the rostral migratory stream toward the olfactory bulb. Our results with iguana and MZovl mutants argue against an analogous role for floor plate cilia in the caudal migration of FBM neurons.
If cilia are not involved, how might Vangl2 regulate the properties of floor plate cells to regulate migration? We found that a GFP-Vangl2 fusion protein is enriched in the basolateral, but not the apical, membranes of floor plate cells. The differential distribution of GFP-Vangl2 on floor plate membranes is likely to reflect the distribution of endogenous Vangl2 since the fusion protein is biologically active (i.e., able to rescue FBM neuron migration in vangl2 mutants), and its expression is controlled by vangl2 promoter elements. Since Vangl2 physically interacts with Wnt/PCP molecules like Prickle and Dishevelled (Bastock et al., 2003; Jenny et al., 2003; Torban et al., 2004), these molecules may also exhibit differential subcellular localization in floor plate cells. However, neither Prickle nor Dishevelled is likely to be involved in the floor plate-mediated regulation of neuronal migration since prickle genes are not expressed in the floor plate (Carreira-Barbosa et al., 2003; Tissir and Goffinet, 2006; Rohrschneider et al., 2007) (this report), and Dishevelled-mediated signaling is largely dispensable for migration (Jessen et al., 2002; Glasco et al., 2012).
Our observation that the protrusions of premigratory wildtype FBM neurons in r4 are preferentially directed toward the midline, while those of non-migrating vangl2 mutant neurons are less directed suggests that the floor plate and/or adjacent neuroepithelial cells may be the source of an orientating cue, as suggested previously (Fig. 8F; Mapp et al., 2010). We propose that this putative orientating cue is closely associated with (sequestered by) asymmetrically distributed Vangl2 on floor plate cells to orientate FBM neurons in r4 and to make them competent (primed) to respond to other environmental cues (Step 1). These primed FBM neurons may respond to cell adhesive or cell repulsive cues in a Pk1b- and Fzd3a-dependent manner (Step 2), get excluded from the neuroepithelium in r4, and migrate caudally, as proposed previously (Wada et al., 2006). In vangl2 mutants, the orientating cue is not localized, and the FBM neurons do not become competent (Step 1 fails), resulting in their integration into the neuroepithelium in r4 and consequent failure to migrate caudally. It will be of great interest to identify the putative orientating cue(s), and to elucidate its postulated association with Vangl2.
Supplementary Material
Highlights.
vangl2 largely functions in the floor plate for facial motor neuron migration.
Floor plate cilia are dispensable for neuronal migration.
Vangl2 is enriched in the basolateral membranes of floor plate cells.
Facial motor neurons fail to migrate in a mouse mutant lacking the floor plate.
Acknowledgments
We thank members of the Chandrasekhar lab for discussion and fish care. We thank Oni Mapp and Victoria Prince for the Tg(zCREST:mRFP) fish, Tom Schilling and Brian Ciruna for TARAM-Ad and mRFP cDNAs, respectively, and Marnie Halpern for advice on floor plate transplants. This work was supported by an NIH predoctoral fellowship 1F31NS063513 (DG), and NIH grants HD057015 (MM) and NS040449 (AC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akitake CM, Macurak M, Halpern ME, Goll MG. Transgenerational analysis of transcriptional silencing in zebrafish. Dev Biol. 2011;352:191–201. doi: 10.1016/j.ydbio.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa K, Suster ML, Mizusawa K, Nagayoshi S, Kotani T, Urasaki A, Kishimoto Y, Hibi M, Kawakami K. Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc Natl Acad Sci U S A. 2008;105:1255–1260. doi: 10.1073/pnas.0704963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC. Emerging epilepsy models: insights from mice, flies, worms and fish. Curr Opin Neurol. 2007;20:164–168. doi: 10.1097/WCO.0b013e328042bae0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- Bingham S, Higashijima S, Okamoto H, Chandrasekhar A. The Zebrafish trilobite gene is essential for tangential migration of branchiomotor neurons. Dev Biol. 2002;242:149–160. doi: 10.1006/dbio.2001.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovina A, Superina S, Voskas D, Ciruna B. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat Cell Biol. 2010;12:407–412. doi: 10.1038/ncb2042. [DOI] [PubMed] [Google Scholar]
- Brand M, Heisenberg CP, Warga RM, Pelegri F, Karlstrom RO, Beuchle D, Picker A, Jiang YJ, Furutani-Seiki M, van Eeden FJ, et al. Mutations affecting development of the midline and general body shape during zebrafish embryogenesis. Development. 1996;123:129–142. doi: 10.1242/dev.123.1.129. [DOI] [PubMed] [Google Scholar]
- Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, Tada M. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–4046. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar A. Turning heads: development of vertebrate branchiomotor neurons. Dev Dyn. 2004;229:143–161. doi: 10.1002/dvdy.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekhar A, Schauerte HE, Haffter P, Kuwada JY. The zebrafish detour gene is essential for cranial but not spinal motor neuron induction. Development. 1999;126:2727–2737. doi: 10.1242/dev.126.12.2727. [DOI] [PubMed] [Google Scholar]
- Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–224. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp AJ, Harding BN. Neuronal migration disorders in humans and in mouse models--an overview. Epilepsy Res. 1999;36:133–141. doi: 10.1016/s0920-1211(99)00047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz C, Ribes V, Kutejova E, Cayuso J, Lawson V, Norris D, Stevens J, Davey M, Blight K, Bangs F, et al. Foxj1 regulates floor plate cilia architecture and modifies the response of cells to sonic hedgehog signalling. Development. 2010;137:4271–4282. doi: 10.1242/dev.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubedo N, Cerdan E, Sapede D, Rossel M. CXCR4 and CXCR7 cooperate during tangential migration of facial motoneurons. Mol Cell Neurosci. 2009;40:474–484. doi: 10.1016/j.mcn.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Muirhead KA, Feng F, Gray BD, Ohlsson-Wilhelm BM. Diffusion and imaging properties of three new lipophilic tracers, NeuroVue Maroon, NeuroVue Red and NeuroVue Green and their use for double and triple labeling of neuronal profile. Brain Res Bull. 2005;66:249–258. doi: 10.1016/j.brainresbull.2005.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilland E, Baker R. Evolutionary patterns of cranial nerve efferent nuclei in vertebrates. Brain Behav Evol. 2005;66:234–254. doi: 10.1159/000088128. [DOI] [PubMed] [Google Scholar]
- Glasco DM, Sittaramane V, Bryant W, Fritzsch B, Sawant A, Paudyal A, Stewart M, Andre P, Cadete Vilhais-Neto G, Yang Y, et al. The mouse Wnt/PCP protein Vangl2 is necessary for migration of facial branchiomotor neurons, and functions independently of Dishevelled. Dev Biol. 2012;369:211–222. doi: 10.1016/j.ydbio.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll MG, Anderson R, Stainier DY, Spradling AC, Halpern ME. Transcriptional silencing and reactivation in transgenic zebrafish. Genetics. 2009;182:747–755. doi: 10.1534/genetics.109.102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene ND, Gerrelli D, Van Straaten HW, Copp AJ. Abnormalities of floor plate, notochord and somite differentiation in the loop-tail (Lp) mouse: a model of severe neural tube defects. Mech Dev. 1998;73:59–72. doi: 10.1016/s0925-4773(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Halpern ME, Ho RK, Walker C, Kimmel CB. Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell. 1993;75:99–111. [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Haffter P, Heisenberg CP, et al. Mutations affecting morphogenesis during gastrulation and tail formation in the zebrafish, Danio rerio. Development. 1996;123:143–151. doi: 10.1242/dev.123.1.143. [DOI] [PubMed] [Google Scholar]
- Higashijima S, Hotta Y, Okamoto H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J Neurosci. 2000;20:206–218. doi: 10.1523/JNEUROSCI.20-01-00206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschuh J, Wada N, Wada C, Schaffer A, Javidan Y, Tallafuss A, Bally-Cuif L, Schilling TF. Requirements for endoderm and BMP signaling in sensory neurogenesis in zebrafish. Development. 2005;132:3731–3742. doi: 10.1242/dev.01936. [DOI] [PubMed] [Google Scholar]
- Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development. 2009;136:3089–3098. doi: 10.1242/dev.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny A, Darken RS, Wilson PA, Mlodzik M. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. Embo J. 2003;22:4409–4420. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Agathon A, Alexander J, Thisse C, Waldron S, Yelon D, Thisse B, Stainier DY. casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 2001;15:1493–1505. doi: 10.1101/gad.892301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Richardson J, van Eeden F, Ingham PW. Gli2a protein localization reveals a role for Iguana/DZIP1 in primary ciliogenesis and a dependence of Hedgehog signal transduction on primary cilia in the zebrafish. BMC Biol. 2010;8:65. doi: 10.1186/1741-7007-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Mapp OM, Walsh GS, Moens CB, Tada M, Prince VE. Zebrafish Prickle1b mediates facial branchiomotor neuron migration via a farnesylation-dependent nuclear activity. Development. 2011;138:2121–2132. doi: 10.1242/dev.060442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapp OM, Wanner SJ, Rohrschneider MR, Prince VE. Prickle1b mediates interpretation of migratory cues during zebrafish facial branchiomotor neuron migration. Dev Dyn. 2010;239:1596–1608. doi: 10.1002/dvdy.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. Cell Migration in the Forebrain. Annu Rev Neurosci. 2003;26:26. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development. 1998;125:2759–2770. doi: 10.1242/dev.125.15.2759. [DOI] [PubMed] [Google Scholar]
- Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001;10:2593–2601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Parnavelas JG. Modes of neuronal migration in the developing cerebral cortex. Nat Rev Neurosci. 2002;3:423–432. doi: 10.1038/nrn845. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Qu Y, Glasco DM, Zhou L, Sawant A, Ravni A, Fritzsch B, Damrau C, Murdoch JN, Evans S, Pfaff SL, et al. Atypical cadherins Celsr1–3 differentially regulate migration of facial branchiomotor neurons in mice. J Neurosci. 2010;30:9392–9401. doi: 10.1523/JNEUROSCI.0124-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider MR, Elsen GE, Prince VE. Zebrafish Hoxb1a regulates multiple downstream genes including prickle1b. Dev Biol. 2007;309:358–372. doi: 10.1016/j.ydbio.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Roszko I, Sawada A, Solnica-Krezel L. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin Cell Dev Biol. 2009;20:986–997. doi: 10.1016/j.semcdb.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T, Kuroiwa A, Takeda H. A novel sox gene, 226D7, acts downstream of Nodal signaling to specify endoderm precursors in zebrafish. Mech Dev. 2001;107:25–38. doi: 10.1016/s0925-4773(01)00453-1. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Sittaramane V, Sawant A, Wolman MA, Maves L, Halloran MC, Chandrasekhar A. The cell adhesion molecule Tag1, transmembrane protein Stbm/Vangl2, and Lamininalpha1 exhibit genetic interactions during migration of facial branchiomotor neurons in zebrafish. Dev Biol. 2009;325:363–373. doi: 10.1016/j.ydbio.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–382. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song MR, Shirasaki R, Cai CL, Ruiz EC, Evans SM, Lee SK, Pfaff SL. T-Box transcription factor Tbx20 regulates a genetic program for cranial motor neuron cell body migration. Development. 2006;133:4945–4955. doi: 10.1242/dev.02694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C, Thisse B. Antivin, a novel and divergent member of the TGFbeta superfamily, negatively regulates mesoderm induction [In Process Citation] Development. 1999;126:229–240. doi: 10.1242/dev.126.2.229. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Expression of planar cell polarity genes during development of the mouse CNS. Eur J Neurosci. 2006;23:597–607. doi: 10.1111/j.1460-9568.2006.04596.x. [DOI] [PubMed] [Google Scholar]
- Torban E, Wang HJ, Groulx N, Gros P. Independent mutations in mouse Vangl2 that cause neural tube defects in looptail mice impair interaction with members of the Dishevelled family. J Biol Chem. 2004;279:52703–52713. doi: 10.1074/jbc.M408675200. [DOI] [PubMed] [Google Scholar]
- Tsujikawa M, Malicki J. Genetics of photoreceptor development and function in zebrafish. Int J Dev Biol. 2004;48:925–934. doi: 10.1387/ijdb.041890mt. [DOI] [PubMed] [Google Scholar]
- Vanderlaan G, Tyurina OV, Karlstrom RO, Chandrasekhar A. Gli function is essential for motor neuron induction in zebrafish. Dev Biol. 2005;282:550–570. doi: 10.1016/j.ydbio.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Vivancos V, Chen P, Spassky N, Qian D, Dabdoub A, Kelley M, Studer M, Guthrie S. Wnt activity guides facial branchiomotor neuron migration, and involves the PCP pathway and JNK and ROCK kinases. Neural Dev. 2009;4:7. doi: 10.1186/1749-8104-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Iwasaki M, Sato T, Masai I, Nishiwaki Y, Tanaka H, Sato A, Nojima Y, Okamoto H. Dual roles of zygotic and maternal Scribble1 in neural migration and convergent extension movements in zebrafish embryos. Development. 2005;132:2273–2285. doi: 10.1242/dev.01810. [DOI] [PubMed] [Google Scholar]
- Wada H, Tanaka H, Nakayama S, Iwasaki M, Okamoto H. Frizzled3a and Celsr2 function in the neuroepithelium to regulate migration of facial motor neurons in the developing zebrafish hindbrain. Development. 2006;133:4749–4759. doi: 10.1242/dev.02665. [DOI] [PubMed] [Google Scholar]
- Walsh GS, Grant PK, Morgan JA, Moens CB. Planar polarity pathway and Nance-Horan syndrome-like 1b have essential cell-autonomous functions in neuronal migration. Development. 2011;138:3033–3042. doi: 10.1242/dev.063842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. Eugene, OR: University of Oregon; 1995. [Google Scholar]
- Yu X, Ng CP, Habacher H, Roy S. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat Genet. 2008;40:1445–1453. doi: 10.1038/ng.263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.