Abstract
Rationale
Sympathetic nervous system triggered activation of protein kinase A (PKA), which phosphorylates several targets within cardiomyocytes, augments inotropy, chronotropy and lusitopy. An important target of β-adrenergic stimulation is the sarcolemmal L-type Ca2+ channel, CaV1.2, which plays a key role in cardiac excitation-contraction coupling. The molecular mechanisms of β-adrenergic regulation of CaV1.2 in cardiomyocytes, however, are incompletely known. Recently, it has been postulated that proteolytic cleavage at Ala1800 and PKA phosphorylation of Ser1700 are required for β-adrenergic modulation of CaV1.2.
Objectives
To assess the role of Ala1800 in the cleavage of α1C and the role of Ser1700 and Thr1704 in mediating the adrenergic regulation of CaV1.2 in the heart.
Method and Results
Using a transgenic approach that enables selective and inducible expression in mice of FLAG-epitope tagged, dihydropyridine-resistant CaV1.2 channels harboring mutations at key regulatory sites, we show that adrenergic regulation of CaV1.2 current and fractional shortening of cardiomyocytes do not require phosphorylation of either Ser1700 or Thr1704 of the α1C subunit. The presence of Ala1800 and the 1798NNAN1801 motif in α1C are not required for proteolytic cleavage of the α1C C-terminus, and deletion of these residues did not perturb adrenergic-modulation of CaV1.2 current.
Conclusions
These results show that PKA phosphorylation of α1C Ser1700 does not have a major role in the sympathetic stimulation of Ca2+ current and contraction in the adult murine heart. Moreover, this new transgenic approach enables functional and reproducible screening of α1C mutants in freshly isolated adult cardiomyocytes in a reliable, timely and cost-effective manner.
Keywords: Ion channels, molecular electrophysiology, calcium channels, sympathetic nervous system, phosphorylation, adrenergic, transgenic mice, excitation-contraction coupling
INTRODUCTION
Cav1.2 has a key role in cardiac muscle excitation-contraction coupling 1, and in determining the plateau phase of the action potential 2. In pathological conditions, CaV1.2 currents can trigger electrical instability, early after-depolarizations (EADs), arrhythmias, and sudden death frequently in the setting of adrenergic stimulation or decreased repolarizing currents 3, 4. Increased CaV1.2 activity can also lead to Ca2+ overload, which in turn can result in arrhythmogenic delayed after-depolarizations (DADs).
CaV1.2 channels are composed minimally of a pore-forming α1C and regulatory β and α2δ subunits. In the heart, CaV1.2 also associates with large supramolecular complexes that regulate channel trafficking, localization, turnover, and function 5-7. Proteolytic cleavage of the α1C C-terminus, occurring in greater than 80% of cardiac CaV1.2 channels, has been posited to play an essential role in setting the basal activity and enabling the adrenergic stimulation of CaV1.2 8-15.
The molecular mechanisms of β-adrenergic regulation of CaV1.2 in cardiomyocytes are incompletely known. A key obstacle for decades has been the failure to reproducibly reconstitute adrenergic regulation of heterologously expressed CaV1.2. Ser1928, in the α1C subunit, was originally identified as the sole α1C PKA phosphorylation site 11, 16-23. Phosphorylation of this residue, however, is not required for β-adrenergic agonist stimulation of CaV1.2, as shown in guinea pig cardiomyocytes infected with adenovirus expressing a relatively dihydropyridine (DHP)-resistant S1928A-α1C 24, and in α1C S1928A knock-in mice25. Similarly, although β2a Ser459, Ser478 and Ser479 are PKA phosphorylated 26, these sites are not required for β-adrenergic stimulation of CaV1.2 in cardiomyocytes 24, 27, 28. Based upon heterologous expression studies, Ser1700 was recently reported to be the functionally relevant PKA phosphorylation site 15, 29.
Although heterologous expression of CaV1.2 channels has proven useful for investigating biophysical properties, it has not been as successful for exploring physiological modulation, especially as related to cardiomyocytes. Knock-in mice are considered the gold standard, but they are time-consuming and expensive to generate, and a phenotype of heart failure or death during perinatal period may preclude studies at later stages of development 14, 30. Although adenoviruses have been used to express CaV1.2 subunits in cardiomyocytes, creation of adenoviruses encoding α1C is difficult because of the α1C insert size and the cardiomyocytes need to be cultured for extended period, potentially inducing dedifferentiation. Since overexpression of α1C or β subunits reduces the hormonal regulation of the channel 27, 31-33, and can induce cardiac dysfunction or apoptosis 34-37, it is also important to limit the amount of overexpression. To circumvent these problems, which have limited progress in the field, we have developed an approach of using a doxycycline-inducible, tissue-specific, transgenic-mouse-expressing FLAG-epitope-tagged, DHP-resistant α1C. The approach preserves hormonal regulation of Cav1.2 by limiting CaV1.2 over-expression.
Prominent roles for proteolytic cleavage of α1C, at residue Ala1800, and PKA phosphorylation of Ser1700, in the C-terminus of α1C (Fig. 1A), in mediating β-adrenergic-induced enhancement of cardiac CaV1.2 current have been proposed, based upon heterologous expression of CaV1.2 subunits 8, 14, 15. In the absence of proteolytic cleavage at Ala1800, PKA is unable to phosphorylate Ser1700 and upregulate the activity of heterologously expressed CaV1.2 15. Ala-substitution of the neighboring Thr1704, a residue that may be phosphorylated by casein kinase II, reduced heterologously expressed basal CaV1.2 channel activity in unstimulated tsA-201 cells, and when combined with Ala-substitution of Ser1700 more effectively reduced forskolin-induced stimulation of CaV1.2, compared to Ala-substitution of Ser1700 alone. These concepts have not been tested in cardiomyocytes. We tested these predictions in native cardiomyocytes by creating a transgenic mouse expressing three mutations within α1C (S1700A, T1704A, and Δ1798NNAN1801).
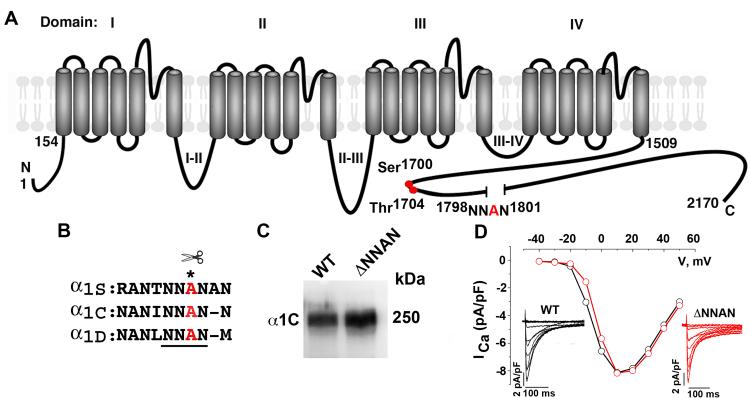
Figure 1. Deletion of proteolytic cleavage site does not affect heterologously expressed CaV1.2 channel expression or function.
(A) Schematic of cardiac α1C subunit topology. The putative proteolytic cleavage site, 1798NNAN1801 is identified. Red circles are putative PKA (Ser1700) and casein kinase II (Thr1704) phosphorylation sites. (B) Highly conserved amino acid sequences surrounding putative proteolytic cleavage site, marked by asterisk. (C) Anti-α1C antibody immunoblot of extracts from WT α1C and ΔNNAN α1C expressing tsA-201 cells. (D) WT (black) and ΔNNAN α1C (red) current-voltage relationships and current traces (inset). Currents elicited by 400-ms test pulses between −60 mV to +60 mV from a holding potential of −70 mV.
METHODS
Reagents
Nisoldipine (Santa Cruz) was dissolved daily in 30 mM ethanol and was protected from light. All other chemicals were acquired from Sigma.
Animals
The pWT α1C and ΔNNAN-S1700A-T1704A constructs were generated by fusing the rabbit CACNA1C cDNA (accession X15539) to the modified murine α-myosin heavy chain (MHC), tetracycline-inducible promoter (“responder” line) vector (gift of Drs. Jeffrey Robbins and Jeffrey Molkentin) 38, 39. A 3X FLAG-epitope was ligated in-frame to the N-terminus of α1C. The α1C subunit was engineered to be DHP-insensitive with the substitutions T1066Y and Q1070M 40, 41. Transgenic founder mice were identified with genomic DNA utilizing polymerase chain reactions. These mice were bred with cardiac specific (αMHC) doxycycline-regulated codon-optimized reverse transcriptional transactivator (rtTA) mice (obtained via MMRRC) 42 to generate double transgenic mice. We selected founder lines that did not express the transgenic α1C in the absence of doxycycline. To induce expression, animals received 0.2 g/kg doxycycline-impregnated food (Bio Serv Cat # S3888) for 1-5 days. The results presented were consistent across all founder lines and gender, and therefore were pooled. The Institutional Animal Care and Use Committee at Columbia University approved all animal experiments.
Immunoblots and immunofluorescence
For immunoblots, cardiomyocytes were isolated 43 from 8-12 week-old non-transgenic and doxycycline-fed transgenic mice. Cardiomyocytes were homogenized in a 1% Triton X-100 buffer containing (in mM): 50 Tris-HCl (pH7.4) 150 NaCl, 10 EDTA, 10 EGTA and protease inhibitors. The lysates were incubated on ice for 30 min, centrifuged at 14K rpm at 4 °C for 10 min and supernatants collected. Proteins were size-fractionated on SDS-PAGE, transferred to nitrocellulose membranes and probed with anti-FLAG (Sigma) or anti-α1C antibodies. Detection was performed with a CCD camera (Carestream Imaging). Image quantification was performed using ImageQuant software. For immunofluorescence, isolated cardiomyocytes were fixed for 15 minutes in 4% paraformaldehyde. Indirect immunofluorescence was performed using a 1:200 rabbit anti-FLAG antibody (Sigma) and 1:200 FITC-labeled goat-anti-rabbit antibody (Sigma). Images were acquired using a confocal microscope.
Cellular electrophysiology
Lipofectamine 2000 (Life Technologies) was used to transfect tsA-201 cells, which were plated onto 12-mm glass coverslips. The experiments were performed 24-48 hours after transfection. The isolated cardiomyocytes 43 and tsA-201 cells were superfused with (in mM) 140 TEA-Cl, 1.8 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES, adjusted to pH 7.4 with CsOH. All experiments were performed at room temperature, 22 ± 1° C. Membrane currents were measured by the whole-cell patch-clamp method using a MultiClamp 700B amplifier (Axon Instruments). The pipette solution contained (in mM) 135 CsCI, 10 EGTA, 1 MgCl2, 2 Mg-ATP, 2.0 CaCl2, and 10 HEPES, adjusted to pH 7.2 with CsOH. Pipette series resistances were usually <1 MΩ after 60% compensation. Leak currents and capacitance transients were subtracted by a P/4 protocol. To measure Ca2+ peak currents, the cell membrane potential was held at −70 mV and stepped to +10 mV for 350 ms every 5-10 seconds. To evaluate the current-voltage relationship for Ca2+ currents, the same protocol was repeated with steps between −50 mV to +50 mV in 10 mV increments.
Fractional shortening
Freshly isolated myocytes were perfused with a Tyrode’s solution containing 1.8 mM CaCl2. Myocytes were field stimulated at 1-Hz. In one series of experiments, nisoldipine (300 nM) was superfused in the absence and presence of isoproterenol (200 nM). In the second series of experiments, cardiomyocytes were placed in a Tyrode’s solution containing 300 nM nisoldipine. A Tyrode’s solution containing 300 nM nisoldipine and 200 nM isoproterenol was superfused. Fractional shortening of sarcomere length was measured using the SarcLen module of Ionoptix.
Statistical analysis
Results are presented as mean ± SEM. For multiple group comparisons, a one-way ANOVA followed by Tukey’s, Sidek’s or Dunnett’s post hoc tests were performed. For comparisons between two groups, an unpaired Student’s t-test was used. Statistical analyses were performed using Prism 6 (Graphpad Software). Differences were considered statistically significant at values of P < 0.05
RESULTS
Generation of inducible, cardiac-specific α1C transgenic mice
We deleted the highly conserved 1798NNAN1801 motif in α1C (Fig. 1B) and co-expressed the cDNA with the β2 subunit in tsA-201 cells. Deletion of this highly conserved region did not affect expression, trafficking to the surface, or the basal electrophysiological characteristics of CaV1.2 (Fig. 1C-D). Since proteolytic cleavage of α1C does not occur when wild-type (WT) α1C is expressed heterologously, the effect of deletion of the putative cleavage site on proteolysis of α1C could not be assessed using this approach (Fig. 1C).
We generated transgenic mice with inducible cardiomyocyte-specific expression of a N-terminal 3X FLAG-epitope-tagged dihydropyridine (DHP)-resistant α1C, designated pseudo-WT, [pWT α1C]) using a bitransgenic tetracycline-regulated system that permits robust expression only when both transgenes, and doxycycline are present (Fig. 2A). The α1C subunit was engineered to be relatively DHP-insensitive with the substitutions T1066Y and Q1070M 40, 41. The IC50 for nisoldipine block of heterologously expressed WT α1C was 12 nM, whereas the IC50 for pWT α1C was 650 nM (Online Fig. I). We selected a concentration of 300 nM nisoldipine as optimal for further experiments since nisoldipine (300 nM) blocked >98% of heterologously expressed WT CaV1.2 current in tsA-201 cells, but only blocked 34.6 ± 2.5% of DHP-insensitive α1C (Online Fig. I).
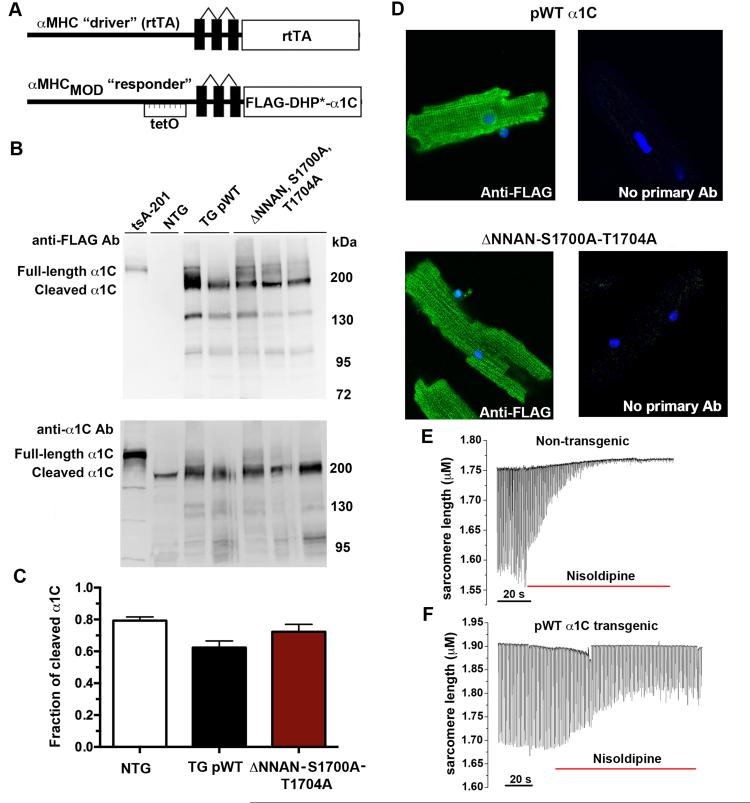
Figure 2. Inducible, cardiac-specific FLAG-tagged α1C-expressing transgenic mice.
(A) Schematic representation of the binary transgene system. The αMHC-rtTA is the standard cardiac-specific reverse tetracycline-controlled transactivator system. The αMHCMOD construct is a modified αMHC promoter containing the tet-operon for regulated expression of FLAG-tagged DHP-resistant (DHP*) α1C. (B) Anti-FLAG antibody (upper) and anti-α1C antibody (lower) immunoblots showing FLAG-epitope tagged α1C expression in tsA-201 cells transfected with FLAG-tagged α1C and expression in isolated cardiomyocytes from either pWT α1C or ΔNNAN-S1700A-T1704A transgenic mice. (C) Bar graph of densitometries of cleaved α1C band divided by truncated + full-length α1C bands. N=4 non-transgenic (NTG) mice; N= 10 pWT α1C mice; N=6 ΔNNAN-S1700A-T1704A mice. P = not significant by Anova. (D) Immunostaining of pWT α1C and ΔNNAN-S1700A-T1704A cardiomyocytes with or without (negative control) anti-FLAG antibody and FITC-conjugated secondary antibody, and nuclear labeling with Hoechst stain. Images obtained with confocal microscope at 40X magnification. (E-F) Time course of changes in sarcomere length after superfusion of nisoldipine (300 nM) containing solution. Cardiomyocytes were field-stimulated at 1-Hz.
Seven pWT α1C founder transgenic lines were originally generated. Two founder lines were lost due to mortality, possibly because of high levels of doxycycline-independent α1C expression. Four founder lines, when crossed with αMHC-rtTA mice, demonstrated doxycycline-induced expression of α1C, assessed by anti-FLAG antibody immunoblots (Fig. 2B, upper; Online Fig II). One transgenic founder line, after crossing with αMHC-rtTA mice, did not demonstrate doxycycline-induced α1C expression. Of 59 pWT α1C bitransgenic mice treated with doxycycline, 18 mice (31%) died within 5 days of doxycycline administration possibly due high levels of doxycycline-dependent α1C expression.
We also generated a transgenic mouse line expressing three mutations within α1C, Ala-substitutions of Ser1700 (S1700A) and Thr1704 (T1704A), and deletion of the 1798NNAN1801 motif (ΔNNAN), in the background of a N-terminal 3X FLAG-epitope tag and DHP-resistance (ΔNNAN-S1700A-T1704A). Six ΔNNAN-S1700-T1704A mutant transgenic founder lines were originally generated. Three founders, when crossed with αMHC-rtTA, demonstrated doxycycline-induced α1C expression (Fig. 2B, upper; Online Fig. II). The other 3 founders, after crossing with α–MHC-rtTA mice, had either no or low levels of doxycycline-induced expression. Of the 35 ΔNNAN-S1700A-T1704A mutant mice treated with doxycycline, 9 mice (26%) died within 5 days, possibly due to high levels of α1C expression. The 41 pWT α1C transgenic mice and the 26 ΔNNAN-S1700A-T1704A mutant transgenic mice form the basis of this study.
Confirming the expression of transgene, immunofluorescence staining of fixed cardiomyocytes from pWT and ΔNNAN-S1700A-T1704A mutant transgenic mice with an anti-FLAG antibody showed a membrane distribution of expressed α1C subunits consistent with t-tubular localization (Fig. 2D). No staining was detected in cardiomyocytes when the anti-FLAG antibody was omitted.
Cardiomyocyte contraction requires Ca2+ influx via CaV1.2, which triggers sarcoplasmic reticulum (SR) Ca2+ release. Superfusion of nisoldipine inhibited the contraction of non-transgenic cardiomyocytes to electric field stimulation at 1-Hz (Fig. 2E). In cardiomyocytes isolated from pWT α1C transgenic mice, the effect of nisoldipine was greatly diminished (Fig. 2F). This indicates that the transgenic channels are correctly localized in the t-tubule and can initiate excitation-contraction coupling.
Proteolytic processing of transgenic channels
Expression of cDNA encoding FLAG-tagged α1C in tsA-201 cells migrated as full-length α1C without evidence of proteolytic processing, detected by immunoblots using anti-FLAG and anti-α1C antibodies (Figs. 1C, 2B). In cardiomyocytes isolated from non-transgenic mice (C57Bl/6), native α1C was detected as a full-length ~240 kDa band and a cleaved ~210 kDa band, using an anti-α1C antibody created against an internal epitope within the intracellular loop of domains II and III. Native α1C in non-transgenic mice cannot be detected using an anti-FLAG antibody (Fig. 2B). Both the pWT α1C transgenic channels and the transgenic channels with a deletion of 1798NNAN1801 were proteolytically cleaved, detected using the anti-FLAG antibody (Fig. 2B, Online Fig. II). The ratios of cleaved to full-length pWT and ΔNNAN transgenic α1C were 62% ± 4% and 72% ± 5% respectively, not significantly different than the 79% ± 5% cleavage of the native α1C (Fig. 2C). Since deletion of the putative proteolytic cleavage site had no effect on the ratio of truncated to full-length α1C in cardiomyocytes, we can conclude that the NNAN motif and Ala1800 are not required for post-translational cleavage of α1C.
Functional, inducible expression of pWT and mutant DHP-insensitive transgenic α1C in cardiomyocytes
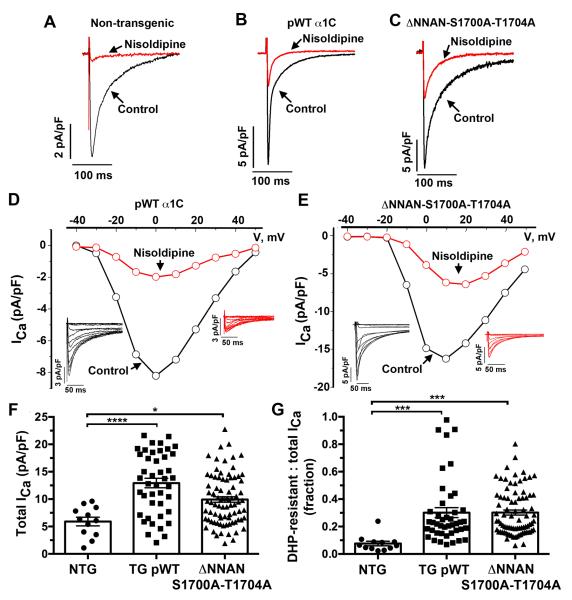
We measured CaV1.2 currents in adult cardiomyocytes from non-transgenic and transgenic mice (Fig. 3A-E). The mean current density was significantly larger in the doxycycline-fed transgenic mice than in the non-transgenic mice (5.9 ± 0.8 pA/pF [n=12] in non-transgenic cardiomyocytes, 12.9 ± 0.9 pA/pF in pWT α1C cardiomyocytes [n=43, P≤0.0001 compared to non-transgenic], and 9.9 ± 0.5 pA/pF in ΔNNAN-S1700A-T1704A mutant cardiomyocytes [n=82, P<0.05 compared to non-transgenic]) (Fig. 3F). Nisoldipine (300 nM) inhibited 92.4% + 1.6% of endogenous peak Ca2+ current in cardiomyocytes isolated from non-transgenic mice (n=12), but 70% + 3.6% of peak current in cardiomyocytes isolated from doxycycline-fed pWT α1C transgenic mice (n= 43, P ≤ 0.001 compared to non-transgenic) and 70% + 1.8% of peak current in the cardiomyocytes isolated from doxycycline-fed ΔNNAN-S1700A-T1704A mutant transgenic mice (n= 82, P ≤ 0.001 compared to non-transgenic). In other words, approximately 30% of the peak current in the cardiomyocytes isolated from doxycycline-treated transgenic mice was insensitive to nisoldipine (Fig. 3G). The voltage dependence of CaV1.2 activation for endogenous, transgenic pWT and ΔNNAN-S1700A-T1704A α1C were equivalent (Fig. 3D-E), implying that at least under basal conditions, the modulation of transgenic CaV1.2 channels by accessory proteins was similar to endogenous CaV1.2 channels.
Figure 3. Dihydropyridine-resistant currents in transgenic mice.
(A-C) Exemplar whole-cell CaV1.2 currents recorded from pulses from −70 mV to +10 mV before (black traces) and 3 minutes after (red traces) of 300 nM nisoldipine. (D-E) Current-voltage relationships of pWT α1C (D) and ΔNNAN-S1700A-T1704A CaV1.2 (E) acquired before (black traces) and 3 minutes after superfusion of 300 nM nisoldipine. Insets: Series of whole-cell CaV1.2 currents recorded from a series of pulses between −40 mV and + 50 mV from a holding potential of −70 mV in the absence of nisoldipine (black traces) and 3 minutes after 300 nM nisoldipine (red traces). (E-F) Combined bar graph and column scatter plot for total peak current density (pA/pF) and peak DHP-resistant current density (pA/pF). Bar graphs are mean + SEM. ***P< 0.0001, *** P<0.001, * P<0.05 by one-way Anova and Sidak’s post-hoc test.
Adrenergic-modulation of CaV1.2 in WT α1C transgenic mice
In cardiomyocytes isolated from non-transgenic mice, we measured the effects of the β-adrenergic agonist, isoproterenol, in the presence of nisoldipine. Isoproterenol (200 nM) increased the small amount of residual CaV1.2 current by a mean of 2.5 ± 0.2- fold (Fig. 4A, F). Other groups have shown a similar response to isoproterenol stimulation in adult murine cardiomyocytes, with a range of 1.6 to 2.8-fold increase in basal currents 25, 28, 32, 44, 45.
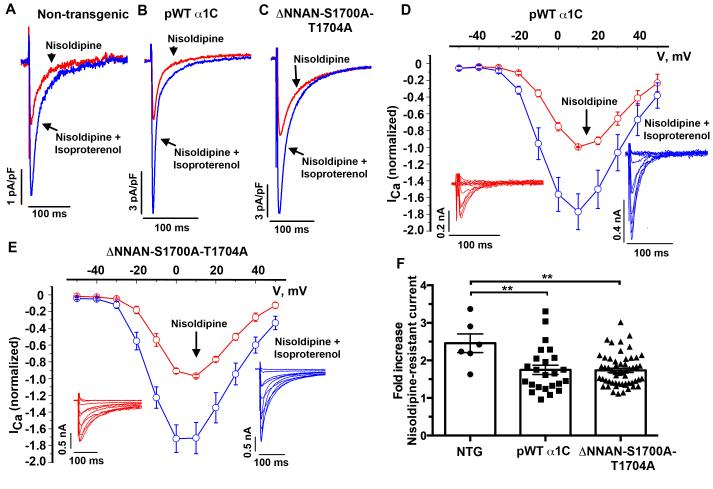
Figure 4. β-adrenergic stimulation of CaV1.2 current does not require phosphorylation of Ser1700.
(A-C) Exemplar whole-cell CaV1.2 currents recorded from pulses from −70 mV to +10 mV before (red traces) and 3 minutes after (blue traces) superfusion of 200 nM isoproterenol, in the presence of nisoldipine. (D-E) Ca2+ current-voltage relationships before (red trace) and after (blue trace) 200 nM isoproterenol, in the presence of 300 nM nisoldipine in cardiomyocytes isolated from pWT α1C (N=4) and ΔNNAN-S1700A-T1704A mice (N=8). Mean ± SEM. Insets: Series of whole-cell CaV1.2 currents recorded from a series of pulses between −50 mV to +50 mV from a holding potential of −70 mV in the presence of nisoldipine, before (red trace) and 3 minutes after (blue trace) 200 nM isoproterenol. (F) Combined bar and column scatter plot depicting the fold increase in peak current caused by isoproterenol. Bar graphs are mean + SEM. **P<0.01 by Anova and Tukey’s post-hoc test. N= 6 non-transgenic cardiomyocytes, N= 24 pWT α1C, N= 56 ΔNNAN-S1700A-T1704A cardiomyocytes.
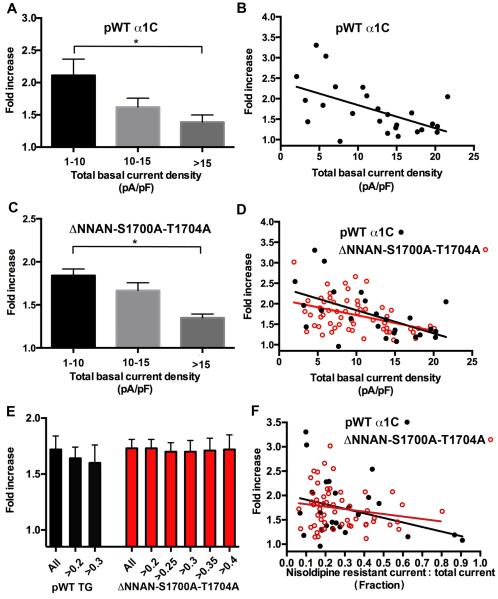
In the cardiomyocytes isolated from pWT α1C transgenic mice, isoproterenol increased the nisoldipine-insensitive peak current by a mean of 1.7 ± 0.1-fold (Fig. 4B, D, F) (P ≤ 0.01 compared to non-transgenic). In cardiomyocytes with a basal current density before nisoldipine of less than 10 pA/pF, which is similar to the basal current density of cardiomyocytes from non-transgenic mice, isoproterenol increased CaV1.2 currents by 2.1 ± 0.3-fold (Fig. 5A) (P=not significant compared to non-transgenic). In cardiomyocytes with peak CaV1.2 currents greater than 15 pA/pF, in contrast, isoproterenol increased CaV1.2 currents by only 1.4 ± 0.1-fold (Fig. 5A) (P<0.05). Across the broad range of basal current densities, the effect of isoproterenol on the nisoldipine-resistant current was inversely correlated with the basal total CaV1.2 current (Fig. 5B). The diminished adrenergic-modulation of the transgenic pWT CaV1.2 current compared to endogenous CaV1.2 is likely due to the increased basal CaV1.2 current density in the transgenic cardiomyocytes. Cardiomyocytes may have a limited number of permissive sites on the membrane where PKA-mediated upregulation of CaV1.2 current can occur and channels in excess of this limited number may be less responsive to β-adrenergic stimulation, thereby diluting the overall fold-increase in CaV1.2 currents 27.
Figure 5. Analysis of isoproterenol’s effects on transgenic Ca2+ currents.
(A, C) Bar graphs of isoproterenol-induced increase in nisoldipine-resistant current binned by total basal current density before nisoldipine for pWT α1C (A) and ΔNNAN-S1700A-T1704A transgenic mice (C). Mean ± SEM. * P<0.05 by Anova and Tukey’s post hoc test. For pWT α1C, N= 9 cardiomyocytes for 1-10 pA/pF, N=9 cardiomyocytes for 10-15 pA/pF, N=8 cardiomyocytes for >15 pA/pF. For ΔNNAN-S1700A-T1704A, N= 33 cardiomyocytes for 1-10 pA/pF, N=17 for 10-15 pA/pF, N=6 for >15 pA/pF. (B, D) Graphs of isoproterenol-induced increase in nisoldipine-resistant current stratified by total basal current density before nisoldipine for pWT α1C (B), and pWT α1C and ΔNNAN-S1700A-T1704A transgenic mice (D). Lines fitted by linear regression. The differences between the slopes and intercepts of pWT α1C and ΔNNAN-S1700A-T1704A are not significant. (E) Bar graph of isoproterenol-induced increase in nisoldipine-resistant current binned by fraction of nisoldipine-resistant current for pWT α1C and ΔNNAN-S1700A-T1704A mice. Mean ± SEM. P= not significant. (F) Graph of isoproterenol-induced increase in nisoldipine-resistant current stratified by fraction of nisoldiipine-resistant current for pWT α1C and ΔNNAN-S1700A-T1704A mice. The differences between the slopes and intercepts of pWT α1C and ΔNNAN-S1700A-T1704A are not significant.
Phosphorylation of Ser1700 and Thr1704 are not required for isoproterenol- and forskolin-induced stimulation of CaV1.2 currents
Freshly isolated cardiomyocytes were isolated from doxycycline-treated ΔNNAN-S1700A-T1704A transgenic mice. In the presence of nisoldipine, isoproterenol increased peak CaV1.2 current by a mean of 1.7 ± 0.1-fold, identical to the isoproterenol-induced augmentation of current in pWT α1C transgenic cardiomyocytes (P= not significant, pWTα1C vs. ΔNNAN-S1700A-T1704A) (Fig. 4C, E-F). In the presence of nisoldipine, forskolin increased peak CaV1.2 current by a mean of 1.9 ± 0.1-fold increase in cardiomyocytes isolated from the ΔNNAN-S1700A-T1704A mice, nearly identical to the 1.8 ± 0.1-fold increase in pWT α1C cardiomyocytes (Online Fig. III).
Similar to the pWT α1C transgenic mice, the magnitude of isoproterenol-induced increase in nisoldipine-insensitive Ca2+ current was inversely correlated with the basal total CaV1.2 current (Fig. 5C, D). The slopes and intercepts of the two linear regression lines describing the relationship of total basal current density and response to isoproterenol of pWT and ΔNNAN-S1700A-T1704A α1C were not statistically different (Fig. 5D). In cardiomyocytes with a total basal CaV1.2 current density less than 10 pA/pF, isoproterenol caused a 1.8 ± 0.1-fold increase in CaV1.2 current (P= not significant compared to pWT TG α1C). In cardiomyocytes with basal CaV1.2 current density greater than 15 pA/pF, isoproterenol increased CaV1.2 currents by 1.3 ± 0.1 fold (P= not significant compared to pWT α1C). Stratifying the magnitude of the isoproterenol effect by the fraction of nisoldipine-resistant current also demonstrated that the increase in Ca2+ current was equivalent for WT α1C and ΔNNAN-S1700A-T1704A α1C (Fig. 5E-F). Thus, phosphorylation of Ser1700 or Thr1704 is not required for isoproterenol or forskolin-induced modulation of CaV1.2 current.
Isoproterenol-induced modulation of fractional shortening is preserved in cardiomyocytes isolated from ΔNNAN-S1700A-T1704A mutant mice
We incubated cardiomyocytes for at least 2 minutes in the superfusion solution containing 300 nM nisoldipine, in order to ensure that all cardiomyocytes were exposed to nisoldipine, In non-transgenic cardiomyocytes, >95% of the cardiomyocytes failed to contract to electric field stimulation at 1-Hz, and in the remaining cardiomyocytes, contraction was reduced by 80% (IVFig. 4A-B). Isoproterenol increased the fractional shortening of the myocytes by 1.5-fold, both in the absence and presence of 300 nM nisoldipine (Online Fig. IVA-B). The cardiomyocytes isolated from both FLAG-tagged pWT and FLAG-tagged ΔNNAN-S1700A-T1704A DHP-resistant transgenic mice were relatively resistant to the effects of nisoldipine (Online Fig. IVA, C). Greater than 90% of cardiomyocytes demonstrated sustained contraction to electric field stimulation at 1-Hz. Isoproterenol increased the fractional shortening of myocytes, in the presence of nisoldipine, in both pWT and ΔNNAN-S1700A-T1704A transgenic lines by 1.6 and 1.7-fold respectively (Online Fig. IVA, C). Thus, phosphorylation of either Ser1700 or Thr1704 is not required for β-adrenergic modulation of excitation-contraction coupling in murine cardiomyocytes.
DISCUSSION
In this study, we have developed an approach to efficiently and reliably probe molecular aspects of CaV1.2 regulation within the context of freshly isolated cardiomyocytes, approximating the ease and power of a heterologous expression system. In prior studies, overexpression of α1C or β subunits markedly reduced the β-adrenergic regulation of the channel, and induced cardiac dysfunction or apoptosis 31, 32, 34-36. To circumvent these problems, we created inducible, tissue-specific, transgenic-mice expressing, DHP-resistant, FLAG-epitope-tagged α1C. This approach preserves hormonal regulation of CaV1.2 by limiting its over-expression. The channels containing the transgenic α1C are transported appropriately to the dyad and can initiate excitation-contraction coupling.
Using this newly developed approach, we now show that β-adrenergic regulation of cardiac CaV1.2 channels is unaltered by Ala-substitution of Ser1700 or Thr1704, indicating that these sites are dispensable for this purpose in adult cardiomyocytes. Ser1700 was recently reported to be the functionally relevant PKA site in heterologously expressed CaV1.2 15, 29. Phosphorylation of Thr1704, a consensus site for casein kinase II, increases the basal activity of heterologously expressed CaV1.2 15. It may also play a role in adrenergic-modulation of CaV1.2, since forskolin-induced stimulation of heterologously expressed CaV1.2 was more attenuated with the double mutant S1700A-T1704A than for S1700A alone 15. Although Ser1928 is PKA phosphorylated 11, 16-23, it is not required for β-adrenergic stimulation of CaV1.2 24,25, and forskolin-induced stimulation of the heterologously expressed triple mutant S1700A-T1704A-S1928A was not different than the double mutant S1700A-T1704A 15. It is based upon these experiments 15 that we chose the S1700A-T1704A mutations for testing in transgenic mice. We were unable to assess the role of Thr1704 on basal activity in cardiomyocytes, however, since the basal activity of heterologously expressed CaV1.2 was determined by comparing the coupling efficiency of pore opening to gating charge movement 15.
β-adrenergic stimulation of CaV1.2 currents is robust in doxycycline-regulated transgenic mice
The isoproterenol-induced increase in current in the cardiomyocytes from transgenic mice is similar to previously reported studies. Schwartz and colleagues reported that isoproterenol (100 nM) induced a 1.7 ± 0.2-fold increase in peak Ca2+ current, but only a 1.2 ± 0.1-fold increase in cardiomyocytes isolated from α1C over-expressing transgenic mice 32. Moosmang and Hofmann reported that isoproterenol (100 nM) increased peak current by 1.9 ± 0.25-fold in WT mice and 1.8 ± 0.25 fold in mice with a deletion of the β-subunit C-terminus at Pro501 28. McKnight, Santana and Catterall reported an approximate 2-fold increase in CaV1.2 currents by isoproterenol (100 nM) in WT and AKAP5 knock-out mice 44. Chen and Houser reported that isoproterenol increased CaV1.2 currents in WT mice by 1.6-fold, but isoproterenol did not increase the current amplitude in transgenic mice overexpressing the β2a subunit 45. Thus, by limiting over-expression of α1C and only inducing expression of α1C for 1-5 days, we have developed a highly reliable system that can accurately and efficiently report the functional effects of mutations.
A new approach to study the regulation of CaV1.2 in cardiomyocytes
Although useful for investigating biophysical properties, heterologous expression systems have not been successful for exploring physiological modulation, especially as related to cardiomyocytes 14, 27, 30, 46. Compared to creating a knock-in mouse, expressing transgenic DHP-resistant α1C mutants in the heart is rapid and cost-effective, and multiple sites within α1C can be mutated at one time, regardless of intron/exon boundaries. There are, however, drawbacks using the approach. Transgenic expression naturally increases the basal current density, potentially disrupting normal stoichiometry and regulation. In the case of β-adrenergic modulation of CaV1.2, the magnitude of β-adrenergic stimulation is reduced with increased basal current density. Reducing the dynamic range of modulation could theoretically minimize the effects of the mutations on β-adrenergic regulation of CaV1.2. Stratifying the magnitude of β-adrenergic-mediated upregulation of CaV1.2 current by total basal current density attenuates this confounding variable.
With or without stratification by basal current density, we found that acute β-adrenergic stimulation of CaV1.2 is not significantly altered by Ala-substitution of Ser1700, implying that phosphorylation of Ser1700 is not the primary mechanism for β-adrenergic regulation of CaV1.2. Could phosphorylation of Ser1700 play a small, secondary role in mediating β-adrenergic regulation of CaV1.2, especially under conditions of relatively low basal current density at which the effect of β-adrenergic stimulation is greatest? At low basal current density, the mean increase in current for cardiomyocytes isolated from pWT α1C transgenic mice was 2.11 ± 0.25-fold, whereas for cardiomyocytes from the ΔNNAN-S1700A-T1704A transgenic mice, the mean increase was 1.84 ± 0.25-fold, a non-significant relative difference of 13%. In this low basal current density group, the current density of the cardiomyocytes from the ΔNNAN-S1700A-T1704A transgenic mice was slightly higher than pWT α1C transgenic mice (6.5 pA/pF vs. 5.5 pA/pF), which may have contributed to the slightly lower increase β-adrenergic stimulation in the ΔNNAN-S1700A-T1704A transgenic mice.
Assuming 7% of endogenous current is not blocked by nisoldipine (Fig. 2) and 65% of DHP-insensitive transgenic channels are not blocked by nisoldipine (Online Fig. I), the maximal contamination of nisoldipine-resistant currents by endogenous channels would be ~8% at 40% nisoldipine-resistant current to total current and ~14% at 30% nisoldipine-resistant current to total current (See Online Methods). At 40% fractional nisoldipine resistance in the cardiomyocytes isolated from ΔNNAN-S1700A-T1704A mice, the effects of β-adrenergic stimulation are identical to cardiomyocytes isolated from pWT α1C mice (Fig. 5E). Taken together, these findings imply that phosphorylation of Ser1700 and Thr1704 cannot be the primary mechanism by which β-adrenergic agonists activate CaV1.2 in the adult cardiomyocytes.
Proteolytic cleavage does not require the conserved motif 1798NNAN1801
Since proteolytic cleavage cannot be reconstituted in heterologous expression, there is no effective way to study the process, other than in native tissues. Indirect evidence, consisting of mass spectrometric analysis of the skeletal muscle α1S proteolytic peptides and sequence alignments of α1S and α1C, was used to identify Ala1800 as the putative proteolytic site in α1C 8. Deletion of Ala1800 and the immediately adjacent conserved residues did not alter the proteolytic cleavage of α1C, suggesting that either Ala1800 is not the site in cardiomyocytes or that there is redundancy. Within the region, there are other similar motifs including 1794NANI1797, which would combine with Asn1802 after 1798NNAN1801 is deleted to form a 1794NANIN motif. Whether cleavage could occur at Ala1795 in ΔNNAN transgenic mouse is a question for future study.
In summary, we have developed an approach to reproducibly and efficiently test informative mutants of CaV1.2 in cardiomyocytes using a transgenic mouse approach. By limiting over-expression of the CaV1.2 α1C subunit, we can reliably assess sympathetic regulation of CaV1.2. These data demonstrate that phosphorylation of Ser1700 and Thr1704 are not the primary mechanisms mediating β-adrenergic modulation of both Ca2+ current and excitation-contraction coupling in adult cardiomyocytes.
Supplementary Material
Novelty and Significance.
What Is Known?
The L-type Ca2+ channel (Cav1.2) plays a key role in cardiac excitation-contraction coupling and it is an important target of the sympathetic nervous system.
It has been suggested that proteolytic cleavage of α1C, at residue Ala1800 and protein kinase A (PKA) phosphorylation of Ser1700 mediate β–adrenergic-induced enhancement of cardiac CaV1.2 current, but these concepts have not been tested in cardiomyocytes
Although heterologous expression of CaV1.2 channels has proven useful for investigating biophysical properties, it has not been as successful for exploring physiological modulation, especially as related to cardiomyocytes.
What New Information Does This Article Contribute?
Selective and inducible expression in mice of FLAG-epitope tagged, dihydropyridine (DHP)-resistant CaV1.2 channels harboring mutations at key regulatory sites can be used to assess the properties of α1C mutants in freshly isolated adult cardiomyocytes
Adrenergic regulation of CaV1.2 current and fractional shortening of cardiomyocytes do not require phosphorylation of either Ser1700 or Thr1704 of the α1C subunit
Deletion of 1798NNAN1801, the previously proposed cleavage site, does not prevent distal α1C C-terminus proteolysis.
Excitation-contraction coupling is controlled in part through the precise regulation of Ca2+ influx by several neurohormonal and second-messenger systems, including the β-adrenergic/PKA signaling pathway; however, the molecular mechanisms of β-adrenergic regulation of CaV1.2 in cardiomyocytes are incompletely understood. .A key obstacle has been the failure to reproducibly reconstitute adrenergic regulation in heterologously expressed CaV1.2. To circumvent this problem, we used doxycycline-inducible, cardiac-specific, transgenic-mice-expressing FLAG-epitope-tagged, DHP-resistant α1C. In this system, we examined the proposed roles of proteolytic cleavage of α1C, at residue Ala1800, and PKA phosphorylation of Ser1700 in mediating β-adrenergic-induced enhancement of cardiac CaV1.2 current. In addition, we tested these predictions in native cardiomyocytes by creating a transgenic mouse expressing three mutations within α1C (S1700A, T1704A, and Δ1798NNAN1801). We found that in cardiomyocytes, the NNAN motif is not required for cleavage of α1C, and that Ser1700 and Thr1704 are not required for the β-adrenergic modulation of both Ca2+ current and excitation-contraction coupling.
Acknowledgments
SOURCES OF FUNDING This work was supported by NHLBI R01 68093, R01HL113136, an American Heart Association Founder’s Affiliate Grant-in-Aid, and the Arlene and Arnold Goldstein Family Foundation. T.S. was supported by a fellowship from the Sarnoff Cardiovascular Research Foundation. JPM is supported by NHLBI K08 105801. RLW is supported by NHLBI T32 HL007854.
Nonstandard Abbreviations and Acronyms
- Ala
alanine
- DHP
dihydropyridine
- DAD
delayed after-depolarization
- EAD
early after-depolarization
- MHC
myosin heavy chain
- PKA
protein kinase A
- pWT
pseudo-wild-type
- rtTA
reverse transcriptional transactivator
- Ser
serine
- TG
transgenic
- WT
wild-type
Footnotes
DISCLOSURES None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fabiato A, Fabiato F. Calcium and cardiac excitation-contraction coupling. Annu Rev Physiol. 1979;41:473–484. doi: 10.1146/annurev.ph.41.030179.002353. [DOI] [PubMed] [Google Scholar]
- 2.Beeler GW, Jr., Reuter H. Membrane calcium current in ventricular myocardial fibres. J Physiol. 1970;207:191–209. doi: 10.1113/jphysiol.1970.sp009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marban E, Robinson SW, Wier WG. Mechanisms of arrhythmogenic delayed and early afterdepolarizations in ferret ventricular muscle. J Clin Invest. 1986;78:1185–1192. doi: 10.1172/JCI112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.January CT, Riddle JM. Early afterdepolarizations: Mechanism of induction and block. A role for l-type ca2+ current. Circ Res. 1989;64:977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- 5.Catterall WA. Structure and regulation of voltage-gated ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 6.Bodi I, Mikala G, Koch SE, Akhter SA, Schwartz A. The l-type calcium channel in the heart: The beat goes on. J Clin Invest. 2005;115:3306–3317. doi: 10.1172/JCI27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulme JT, Konoki K, Lin TW, Gritsenko MA, Camp DG, 2nd, Bigelow DJ, Catterall WA. Sites of proteolytic processing and noncovalent association of the distal c-terminal domain of cav1.1 channels in skeletal muscle. Proc Natl Acad Sci U S A. 2005;102:5274–5279. doi: 10.1073/pnas.0409885102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Jongh KS, Warner C, Colvin AA, Catterall WA. Characterization of the two size forms of the alpha 1 subunit of skeletal muscle l-type calcium channels. Proc Natl Acad Sci U S A. 1991;88:10778–10782. doi: 10.1073/pnas.88.23.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Jongh KS, Merrick DK, Catterall WA. Subunits of purified calcium channels: A 212-kda form of alpha 1 and partial amino acid sequence of a phosphorylation site of an independent beta subunit. Proc Natl Acad Sci U S A. 1989;86:8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the alpha 1 subunit of the cardiac l-type calcium channel by adenosine 3′,5′-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 12.Gao T, Puri TS, Gerhardstein BL, Chien AJ, Green RD, Hosey MM. Identification and subcellular localization of the subunits of l-type calcium channels and adenylyl cyclase in cardiac myocytes. J Biol Chem. 1997;272:19401–19407. doi: 10.1074/jbc.272.31.19401. [DOI] [PubMed] [Google Scholar]
- 13.Hulme JT, Yarov-Yarovoy V, Lin TW, Scheuer T, Catterall WA. Autoinhibitory control of the cav1.2 channel by its proteolytically processed distal c-terminal domain. J Physiol. 2006;576:87–102. doi: 10.1113/jphysiol.2006.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Westenbroek RE, Yu FH, Clark JP, 3rd, Marshall MR, Scheuer T, Catterall WA. Deletion of the distal c terminus of cav1.2 channels leads to loss of beta-adrenergic regulation and heart failure in vivo. J Biol Chem. 2011;286:12617–12626. doi: 10.1074/jbc.M110.175307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal. 2010;3:ra70. doi: 10.1126/scisignal.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitterdorfer J, Froschmayr M, Grabner M, Moebius FF, Glossmann H, Striessnig J. Identification of pka phosphorylation sites in the carboxyl terminus of l-type calcium channel alpha 1 subunits. Biochemistry. 1996;35:9400–9406. doi: 10.1021/bi960683o. [DOI] [PubMed] [Google Scholar]
- 17.Perets T, Blumenstein Y, Shistik E, Lotan I, Dascal N. A potential site of functional modulation by protein kinase a in the cardiac ca2+ channel alpha 1c subunit. FEBS Lett. 1996;384:189–192. doi: 10.1016/0014-5793(96)00303-1. [DOI] [PubMed] [Google Scholar]
- 18.Gao T, Yatani A, Dell’Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. Camp-dependent regulation of cardiac l-type ca2+ channels requires membrane targeting of pka and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 19.Hall DD, Davare MA, Shi M, Allen ML, Weisenhaus M, McKnight GS, Hell JW. Critical role of camp-dependent protein kinase anchoring to the l-type calcium channel cav1.2 via a-kinase anchor protein 150 in neurons. Biochemistry. 2007;46:1635–1646. doi: 10.1021/bi062217x. [DOI] [PubMed] [Google Scholar]
- 20.Hall DD, Feekes JA, Arachchige Don AS, Shi M, Hamid J, Chen L, Strack S, Zamponi GW, Horne MC, Hell JW. Binding of protein phosphatase 2a to the l-type calcium channel cav1.2 next to ser1928, its main pka site, is critical for ser1928 dephosphorylation. Biochemistry. 2006;45:3448–3459. doi: 10.1021/bi051593z. [DOI] [PubMed] [Google Scholar]
- 21.Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW. A beta2 adrenergic receptor signaling complex assembled with the ca2+ channel cav1.2. Science. 2001;293:98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- 22.Hell JW, Yokoyama CT, Breeze LJ, Chavkin C, Catterall WA. Phosphorylation of presynaptic and postsynaptic calcium channels by camp-dependent protein kinase in hippocampal neurons. EMBO J. 1995;14:3036–3044. doi: 10.1002/j.1460-2075.1995.tb07306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hulme JT, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation of serine 1928 in the distal c-terminal domain of cardiac cav1.2 channels during beta1-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103:16574–16579. doi: 10.1073/pnas.0607294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganesan AN, Maack C, Johns DC, Sidor A, O’Rourke B. Beta-adrenergic stimulation of l-type ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of alpha1c but not serine 1928. Circ Res. 2006;98:e11–18. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemke T, Welling A, Christel CJ, Blaich A, Bernhard D, Lenhardt P, Hofmann F, Moosmang S. Unchanged beta-adrenergic stimulation of cardiac l-type calcium channels in ca v 1.2 phosphorylation site s1928a mutant mice. J Biol Chem. 2008;283:34738–34744. doi: 10.1074/jbc.M804981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerhardstein BL, Puri TS, Chien AJ, Hosey MM. Identification of the sites phosphorylated by cyclic amp-dependent protein kinase on the beta 2 subunit of l-type voltage-dependent calcium channels. Biochemistry. 1999;38:10361–10370. doi: 10.1021/bi990896o. [DOI] [PubMed] [Google Scholar]
- 27.Miriyala J, Nguyen T, Yue DT, Colecraft HM. Role of cavbeta subunits, and lack of functional reserve, in protein kinase a modulation of cardiac cav1.2 channels. Circ Res. 2008;102:e54–64. doi: 10.1161/CIRCRESAHA.108.171736. [DOI] [PubMed] [Google Scholar]
- 28.Brandmayr J, Poomvanicha M, Domes K, Ding J, Blaich A, Wegener JW, Moosmang S, Hofmann F. Deletion of the c-terminal phosphorylation sites in the cardiac beta-subunit does not affect the basic beta-adrenergic response of the heart and the ca(v)1.2 channel. J Biol Chem. 2012;287:22584–22592. doi: 10.1074/jbc.M112.366484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emrick MA, Sadilek M, Konoki K, Catterall WA. Beta-adrenergic-regulated phosphorylation of the skeletal muscle ca(v)1.1 channel in the fight-or-flight response. Proc Natl Acad Sci U S A. 2010;107:18712–18717. doi: 10.1073/pnas.1012384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domes K, Ding J, Lemke T, Blaich A, Wegener JW, Brandmayr J, Moosmang S, Hofmann F. Truncation of murine cav1.2 at asp-1904 results in heart failure after birth. J Biol Chem. 2011;286:33863–33871. doi: 10.1074/jbc.M111.252312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beetz N, Hein L, Meszaros J, Gilsbach R, Barreto F, Meissner M, Hoppe UC, Schwartz A, Herzig S, Matthes J. Transgenic simulation of human heart failure-like l-type ca2+-channels: Implications for fibrosis and heart rate in mice. Cardiovasc Res. 2009;84:396–406. doi: 10.1093/cvr/cvp251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muth JN, Yamaguchi H, Mikala G, Grupp IL, Lewis W, Cheng H, Song LS, Lakatta EG, Varadi G, Schwartz A. Cardiac-specific overexpression of the alpha(1) subunit of the l-type voltage-dependent ca(2+) channel in transgenic mice. Loss of isoproterenol-induced contraction. J Biol Chem. 1999;274:21503–21506. doi: 10.1074/jbc.274.31.21503. [DOI] [PubMed] [Google Scholar]
- 33.Groner F, Rubio M, Schulte-Euler P, Matthes J, Khan IF, Bodi I, Koch SE, Schwartz A, Herzig S. Single-channel gating and regulation of human l-type calcium channels in cardiomyocytes of transgenic mice. Biochem Biophys Res Commun. 2004;314:878–884. doi: 10.1016/j.bbrc.2003.12.174. [DOI] [PubMed] [Google Scholar]
- 34.Tang M, Zhang X, Li Y, Guan Y, Ai X, Szeto C, Nakayama H, Zhang H, Ge S, Molkentin JD, Houser SR, Chen X. Enhanced basal contractility but reduced excitation-contraction coupling efficiency and beta-adrenergic reserve of hearts with increased cav1.2 activity. Am J Physiol Heart Circ Physiol. 2010;299:H519–528. doi: 10.1152/ajpheart.00265.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, Berretta R, Potts ST, Marsh JD, Houser SR. Ca2+ influx-induced sarcoplasmic reticulum ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Nakayama H, Zhang X, Ai X, Harris DM, Tang M, Zhang H, Szeto C, Stockbower K, Berretta RM, Eckhart AD, Koch WJ, Molkentin JD, Houser SR. Calcium influx through cav1.2 is a proximal signal for pathological cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2011;50:460–470. doi: 10.1016/j.yjmcc.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S, Ziman B, Bodi I, Rubio M, Zhou YY, D’Souza K, Bishopric NH, Schwartz A, Lakatta EG. Dilated cardiomyopathy with increased sr ca2+ loading preceded by a hypercontractile state and diastolic failure in the alpha(1c)tg mouse. PLoS One. 2009;4:e4133. doi: 10.1371/journal.pone.0004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res. 2003;92:609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- 39.Hambleton M, York A, Sargent MA, Kaiser RA, Lorenz JN, Robbins J, Molkentin JD. Inducible and myocyte-specific inhibition of pkcalpha enhances cardiac contractility and protects against infarction-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H3768–3771. doi: 10.1152/ajpheart.00486.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He M, Bodi I, Mikala G, Schwartz A. Motif iii s5 of l-type calcium channels is involved in the dihydropyridine binding site. A combined radioligand binding and electrophysiological study. J Biol Chem. 1997;272:2629–2633. doi: 10.1074/jbc.272.5.2629. [DOI] [PubMed] [Google Scholar]
- 41.Hockerman GH, Peterson BZ, Sharp E, Tanada TN, Scheuer T, Catterall WA. Construction of a high-affinity receptor site for dihydropyridine agonists and antagonists by single amino acid substitutions in a non-l-type ca2+ channel. Proc Natl Acad Sci U S A. 1997;94:14906–14911. doi: 10.1073/pnas.94.26.14906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valencik ML, McDonald JA. Codon optimization markedly improves doxycycline regulated gene expression in the mouse heart. Transgenic Res. 2001;10:269–275. doi: 10.1023/a:1016601928465. [DOI] [PubMed] [Google Scholar]
- 43.O’Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 44.Nichols CB, Rossow CF, Navedo MF, Westenbroek RE, Catterall WA, Santana LF, McKnight GS. Sympathetic stimulation of adult cardiomyocytes requires association of akap5 with a subpopulation of l-type calcium channels. Circ Res. 2010;107:747–756. doi: 10.1161/CIRCRESAHA.109.216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W, Zhang H, Gao H, Kubo H, Berretta RM, Chen X, Houser SR. {beta}1-adrenergic receptor activation induces mouse cardiac myocyte death through both l-type calcium channel-dependent and -independent pathways. Am J Physiol Heart Circ Physiol. 2010;299:H322–331. doi: 10.1152/ajpheart.00392.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meissner M, Weissgerber P, Londono JE, Prenen J, Link S, Ruppenthal S, Molkentin JD, Lipp P, Nilius B, Freichel M, Flockerzi V. Moderate calcium channel dysfunction in adult mice with inducible cardiomyocyte-specific excision of the cacnb2 gene. J Biol Chem. 2011;286:15875–15882. doi: 10.1074/jbc.M111.227819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.