Abstract
This study determined effects of community-based adapted tango upon spatial cognition and disease severity in Parkinson’s disease (PD) while controlling for the effects of social interaction. Thirty-three individuals with mild-moderate PD (stage I–III) were assigned to twenty, 90-minute Tango (n=24) or Education (n=9) lessons over 12 weeks. Disease severity, spatial cognition, balance, and fall incidence were evaluated pre-, post-, and 10–12 weeks post-intervention. T-tests and ANOVAs evaluated differences. Twenty-three Tango and 8 Education participants finished. Tango participants improved on disease severity (p=0.008), and spatial cognition (p=0.021) compared to Education participants. Tango participants also improved in balance (p=0.038), and executive function (p=0.012). Gains were maintained 10–12 weeks post-intervention. Multimodal exercise with structured syllabi may improve disease severity and spatial cognition.
Keywords: dance, spatial cognition, balance, Parkinson’s disease, exercise
Introduction
The costs of Parkinson’s disease (PD) are formidable (more than $34 billion/year) and increasing (Noyes, Liu, Li, Holloway, & Dick, 2006). Difficulty turning and dual-tasking, postural instability, and gait impairment rob individuals with PD of their quality of life (QOL) (Muslimovic, Post, Speelman, Schmand, & de Haan, 2008). Individuals with PD also commonly experience cognitive impairment in domains of spatial cognition (Possin, Filoteo, Song, & Salmon, 2008), and executive function (Hausdorff et al., 2006), which further affects mobility, increasing fall risk (Camicioli & Majumdar, 2010). PD impairment-targeted exercise programs may improve motor function (Kadivar, Corcos, Foto, & Hondzinski, 2011), reduce fall rates (Protas et al., 2005) and improve cognition (Cruise et al., 2011).
A program of Argentine tango dance (adapted tango), adapted for PD motor impairments, has improved balance, gait, and QOL, in comparison to other ballroom dances, non-partnered dancing and PD-tailored exercise programs (Hackney & Earhart, 2009b, 2010a; Hackney, Kantorovich, & Earhart, 2007; Hackney, Kantorovich, Levin, & Earhart, 2007). However, adapted tango’s effects upon spatial cognition, which is the knowledge involving interrelationships of people, objects and space (Devlin, 2001), have not previously been examined.
Because spatial cognition is supported by brain structures particularly vulnerable to normal cerebral aging, (Klencklen, Despres, & Dufour, 2012) it is especially vulnerable to the neurodegenerative processes occurring in PD (Possin, 2010). Spatial cognition is critical for forming cognitive maps of spatial relationships used in the successful navigation of dynamic environments, and therefore underlies mobility and orientation (Klencklen et al., 2012). Thus, unique therapies tailored to enhance spatial function in individuals with PD are necessary. Tango dancing involves consistent attention to spatial relationships of posture, step patterns, the partner, and the path traveled within the room. Tango might offer a cogent opportunity for learning coordinate relationships between people, places and objects, which is crucial for encoding spatial information in memory. Potentially, tango could aid spatial cognition in those with PD because the dance requires participants to learn, store in memory, recall, use, and be cognizant of spatial postures, relationships, patterns, and paths.
Little research has investigated effects of non-pharmacologic therapies for enhancing cognition in those with PD (Hindle, Petrelli, Clare, & Kalbe, 2013). Tanaka and colleagues demonstrated an improvement on executive function in PD as a result of 180 minutes per week of multimodal (primarily aerobic) training (Tanaka et al., 2009), —a dose which also meets recommendations that older adults exceed 150 minutes of weekly exercise (Chodzko-Zajko et al., 2009). Here we implemented this 180-minute dose in the form of twenty, 90-minute biweekly sessions of adapted tango for individuals with PD. This dose, not examined in prior studies of adapted tango, was designed to increase learning time for individuals with PD to overcome well-documented motor learning challenges (Stephan, Meier, Zaugg, & Kaelin-Lang, 2011), which many participants have claimed to face in previous studies (Hackney & Earhart, 2010b). Additionally, given the retained yet slowed ability to learn experienced by those with PD (Nieuwboer, Rochester, Muncks, & Swinnen, 2009), the increased learning time may be particularly important to the encoding, storage, and recall processes related to learning spatial relationships. To be able to rule out the effects of social engagement and group learning as responsible for changes in cognition, mobility and disease severity, we assigned participants to either an interactive health education lecture series (Education) control group or adapted tango (Tango).
This study aimed to examine the effects of Adapted Tango on spatial cognition as well as disease severity in individuals with PD while controlling for social interaction. Primary end points were spatial cognition, executive function, disease severity, and everyday fall incidence, mobility and QOL. We also examined gain retention 10–12 weeks post for the first time. We hypothesized that if improvement in spatial cognition is related to the learning, storage and recall of paths, body postures, steps and spatial relationships, then those in adapted tango would improve more than those in Education in spatial cognition. We also expected that the Tango group would improve more than Education on disease severity and fall incidence, mobility and QOL, with retention of gains at 10–12 weeks post.
Methods
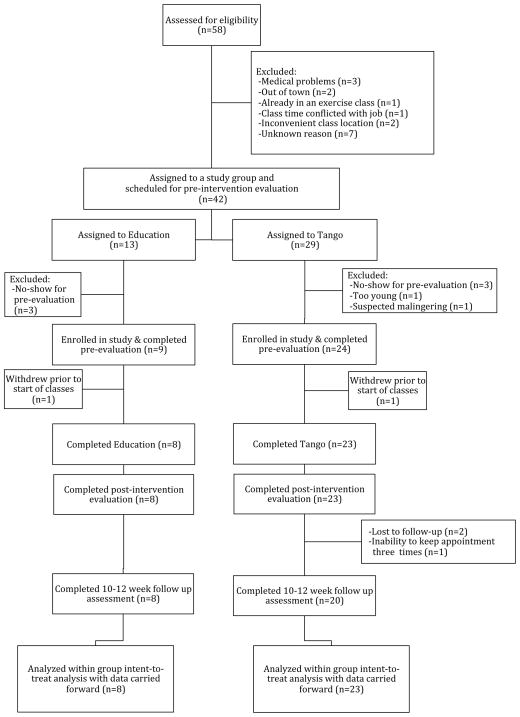
The Institutional Review Board at Emory University School of Medicine approved this work. Participants provided written informed consent before participating. Figure 1 describes participation throughout the trial.
Figure 1.
Consort Diagram of Participation.
Participants
Participants were recruited through flyers, referral, PD newsletters, support groups, and websites. Thirty-four participants with a diagnosis of idiopathic “definite PD” (Racette, Rundle, Parsian, & Perlmutter, 1999), and who had demonstrated benefit from anti-parkinsonian medication were recruited. Participants (Hoehn and Yahr (H&Y) stages I–III) had no history of other neurological insult, were 50 years or older, and could walk 3+ meters with or without assistance. Four Education and six Tango participants reported habitual use of an assistive device such as a cane or walker. At the first visit, to characterize participants, they underwent measures of disease severity, depression, the ability to perform ADLs, cognitive impairment, and fall risk with standardized measures: the Unified Parkinson’s Disease Rating Scale motor subscale III (UPDRS-III) (Fahn & Elton, 1987), Beck Depression Inventory-II (BDI-II) (Beck, Steer, Ball, & Ranieri, 1996; Silberman et al., 2006), Composite Physical Function Index (CPF) (Rikli & Jones, 2001), Montreal Cognitive Assessment (MoCA) (Chou et al., 2010), and Berg Balance Scale (BBS) (Berg, Wood-Dauphinee, & Williams, 1995) (Table 1).
Table 1.
Baseline Participant Demographics & Characteristics
| Lecture Series n=9 | Tango n=24 | pa | |
|---|---|---|---|
| Sex | 1 F; 8 M | 12 F; 12 M | 0.056* |
| UPDRS Motor Subscale-III | 27.2 (7.0) | 28.1 (6.9) | 0.747 |
| Hoehn & Yahr Stage (Median (1st, 3rd Quartiles)) | 2.0 (2.0, 2.0) | 2.3 (2.0, 2.6) | 0.438 |
| Age (years) | 74.4 (6.5) | 68.4 (7.5) | 0.041 |
| Duration of PD (years) | 7.2 (4.9) | 7.0 (5.5) | 0.900 |
| Education (years) | 16.4 (1.3) | 16.5 (2.1) | 0.941 |
| Freezer (#yes/#no) | 3/6 | 8/16 | 1.000* |
| Number of Comorbidities | 3.1 (1.2) | 3.3 (1.7) | 0.775 |
| Number of Prescription Medications | 7.8 (6.6) | 5.4 (4.5) | 0.343^ |
| Use of Assistive Device (#yes/#no) | 4/5 | 6/18 | 0.3999* |
| Hx of ≥1 falls in Past Year (#yes/#no) | 5/4 | 15/9 | 1.000* |
| Fear of Falling§ | 3.1 (1.4) | 3.0 (1.5) | 0.907 |
| Quality of Life§ | 5.1 (1.1) | 5.2 (0.9) | 0.752 |
| Beck Depression Inventory-II (/63) | 12.1 (9.6) | 12.1 (5.5) | 0.992 |
| Composite Physical Function (/24) | 20.2 (4.7) | 20.3 (4.9) | 0.971 |
| Montreal Cognitive Assessment (/30) | 26.0 (2.8) | 24.9 (3.3) | 0.384 |
| Berg Balance Scale (/56) | 48.1 (13.2) | 51.1 (4.6) | 0.325 |
Unless otherwise noted, values are given in means and standard deviations (M (SD)). Abbreviations: PD, Parkinson’s Disease; UPDRS, Unified Parkinson’s Disease Rating Scale; Hx, History; SD, Standard Deviation.
Independent t-tests compared groups.
Fishers Exact Test.
Equal variance not assumed.
Rating worry about falling / quality of life from 1 (low) to 7 (high).
Outcome Measures
Participants underwent 3 evaluations: within 1 week before (pre-test), 1 week after (post-test) and 10–12 weeks after (follow-up) Education or Tango classes. Participants were tested “ON” medications at a participant-preferred time of day to reduce medication-related performance fluctuations. Trained raters administered measures according to standard procedures. All assessments were videotaped for blinded ratings by third year doctorate of physical therapy students and a medical student. During the study (6 months total), participants were instructed not to change habitual exercise routines and were provided with an event calendar to record adverse events, including any event that could be considered a “fall”, i.e., a slip or trip, in which they unexpectedly lost their balance and landed on the ground or a lower level. Every two weeks, staff reminded participants to use their calendars. At post and follow-up evaluations, investigators reviewed calendars and questioned participants about medical/exercise changes and any reported adverse events.
Primary Endpoints
Cognitive measures included: 1) the MoCA (Chou et al., 2010)—an assessment of global cognition/executive function, 2) the Reverse Corsi Blocks (Kessels, van den Berg, Ruis, & Brands, 2008)—a visuospatial memory assessment, and 3) the Brooks Spatial Task (Brooks) (Brooks, 1967)—a spatial cognition task using mental imagery. The Brooks task required participants to remember the placement of numbers on a verbally-described 4×4 matrix, i.e., participants were asked to visualize the numbers in their mind and then repeat the location of the numbers to the rater. Participants began with a span of three instructions (the placement of 3 numbers) and progressed up to 8 instructions (8 numbers). All levels were completed regardless of performance on preceding levels. The percentages correct (out of 50 items) were considered for analyses. Because the Brooks has been rarely used previously in individuals with PD, correlations were conducted, which demonstrated validity between Brooks and the MoCA (r=0.72) and Corsi Blocks (r=0.61). The Brooks is stable over a one month period in those with PD (n=7, ICC=0.721, p=0.015, unpublished data).
Standard measures of disease severity, mobility, and balance included: UPDRS-III (Fahn & Elton, 1987), Fullerton Advanced Balance Scale (FAB) (Hernandez & Rose, 2008; Klein, Fiedler, & Rose, 2011; Rose, Lucchese, & Wiersma, 2006), Four-Square Step Test (Dite & Temple, 2002), Single/Dual timed up and go (Shumway-Cook, Brauer, & Woollacott, 2000) with single task (TUG), dual-cognitive (Cognitive-TUG: counting backward by 3s from a randomly generated number between 20 and 100) and dual-manual (Manual-TUG: carrying full cup of water) conditions and everyday fall incidence outside of class. Because the BBS is prone to ceiling effects in higher functioning community dwelling older adults (Pardasaney et al., 2012) and screening this sample with the BBS revealed these participants were relatively high functioning in balance, the more challenging FAB was used to increase sensitivity to detect changes in higher functioning individuals. Because the FAB has rarely been used in individuals with PD, correlations were conducted between the BBS and the FAB, which demonstrated validity (r=0.78).
Psychosocial questionnaires included: PD Questionnaire-39 (Peto, Jenkinson, Fitzpatrick, & Greenhall, 1995), Freezing of Gait Questionnaire (FOGQ) (Giladi et al., 2000), and Short Form health survey-12 (SF-12) including the Physical (PCS) and Mental Composite (MCS) scales (Ware, Kosinski, & Keller, 1996). Using the FOGQ, participants were classified as freezers if they scored > 1 on item 3 (Giladi et al., 2000), indicating freezing of more than once per week (Nieuwboer et al., 2007).
Secondary Endpoints
Secondary endpoints included adverse events during class and participant attrition, which were tracked by a trained medical student. To assess participant satisfaction, an exit questionnaire, cited previously (Hackney & Earhart, 2009a, 2010a; Hackney, Hall, Echt, & Wolf, 2013) was administered at post-testing only.
Intervention
Participants were assigned to twenty, 90-minute lessons in Tango or Education that took place at two senior independent living communities. Participants were not allowed to attend more than 20 classes and were instructed not to participate in Tango or Education seminars between the conclusion of their 20 lessons and their 10–12 week follow-up visit. Additionally, for safety purposes and to control Tango dose, Tango participants were instructed throughout the study not to practice Tango outside of class at their home or other location. Due to scheduling and physical constraints of the senior community living centers hosting the programs, Education started one week earlier than Tango and was limited in its enrollment as the available space could comfortably accommodate at most 10 individuals with PD. Therefore, recruitment occurred as such: participants were recruited and assigned sequentially first to Education and then to Tango. Class instructors and moderators for both Tango and Education were not involved in participant recruitment, screening, assessment, or class-assignment.
Tango
Nine dance instructors without clinical qualifications participated in a 12-hour workshop on adapted tango methods, PD-specific motor impairments, and fall prevention, followed by an additional 3 hours of individual training from the senior author. Teachers were given an adapted tango manual, prepared for this study from former work about a 20h program (Hackney & Earhart, 2010b) as well as a 30h program (Hackney, Hall, Echt, & Wolf, 2011; Hackney et al., 2013). The manual delineates: PD motor impairments, fall risk and prevention, partnering enhancement and rhythmic entrainment, and a 20-class syllabus and format (Hackney et al., 2011, 2013). Teacher-trainees had 2–25 years of experience teaching dance and >1 year working with older adults in a fitness/dance setting. Based upon their demonstrated proficiency with safety concepts and adapted tango methods, four teacher-trainees were selected to teach Tango.
Tango classes strictly followed the manual’s syllabus (Hackney & Earhart, 2010b; Hackney et al., 2011, 2013), which presents step-progression on a specific timeline. Classes began with practice of previously learned steps and a 20-minute standing warm-up followed by partnering and rhythmic enhancement exercises. Next, novel step elements were introduced and amalgamated to previously learned steps. Everyone lead and followed all step patterns with multiple partners. Participants with PD always danced with individuals without PD: caregivers, friends, and relatives as well as several graduate and undergraduate student volunteers.
Volunteers received training in human research, falls detection/prevention, and adapted tango methods. Volunteers were initially partnered with individuals at low fall risk (as per their BBS score (greater than 46 points) and Hoehn and Yahr staging (2 or better)), and observed for proficiency before partnering individuals at higher fall risk. In efforts to maximize safety through appropriate pairings, investigators identified participants at high fall risk to the teachers prior to the first class. The senior author and a trained medical student monitored adherence to the syllabus on a near-daily basis. Adverse events during class, and participant attrition were tracked through records corroborated by adapted tango teachers and the medical student.
Education
Highly diverse health-related topics were delivered in a seminar designed to encourage extensive interaction and socialization. In 90-minute sessions, medical students and professors from local universities delivered one hour of lecture/discussion followed by one half hour of partnered and group learning through structured activities, question and answers, and further discussion. These seminars, on physical, mental and social wellbeing as well as contemporary scientific advances, included some PD-related information, and were moderated by a graduate student and several undergraduate students. Adverse events and participant attrition were tracked through careful records maintained by the moderators.
Statistical Analysis
Descriptive statistics were calculated. Independent samples t-tests compared groups. Fisher’s exact test compared groups for categorical variables. The last data point was carried forward for all participants who did not complete all evaluations, including those who had only baseline measures. Outcome measures were compared with 2×3 repeated measures (RM) analyses of variance (ANOVAs) with two groups (Education and Tango) as the between factor and three time-points (pre, post, and follow-up) as the within factor. RM multivariate ANOVAs were used for psychosocial measures. Pairwise comparisons examined changes within groups over time, when main effects were detected. Because the study was underpowered to determine all main effects, some planned comparisons within groups were conducted on main effects of time. To determine validity of novel measures, Pearson correlations were calculated between: FAB and BBS and between the Brooks task and both MoCA and Corsi. The significance level was α=0.05; however, in order to control the rate of incorrectly rejected null hypotheses, the False Discovery Rate (FDR) method was used in a procedure that corrected for multiple comparisons. Given 14 outcome measures, the new significance level for the ANOVAs was set at p<0.027. For post-hoc tests examining within-group changes from pre to post and from post to follow-up, the FDR correction was p<0.038, given the two tests performed. Analyses were performed using SPSS (version 19).
Results
Thirty-three participants enrolled (Tango, n=24; Education, n=9). Twenty-three Tango and 8 Education participants completed 20 lessons. Figure 1 describes participation throughout the trial. There were no significant differences between groups related to measures of PD (duration of PD, UPDRS, H&Y Staging, freezing status), but Education was slightly older than Tango (Table 1), therefore age was used as a covariate in analyses. These participants experienced mild-moderate PD, had completed post-secondary education, experienced minimal to mild depression, were mildly afraid of falling, had generally high quality of life and were relatively high functioning in completing ADLs. Table 2 lists performance on motor, cognitive and psychosocial measures for both groups.
Table 2.
Motor, Cognitive & Psychosocial Measures
| Education | Tango | |
|---|---|---|
| Motor and Disease Severity
| ||
| Unified Parkinson’s Disease Rating Scale -motor subscale III*a (/108) | ||
| Pre | 27.2 (7.0) [18.5–40.5] | 28.1 (6.9) [9.0–41.0] |
| Post | 29.3 (7.5) [19.0–41.0] | 24.0 (7.9) [11.0–38.0] |
| Follow-up | 29.5 (6.7) [19.5–41.0] | 25.9 (7.5) [7.0–41.0] |
| Fullerton Advanced Balance Scale (/40) | ||
| Pre | 28.6 (3.7) [23.0–34.0] | 28.7 (5.2) [21.0–36.0] |
| Post | 29.8 (4.6) [23.0–36.0] | 31.3 (4.5) [21.0–38.0] |
| Follow-up | 28.6 (3.2) [23.0–33.0] | 30.4 (6.1) [14.0–38.0] |
| Timed Up and Go Baseline (s) | ||
| Pre | 9.5 (1.7) [7.6–12.7] | 10.1 (3.1) [6.7–19.3] |
| Post | 10.5 (1.7) [9.0–14.0] | 10.3 (3.2) [6.4–19.3] |
| Follow-up | 10.1 (1.4) [8.1–12.8] | 10.3 (3.6) [6.2–19.9] |
| Timed Up and Go Cognitive (s) | ||
| Pre | 13.4 (2.4) [10.3–17.6] | 14.6 (8.1) [6.9–42.6] |
| Post | 14.7 (3.4) [9.8–19.8] | 14.3 (7.4) [7.4–42.6] |
| Follow-up | 12.5 (2.0) [9.1–14.3] | 14.4 (8.3) [7.0–42.6] |
| TUGc Correct Response Rate (correct numbers given/total numbers given/s) | ||
| Pre | 0.065 (0.03) [0.054–0.097] | 0.081 (0.03) [0.025–0.144] |
| Post | 0.071 (0.02) [0.051–0.102] | 0.074 (0.03) [0.031–0.135] |
| Follow-up | 0.076 (0.01) [0.060–0.097] | 0.080 (0.04) [0.017–0.143] |
| Timed Up and Go Manual (s) | ||
| Pre | 12.5 (1.5) [10.4–15.0] | 12.8 (4.1) [7.5–25.5] |
| Post | 14.2 (2.6) [11.1–19.1] | 13.7 (5.1) [7.1–26.8] |
| Follow-up | 13.5 (1.8) [11.6–16.4] | 13.3 (4.7) [7.4–25.5] |
| Four Square^ (s) | ||
| Pre | 12.1 (2.0) [9.1–15.3] | 13.2 (5.1) [8.3–28.0] |
| Post | 11.8 (1.7) [8.9–13.7] | 13.2 (5.4) [7.9–27.9] |
| Follow-up | 10.1 (1.7) [8.1–11.5] | 11.5 (5.8) [6.2–28.9] |
|
| ||
| Cognitive
| ||
| Brooks Spatial Task* (% correct) | ||
| Pre | 65.3 (12.1) [48.0–86.0] | 60.4 (20.4) [4.0–90.0] |
| Post | 65.3 (14.4) [46.0–88.0] | 66.8 (21.0) [4.0–96.0] |
| Follow-up | 60.4 (19.3) [36.0–90.0] | 68.4 (18.2) [16.0–96.0] |
| Montreal Cognitive Assessment^ (/30) | ||
| Pre | 26.0 (2.8) [21.0–29.0] | 24.9 (3.3) [20.0–30.0] |
| Post | 26.3 (2.3) [22.0–30.0] | 26.1 (3.3) [17.0–30.0] |
| Follow-up | 27.4 (2.5) [22.0–30.0] | 26.3 (3.9) [16.0–30.0] |
| Corsi span (/9) | ||
| Pre | 4.0 (0.7) [3.0–5.0] | 4.4 (1.4) [2.0–8.0] |
| Post | 4.4 (0.5) [4.0–5.0] | 4.8 (1.4) [3.0–9.0] |
| Follow-up | 4.7 (1.0) [3.0–6.0] | 4.5 (1.4) [3.0–8.0] |
|
| ||
| Psychosocial
| ||
| Freezing of Gaita (/24) | ||
| Pre | 7.1 (6.6) [1.0–20.0] | 5.4 (5.1) [0.0–14.0] |
| Post | 5.7 (4.1) [1.0–14.0] | 4.7 (4.2) [0.0–14.0] |
| Follow-up | 6.2 (4.7) [1.0–16.0] | 5.5 (4.6) [0.0–15.0] |
| SF-12 Physical Composite Scoreb (/100) | ||
| Pre | 41.5 (9.3) [28.2–55.4] | 42.2 (11.0) [17.0–58.3] |
| Post | 41.8 (8.8) [30.6–54.3] | 40.4 (11.2) [17.0–54.1] |
| Follow-up | 40.8 (11.5) [23.2–57.0] | 41.3 (12.6) [9.2–59.9] |
| SF-12 Mental Composite Scoreb (/100) | ||
| Pre | 45.1 (15.2) [16.5–62.1] | 50.0 (8.5) [33.0–65.7] |
| Post | 50.4 (11.3) [31.1–64.1] | 50.7 (7.3) [34.5–65.7] |
| Follow-up | 50.5 (12.3) [31.1–64.7] | 50.0 (9.1) [31.6–68.4] |
| Parkinson’s Disease Questionnaire 39-Summary Indexa (/100) | ||
| Pre | 18.3 (10.0) [8.9–42.2] | 19.3 (9.6) [7.6–42.6] |
| Post | 17.7 (10.2) [7.7–38.0] | 19.5 (11.1) [2.5–44.0] |
| Follow-up | 19.3 (8.1) [10.7–37.0] | 18.7 (9.4) [4.3–40.2] |
Values presented as mean (standard deviation) [range].
Significant group by time interaction, p< 0.05
Significant main effect of time, p< 0.05
Lower score indicates less symptomatology.
Higher score indicates greater physical / mental well-being.
Cognition
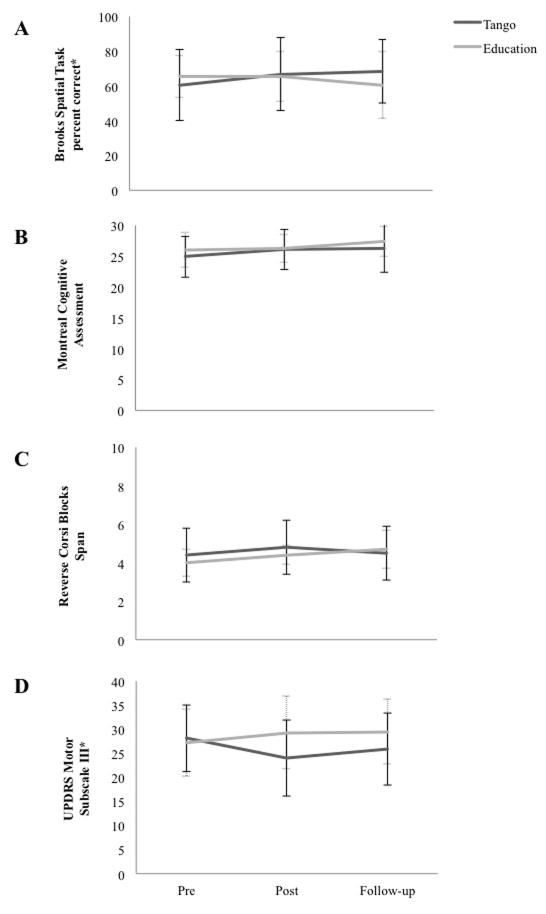
There was a group x time interaction on the Brooks (F(2,60)=4.131, p=0.021, ηp2=0.121) (Figure 2A). Tango improved (F(2,22)=5.457, p=0.012), between pre and post (p=0.017), and maintained at follow-up (p=0.484); whereas Education did not (F(2,7)=0.620, p=0.565). There was a main effect of time in the MoCA (F(2,62)=4.753, p=0.012, ηp2=0.133), but no group x time interaction (Figure 2B). There was a trend toward an effect of time on Corsi Blocks (F(2,62)=2.874, p=0.064, ηp2=0.085), but no group x time interaction (Figure 2C).
Figure 2.
Between-Group Differences in Measures of Cognition and Disease Severity. Values plotted are means ± standard deviations at pre, post, and follow-up. Values for Education are shown in grey with dashed and capped error bars, and values for Tango are shown in black with black and capped error bars. *Denotes a significant group by time interaction, p< 0.05. (A) Brooks Spatial Task Percentage Correct*. (B) Montreal Cognitive Assessment. (C) Reverse Corsi Blocks span. (D) Unified Parkinson’s Disease Rating Scale-Motor Subscale III*.
Disease Severity and Motor
There was a group x time interaction on the UPDRS-III (F(2,60)=5.297, p=0.008, ηp2=0.148) (Figure 2D). Tango improved (F(2,22)=4.919, p=0.017) between pre and post (p=0.017) and maintained at follow-up (p=0.364); whereas Education showed a trend towards increased disease severity over time (F(2,7)=4.249, p=0.062). There was a main effect of time on the FAB (F(2,56)=3.463, p=0.038, ηp2=0.120). Planned comparisons revealed Tango improved on the FAB from pre to post (p=0.004) and maintained gains at follow-up (p=0.195); whereas Education did not (p=0.265) (Table 2). There was a main effect of time on the Four Square Step Test (F(2,56)=4.438, p=0.031, ηp2=0.137). Planned comparisons revealed no significant changes in either group as a result of time (Tango: p=0.196, Education: (p=0.134). There were no main effects of time on the TUG, (F(2,58)=0.750, p=0.477, ηp2=0.025), Cognitive-TUG (F(2,58)=1.145 p=0.325, ηp2=0.038) or Manual-TUG (F(2,58)=1.937 p=0.164, ηp2=0.063). There were no main effects of time or group on Correct Response Rate of the counting backwards by 3s task during the Cognitive-TUG (p>0.05). (Table 2)
Psychosocial
On the PDQ39-SI, FOG, PCS and MCS, there were no main effects (F(8,112)=0.662; p=0.724, ηp2=0.045) (Table 2).
Everyday Falls
In this sample, 47 falls were reported in the year before the study, 14 falls occurred outside of class time during the 3 months interventional period, and 28 falls during the 10–12 weeks before the follow-up evaluation. The number of fallers versus non-fallers was not significantly different between groups. Tango was 1.42 times (CI 0.02–111.5, φ=0.066, p=0.724) more likely than Education to experience decreased or no change in fall incidence during the follow-up period compared to the year before the program. Among participants who improved balance, 66% of Tango and 50% of Education experienced decreased or no change in fall incidence.
Secondary Endpoints
Safety: Fall Incidence within Class
Two non-injurious falls—involving two self-reported frequent fallers—occurred out of 48 offered tango classes. After each incident the involved participant was eager to resume dancing in the same class session, after 10–15 minutes mandated rest. Root cause analysis determined reasons for falls that occurred and systematic changes were implemented to prevent future events. No falls occurred during Education classes.
Attrition/Participant Satisfaction
Attrition rates were 11% for Education and 0% for Tango. The Exit Questionnaire showed both groups strongly agreed they enjoyed the program. Tango strongly agreed they would continue and noted balance, walking, mood, coordination, strength, and endurance improvements. Both groups agreed they were more physically and mentally active. There were no statistically significant differences between groups.
Discussion
Twenty-three individuals with mild-moderate PD participated in 30 hours of adapted tango and improved in spatial cognition and disease severity in comparison to an Education control group. Improvements in disease severity and mobility were comparable to prior studies (Duncan & Earhart, 2012; Hackney & Earhart, 2009a, 2009b, 2010a). Here, with an Education control, we have demonstrated that partnered, social interaction and group learning are improbably responsible for gains. Importantly, evaluations at 10–12 weeks post, a previously uninvestigated interval, demonstrated gain-retention. Despite 50% higher dosage compared to former work, participant satisfaction for Education and Tango was high (Figure 2) and attrition was low.
Tango’s improvement and retention of gains on the Brooks spatial test, may suggest that Tango enhances cognition. While social interaction and group learning can play an important role in gains from rehabilitation (Glass, de Leon, Marottoli, & Berkman, 1999), the significant improvement in Tango compared to Education, another highly interactive and social group activity, suggests motor training inherent to adapted tango could underlie observed changes. This study’s design does not permit the demonstration that elements unique to Tango were responsible for gains observed in spatial or other cognitive domains. The possibility exists that cognitive gains noted in Tango occurred because aerobic exercise has beneficial effects upon cognition (Kraft, 2012; Ratey & Loehr, 2011). Tango could be considered light-moderate exercise because participants are stepping at 60–120 beats/minute (typical tempi of tango music) and expending at least 3 Metabolic Equivalent of Task (METs) per minute during typical tango dancing (Heyward, 2010). Further, the novel dose —30 hours over 10–12 weeks—may have affected cognitive and motor function merely because participants exceeded weekly exercise dosage recommendations for deconditioned older adults with chronic illness (Chodzko-Zajko et al., 2009). However, prior work has demonstrated Tango’s improvement upon PD mobility and QOL versus other partnered dances’ steps and structure (Hackney & Earhart, 2009a), non-partnered dance (Hackney & Earhart, 2010a) and generalized exercise (Hackney, Kantorovich, Levin, et al., 2007), while here partnered/social learning and interaction have been shown to be improbably responsible for gains. Thus, adapted tango elements, including structured motor components that engage memory of steps and directions whilst encouraging keen awareness of spatial relationships, could have contributed through some currently undetermined mechanism, to gains in cognition. Further study into application of therapies like adapted tango for improving cognition is warranted and definitely needed (Hindle et al., 2013) given the prevalence of mild cognitive impairment in PD, which affects the spectrum of mental function.
Through the Exit questionnaire, the program satisfaction instrument, both Education and Tango reported high satisfaction with classes, and indicated spheres related to QOL were enhanced. However, no significant changes were observed in psychosocial function in either group, potentially because, similar to motor domains, the sample demonstrated high baseline psychosocial functioning. Both groups began and remained in the average range for healthy individuals without PD on measures of physical (SF-12 PCS) and mental (SF-12 MCS) well-being (Ware et al., 1996). This sample’s global QOL (PDQ39-SI) was also substantially better than that typically noted in individuals with mild-to-moderate PD (Damiano et al., 2000).
Within Tango, we observed little change in fall incidence outside class. However, other exercise studies for those with PD (Allen, Sherrington, Paul, & Canning, 2011; Goodwin et al., 2011; Tomlinson et al., 2012) and older adults (Faber, Bosscher, Chin, & van Wieringen, 2006; Nowalk, Prendergast, Bayles, D’Amico, & Colvin, 2001; Rosendahl, Gustafson, Nordin, Lundin-Olsson, & Nyberg, 2008; Wolf et al., 2003) have also reported little or no change in fall incidence. Nevertheless, similar to findings from institution-based studies (Duncan & Earhart, 2012; Hackney & Earhart, 2009b; Hackney, Kantorovich, Levin, et al., 2007), a minimal clinically important difference (CID) (between 2.3 and 2.7 points) (Shulman et al., 2010) in disease severity was noted on the motor subscale of the UPDRS for Tango (maintained at follow-up). While this change was small, it may impact the ability of an individual with PD to perform functional activities. Furthermore, the CID for the UPDRS has been used to evaluate drug therapy and weigh the risks and benefits of an intervention. The low side-effect profile of adapted tango compared with many of the drugs noted to have achieved a CID could be a compelling reason for clinicians to “prescribe” adapted tango therapy for individuals with PD. Finally, Tango demonstrated balance improvement on the FAB—also noted in individuals with PD who participated in modern dance (Batson, 2010). Although clinical significance of FAB changes cannot currently be determined, the FAB scale allowed greater sensitivity to detect balance changes in higher functioning participants, who would have reached a ceiling with the BBS—on which improvements were previously noted (Hackney & Earhart, 2009a, 2010a; Hackney, Kantorovich, Levin, et al., 2007). The participant makeup of this study highlights the fact that therapies targeting higher functioning individuals in earlier stages of PD are imperative (Hirsch, 2009). While gains in balance and correspondingly, fall risk, are encouraging, further research to improve exercise programming to decrease fall incidence is needed.
It must be emphasized that fall occurrence within exercise classes for individuals with PD is a real possibility (Li et al., 2012) and therefore, must be anticipated. In this study, though 2 falls occurred during Tango, no injuries resulted and participants continued therapy. The results and risks observed in this study are generalizable only for the specific PD population characterized (in Table 1) and studied here. While other populations may benefit from Tango as well, the PD population identified here is appropriate for and may benefit from the adapted tango intervention.
Limitations
The study was limited by small sample size, particularly in Education. A slight age difference between those in Education and Tango may have impacted outcomes; however, age was co-varied, and the groups were well matched on disease severity, duration with and stage of PD, factors which likely affect motor and cognitive function more. Also, participants’ knowledge of their class assignment and any potential bias towards one treatment or another may have impacted performance on outcome measures. A major limitation is that some raters were not blinded to class assignment at post-testing. The two treatments took place at two comparable, but nevertheless different community living centers because of scheduling and space constraints. Education began one week earlier than Tango and spaces in the Education program were filled before those in Tango. These personnel, location and scheduling differences may have affected treatment effectiveness and enthusiasm, in currently unknown ways. Further, this study’s findings can only be generalized to outcome performance while participants are ON medications. Future studies must evaluate the effects of adapted tango while participants are OFF medications. Some evidence implies that those with Right-side versus Left-side dominant disease are differentially affected by impairments of specific cognitive domains, (Verreyt, Nys, Santens, & Vingerhoets, 2011) which suggests that further research with respect to side of disease onset and treatment outcomes is necessary. This study’s outcomes should be considered in light of hypothesis testing and effect size generation, for adequately powering future, larger studies that will more definitively determine the effects adapted tango may have on cognition, as well as mobility.
Conclusions
Currently, few therapies have been indicated to specifically target and benefit aspects of cognition in those with PD (Hindle et al., 2013). Here, findings of enhanced cognition with retention of gains at 10–12 weeks post in those who participated in tango are therefore, encouraging. Further, because exercise may serve as a disease modifying intervention (Ahlskog, 2011), potentially preventive therapies like adapted tango should be promoted in higher-functioning individuals, perhaps even slowing disease progression, as noted here. Although traditional exercise programs suffer more than 30% attrition (Jancey et al., 2007), successful models for PD exercise programs now exist (Corcos et al., 2013). When examined in future, larger randomized controlled trials, the adapted tango program, with already encouraging compliance rates, could benefit from methodology employed in these successful PD exercise programs. Adapted tango with high retention and demonstrated efficacy should be pursued as a potential means for improving PD motor and cognitive impairments and possibly slowing disease progression.
Acknowledgments
We would like to thank the teachers, Ronda and Manuel Patino, and Gabriela Lopez of Tango Rio, and Erik Renz. We also acknowledge volunteers Allison Bascas, Marco Coelho, Dabin Choi, Carly DiLeo, Margaret Fang, Dana Heyl, Kevin Huang, Twinkle Mehta, Malije Obi, Mina Taheb, Diana Tiwari, Kedra Woodard. We acknowledge Medlock Gardens retirement community for providing space for the adapted tango workshop, Clairmont Oaks retirement community and Wesley Woods Towers for providing space for the interventions and we thank the administrative staff of each institution. We acknowledge Dr. William De L’Aune for statistical assistance. The Dan and Merrie Boone Foundation, the Emory Center for Injury Control, and the Emory Center for Health in Aging supported the study. This study was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Emory School of Medicine Discovery Program supported KE McKee, and a Department of VA R&D Service Career Development Award (E7108M) supported ME Hackney.
References
- Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77(3):288–294. doi: 10.1212/WNL.0b013e318225ab66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NE, Sherrington C, Paul SS, Canning CG. Balance and falls in Parkinson’s disease: a meta-analysis of the effect of exercise and motor training. Mov Disord. 2011;26(9):1605–1615. doi: 10.1002/mds.23790. [DOI] [PubMed] [Google Scholar]
- Batson G. Feasibility of an Intensive Trial of Modern Dance for Adults with Parkinson Disease. Complementary Health Practice Review. 2010;15(2):65–83. [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Berg K, Wood-Dauphinee S, Williams JI. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med. 1995;27(1):27–36. [PubMed] [Google Scholar]
- Brooks LR. The suppression of visualization by reading. Q Exp J Psychol. 1967;19(4):289–299. doi: 10.1080/14640746708400105. [DOI] [PubMed] [Google Scholar]
- Camicioli R, Majumdar SR. Relationship between mild cognitive impairment and falls in older people with and without Parkinson’s disease: 1-Year Prospective Cohort Study. Gait Posture. 2010;32(1):87–91. doi: 10.1016/j.gaitpost.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- Chou KL, Amick MM, Brandt J, Camicioli R, Frei K, Gitelman D, Uc EY. A recommended scale for cognitive screening in clinical trials of Parkinson’s disease. Mov Disord. 2010;25(15):2501–2507. doi: 10.1002/mds.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcos DM, Robichaud JA, David FJ, Leurgans SE, Vaillancourt DE, Poon C, Comella CL. A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord. 2013 doi: 10.1002/mds.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruise KE, Bucks RS, Loftus AM, Newton RU, Pegoraro R, Thomas MG. Exercise and Parkinson’s: benefits for cognition and quality of life. Acta Neurol Scand. 2011;123(1):13–19. doi: 10.1111/j.1600-0404.2010.01338.x. [DOI] [PubMed] [Google Scholar]
- Damiano AM, McGrath MM, Willian MK, Snyder CF, LeWitt PA, Reyes PF, Means ED. Evaluation of a measurement strategy for Parkinson’s disease: assessing patient health-related quality of life. Qual Life Res. 2000;9(1):87–100. doi: 10.1023/a:1008928321652. [DOI] [PubMed] [Google Scholar]
- Devlin Ann S. Mind and Maze: Spatial Cognition and Environmental Behavior. Westport, CT: Greenwood Press; 2001. [Google Scholar]
- Dite W, Temple VA. A clinical test of stepping and change of direction to identify multiple falling older adults. Arch Phys Med Rehabil. 2002;83(11):1566–1571. doi: 10.1053/apmr.2002.35469. [DOI] [PubMed] [Google Scholar]
- Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. 2012;26(2):132–143. doi: 10.1177/1545968311421614. [DOI] [PubMed] [Google Scholar]
- Faber MJ, Bosscher RJ, Chin A, Paw MJ, van Wieringen PC. Effects of exercise programs on falls and mobility in frail and pre-frail older adults: A multicenter randomized controlled trial. Arch Phys Med Rehabil. 2006;87(7):885–896. doi: 10.1016/j.apmr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinson’s Disease. Vol. 2. Florham Park, NJ: Macmillan Healthcare Information; 1987. [Google Scholar]
- Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat Disord. 2000;6(3):165–170. doi: 10.1016/s1353-8020(99)00062-0. [DOI] [PubMed] [Google Scholar]
- Glass TA, de Leon CM, Marottoli RA, Berkman LF. Population based study of social and productive activities as predictors of survival among elderly Americans. BMJ. 1999;319(7208):478–483. doi: 10.1136/bmj.319.7208.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin VA, Richards SH, Henley W, Ewings P, Taylor AH, Campbell JL. An exercise intervention to prevent falls in people with Parkinson’s disease: a pragmatic randomised controlled trial. J Neurol Neurosurg Psychiatry. 2011;82(11):1232–1238. doi: 10.1136/jnnp-2011-300919. [DOI] [PubMed] [Google Scholar]
- Hackney ME, Earhart GM. Effects of dance on movement control in Parkinson’s disease: a comparison of Argentine tango and American ballroom. J Rehabil Med. 2009a;41(6):475–481. doi: 10.2340/16501977-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney ME, Earhart GM. Short duration, intensive tango dancing for Parkinson disease: an uncontrolled pilot study. Complement Ther Med. 2009b;17(4):203–207. doi: 10.1016/j.ctim.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney ME, Earhart GM. Effects of dance on gait and balance in Parkinson’s disease: a comparison of partnered and nonpartnered dance movement. Neurorehabil Neural Repair. 2010a;24(4):384–392. doi: 10.1177/1545968309353329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney ME, Earhart GM. Recommendations for implementing partnered dance classes for persons with Parkinson Disease. Am J Dance Ther. 2010b;31(1):41–45. doi: 10.1007/s10465-010-9086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney ME, Hall CD, Echt KV, Wolf SL. Application of Adapted Tango as Therapeutic Intervention for Patients With Chronic Stroke. J Geriatr Phys Ther. 2011 doi: 10.1519/JPT.0b013e31823ae6ea. [DOI] [PubMed] [Google Scholar]
- Hackney ME, Hall CD, Echt KV, Wolf SL. Dancing for balance: feasibility and efficacy in oldest-old adults with visual impairment. Nurs Res. 2013;62(2):138–143. doi: 10.1097/NNR.0b013e318283f68e. [DOI] [PubMed] [Google Scholar]
- Hackney ME, Kantorovich S, Earhart GM. A study on the effects of Argentine tango as a form of partnered dance for those with Parkinson disease and the healthy elderly. American Journal of Dance Therapy. 2007;29(2):109–127. [Google Scholar]
- Hackney ME, Kantorovich S, Levin R, Earhart GM. Effects of tango on functional mobility in Parkinson’s disease: a preliminary study. J Neurol Phys Ther. 2007;31(4):173–179. doi: 10.1097/NPT.0b013e31815ce78b. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Doniger GM, Springer S, Yogev G, Simon ES, Giladi N. A common cognitive profile in elderly fallers and in patients with Parkinson’s disease: the prominence of impaired executive function and attention. Exp Aging Res. 2006;32(4):411–429. doi: 10.1080/03610730600875817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez D, Rose DJ. Predicting which older adults will or will not fall using the Fullerton Advanced Balance scale. Arch Phys Med Rehabil. 2008;89(12):2309–2315. doi: 10.1016/j.apmr.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Heyward Vivian H. Advanced Fitness Assessment and Exercise Prescription. 6. Champaign, IL: Human Kinetics; 2010. [Google Scholar]
- Hindle JV, Petrelli A, Clare L, Kalbe E. Nonpharmacological enhancement of cognitive function in Parkinson’s disease: A systematic review. Mov Disord. 2013 doi: 10.1002/mds.25377. [DOI] [PubMed] [Google Scholar]
- Hirsch MA. Community-based rehabilitation for Parkinson’s disease: from neurons to neighborhoods. Parkinsonism Relat Disord. 2009;15(Suppl 3):S114–117. doi: 10.1016/s1353-8020(09)70795-3. [DOI] [PubMed] [Google Scholar]
- Jancey J, Lee A, Howat P, Clarke A, Wang K, Shilton T. Reducing attrition in physical activity programs for older adults. J Aging Phys Act. 2007;15(2):152–165. doi: 10.1123/japa.15.2.152. [DOI] [PubMed] [Google Scholar]
- Kadivar Z, Corcos DM, Foto J, Hondzinski JM. Effect of step training and rhythmic auditory stimulation on functional performance in Parkinson patients. Neurorehabil Neural Repair. 2011;25(7):626–635. doi: 10.1177/1545968311401627. [DOI] [PubMed] [Google Scholar]
- Kessels RP, van den Berg E, Ruis C, Brands AM. The backward span of the Corsi Block-Tapping Task and its association with the WAIS-III Digit Span. Assessment. 2008;15(4):426–434. doi: 10.1177/1073191108315611. 1073191108315611 [pii] [DOI] [PubMed] [Google Scholar]
- Klein PJ, Fiedler RC, Rose DJ. Rasch Analysis of the Fullerton Advanced Balance (FAB) Scale. Physiother Can. 2011;63(1):115–125. doi: 10.3138/ptc.2009-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klencklen G, Despres O, Dufour A. What do we know about aging and spatial cognition? Reviews and perspectives. Ageing Res Rev. 2012;11(1):123–135. doi: 10.1016/j.arr.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Kraft E. Cognitive function, physical activity, and aging: possible biological links and implications for multimodal interventions. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2012;19(1–2):248–263. doi: 10.1080/13825585.2011.645010. [DOI] [PubMed] [Google Scholar]
- Li F, Harmer P, Fitzgerald K, Eckstrom E, Stock R, Galver J, Batya SS. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med. 2012;366(6):511–519. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ. Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology. 2008;70(23):2241–2247. doi: 10.1212/01.wnl.0000313835.33830.80. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Kwakkel G, Rochester L, Jones D, van Wegen E, Willems AM, Lim I. Cueing training in the home improves gait-related mobility in Parkinson’s disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78(2):134–140. doi: 10.1136/jnnp.200X.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwboer A, Rochester L, Muncks L, Swinnen SP. Motor learning in Parkinson’s disease: limitations and potential for rehabilitation. Parkinsonism Relat Disord. 2009;15(Suppl 3):S53–58. doi: 10.1016/s1353-8020(09)70781-3. [DOI] [PubMed] [Google Scholar]
- Nowalk MP, Prendergast JM, Bayles CM, D’Amico FJ, Colvin GC. A randomized trial of exercise programs among older individuals living in two long-term care facilities: the FallsFREE program. J Am Geriatr Soc. 2001;49(7):859–865. doi: 10.1046/j.1532-5415.2001.49174.x. [DOI] [PubMed] [Google Scholar]
- Noyes K, Liu H, Li Y, Holloway R, Dick AW. Economic burden associated with Parkinson’s disease on elderly Medicare beneficiaries. Mov Disord. 2006;21(3):362–372. doi: 10.1002/mds.20727. [DOI] [PubMed] [Google Scholar]
- Pardasaney PK, Latham NK, Jette AM, Wagenaar RC, Ni P, Slavin MD, Bean JF. Sensitivity to change and responsiveness of four balance measures for community-dwelling older adults. Phys Ther. 2012;92(3):388–397. doi: 10.2522/ptj.20100398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res. 1995;4(3):241–248. doi: 10.1007/BF02260863. [DOI] [PubMed] [Google Scholar]
- Possin KL. Visual spatial cognition in neurodegenerative disease. Neurocase. 2010;16(6):466–487. doi: 10.1080/13554791003730600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possin KL, Filoteo JV, Song DD, Salmon DP. Spatial and object working memory deficits in Parkinson’s disease are due to impairment in different underlying processes. Neuropsychology. 2008;22(5):585–595. doi: 10.1037/a0012613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protas EJ, Mitchell K, Williams A, Qureshy H, Caroline K, Lai EC. Gait and step training to reduce falls in Parkinson’s disease. NeuroRehabilitation. 2005;20(3):183–190. [PubMed] [Google Scholar]
- Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson’s disease. Am J Med Genet. 1999;88(5):539–543. [PubMed] [Google Scholar]
- Ratey JJ, Loehr JE. The positive impact of physical activity on cognition during adulthood: a review of underlying mechanisms, evidence and recommendations. Rev Neurosci. 2011;22(2):171–185. doi: 10.1515/rns.2011.017. [DOI] [PubMed] [Google Scholar]
- Rikli R, Jones C. Senior Fitness Test Manual. Champaign, IL: Human Kinetics; 2001. [Google Scholar]
- Rose DJ, Lucchese N, Wiersma LD. Development of a multidimensional balance scale for use with functionally independent older adults. Arch Phys Med Rehabil. 2006;87(11):1478–1485. doi: 10.1016/j.apmr.2006.07.263. [DOI] [PubMed] [Google Scholar]
- Rosendahl E, Gustafson Y, Nordin E, Lundin-Olsson L, Nyberg L. A randomized controlled trial of fall prevention by a high-intensity functional exercise program for older people living in residential care facilities. Aging Clin Exp Res. 2008;20(1):67–75. doi: 10.1007/BF03324750. [DOI] [PubMed] [Google Scholar]
- Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified Parkinson’s disease rating scale. Arch Neurol. 2010;67(1):64–70. doi: 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80(9):896–903. [PubMed] [Google Scholar]
- Silberman CD, Laks J, Capitao CF, Rodrigues CS, Moreira I, Engelhardt E. Recognizing depression in patients with Parkinson’s disease: accuracy and specificity of two depression rating scale. Arq Neuropsiquiatr. 2006;64(2B):407–411. doi: 10.1590/s0004-282x2006000300011. [DOI] [PubMed] [Google Scholar]
- Stephan MA, Meier B, Zaugg SW, Kaelin-Lang A. Motor sequence learning performance in Parkinson’s disease patients depends on the stage of disease. Brain Cogn. 2011;75(2):135–140. doi: 10.1016/j.bandc.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Quadros AC, Jr, Santos RF, Stella F, Gobbi LT, Gobbi S. Benefits of physical exercise on executive functions in older people with Parkinson’s disease. Brain Cogn. 2009;69(2):435–441. doi: 10.1016/j.bandc.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Patel S, Meek C, Clarke CE, Stowe R, Shah L, Ives N. Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane Database Syst Rev. 2012;8:CD002817. doi: 10.1002/14651858.CD002817.pub3. [DOI] [PubMed] [Google Scholar]
- Verreyt N, Nys GM, Santens P, Vingerhoets G. Cognitive differences between patients with left-sided and right-sided Parkinson’s disease. A review. Neuropsychol Rev. 2011;21(4):405–424. doi: 10.1007/s11065-011-9182-x. [DOI] [PubMed] [Google Scholar]
- Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Sattin RW, Kutner M, O’Grady M, Greenspan AI, Gregor RJ. Intense tai chi exercise training and fall occurrences in older, transitionally frail adults: a randomized, controlled trial. J Am Geriatr Soc. 2003;51(12):1693–1701. doi: 10.1046/j.1532-5415.2003.51552.x. 51552 [pii] [DOI] [PubMed] [Google Scholar]