Abstract
Purpose
To test the sensitivity and reproducibility of a 25-gauge force-sensing micropick during microsurgical maneuvers that are below tactile sensation.
Methods
Forces were measured during membrane peeling in a “raw egg” and the chick chorioallantoic membrane models (N = 12) of epiretinal membranes. Forces were also measured during posterior hyaloid detachment and creation of retinal tears during vitrectomy in live rabbits (n = 6).
Results
With the raw egg model, 0.5 ± 0.4 mN of force was detected during membrane peeling. In the chorioallantoic membrane model, delaminating the upper membrane produced 2.8 ± 0.2 mN of force. While intentionally rupturing the lower membrane to simulate a retinal tear, 7.3 ± 0.5 mN (range, 5.1–9.2 mN; P < 0.001) of force was generated while peeling the upper membrane. During vitrectomy, the minimum force that detached the posterior hyaloid was 6.7 ± 1.1 mN, which was similar to the force of 6.4 ± 1.4 mN that caused a retinal tear. The rate of force generation, as indicated by the first derivative of force generation, was 3.4 ± 1.2 mN/second during posterior hyaloid detachment, compared with 7.7 ± 2.4 mN/second during the creation of a retinal tear (P = 0.04).
Conclusion
Force-sensing microsurgical instruments can detect forces below tactile sensation, and importantly, they can distinguish the forces generated during normal maneuvers from those that cause a surgical complication.
Keywords: force-sensing micropick, membrane peeling, vitrectomy
Many clinical procedures involve intervention and manipulation of extremely small, delicate tissue structures. Vitreoretinal surgery is a prime example of the requirement for micron-scale maneuvers. The manipulation of vitreoretinal structures inside the eye poses enormous challenges because of tissue fragility, surgical inaccessibility, suboptimal visualization, and the potential for irreversible tissue damage resulting from unintentional movement.
Epiretinal membrane (ERM) peeling is a prototypical task where delaminating a thin membrane off of the retina with a microforceps is well below the threshold of human tactile perception.1 Imprecise surgical movements or unexpected patient movement can induce excessive forces that lead to tissue injury, such as retinal hemorrhage and tearing, with potential irreversible vision loss. Vitreoretinal surgeons visually monitor local surface deformation as a surrogate for tactile sensation during membrane peeling. The imperceptible tactile sensation, subtle visual cues that substitute for force generation, and the need for very precise, minute visuomotor reflexes makes membrane peeling one of the most difficult ophthalmologic surgical tasks to perform. Surgical instruments that can detect forces during a surgical maneuver could enable the surgeon to complete the task with greater ease and fewer complications.
Previous force-sensing microsurgical instruments had force-sensing elements built into the handle of the instrument.2-4 Because of this handle-mounted tool force sensor design, these tools do not give useful information for vitreoretinal surgery because the forces generated by interactions between the tool shaft and sclera at the sclerotomy interfered with the accurate measurement of tool-to-tissue interaction forces inside the eye (e.g., at the retina). Jagtap and Riviere reported exactly this problem when using a handle-based force-sensing tool to measure retinal microsurgery forces and went on to state, “It seems likely that discrimination between forces applied at the tool tip and forces due to contact with the sclera seems likely to be a significant challenge in the development of useful force feedback for vitreoretinal microsurgery.”3 We have recently addressed this limitation by incorporating force-sensing elements into the section of the instrument shaft that is located inside the eye.5
We have hypothesized that new technology that measures force generation during a surgical procedure will improve the performance of surgeon and diminish surgical complication. To provide benefit to the surgeon, both the forces that are generated during any surgical maneuver and a library of forces that induce injury are necessary. To achieve this goal, a significant engineering effort has been put into designing and optimizing the sensor-integrated tool tip that would be suitable for use in a live animal eye. The new probe tip, although optimal bonding and positioning of the tool shift, and improved uniformity between the three fiber sensors show greater robust-ness, with improved linearity and reliability compared with the previous version. In this study, therefore, we sought to test whether our newly designed force-sensing instrument can reliably detect extremely delicate (millinewton scale) forces in suitable membrane peeling phantom systems and distinguish the forces generated between normal surgical maneuvers and a known complication. We also tested this instrument during vitrectomy in a live rabbit eye to demonstrate that contact with the sclera did not interfere with force measurements during 1) safe delamination of the posterior hyaloid from the retina, and 2) creation of a retinal tear.
Methods
Microforce-Sensing Instrument Design
Vitreoretinal microsurgical applications introduce certain limitations on the design of force sensors by demanding specific characteristics. Submillinewton accuracy is required to sense and track forces that are routinely <7.5 mN1,3 in a small-gauge instrument, such as 23 or 25 gauge. The force sensor must be able to obtain measurements at the instrument’s tip when it is inside the eye. The conceptual design of our force-sensing tool has been previously reported.5 The force-sensing system relies on fiber Bragg grating strain sensors incorporated into the tool shaft.6 Our studies have shown that the positioning of the fiber sensors, groove depth, relative position between the fibers, sensor uniformity, and the bonding of the fiber to the probe tip are critical factors that provide reliable force measurement data. For this study, we selected 3 closely matched fiber Bragg grating sensors (Micron Optics, Inc, Atlanta, GA) having standard 125 μm in diameter for the tool tip fabrication.
Three fiber Bragg grating optical strain–sensing fibers were integrated into a 50-mm-long titanium wire of 0.5 diameter. Three square grooves (160 × 160 μm) along the wire were cut to position the fiber sensors. A medical device super glue (Loctite 4014; Henkel, Dusseldorf, Germany) was used to bond the fiber to the wire. The 3-mm-long pick was created by bending a 1.5-inch 25-gauge needle tip 45°. The pick was attached to the distal end of the titanium wire, which serves as the tool shaft. The sensor is monitored by an optical-sensing interrogator (Model sm 130-700; Micron Optics, Inc), which has a resolution of 0.001 nm and a scan frequency of 2 kHz, with 4 channels. The sensor data were collected and processed at 1 kHz over TCP/IP on a local 100 Mb Ethernet network. This instrument detects forces in two degrees of freedom, in the x and y planes but not the axial z plane.
Calibration of the Force Sensor
Calibration of the microforce sensor was performed in an electrically shielded analytical balance (Sartorius 1601 from Data Weighing Systems, Inc, Elk Grove, IL), as previously described.5
Raw Egg Membrane Peeling Phantom
The raw egg membrane peeling model uses chicken eggs bought in any supermarket. The egg is cracked in half, and the yolk was removed. The shell membrane was used for “membrane peeling” in this model. A drop of food coloring was dropped on top of the membrane to better visualize the membrane. A FL2-08S2M/C camera (Point Grey Research, Inc, British Columbia, Canada) with video recording capability was mounted on the operating microscope (OPMI MD; Carl Zeiss Optical, Inc, Dublin, CA) to record the experimental task. When peeling the shell membrane, the surgeon attempted to maintain a steady force and peeling velocity without tearing the membrane. The force measurement represents an average of the forces measured during the membrane peeling.
Chick Chorioallantoic Membrane Phantom
The chick chorioallantoic membrane (CAM) from Day 11 to Day 15 eggs has previously been reported to serve as an excellent phantom of the retina that is suitable for vitreoretinal surgery testing.7 An opening of the size of a fully dilated human pupil was made at the top of the distal portion of the egg shell, where the allantoic vesicle lies (a space between the CAM and the shell membrane). Saline was then placed in the egg to keep the membranes moist. The inner shell membrane (ISM), which mimics an ERM, is attached to the CAM, which mimics the neurosensory retina. Two experimental strategies were performed. The first experiment peeled the ISM without rupturing the lower CAM, which simulates membrane peeling without retinal injury. The second experiment intentionally ruptured the lower CAM while peeling the upper ISM to simulate a retinal tear. The membrane peeling forces were measured, and the procedures were recorded in a video, as described above.
Rabbit Vitrectomy
The use of animals in this study adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and the study was approved by the Johns Hopkins Animal Care and Use Committee. New Zealand albino rabbits (n = 6) were anesthetized with an intramuscular injection of a mixture of ketamine hydrochloride of 50 mg/kg body weight (Fort Dodge Laboratories, Fort Dodge, IA) and acepromazine of 10 mg/kg (Fort Dodge Laboratories). The pupils were pharmacologically dilated with 1 drop each of 1% tropicamide ophthalmic solution (Alcon Laboratories, Fort Worth, TX) and 2.5% phenylephrine hydrochloride ophthalmic solution (Akorn, Inc, Buffalo Grove, IL). The surgical eye was topically anesthetized with 1 drop of proparacaine (Wilson Ophthalmic, Mustang, OK) and prepped for the surgical procedure by irrigation with 5% propidium iodide sterile ophthalmic prep solution (Betadine; Alcon Laboratories) followed by surgical draping. A standard 3-port vitrectomy and lensectomy were performed using a 23-gauge system (Alcon Laboratories) and the Accurus Vitrectomy system (Alcon Laboratories). Forces were measured with the micropick force-sensing instrument when detaching the posterior hyaloid from the optic nerve and inducing a retinal tear in the posterior segment. The vitectomies were recorded using a Zeiss OPMI Lumera 700 microscope equipped with an experimental HD stereo video adapter developed by Zeiss (Carl Zeiss Meditec AG, Oberkochen, Germany).
Results
Force Sensor Can Detect Minute Forces Generated During Phantom Membrane Peeling
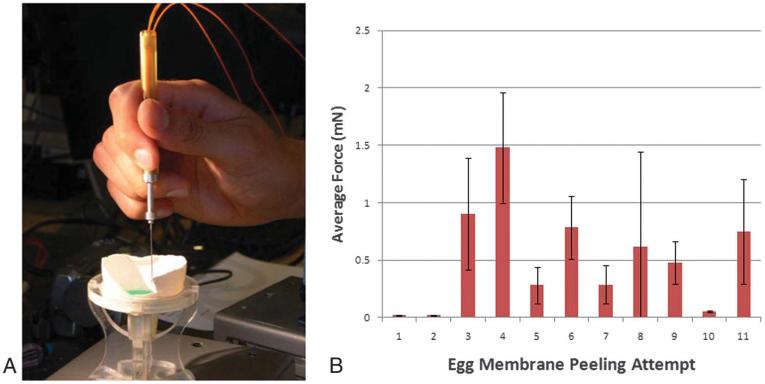
We previously reported on the engineering design for the force-sensing micropick and demonstrated that measuring small forces during microsurgical maneuvers in the raw egg shell and CAM models are possible. However, the sensitivity and reproducibility are unknown.5 We first used the chick eggshell membrane model to test the sensitivity of the force sensor while the surgeon peeled the inner membrane from the eggshell. The surgeon in these experiments underwent an initial training period (n = 20 attempts) where the objective was to perform the peeling with the instrument in a vertical position so that the forces measured would be in the x–y plane. The surgeon engaged the membrane edge with the micropick and delaminated the membrane from the shell while maintaining the instrument shaft in a vertical position (Figure 1A). The goal was to maintain a steady force and peeling velocity without tearing the membrane. The minimum force generated during the membrane peeling was 0.2 mN, while the average force generated during peeling of the membrane from the shell was 0.5 ± 0.4 mN (range, 0.2–1.5 mN; n = 11), as shown in Figure 1B. The standard deviation serves as a quantitative indicator of the variability of forces generated during membrane peeling.
Fig. 1.
A. Force-sensing micropick peeling the raw egg shell membrane. B. Graph of average forces ± standard deviation measured during raw egg shell membrane peeling attempt.
Force Sensor Can Measure the Difference in Forces Between Uncomplicated and Complicated Membrane Peeling
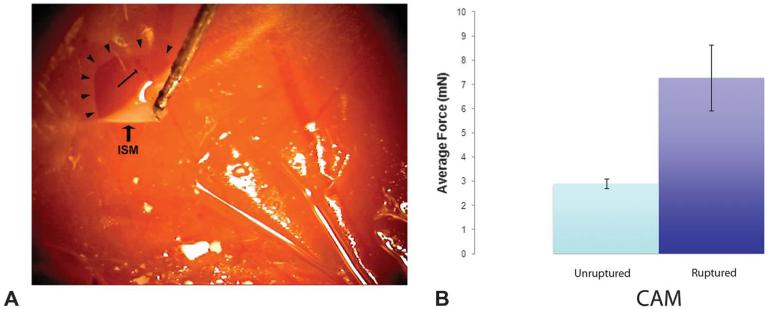
The CAM model has been previously used to mimic vitreoretinal surgery.7 The CAM mimics the neurosensory retina because it is similar in thickness (approximately 100 μm) and has its own vasculature. If sufficiently traumatized, the CAM will bleed or tear. The ISM is adherent to the CAM, and therefore, it simulates an ERM. The surgeon (S.S.) underwent a training session to learn how to delaminate the membrane without traumatizing the underlying CAM (n = 10) and then by delaminating the membrane while trying to intentionally tear the CAM (n = 10). Next, we measured the forces generated during membrane peeling under 2 conditions (Figure 2). First, when nontraumatically delaminating the ISM from the CAM, the maximum force generated during the peeling was 4.1 mN while the average force was 2.8 ± 0.2 mN (range, 1.3–4.1 mN; n = 6). In the second set of experiments, the CAM was intentionally injured during delamination of the ISM from the CAM by increasing the velocity of membrane peeling. Here, the minimum force that created an injury to the underlying CAM during membrane peeling was 5.1 mN, which is greater than the maximum force generated during nontraumatic delamination, while the average force was 7.3 ± 0.5 mN (range, 5.1–9.2 mN; n = 6). This difference in force generation was significant (P < 0.0001).
Fig. 2.
A. Force-sensing micropick peeling the ISM (arrow-heads) in the CAM model. Underlying blood vessel (arrow) is intact after peeling. B. Graph of forces generated while peeling the ISM with and without injuring the underlying CAM.
Force Sensor Can Detect Forces During Vitrectomy in the Rabbit Eye
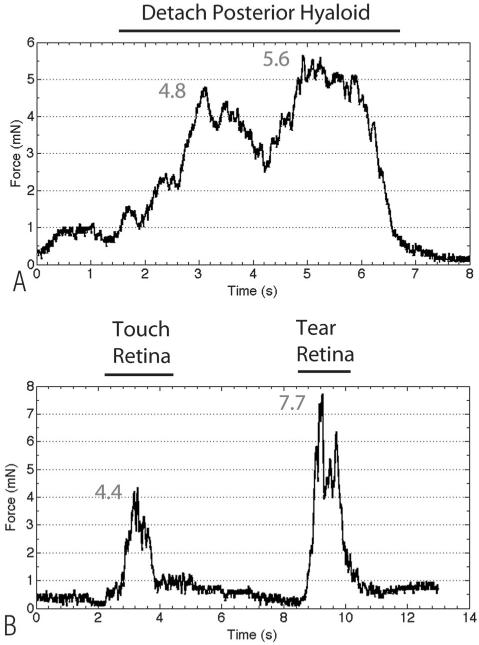
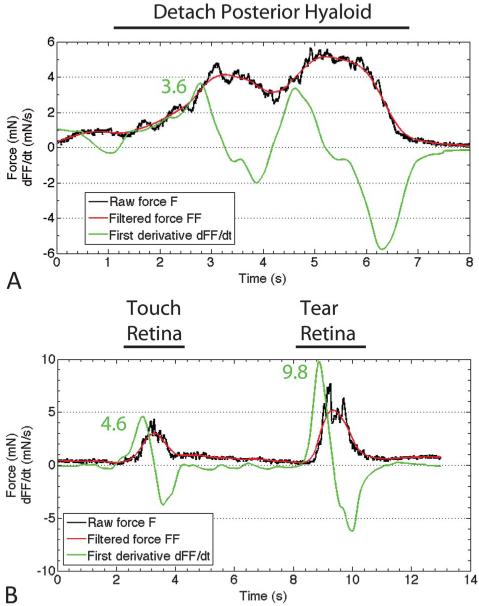
The previous two experiments demonstrate that the force-sensing instrument can reliably detect very small forces during a prototypical surgical maneuver such as membrane peeling and that force differences can be detected between a normal maneuver and during a surgical complication, such as a retinal tear. This force sensor was designed to incorporate force-sensing elements into the section of the instrument shaft that is located inside the eye to eliminate the influence of forces generated between the instrument shaft and sclerotomy. To test this new instrument design inside the eye, we measured forces inside the living rabbit eye during vitrectomy. Our goal was to detect forces generated during prototypical vitreoretinal surgical maneuvers and during creation of a surgical complication. After a core vitrectomy, the micropick was calibrated to zero inside the eye. We then detached the posterior hyaloid from the optic nerve using the micropick. We performed the maneuver 12 times and selected the 3 smallest forces that successfully detached the hyaloid to determine the minimum force that can accomplish this goal. The average force of the 3 smallest measured forces to successfully detach the hyaloid was 6.7 ± 1.1 mN. Figure 3A illustrates the force profile during hyaloid detachment. We next wanted to quantify the minimum force required to generate a retinal tear. In 6 rabbits, we induced 25 retinal tears and selected the 3 smallest forces, which was 6.4 ± 1.4 mN. Figure 3B shows a representative force profile of a retinal tear. Compared with detaching the posterior hyaloid, a rapid increase in force is seen during the initiation of the retinal tear, with plateauing of force until the retina was torn, when there was a sharp decline in force. To demonstrate the difference in the rate of force generation between detaching the posterior hyaloid and creating a retinal tear, we calculated the derivative (dFF/dt), as illustrated in Figure 4. The dFF/dt is calculated by first removing high-frequency noise by filtering the raw force (F) using a moving average filter to generate the filtered force (FF). The filtered force is then used to calculate the first time derivative dFF/dt. The average dFF/dt for the posterior hyaloid stripping was 3.4 ± 1.2 mN/second, while the average dFF/dt for a retinal tear was 7.7 ± 2.4 mN/second (P = 0.04). We observed extremely high forces in excess of 30 mN when the lower instrument shaft that detected the forces was pulled away from the retina so that it came in contact with the sclera at the sclerotomy, without performing any surgical maneuver.
Fig. 3.
A. Representative tracing of the raw forces measured by the force-sensing micropick during posterior hyaloid detachment. A gradual increase in force was seen, with a general plateau phase, followed by a rapid decrease when the hyaloid was separated from the optic nerve. B. Representative tracing of the forces measured during creation of a retinal tear. From 2 seconds to 4 seconds, a maximum force of 4.4 mN was generated when the instrument was touching the retina. From 8 seconds to 11 seconds, when a retinal tear was created, there was a rapid increase in force, a short plateau phase, and a rapid decrease in force when the retina was torn.
Fig. 4.
A. Representative tracing of the first derivative of the smoothened force during posterior hyaloid detachment. The black tracing is the raw forced data curve, the red tracing is the smoothened force data curve, and the green line is the first derivative of the smoothened force. The peak first derivative force was 3.6. B. Representative tracing of the first derivative of the smoothened force during creation of a retinal tear. The peak first derivative force was 4.6 for touching the retina and 9.8 for causing a retinal tear.
Discussion
We have transformed a common surgical instrument into a “smart” instrument that can detect forces that are below human tactile sensation. By incorporating fiber Bragg grating sensors into the instrument shaft near the tool tip, a surgical micropick is able to reliably detect a force as small as 0.1 mN.5 This is roughly half the equivalent force of picking up a 1 × 1 inch piece of paper. In two different phantom models of ERM peeling, we detected very small forces that were reliable and reproducible while delaminating “membranes.” The egg shell model illustrated the high degree of sensitivity of the force sensor because it measured forces (i.e., 0.5 mN) far below that reported for manipulating the retina (i.e., 7.5 mN).1 Differences in force required to delaminate the ISM in the CAM model were predictive of whether uncomplicated or complicated membrane peeling (with underlying tissue injury) had occurred. These experiments demonstrate that small force differences predictive of tissue injury are measurable.
We further validated the force sensor during a vitrectomy in live rabbit eyes. We reliably measured the forces generated during hyaloid detachment and while intentionally creating a retinal tear. This magnitude is below the human tactile perceptual thresholds, and this is in the range of previously reported manipulation of the retina using an “open sky” technique in the porcine cadaver eye so that the measurements were not influenced by forces generated by the instrument shaft touching the eye wall.1 In contrast to our results, the measured minimum force reported during internal limiting membrane peeling in a live rabbit eye during vitrectomy in a previous study was 54 mN.3 The authors of this previous study attributed the high measured forces to contact between the tool and the sclerotomy site. When the region of the instrument shaft that detected force came in contact with the sclera at the sclerotomy, the forces increased to this range without any intentional surgical maneuver. Therefore, our measurements suggest that the new instrument design is less affected by forces resulting from the interaction of the instrument shaft with the sclera at the sclerotomy.
We were surprised that the minimal force required to detach the hyaloid and create a retinal tear were similar. Perhaps, not surprising was the difference that we observed in the force generation profile. To induce a posterior hyaloid detachment, the force profile showed a gradual increase in force before a plateau phase and finally a decrease in force after the hyaloid was detached. In contrast, during creation of a retinal tear, we observed a steep initial increase in force, and then a short plateau phase before a decrease in force when the retina tore. The dFF/dt calculation quantifies this difference. Further investigation of the force profile will be necessary to understand the full implications of the forces that are associated with both normal and damaging maneuvers.
With further understanding of the forces required both to successfully complete a surgical task and those that induce tissue injury, a library of safe and dangerous forces for any intraocular surgical maneuver can be developed that would serve as a framework for developing enabling systems that preemptively warn the surgeon of imminent tissue injury and allow the surgeon to minimize the risk of inducing a complication. For example, retinal tears are a rare but recognized complication during ERM peeling. Because the forces of typical retinal manipulation and that of creating a retinal tear are both below tactile sensation, quantitative information communicated to the surgeon during this maneuver could prevent a retinal tear during ERM peeling. Technology such as this, while originally developed for vitreoretinal surgery, is applicable to other intraocular or microsurgical procedures where the forces generated are below human tactile sensation.
Currently, the surgeon indirectly assesses the relative forces applied to a tissue by interpretation of secondary visual cues, such as the changing light reflections from deforming tissue. This type of sensory substitution requires significant experience and concentration common to only the expert surgeon. To be valuable, this novel real-time and quantitative force-sensing information must be communicated to the operating surgeon. Visual, tactile, or auditory feedback is an effective means of communicating forces to a surgeon in a given situation. Visual feedback may be provided by image injection into the surgeon’s view through the operating microscope in some situations, but this could distract the surgeon’s attention away from the surgical task. Tactile feedback carries the risk of introducing unwanted instrument movement. Similar to our own experience, Kitagawa et al8 showed that auditory feedback representing force in a complex surgical task improves robot-assisted performance and suggested that real-time auditory feedback is an effective way to inform the surgeon. We believe that informing the surgeon of the forces being generated through auditory feedback would be a suitable strategy without confusing the surgeon, just as auditory feedback provides the anesthesiologist with a patient’s pulse rate. Accordingly, we have begun to experiment with auditory substitution feedback in conjunction with our new force-sensing tools.9
We considered testing the instrument on human cadaver eyes, but because of tissue degradation and other factors that could alter tissue forces, we did not believe that these experiments would significantly enhance our understanding of this technology at this time. The current instrument measures forces with two degrees of freedom and lacks force sensing in the axial or z axis direction along the tool shaft. As such, our experiments were conducted by keeping the instrument tip somewhat perpendicular to the tissue in order the measure the forces. With this instrument, we can accurately measure forces within a 1.5% error at a 10° angle between instrument shaft and tissue plane. For a 20 mN force, the error introduced would be 0.3 mN, which is equivalent to our 0.1 mN instrument sensitivity. With intraocular surgery, this instrument position is not feasible to complete many tasks. Incorporation of a sensing capability in the z axis would allow us to measure forces in the z axis, so that instrument position would be less of a factor influencing the accuracy of measured forces. Our group has developed a prototype three degrees of freedom sensing tool that we hope to test and report in the near future.
This project is part of a comprehensive program that has been developed by computer scientists, bioengineers, and vitreoretinal surgeons at our institution. The goal is to provide enabling technology that will make surgery safer and easier while improving outcomes. We want to make the average surgeon accomplished and to allow the excellent surgeon to achieve the heretofore unachievable. We believe that these concepts apply not only to ophthalmologic surgery but also to other microsurgical disciplines such as neurosurgery, otolaryngology, and vascular surgery.
Acknowledgments
Supported in part by the U.S. National Science Foundation under Cooperative Agreement EEC9731478, the National Institutes of Health (BRP 1 R01 EB 007969-01 A1), the ARCS Foundation, Research to Prevent Blindness, and Johns Hopkins internal funds. Partial equipment support was provided by Alcon and Carl Zeiss Meditec.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Gupta PK, Jensen PS, de Juan EJ. Surgical forces and tactile perception during retinal microsurgery. MICCAI’99 LNCS. 1999;1679:1218–1225. [Google Scholar]
- 2.Berkelman P, Whitcomb L, Taylor R, Jensen P. A miniature microsurgical instrument tip force sensor for enhanced force feedback during robot-assisted manipulation. IEEE Transact. 2003;19:917–921. [Google Scholar]
- 3.Jagtap AS, Riviere CN. Applied force during vitreoretinal microsurgery with handheld instruments. Conf Proc IEEE Eng Med Biol Soc. 2004;4:2771–2773. doi: 10.1109/IEMBS.2004.1403792. [DOI] [PubMed] [Google Scholar]
- 4.Kumar R, Berkelman P, Gupta P, et al. Preliminary experiments in Cooperative Human/Robert Force Control for robot assisted microsurgical manipulation. IEEE ICRA. 2000;1:610–617. [Google Scholar]
- 5.Iordachita I, Sun Z, Balicki M, et al. A sub-millimetric, 0.25 mN resolution fully integrated fiber-optic force-sensing tool for retinal microsurgery. Int J Comput Assist Radiol Surg. 2009;4:383–390. doi: 10.1007/s11548-009-0301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill KO, Meltz G. Fiber Bragg grating technology fundamentals and overview. J Lightwave Technol. 1997;15:1263–1276. [Google Scholar]
- 7.Leng T, Miller JM, Bilbao KV, et al. The chick chorioallantoic membrane as a model tissue for surgical retinal research and simulation. Retina. 2004;24:427–434. doi: 10.1097/00006982-200406000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Kitagawa M, Dokko D, Okamura AM, Yuh DD. Effect of sensory substitution on suture-manipulation forces for robotic surgical systems. J Thorac Cardiovasc Surg. 2005;129:151–158. doi: 10.1016/j.jtcvs.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 9.Balicki M, Uneri A, Iordachita I, et al. Micro-force sensing in robot assisted membrane peeling for vitreoretinal surgery. Med Image Comput Comput Assist Interv. 2010;13:303–310. doi: 10.1007/978-3-642-15711-0_38. [DOI] [PMC free article] [PubMed] [Google Scholar]