Abstract
The purpose of this study was to determine how dexamethasone (DEX) regulates the expression and activity of αvβ3 integrin. FACS analysis showed that DEX treatment induced expression of an activated αvβ3 integrin. Its expression remained high as long as DEX was present and continued following DEX removal. FACS analysis showed that the upregulation of αvβ3 integrin was the result of an increase in the expression of the β3 integrin subunit. By real time qPCR, DEX treatment induced a 6.2-fold increase (p<0.04) in β3 integrin mRNA by day 2 compared to control and remained elevated for 6 days of treatment and then an additional 10 days once the DEX was removed. The increase in β3 integrin mRNA levels required only 1 day of DEX treatment to increase levels for 4 days in the absence of DEX. In contrast, DEX did not alter β1 integrin mRNA or protein levels. The DEX-induced upregulation of β3 integrin mRNA was partly due to an increase in its half-life to 60.7 h from 22.5 h in control cultures (p<0.05) and could be inhibited by RU486 and cycloheximide, suggesting that DEX-induced de novo protein synthesis of an activation factor was needed. The calcineurin inhibitors cyclosporin A (CsA) and FK506 inhibited the DEX induced increase in β3 integrin mRNA. In summary, the DEX-induced increase in β3 integrin is a secondary glucocorticoid response that results in prolonged expression of αvβ3 integrin and the upregulation of the β3 integrin subunit through the calcineurin/NFAT pathway.
Keywords: cytoskeleton, integrin, calcineurin, glucocorticoids, glaucoma, trabecular meshwork
1. Introduction
The αvβ3 integrin has been shown to be involved in the pathogenesis of a number of diseases including cancer, diabetes and osteoporosis [1–6]. More recently, αvβ3 integrin has been implicated as having a role in steroid-induced glaucoma (SIG) [7–9]. SIG results from the long term use of glucocorticoids (GCs) such as dexamethasone (DEX), most commonly through topical ocular application. About 30–40% of the normal population exhibit elevated intraocular pressure (IOP) when treated with GCs [10, 11] with about 6% of these patients developing SIG. The cause of this increase in IOP is believed to be a restriction in the movement of aqueous humor through the trabecular meshwork (TM) of the anterior segment of the eye.
A number of cytoskeletal events known to be regulated by integrins have been implicated in glaucoma [12, 13] including changes in contractility and a reorganization of the actin cytoskeleton into a unique structure of cross linked actin networks called CLANs. CLANs are believed to alter the contractile properties of TM cells by making them more rigid and unable to respond to pressure changes in the eye [14]. They are observed in higher frequency in glaucomatous and DEX-treated tissues [15] and DEX-treated TM cell cultures [7, 16]. In TM cell cultures, these networks can be formed by the activation of an αvβ3 integrin/Src/Trio/Rac1 signaling pathway [7–9] that may involve CD47 and requires crosstalk with a β1 integrin/Src/PI-3 kinase pathway. Inhibition of this pathway impairs CLAN formation in DEX-treated cells suggesting that αvβ3 integrin signaling may be responsible for some of the cellular changes observed in glaucoma. Recent work supports this idea and shows that DEX increases the expression of αvβ3 integrin in cells from the TM [7].
Despite the preponderance of studies implicating αvβ3 integrin in disease processes we know very little about how the expression of αvβ3 integrin is regulated. In bone marrow macrophages, IL-4 and to a lesser extent IL-6, GM-CSF and TNFα, increase β3 integrin mRNA [17]. During angiogenesis, the Foxc1 and Foxc2 transcription factors has been shown to regulate expression of the β3 integrin subunit in endothelial cells via Forkhead-binding elements in the promoter region of the β3 integrin subunit [18, 19]. In osteoblasts, β3 integrin expression is transiently increased by DEX [20]. Studies show that expression of the β3 integrin subunit in osteoclasts is regulated by calcineurin and the transcription factor NFATc1 [21, 22]. The NFAT family of transcription factors is activated through dephosphorylation by calcineurin. Calcineurin is a ubiquitously expressed serine/threonine phosphatase that is activated by Ca2+ ion and calmodulin binding. To date, calcineurin is the only protein phosphatase that dephosphorylates NFAT [23], making it essential for the activation of genes by NFATs.
In this study we used human trabecular meshwork cells, which are specialized smooth muscle-like cells in the eye that regulate the contractile properties of the anterior segment of the eye, to study how DEX regulates αvβ3 integrin expression. Our studies show that DEX increased transcription of β3 integrin mRNA through a secondary glucocorticoid response mechanism and required de novo protein synthesis. This increase was sensitive to the immunosuppressive drugs cyclosporine A (CsA) and FK506 indicating that calcineurin may be involved. Furthermore, we show that the increased transcription of β3 integrin mRNA resulted in increased protein expression of the β3 integrin subunit that persisted even after removal of DEX and that the αvβ3 integrin was in an active conformation. These results suggest that induction of β3 integrin by DEX occurs at both the transcriptional and protein levels and may result in the dysregulation of an activated αvβ3 integrin signaling pathway that can lead to the cytoskeleton changes (i.e., CLANs) observed in glaucoma. Understanding how DEX affects TM cells in the eye is important since many systemic steroid treatments can lead to increases in intraocular pressure and glaucoma.
2. Materials and Methods
2.1. Materials
For western blotting, the primary antibodies used were: β3 integrin mAb (EP2417Y, Abcam; 1:500), β1 integrin mAb (HB1.1, Millipore; 1:1000), FKBP51 (also known as FKBP5; 1:1000) pAb (Sigma-Aldrich) and Succinate dehydrogenase complex, subunit A (SDHA) mAb (2E3, Abcam; 1:2000). Secondary antibodies used were goat anti-mouse or anti-rabbit HRP conjugated Ab (Santa Cruz; 1:5000). Antibodies used for FACS were: mouse IgG1 (BD Biosciences; 1:100), αvβ3 integrin mAb (LM609, Millipore; 1:100), an activated β3 integrin mAb (CRC54, Abcam; 1:100) and goat anti-mouse Alexa 488 conjugated Ab (Life Technologies; 1:400). All inhibitors were obtained from Sigma-Aldrich, Co.
2.2. Cell Culture
The N27TM-2 cell strain of human trabecular meshwork (HTM) cells were isolated from cadaver eyes of a 27-year old donor and cultured as previously described [24] and used between passages 7–8. One week after reaching confluency, cells were treated with either 500 nM DEX or 0.1% ethanol (EtOH; vehicle control). In some experiments, cells were incubated with the RNA polymerase II inhibitor actinomycin D (5 μg/ml). In other experiments, the glucocorticoid inhibitor RU486 (mifepristone; 2.5, 10 or 25 μg/ml), cycloheximide (25 μg/ml) or CsA or FK506 (1 or 10 μM) was added 1 h prior to the addition of DEX or EtOH and incubated for 2 days.
2.3. Cell Spreading Assay
The cell spreading assay was done as previously described [7]. Briefly, cells were spread for 1.5 h on coverslips precoated with 20 nM fibronectin and co-labeled with anti-αvβ3 integrin mAb and Alexa 488 conjugated phalloidin (Life Technologies) as described [9]. Images were captured with an Axioplan 2 epifluorescence microscope (Carl Zeiss, Inc.) equipped with an Axiocam HRm digital camera using AxioVision image acquisition software.
2.4. Immunoblotting
HTM cells were washed and lysed with lysis buffer (25 mM Hepes, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM NaF, 1% NP-40, 0.25% deoxycholate, HALT phosphatase inhibitor cocktail and HALT protease inhibitor cocktail (Thermo Fischer Scientific, Inc.). The cellular debris in the cell lysate was removed by centrifugation at 10,000 × g. A bicinchoninic acid (BCA) assay (Pierce) was done to determine protein concentration and the lysate (10 μg) was separated on a 10% SDS-PAGE and transferred to Immobilon-P (Millipore Corp.). The membrane was blocked in 3% bovine serum albumin (BSA)/tris buffered saline (TBS) or 5% milk/TBS (FKBP51 pAb) overnight at 4°C and incubated with the primary antibody in 1% BSA/TBS/0.1% Tween-20 or 5% milk/TBS/0.1% Tween-20 for 1 h. Membranes were washed with TBS/0.1% Tween-20 and incubated for 1 h with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG. Bound antibody was detected with the ECL Plus Western blotting detection kit (Amersham Biosciences, Piscataway, NJ).
2.5. Flow Cytometry
HTM cells treated with DEX or EtOH were lifted from the plate with Cell Dissociation Buffer (Sigma), washed with ice cold PBS and blocked for 30 min on ice with 2% BSA in PBS (blocking buffer). Cells were labeled with the primary antibodies for 1 h on ice in blocking buffer and then incubated on ice for 30 min with the secondary antibody. Cells were washed then resuspended in 1% paraformaldehyde in PBS. Flow cytometry was done with a FACSCalibur (BD Biosciences) using FlowJo software (Tree Star, Inc.) for analysis.
2.6. RNA Isolation, Reverse Transcription and Real-Time (RT)-qPCR
Total RNA was isolated using the QIAshredder and RNeasy Plus Mini Kits (Qiagen Inc., Valencia, CA) and RNA concentration was determined using a NanoDrop spectrophotometer. Total RNA (1 μg) was reverse transcribed and RT-qPCR experiments using the synthesized cDNA were performed as previously described [25]. Data were normalized to no treatment and fold change compared to the β1 integrin housekeeping gene was determined according to Pfaffl [26]. Primers pairs used were: β1 integrin forward 5′-GTGGAGAATCCAGAGTGTCCCA-3′ and reverse 5′-GACCACAGTTGTTACGG-3′, β3 integrin forward 5′-GTGACCTGAAGGAGAATCTGC-3′ and reverse 5′-TTCTTCGAATCATCTGGCC-3′, and FKBP51 forward 5′-CTCCCTAAAATTCCCTCGAATGC-3′ and reverse 5′-CCCTCTCCTTTCCGTTTGGTT-3′.
2.7. Rate of RNA Synthesis
Nascent RNA synthesis was determined using the Click-iT® Nascent RNA Capture Kit (Life Technologies). Cells were treated with DEX or EtOH for 2 days after which cells were labeled by incubation with 0.1 mM 5-ethynyl uridine (EU) for 1 h in the presence of DEX or EtOH. Cells were collected and the RNA was isolated as described above. EU labeled RNA (1 μg total RNA) was then biotinylated and isolated using Dynabeads® MyOne™ Streptavidin T1 magnetic beads according to the manufacturer’s instructions. RT-qPCR was performed as above using primers for β3 integrin.
2.8. Data Analysis
Data are presented as mean ± S.E.M. Statistical comparisons were done using the Student T-test and a p value < 0.05 was considered significant. Relative quantification of the RT-qPCR data was performed according to Pfaffl [26], using β1 integrin for normalization.
3. Results
3.1. DEX increases β3 integrin protein and mRNA levels
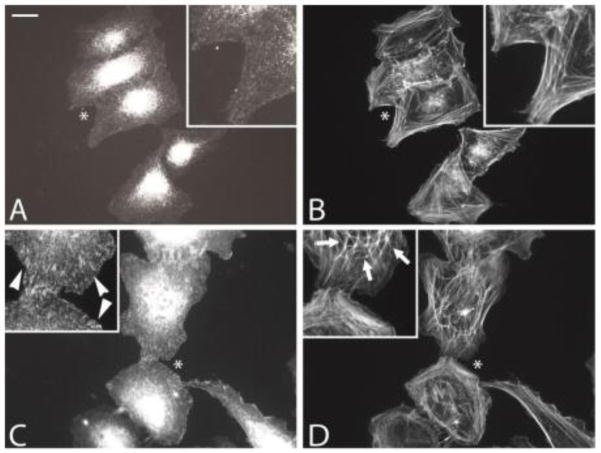
Fig. 1 shows that treating HTM cells with DEX increases the level of αvβ3 integrin in focal adhesions (Fig. 1A vs. 1C). When stained with phalloidin, a subset of the cells incubated with DEX also exhibited the distinctive geodesic dome actin structure called CLANs thought to be involved in glaucoma and caused by αvβ3 integrin signaling (Fig. 1B vs. 1D).
Fig. 1. Localization of αvβ3 integrin in DEX and EtOH treated HTM cells.
HTM cells treated with EtOH (A, B) or DEX (C, D) for 4 days were spread on fibronectin-coated coverslips for 1.5 h prior to fixation. The cells were labeled with mAb LM609 against αvβ3 integrin (A, C) and phalloidin (B, D). Asterisks indicate areas enlarged in the insets; Arrowheads indicate αvβ3 integrin-positive adhesions; arrows indicate part of a CLAN. Scale bar = 20 μm.
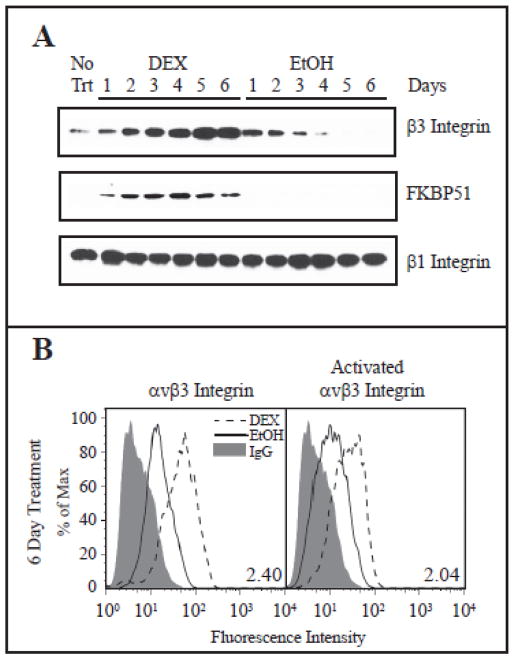
To understand how DEX affected the expression of αvβ3 integrin in HTM cells that leads to CLAN formation, western blots analysis were carried out to detect changes in αvβ3 integrin expression over 6 days of DEX treatment. This prolonged treatment was chosen in order to correspond with the time frame needed to observe CLAN formation in vitro [27] and the time course of patient treatment. As shown in Fig. 2A, DEX induced a time dependent increase in the expression of the β3 integrin subunit starting 2 days after initiation of DEX treatment. By day 5, the β3 integrin subunit level appeared to plateau. This increase was not seen in the EtOH treated controls. Rather, β3 integrin levels appear to diminish the longer the EtOH treated cells were in culture. (Fig. 2A). The DEX-induced increase was unique to the β3 integrin subunit as no changes were observed in β1 integrin levels. This upregulation of expression was not limited to this cell strain and was observed in three other HTM cell strains (data not shown). As a positive control we also examined the levels of the FK506-binding protein FKBP51, which is known to be induced by DEX in several cell types [28, 29], including TM cells [30]. As expected, FKBP51 also showed a DEX induced increase in expression. However, this increase occurred after one day of treatment compared to the 2 days needed to increase β3 integrin. FACS analysis verified the western blot analysis and showed that DEX treatment increased the expression of β3 integrin at the cell surface 2.4 fold compared to EtOH (Fig. 2B). Although αvβ3 integrin had an active conformation in both DEX-treated and ETOH-treated cells, as determined with the CRC54 mAb that detects activated αvβ3 integrin (Fig. 2B, right panel), the level of activated αvβ3 integrin was ~2 fold greater in the DEX-treated cells compared to the EtOH-treated cells. In contrast, the level of the αv integrin subunit was not changed by DEX, as determined by FACS (data not shown).
Fig. 2. DEX induced β3 integrin expression and activation.
(A) HTM cells were treated with DEX or EtOH for 6 days and cell lysates were analyzed for levels of β3 integrin, β1 integrin and FKBP51. SDHA was included as a loading control. Blots are representative of 3 biological replicates. (B) Cells were treated with DEX or EtOH for 6 days. FACS analysis was done using the mAb LM609 against αvβ3 integrin (left) or mAb CRC54 which detects activated αvβ3 integrin (right). The fold change in geometric mean of DEX vs. EtOH is indicated the panels for each antibody. Data representative of 2 biological replicates.
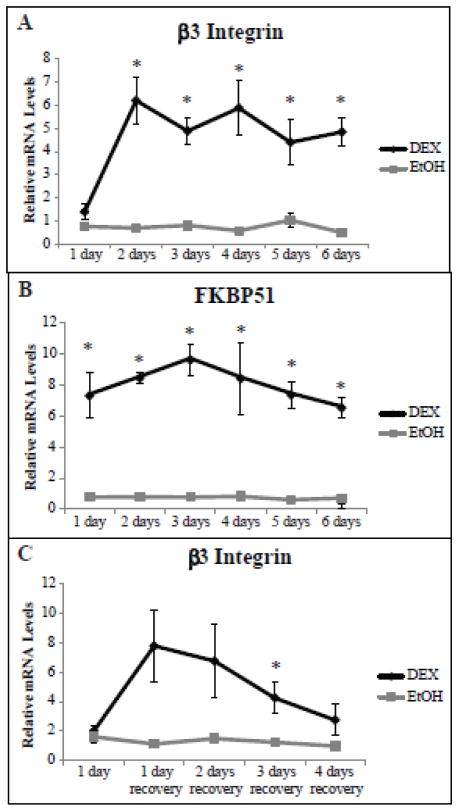
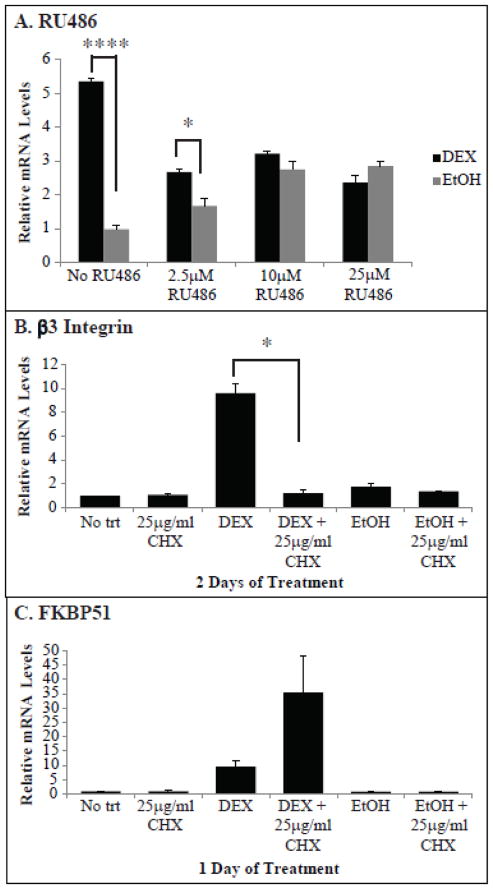
Using RT-qPCR, we found that DEX increased the expression of β3 integrin mRNA by 6.2 fold (p<0.04) after 2 days of DEX treatment compared to the EtOH controls. The mRNA levels remained elevated throughout the 6 day treatment (Fig. 3A). In contrast, β1 integrin mRNA was unaffected by DEX (data not shown) and therefore was used as a housekeeping gene to control for normalization. As expected, FKBP51 mRNA levels also increased by 7.3 fold (p<0.04) and remained elevated throughout treatment (Fig. 3B). The increase in FKBP51 mRNA levels appeared after only 1 day of DEX treatment compared to 2 days for β3 integrin mRNA.
Fig. 3. DEX treatment increases β3 integrin mRNA levels.
RT-qPCR was performed on HTM cells treated with DEX or EtOH. Ct values were corrected for primer efficiency and compared to cells with no treatment as described by Pfaffl [26]. Data was then normalized to the β1 integrin housekeeping gene. Level of β3 integrin mRNA (A) and FKBP51 mRNA (B) expression in the presence of DEX or EtOH for 6 days. Level of β3 integrin mRNA (C) in HTM cells treated with DEX or EtOH for 1 day only followed by media alone for 4 days. DEX significantly different from EtOH *p<0.05. n = 4 biological replicates (A, B), n = 3 biological replicates (C).
To determine if 2 days of DEX treatment was needed to increase β3 integrin mRNA levels, cultures were treated with DEX for 1 day only. As shown in Fig. 3C, β3 integrin mRNA levels still increased on day 2 even though DEX was not present on the second day. However, in the absence of any additional DEX, the mRNA levels started to decrease and by day 5 the levels of β3 integrin mRNA returned to baseline.
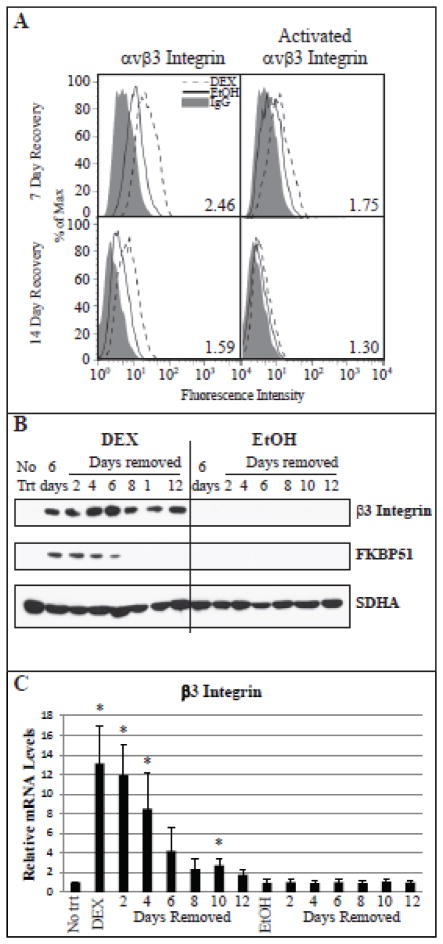
Since some individuals treated with glucocorticoids go on to develop glaucoma even after glucocorticoid treatment has stopped, we asked what happens to αvβ3 integrin expression once DEX is removed. FACs analysis (Fig. 4A, left panels) showed that β3 integrin expression remained 1.59 fold higher 14 days after removal of DEX compared to EtOH. Interestingly, there was still an elevation (~1.75 fold) in the level of activated αvβ3 integrin in the DEX-treated cells compared to the EtOH treated cells 7 days after the removal of the DEX (Fig. 4A, right panels). By day 14, little of the αvβ3 integrin in the DEX-treated cells was activated, although protein expression was still upregulated. Western blot analysis of lysate from cells treated with DEX followed by DEX removal verified the FACS analysis and showed that total β3 integrin expression is still elevated 12 days after DEX removal (Fig. 4B). The increase in β3 integrin levels coincided with its mRNA levels which remained elevated for up to 10 days after removal of DEX from the medium (Fig. 4C). In contrast, FKBP51 protein levels returned to EtOH control levels 8 days after removing DEX. Similar results were seen at the mRNA level for FKBP51.
Fig. 4. Elevated levels of β3 integrin persists following the removal of DEX.
(A) Cells were treated with DEX or EtOH for 6 days after which cells were incubated with media alone for 7 days (upper panels) or 14 days (lower panels). FACS analysis was done with the mAb LM609 against αvβ3 integrin (left) or mAb CRC54 which detects activated αvβ3 integrin (right). The fold change in geometric mean fold of DEX vs. EtOH is indicated in each of the panels for each antibody. Data representative of 2 biological replicates. (B and C) HTM cells were treated with DEX or EtOH for 6 days followed by media alone for 12 days. (B) Lysate was collected every other day after DEX or EtOH removal and analyzed for the expression of β3 integrin and FKBP51. SDHA was included as a loading control. Blots representative of 2 biological replicates. (C) Cells were collected every other day after DEX or EtOH removal and total RNA was analyzed by RT-qPCR. Ct values were corrected for primer efficiency and compared to cells with no treatment as described by Pfaffl [26]. Data was then normalized to the β1 integrin housekeeping gene. DEX significantly different from equivalent EtOH control, *p<0.05. n = 3 biological replicates.
3.2. β3 integrin mRNA half-life and synthesis rate increase with DEX
We next used actinomycin D to determine if the accumulation of β3 integrin mRNA could also be attributed to an increase in mRNA half-life. Cells were treated for two days with DEX or EtOH after which a final concentration of 5 μg/ml actinomycin D was added to fresh media containing DEX and EtOH for 4, 8, 12 or 24 h. Fig. 5A shows that in the presence of actinomycin D there was no significant decrease in the β3 integrin mRNA compared to the zero time at 4, 8 or 12 h. However, β3 integrin mRNA levels were significantly different from time zero at 24 h (p<0.025). In contrast, there was a significant decrease in the level of β3 integrin mRNA in the EtOH treated controls over the entire 24 h. The half-life in DEX and EtOH treated cells was 60.7 and 22.5 h respectively (p<0.05 overall). This suggests that the half-life of the β3 integrin mRNA was increased in the presence of DEX. In addition to mRNA half-life, DEX also increased β3 integrin mRNA synthesis. Cells treated with DEX or EtOH for 2 days were labeled with 5-ethynyl uridine (EU) for 1 h to detect nascent RNA. Fig. 5B shows a 5.9 fold increase in nascent β3 integrin mRNA compared to EtOH treated cells (p<0.025).
Fig. 5. DEX treatment increased β3 integrin mRNA stability and synthesis.
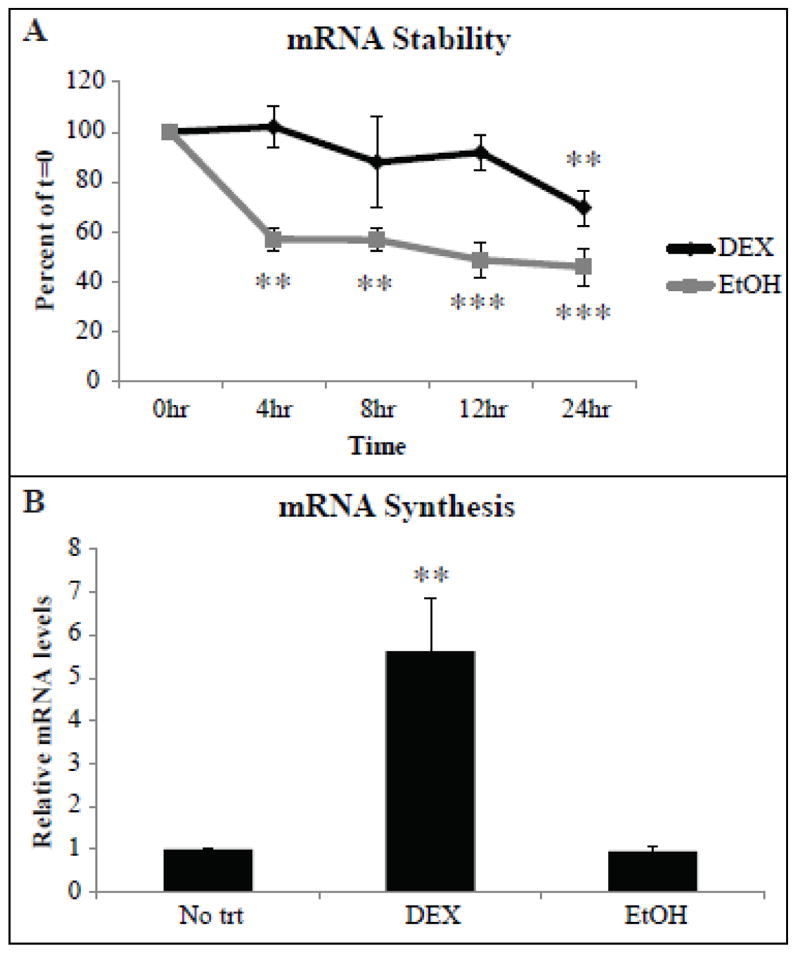
(A) HTM cells were treated with DEX or EtOH for 2 days after which 5 μg/ml actinomycin D was added to cells in the presence of DEX or EtOH. RNA was isolated from cells after 4, 8, 12 or 24 h of actinomycin D treatment. RT-qPCR was performed and the average mRNA half- life was determined from the slopes of the linear regression lines from each experiment. Data from cells treated with DEX or EtOH with actinomycin D was normalized to αvβ3 integrin mRNA levels after 2 days of DEX or EtOH alone respectively (t=0). Significantly different from 0 h, **p<0.025, ***p<0.02. DEX significantly different from EtOH overall p<0.05. (B) HTM cells were treated with DEX or EtOH for 2 days after which nascent RNA was labeled with 5-ethynyl Uridine (EU). After 1 h, EU labeled RNA was isolated using the Click-iT® Nascent RNA Capture Kit from Invitrogen (see Methods). Relative mRNA levels compared to No trt was determined. DEX significantly different from EtOH, **p<0.025. n = 4 biological replicates (A) or 3 biological replicates (B).
3.3. The increase in β3 integrin mRNA requires glucocorticoid receptor signaling
The delay in β3 integrin mRNA induction by DEX suggests that this is a secondary glucocorticoid response and that another factor induced by DEX is needed for the increase seen in β3 integrin mRNA levels. To test if the DEX induced increase in αvβ3 integrin is mediated through glucocorticoid receptor signaling, cultures were treated with the glucocorticoid receptor antagonist RU486. The RU486-glucocorticoid receptor complex interacts with DNA at a much lower affinity than that of the dexamethasone-GR complex and can act as an agonist when other transcriptional coactivators are activated or in specific cells lines[31–33]. For this experiment, RU486 was added 1 h prior to the addition of DEX or EtOH, to determine if a DEX-induced factor was responsible for the increase in β3 integrin mRNA. In the absence of RU486, DEX increased β3 integrin mRNA by 5.4 fold on the second day of treatment (p<0.001; Fig. 6A). Preincubation with RU486 eliminated this increase in β3 integrin mRNA, suggesting that the increase was due to a glucocorticoid signaling event. RU486 by itself was able to increase β3 integrin mRNA in the EtOH controls, but not to the same extent as DEX alone.
Fig. 6. RU486 and CHX prevented the induction of β3 integrin mRNA by DEX.
RT-qPCR was performed on HTM cells pretreated for 1 h with 2.5, 10 or 25 μM RU486 (A) or 25 μg/ml CHX (B, C) prior to the addition of DEX or EtOH for 2 days (A, B) or 1 day (C). Fresh media containing inhibitor and DEX or EtOH was added after 1 day (A, B). Ct values were corrected for primer efficiency and compared to cells with no treatment as described by Pfaffl [26]. Data was then normalized to the β1 integrin housekeeping gene. Significantly different as indicated with brackets, *p<0.05, ****p<0.001. n = 3 biological replicates.
3.4. DEX induces β3 integrin through a secondary glucocorticoid response mechanism
To demonstrate that this is a secondary glucocorticoid response and required de novo protein synthesis expression for induction [34], cultures were pretreated with cycloheximide. As shown in Fig. 6B, treatment with cycloheximide caused a significant decrease in the level of β3 integrin mRNA induction observed at day 2 (p<0.05). In contrast, pre-treatment with cycloheximide had no inhibitory effect on the DEX induced increase in FKBP51 mRNA levels observed after one day of DEX treatment (Fig. 6C). These results indicate that transcription of the FKBP51 mRNA is directly induced by DEX whereas transcription of the β3 integrin mRNA required translation of a DEX-induced protein.
3.5. Calcineurin regulates β3 integrin
To understand the mechanisms by which DEX induces β3 integrin mRNA expression we treated the cells with CsA and FK506. CsA and FK506 are immunosuppressants that inhibit calcineurin, a phosphatase that activates the NFAT family of transcription factors. [23] NFATc1 had previously been shown to directly induce β3 integrin expression in osteoclasts [21]. FK506 has also been shown to suppress endogenous expression of NFATc1 [35]. Figs. 7A and B show that in cells pretreated with either CsA or FK506, the DEX-induced increase in β3 integrin mRNA was significantly inhibited suggesting that calcineurin may play a role in regulating β3 integrin mRNA levels. Only CsA, however, completely inhibited the increase in β3 integrin mRNA back to the control level. This was not completely unexpected since several studies have shown that these compounds exhibit different efficiencies at inhibiting calcineurin [36, 37] which may be due to the levels of their respective target immunophilins.
Fig. 7. CsA and FK506 prevented the induction of β3 integrin mRNA by DEX.
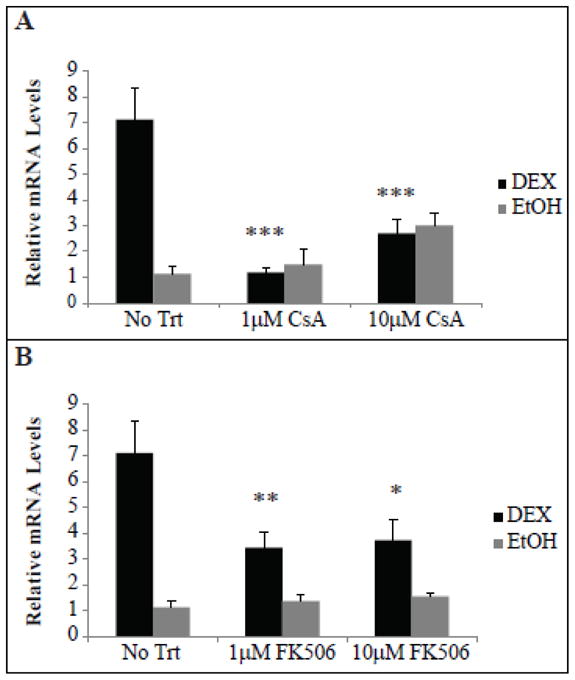
RT-qPCR was performed on HTM cells pretreated for 1 h with 1 or 10 μM CsA (A) or FK506 (B) prior to the addition of DEX or EtOH for 2 days. Fresh media containing inhibitor and DEX or EtOH was added after 1 day. Ct values were corrected for primer efficiency and compared to cells with no treatment as described by Pfaffl [26]. Data was then normalized to the β1 integrin housekeeping gene. Significantly different from DEX alone (no trt), *p<0.05, **p<0.025, ***p<0.02. n = 4 biological replicates (A, B) or 3 biological replicates (C).
4. Discussion
In this paper we demonstrate that the upregulation of β3 integrin mRNA and protein levels by DEX is a secondary glucocorticoid response that is regulated by calcineurin. This novel induction of β3 integrin expression appeared to be triggered by a classic GR-mediated pathway since it could be inhibited by RU486. The increase in β3 integrin expression was due both to an increase in the half-life of the β3 integrin mRNA as well as an increase in transcription. It resulted in an expression of the β3 integrin subunit that persisted for days even after the removal of DEX. Thus, DEX appears to specifically trigger a long-term dysregulation of αvβ3 integrin signaling in trabecular meshwork cells which could explain the cytoskeleton changes associated with glaucoma.
The DEX-induced upregulation of β3 integrin in HTM cells differed from that previously reported in osteoblasts [20]. In those studies, β3 integrin was transiently expressed. In comparison, the upregulation of β3 integrin persisted in HTM cells for 2 weeks even after the removal of DEX. The most likely explanation for this difference is that the upregulation of β3 integrin mRNA in osteoblasts involved specific palindromic sequences called glucocorticoid response elements (GREs) in the 5′ region of the β3 integrin promoter suggesting a direct interaction between the GR receptor and the GREs in the promoter. In contrast, this interaction with the GREs did not appear to function in HTM cells, since the DEX-induced upregulation of the β3 integrin subunit could be inhibited with cycloheximide indicating the need for factor(s) to be synthesized prior to the upregulation of the β3 subunit.
This DEX induced increase in β3 integrin mRNA levels may be unique to HTM cells and is similar to that observed for the glucocorticoid response protein called myocilin [38, 39]. Myocilin is a secreted glycoprotein that is mutated in some juvenile open-angle glaucoma [40–42]. Like the β3 integrin gene, myocilin also contains GREs and the upregulation of its expression is also due to a secondary glucocorticoid response [38, 39]. Why the GREs in myocilin and the β3 integrin subunit are not utilized by the GR receptor is not clear. There are a number of factors which are responsible for mediating the interactions between the GREs and the GR receptor [43] including the multi-subunit complexes that remodel chromatin, target the initiation sites and stabilize the RNA polymerase II machinery.
It is presently unclear which factor(s) are involved in the upregulation of β3 integrin. One possible mechanism may be that the GR is exerting its effect through an indirect non-DNA binding mechanism termed transrepression [44]. In this mechanism, transcriptional modulation of the β3 integrin subunit could be achieved by cross-talk between the GR and other transcription factors such as NF-κB, AP-1 and members of the STAT family. However, because it took 2 days to see the upregulation and it required de novo protein synthesis, we tend to favor the idea that DEX induced the expression of a soluble factor which in turn activated calcineurin. In support of this idea, recent proteomic studies have shown that phosphatidylinositide 3-kinase (PI-3K) and protein kinase C (PKC), both of which modulate calcineurin signaling [23], are upregulated in HTM cells in response to DEX [25]. It was not surprising that calcineurin was involved. Previous studies have shown that the regulation of β3 integrin subunit involves NFATc1 [21, 45] which is a well-established target of calcineurin [23]. Interestingly, in bone marrow cells NFATc1 expression is upregulated in the cytoplasm after one day of treatment with RANKL, a factor that induces osteoclast formation, and detected in the nucleus after 2 days of treatment [46, 47]. Whether NFATc1 is upregulated by DEX in TM cells is unknown.
Calcineurin, has been shown to regulate the organization of the cytoskeleton. The involvement of calcineurin fits with the observations that in HTM cell cultures and in anterior segments, DEX induces the reorganization of the actin cytoskeleton into CLANs [7, 16]. This unique actin structure is thought to make the cells more rigid so they cannot change shape in response to pressure changes. This, in turn, causes the TM to become more rigid which could restrict the outflow of aqueous humor from the eye [14, 48].
Interestingly, the DEX treatment also increased the stability of the β3 integrin mRNA. The regulation of mRNA stability is a powerful mechanism to alter long term gene expression and has been shown to be controlled by hypoxia, inflammation, cancer and aging [49]. In terms of steroid induced glaucoma or primary open angle glaucoma, this enhanced mRNA stability could have large implications especially since the elevated levels of β3 integrin subunit at the cell surface also appeared to be more stable and more of the αvβ3 integrin was in the activated state. At the present time, however, it is not possible to say whether the increased mRNA expression was due to an increase in mRNA stability or in transcription. This supports previous work from our laboratory which showed that in the presence of DEX, CLAN formation is associated with a change in the conformation of αvβ3 integrin that results in integrin activation. In the absence of DEX, CLANs can be formed simply by activating αvβ3 integrin using either the AP-5 antibody or the thrombospondin peptide 4N1K [8]. Thus, a DEX induced increased in β3 integrin expression and activation could explain the increased frequency of CLAN formation observed in glaucomatous and DEX-treated anterior segments.
In summary, this study suggests some of the long term effects normally attributed to steroid induced glaucoma SIG could be due to the indirect activation of calcineurin that results in a prolonged dysregulation of αvβ3 integrin signaling. Clearly additional studies looking at the other transcriptional factors responsible for the secondary glucocorticoid response and their role in the expression of proteins such as myocilin are warranted, since it may provide insight on how to prevent undesirable steroid induced side effects in the treatment of eye diseases.
Highlights.
Dexamethasone increases β3 integrin mRNA synthesis, stability and protein expression.
The β3 integrin increase occurs through a secondary glucocorticoid response.
The dexamethasone induction of β3 integrin is mediated by calcineurin/NFAT.
Acknowledgments
This work was supported by NEI grants EY017006, EY0020490 (D.M.P.), and a Core grant to the Department of Ophthalmology and Visual Sciences (P30 EY016665).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alghisi GC, Ruegg C. Vascular integrins in tumor angiogenesis: mediators and therapeutic targets. Endothelium. 2006;13:113–135. doi: 10.1080/10623320600698037. [DOI] [PubMed] [Google Scholar]
- 2.Kuphal S, Bauer R, Bosserhoff AK. Integrin signaling in malignant melanoma. Cancer Metastasis Rev. 2005;24:195–222. doi: 10.1007/s10555-005-1572-1. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Park R, Tohme M, Shahinian AH, Bading JR, Conti PS. MicroPET and autoradiographic imaging of breast cancer alpha v-integrin expression using 18F- and 64Cu-labeled RGD peptide. Bioconjug Chem. 2004;15:41–49. doi: 10.1021/bc0300403. [DOI] [PubMed] [Google Scholar]
- 4.Hutchinson JH, Halczenki W, Brashear KM, Breslin MJ, Coleman PJ, Duong le T, Fernandez-Metzler C, Gentile MA, Fisher JE, Hartman GD, Huff JR, Kimmel DB, Leu CT, Meissner RS, Merkle K, Nagy R, Pennypacker B, Perkins JJ, Prueksaritanont T, Rodan GA, Varga SL, Wesolowski GA, Zartman AE, Rodan SB, Duggan ME. Nonpeptide αvβ3 antagonists. 8. In vitro and in vivo evaluation of a potent αvβ3 antagonist for the prevention and treatment of osteoporosis. J Med Chem. 2003;2003:4790–4798. doi: 10.1021/jm030306r. [DOI] [PubMed] [Google Scholar]
- 5.Panchatcharam M, Miriyala S, Yang F, Leitges M, Chrzanowska-Wodnicka M, Quilliam LA, Anaya P, Morris AJ, Smyth SS. Enhanced proliferation and migration of vascular smooth muscle cells in response to vascular injury under hyperglycemic conditions is controlled by β3 integrin signaling. Int J Biochem Cell Biol. 2010;42:965–974. doi: 10.1016/j.biocel.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemmons DR, Maile LA, Ling Y, Yarber J, Busby WH. Role of the integrin αvβ3 in mediating increased smooth muscle cell responsiveness to IGF-1 in response to hyperglycemic stress. Growth Horm IGF Res. 2007;17:265–270. doi: 10.1016/j.ghir.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filla MS, Schwinn MK, Nosie AK, Clark RW, Peters DM. Dexamethasone-associated cross-linked actin network formation in human trabecular meshwork cells involves β3 integrin signaling. Invest Ophthalmol Vis Sci. 2011;52:2952–2959. doi: 10.1167/iovs.10-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filla MS, Schwinn MK, Sheibani N, Kaufman PL, Peters DM. Regulation of cross-linked actin network (CLAN) formation in human trabecular meshwork (HTM) cells by convergence of distinct β1 and β3 integrin pathways. Invest Ophthalmol Vis Sci. 2009;50:5723–5731. doi: 10.1167/iovs.08-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filla MS, Woods A, Kaufman PL, Peters DM. β1 and β3 integrins cooperate to induce syndecan-4-containing cross-linked actin metworks in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006;47:1956–1967. doi: 10.1167/iovs.05-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker B, Mills DW. Corticosteroids and intraocular pressure. Arch Ophthalmol. 1963;70:500–507. doi: 10.1001/archopht.1963.00960050502012. [DOI] [PubMed] [Google Scholar]
- 11.Armaly MF. Effect of corticosteroids on intraocular and fluid dynamics. I. The effect of dexamethasone in the normal eye. Arch Ophthalmol. 1963;70:482–491. doi: 10.1001/archopht.1963.00960050484010. [DOI] [PubMed] [Google Scholar]
- 12.Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000;41:619–623. [PubMed] [Google Scholar]
- 13.Faralli JA, Schwinn MK, Gonzalez JM, Filla MS, Peters DM. Functional properties of fibronectin in the trabecular meshwork. Exp Eye Rese. 2009;88:689–693. doi: 10.1016/j.exer.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark AF, Brotchie D, Read AT, Hellberg P, English-Wright S, Pang IH, Ethier CR, Grierson I. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskeleton. 2005;60:83–95. doi: 10.1002/cm.20049. [DOI] [PubMed] [Google Scholar]
- 15.Clark AF, Miggans ST, Wilson K, Browder S, McCartney MD. Cytoskeletal changes in cultured human glaucoma trabecular meshwork cells. J Glaucoma. 1995;4:183–188. [PubMed] [Google Scholar]
- 16.Clark AF, Wilson K, McCartney MD, Miggans ST, Kunkle M, Howe W. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1994;35:281–294. [PubMed] [Google Scholar]
- 17.Kitazawa S, Ross FP, McHugh K, Teitelbaum SL. Interleukin-4 induces expression of the integrin αvβ3 via transactivation of the β3 gene. J Biol Chem. 1995;270:4115–4120. doi: 10.1074/jbc.270.8.4115. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi H, Kume T. Foxc2 transcription factor as a regulator of angiogenesis via induction of integrin β3 expression. Cell Adh Migr. 2009;3:24–26. doi: 10.4161/cam.3.1.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi H, Sano H, Seo S, Kume T. The Foxc2 transcription factor regulates angiogenesis via induction of integrin β3 expression. J Biol Chem. 2008;283:23791–23800. doi: 10.1074/jbc.M800190200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng SL, Lai CF, Fausto A, Chellaiah M, Feng X, McHugh KP, Teitelbaum SL, Civitelli R, Hruska KA, Ross FP, Avioli LV. Regulation of αvβ3 and αvβ5 integrins by dexamethasone in normal human osteoblastic cells. J Cell Biochem. 2000;77:265–276. doi: 10.1002/(sici)1097-4644(20000501)77:2<265::aid-jcb9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Crotti TN, Flannery M, Walsh NC, Fleming JD, Goldring SR, McHugh KP. NFATc1 regulation of the human β3 integrin promoter in osteoclast differentiation. Gene. 2006;372:92–102. doi: 10.1016/j.gene.2005.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun L, Peng Y, Zaidi N, Zhu L-L, Iqbal J, Yamoah K, Wang X, Liu P, Abe E, Moonga BS, Epstein S, Zaidi M. Evidence that calcineurin is required for the genesis of bone-resorbing osteoclasts. Am J Physiol Renal Physiol. 2007;292:F285–F291. doi: 10.1152/ajprenal.00415.2005. [DOI] [PubMed] [Google Scholar]
- 23.Crabtree GR. Calcium, calcineurin, and the control of transcription. J Biol Chem. 2001;276:2313–2316. doi: 10.1074/jbc.R000024200. [DOI] [PubMed] [Google Scholar]
- 24.Filla MS, David G, Weinreb RN, Kaufman PL, Peters DM. Distribution of syndecans 1–4 within the anterior segment of the human eye: expression of a variant syndecan-3 and matrix-associated syndecan-2. Exp Eye Res. 2004;79:61–74. doi: 10.1016/j.exer.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Clark RW, Nosie AK, Walker T, Faralli JA, Filla MS, Barrett-Wilt G, Peters DM. Comparative genomic andproteomic analysis of cytoskeletal changes in dexamethasone-treated trabecular meshwork cells. Mol Cell Proteomics. 2013;12:194–206. doi: 10.1074/mcp.M112.019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR. Gene structure and properties of TIGR, a olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998;273:6341–6350. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds PD, Roveda KP, Tucker JA, Moore CM, Valentine DL, Scammell JG. Glucocorticoid-resistant B-lymphoblast cell line derived from the Bolivian squirrel monkey (Saimiri boliviensis boliviensis) Lab Anim Sci. 1998;48:364–370. [PubMed] [Google Scholar]
- 29.Baughman G, Wiederrecht GJ, Chang F, Martin MM, Bourgeois S. Tissue distribution and abundance of human FKBP51 and FK506-binding protein that can mediate calcineurin inhibition. Biochem Biophys Res Commun. 1997;232:437–443. doi: 10.1006/bbrc.1997.6307. [DOI] [PubMed] [Google Scholar]
- 30.Nehme A, Lobenhofer EK, Stamer WD, Edelman JL. Glucocorticoids with different chemical structures but similar glucocorticoid receptor potency regulate subsets of common and unique genes in human trabecular meshwork cells. BMC Med Genomics. 2009;2:58. doi: 10.1186/1755-8794-2-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fryer CJ, Kinyamu HK, Rogatsky I, Garabedian MJ, Archer TK. Selective activation of the glucocorticoid receptor by steroid antagonists in human breast cancer and osteosarcoma cells. J Biol Chem. 2000;275:17771–17777. doi: 10.1074/jbc.M908729199. [DOI] [PubMed] [Google Scholar]
- 32.Distelhorst CW, Howard KK. Evidence from pulse-chase labeling studies that the antiglucocorticoid hormone RU486 stabilizes the nonactivated form of the glucocorticoid receptor in mouse lymphoma cells. J Steroid Biochem. 1990;36:25–31. doi: 10.1016/0022-4731(90)90110-e. [DOI] [PubMed] [Google Scholar]
- 33.Bourgeois S, Pfahl M, Baulieu EE. DNA binding properties of glucocorticosteroid receptors bound to the steroid anatagonist RU-486. EMBO J. 1984;3:751–755. doi: 10.1002/j.1460-2075.1984.tb01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clever U, Romball CG. RNA and protein synthesis in the cellular response to a hormone ecdysone. Proc Natl Acad Sci U S A. 1966;56:1470–1476. doi: 10.1073/pnas.56.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zawawi MSF, Dharmapatni AASSK, Cantley MD, McHugh KP, Haynes DR, Crotti TN. Regulation of ITAM adaptor molecules and their receptors by inhibition of calcineurin-NFAT signalling during late stage osteoclast differentiation. Biochem Biophys Res Commun. 2012;427:404–409. doi: 10.1016/j.bbrc.2012.09.077. [DOI] [PubMed] [Google Scholar]
- 36.Liu J, Farmer JDJ, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 37.Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 2006;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 38.Joe MK, Sohn S, Kim TE, Im J, Choi YR, Kee C. Analysis of glucocorticoid-induced MYOC expression in human trabecular meshwork cells. Vis Res. 2011;51:1033–1038. doi: 10.1016/j.visres.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 39.Shepard AR, Jacobson N, Fingert JH, Stone EM, Sheffield VC, Clark AF. Delayed secondary glucocorticoid responsiveness of MYOC in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2001;42:3173–3181. [PubMed] [Google Scholar]
- 40.Alward WL, Fingert JH, Coote MA, Johnson AT, Lerner SF, Junqua D, Durcan FJ, McCartney PJ, Mackey DA, Sheffield VC, Stone EM. Clinical features associated with mutations in the chromosome 1 open-angle glaucoma gene (GLC1A) N Eng J Med. 1998;338:1022–1027. doi: 10.1056/NEJM199804093381503. [DOI] [PubMed] [Google Scholar]
- 41.Fingert JH, Jeon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, Kawase K, Hoh ST, Buys YM, Dickinson J, Hockey RR, Williams-Lyn D, Trope G, Kitazawa Y, Ritch R, Mackey DA, Alward WL, Sheffield VC, Stone EM. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8:899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- 42.Stone EM, Fingert JH, Alward WLM, Nguyen TD, Polansky JR, Sunden SLF, Nishimura D, Clark AF, Nystuen A, Nichols B, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 43.Nicolaides NC, Galata Z, Kino T, Chrousos GP, Charmandari E. The human glucocorticoid receptor: molecular basis of biologic function. Steroids. 2010;75:1–12. doi: 10.1016/j.steroids.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muzikar KA, Nickol NG, Dervan PB. Repression of DNA-biding dependent glucocorticoid receptor-mediated gene expression. Proc Natl Acad Sci U S A. 2009;106:16598–16603. doi: 10.1073/pnas.0909192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crotti TN, Sharma SM, Fleming JD, Flannery MR, Ostrowski MC, Goldring SR, McHugh KP. PU.1 and NFATc1 mediate osteoclastic induction of the mouse β3 integrin promoter. J Cell Physiol. 2008;215:636–644. doi: 10.1002/jcp.21344. [DOI] [PubMed] [Google Scholar]
- 46.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 47.Ishida N, Hayashi K, Hoshijima M, Ogawa T, Koga S, Miyatake Y, Kumegawa M, Kimura T, Takeya T. Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J Biol Chem. 2002;277:41147–41156. doi: 10.1074/jbc.M205063200. [DOI] [PubMed] [Google Scholar]
- 48.Wade NC, Grierson I, O’Reilly S, Hoare MJ, Cracknell KP, Paraoan LI, Brotchie D, Clark AF. Cross-linked actin networks (CLANs) in bovine trabecular meshwork cells. Exp Eye Res. 2009;89:648–659. doi: 10.1016/j.exer.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Ing NH. Steroid hormones regulate gene expression posttranscriptionally by altering the stabilities of messenger RNAs. Biol Reprod. 2005;72:1290–1296. doi: 10.1095/biolreprod.105.040014. [DOI] [PubMed] [Google Scholar]