Abstract
The chloroplast twin arginine translocation (cpTat) system transports highly folded precursor proteins into the thylakoid lumen using the protonmotive force as its only energy source. Hcf106, as one of the core components of the cpTat system, is part of the precursor receptor complex and functions in the initial precursor-binding step. Hcf106 is predicted to contain a single amino terminal transmembrane domain followed by a Pro-Gly hinge, a predicted amphipathic α-helix (APH), and a loosely structured carboxy terminus. Hcf106 has been shown biochemically to insert spontaneously into thylakoid membranes. To better understand the membrane active capabilities of Hcf106, we used solid-state NMR spectroscopy to investigate those properties of the APH. In this study, synthesized peptides of the predicted Hcf106 APH (amino acids 28–65) were incorporated at increasing mol% into 1-palmitoyl-2-oleoyl-sn-glycero-phosphocholine (POPC) and POPC/MGDG (monogalactosyldiacylglycerol; mole ratio 85:15) multilamellar vesicles (MLVs) to probe the peptide-lipid interaction. Solid-state 31P NMR and 2H NMR spectroscopic experiments revealed that the peptide perturbs the headgroup and the acyl chain regions of phospholipids as indicated by changes in spectral lineshape, chemical shift anisotropy (CSA) line width, and 2H order SCD parameters. In addition, the comparison between POPC MLVs and POPC/MGDG MLVs indicated that the lipid bilayer composition affected peptide perturbation of the lipids, and such perturbation appeared to be more intense in a system more closely mimicking a thylakoid membrane.
Keywords: amphipathic helix, membrane active peptide, twin arginine transport, chloroplast TatB
1. Introduction
In plant cells most thylakoid lumen proteins are synthesized in the cytosol as higher molecular weight precursors containing targeting sequences, which allow import into chloroplasts and localization to thylakoid lumen for function [1, 2]. Two thylakoid transport systems, homologous to bacteria export systems, direct precursors to the thylakoid lumen [3]. The chloroplast Sec system transports largely unfolded proteins across the membrane in an ATP dependent manner, while the chloroplast twin arginine translocation (cpTat)2 system delivers highly folded proteins across the membrane utilizing the trans-thylakoidal protonmotive force as its only energy source. Another distinguishing feature of Tat transport system is the presence of a twin arginine amino acid motif in the lumen-targeting signal peptide of the precursor, which gives the pathway its name. The cpTat pathway consists of three core membrane-bound subunits, Tha4, Hcf106, and cpTatC; whereas the homologous proteins in the bacterial Tat system are TatA, TatB and TatC [2]. Sequence analysis predicts that Tha4 (TatA) and Hcf106 (TatB) have similar structure, both consisting of an N-terminal transmembrane domain (TMD), followed by an amphipathic helix (APH) and an unstructured C-terminus (C-tail) [4], however, the APH domain and C-tail region of Hcf106 are approximately two times larger than those of Tha4. The predicted structure for Tha4 has been confirmed by determination of the structure of a truncated version of the homologous protein, TatAd (2–45), from Bacillus subtilis by solution NMR [5] and solid-state NMR [6]. Even though Tha4 and Hcf106 appear to have structural similarities, this has not been investigated directly. In addition the two proteins are functionally distinct such that one cannot functionally replace the other [4]. cpTatC is an integral membrane protein with six transmembrane-spanning helices [7, 8]. Nuclear genes encode all three proteins, and they are synthesized in the cytoplasm as larger molecular weight precursors containing N-terminal amino acid extensions, the stromal-targeting transit peptides. cpTatC is localized to the thylakoid via a stromal intermediate [9], and it cannot integrate into the thylakoid membrane directly. Conversely, Tha4 and Hcf106 do not appear to use a stromal intermediate, and they can integrate directly into thylakoid membranes even in the absence of their signal peptide [4]. Currently how these proteins are able to insert into the membrane spontaneously or unassisted is unknown. Here we investigated interactions of a synthetic peptide corresponding to the APH region of Hcf106 for its ability to interact with multi-lamellar vesicles (MLVs) as insight into the ability to spontaneously insert into thylakoid membranes. Understanding Hcf106 interaction with lipid bilayers may also provide insights into Hcf106 function and activity.
One way to better understand the integration, topology, and structure-function relationships of membrane proteins is to study the interaction between the peptide and lipids by solid-state NMR spectroscopy. Solid-state NMR techniques are widely used to study the structural and dynamic properties of peptides and lipids [10–13]. 31P and 2H solid-state NMR spectroscopy are useful probes to study peptide-lipid interactions from the perspective of lipids. Utilizing 31P as a natural probe, which is sensitive to the conformation of lipid headgroup, lipid phase and electrostatic property, 31P NMR is extensively used to investigate the dynamics and interaction between lipid headgroups with peptides [14–16]. Complementary to 31P NMR, 2H solid-state NMR spectroscopy provides information on the orientation and dynamics of the acyl chains of lipids by incorporating peptides or proteins into acyl-chains deuterated phospholipid bilayers [17–19]. Peptide-lipid interactions are based on the amino acid sequence of proteins and the composition of lipids, and are widely used to address questions such as protein and lipid structure, hydrophobicity and aggregation of proteins, charge distribution on lipid, depth of peptide in the lipid milieu, modulation of interaction by altering the lipid composition [20–23]. Unlike small micelles with high curvature, which could hardly retain native structure of peptides, multilamellar vesicles (MLVs) are more membrane-mimicking systems that are composed of multi-layers of lipid bilayers [24, 25].
Here we use solid-state NMR spectroscopy to investigate the topology of vesicles from the perspective of the lipids, obtaining preliminary topology information of Hcf106 in lipids by studying the peptide-lipid interaction. To simplify the study of this 176 amino acid protein, a peptide encompassing the APH (residues 28–65) of Hcf106 were synthesized by Fmoc-based solid-phase methods, and purified by high-performance liquid chromatography [26]. These peptides were then incorporated into POPC (1-palmitoyl-2-oleoyl-sn-glycero-phosphoatidylcholine) and POPC/MGDG (monogalactosyldiacylglycerol) vesicles at various concentrations. The synthetic phospholipid bilayers mimicked biological membranes providing a controlled environment in which to assay peptide interaction or insertion with the membrane. A peptide from the C terminus of the small subunit of Rubisco (mSSU Rubisco) was predicted to be non-amphipathic by standard algorithms, and was used as a control peptide in this study. Using 31P NMR spectroscopy we showed that Hcf106 APH peptides interact with phospholipid headgroups. In addition 2H NMR spectroscopic measurements revealed the perturbation of the deuterated acyl chains by the presence of peptide and the effect of lipid composition on the peptide-lipid interaction. Further, the order parameter SCD from 2H NMR demonstrated the dynamic characteristics of lipid headgroup and acyl chain and the effect of peptide addition to the vesicles. These experimental results confirmed the membrane-associate activity of the proposed amphipathic helix of Hcf106 and suggested that Hcf106 mostly sat on the membrane surface and perturbed the hydrophobic region of phospholipid to a lesser extent.
2. Materials and Methods
2.1 Materials
Synthetic phospholipids POPC and POPC-d31 were purchased from Avanti Polar Lipids (Alabaster, AL). Natural galactolipid from parsley leaves, MGDG, was purchased from Larodan Fine Chemicals AB (Sweden). All lipids were dissolved in chloroform or chloroform/methanol and stored at −20°C under N2 gas until use. Fmoc amino acids were purchased from Novabiochem (San Diego, CA). Peptide synthesis resin and other chemicals for synthesis were purchased from Applied Biosystems (Foster City, CA). HPLC grade acetonitrile was purchased from VWR (Radnor, PA) and was filtered through a 0.22-mm nylon membrane before use. Water was purified using a Nanopure reverse osmosis system (Millipore, Bedford, MA). Chloroform, piperidine, EDTA, N-[2-hydroxyethyl] piperazine-N″-2-ethane sulfonic acid (HEPES) and 2,2,2 trifluoroethanol (TFE) were purchased from Sigma-Aldrich (Milwaukee, WI). The deuterium-depleted water was purchased from Isotec™ (Miamisburg, OH).
2.2 Synthesis and purification of peptide Hcf106 APH and peptide mSSU Rubisco
Hcf106 APH and mSSU Rubisco were synthesized using Fmoc solid-phase peptide synthesize chemistry on a CEM microwave peptide synthesizer (Matthews, NC). The sequence for the amphipathic helix of Hcf106 is NH2-LAEVARNLGKTLREFQPTIREIQDVSREFKSTLEREIG-COO−, while the sequence for the control peptide mSSU Rubisco is NH2-EHNKSPRYYDGRYWTMWKLPMFGTTDASQVLG-COO−. After the peptides were cleaved from their solid support, they were purified via HPLC on a C18 reverse-phase preparation column. The column was equilibrated with 95% solvent A (100% H2O) and 5% solvent B (60% acetonitrile, 40% H2O for Hcf106 APH; 90% acetonitrile, 10% H2O for mSSU Rubisco), and was eluted with a linear gradient of 5% to 95% solvent B. The buffers also contained 0.1% trifluoroacetic acid (TFA). The purified peptide was lyophilized to remove existing solvent. Purity was confirmed by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry to ensure the purity of the peptides.
2.3 NMR sample preparation
POPC bilayers containing Hcf106 APH peptide of various concentrations were prepared as described [15]. Briefly Hcf106 APH peptide (2.0mg–6.1mg) and POPC bilayers (17.5mg) were dissolved in 2,2,2 trifluoroethanol and chloroform, respectively, and the peptide was added to the lipids at the mol% indicated in the Figure Legends. The mixtures were dried under steady N2 gas and placed in a vacuum desiccator overnight to remove residual solvent. The multi-lamellar vesicles (MLVs) were prepared by suspension of the dried mixture in 95μl HEPES buffer (30mM HEPES, pH 7.0, 5mM EDTA, 20mM NaCl) followed by five cycles of moderate vortexing for two min, freezing with liquid N2 for one min and sonication for five min in a 45°C bath sonicator. While the presence of residual TFA from the cleavage reaction should be minimized, the presence of residual TFA would lower the pH of the buffer, especially given the small volume of dilute buffer added; therefore, the peptide solution may have a pH <7.0. The fully homogenized samples were transferred to NMR sample tubes. Peptide incorporation into POPC/MGDG MLVs (85%:15% ratio) were prepared following the same procedure, maintaining the total lipid amount at 17.5 mg. For the 2H NMR experiments, 5mg POPC-d31 phospholipids replaced the same amount of non-deuterated POPC in the sample to maintain total lipid amount (17.5mg). POPC and POPC/MGDG MLVs containing 6 mol % control peptide from the SSU of Rubisco (5.3mg) were prepared by the same procedure.
2.4 NMR spectroscopy
All NMR data were recorded on a Bruker Avance 500MHz WB NMR spectrometer equipped with a CPMAS probe (Bruker, Billerica, MA). 31P NMR spectra were acquired at 202.4MHz with proton decoupling using a 4 μs 90° pulse and a 4 s recycle delay. For the 31P static NMR spectra 512 scans were taken and the free induction delay was processed using 200 Hz of line broadening. The spectral width was set to 500 ppm. All 31P NMR spectra were referenced by assigning the 85% H3PO4 31P peak to 0 ppm.
2H NMR spectra were performed at 76.77 MHz employing a quadrupolar echo pulse sequence using quadrature detection with complete phase cycling of the pulse pairs. The 90° pulse length was 4 μs, the interpulse delay was 40 μs, the recycle delay was 0.4 s, and the spectral width was set to 100 kHz. For the 2H static NMR spectra 122,880 scans were taken and were processed using 200 Hz of line broadening. Powder-type 2H NMR spectra were deconvoluted (dePaked) using the algorithm of McCabe and Wassall [27] such that the bilayer normal was perpendicular with respect to the direction of the static magnetic field. The quadrupolar splittings were measured from the dePaked spectra, and the 2H order parameters SCD were calculated according to the equation SCDi = Δvi/(3/2(e2qQ/h)), where SCDi is the quadrupolar splitting for a deuteron attached to the ith carbon of the POPC acyl chain, and e2qQ/h is the quadruple coupling constant (168 kHz for deuteron in C-D bonds). The order parameters of the methyl groups at the terminal acyl chain were multiplied by three as the calculated values [28].
3. Results and Discussion
3.1 31P NMR studies indicate Hcf106 APH interaction with MLVs
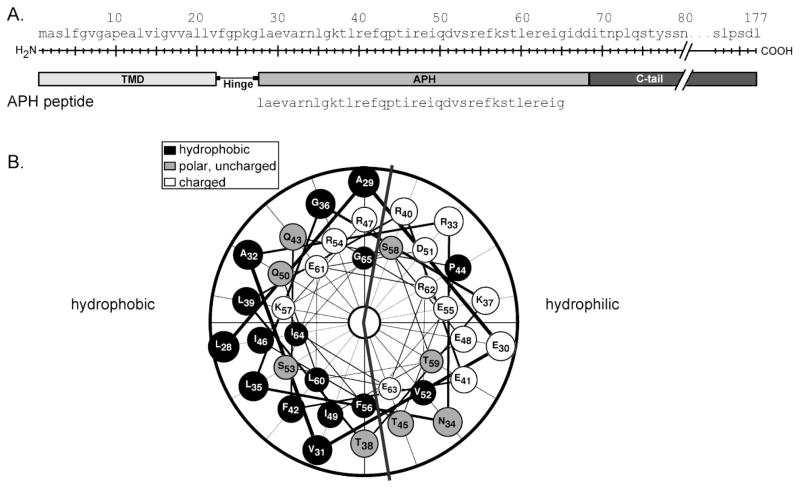
Chloroplast Tat component Hcf106 is predicted to contain a N-terminal proximal amphipathic α-helix (Figure 1A), which has not been characterized experimentally. In addition, previous studies suggest that Hcf106 has the ability to insert into the thylakoid via the spontaneous pathway, requiring no membrane bound protein assistance or additional energy, either in the form of nucleotide triphosphate hydrolysis or transmembrane protonmotive force [4]. To better understand the ability of Hcf106 to interact with membranes, we investigated the membrane interactivity of one of the key predicted membrane active components, the APH, using a synthetic peptide (Figure 1A). Helical wheel projections of the APH peptide used in this study clearly indicate the presence of a hydrophilic face and a hydrophobic face of the helix (Figure 1B).
Figure 1. Schematic of Hcf106 and peptide used in this study.
Peptides were synthesized on a CEM microwave peptide synthesizer (Matthews, NC) using Fmoc solid-phase peptide synthesis chemistry as described in the Materials and Methods. (A) A schematic of Hcf106 showing the different domains. The amino acid sequence of the peptide representing the APH is indicated. (B) Helical wheel plot (DNAStar, Madison, WI) of the amino acids of the Hcf106 APH peptide indicating the hydrophilic and hydrophobic faces.
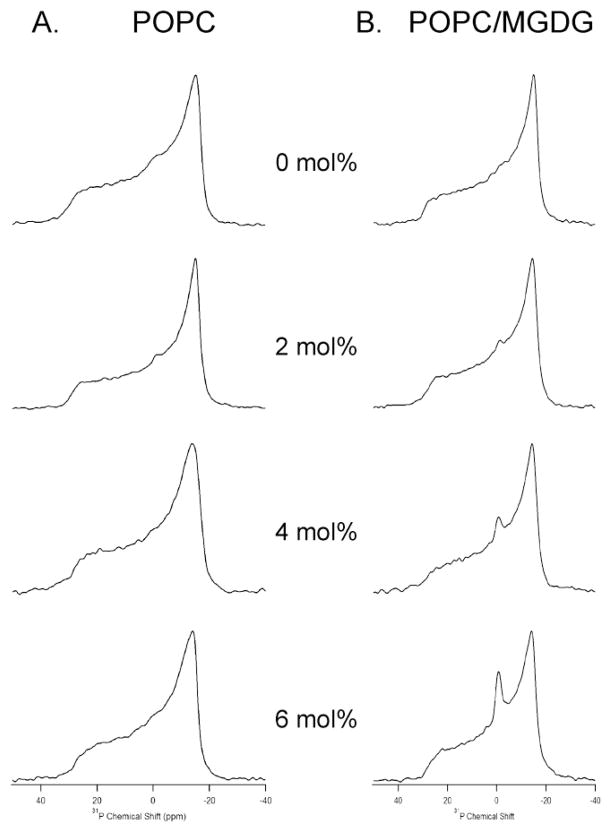
To investigate the nature of lamellar and nonlamellar phase transitions exhibited by the POPC MLVs in the presence of the Hcf106 APH peptide, static 31P NMR spectroscopic measurements were made (Figure 2A). The Hcf106 APH peptide concentration was varied from 0 mol % to 6 mol % with respect to POPC lipids. As expected for POPC bilayers at a temperature above the chain melting transition temperature (Tm) of −2°C [29], all spectra displayed a motionally averaged powder-pattern at 25°C, indicating that the phospholipid bilayers are in the liquid crystalline phase (Figure 2A). Increasing amounts of Hcf106 APH peptide incorporated into POPC MLVs caused a corresponding increase in the static lineshape width of the 31P NMR spectra as compared to the lineshape width of the empty POPC vesicles (Figure 2A). Furthermore, the addition of the peptide caused a corresponding decrease in the chemical shift anisotropy (CSA, σ// − σ⊥) line widths. CSA line widths were measured for control vesicles and vesicles containing 6 mol% APH peptide (Figure 2A) and were found to be 45±1 ppm and 42±1 ppm, respectively. There was a 3 ppm decrease in CSA line width when 6 mol % peptide was added as compared to control POPC bilayers, indicating that the headgroup region of the phospholipid bilayer was perturbed by the addition of Hcf106 APH peptide. This change contrasts directly with a CSA line width measure of 45±1 ppm in the presence of 6 mol% control peptide (Supplemental Figure 1A), which was indistinguishable from control vesicles.
Figure 2. Hcf106 APH preferentially perturbs POPC/MGDG MLVs.
(A) 31P spectra of MLV samples showing the effect of Hcf106 APH on 31P spectra line shape of POPC bilayers as a function of Hcf106 APH peptide concentration. (B) 31P spectra of MLV samples showing the effect of Hcf106 APH on 31P spectra line shape of POPC/MGDG bilayers (85%:15%) as a function of Hcf106 APH concentration at 25°C. MLV samples of different lipids in the absence of Hcf106 APH, and in the presence of 2 mol %, 4 mol %, and 6 mol % Hcf106 APH with respect to lipids were investigated.
The Hcf106 APH peptide is derived from the thylakoid localized Hcf106 protein. Thylakoid membrane has a very high proportion of nonphospholipids to phospholipids as well as a high proportion of the nonbilayer forming lipid, monogalactosyl diacylglycerol (MGDG) that can compose >50% of thylakoid lipid [30]. To more closely mimic the native thylakoid lipid composition, we tested Hcf106 APH interaction with vesicles containing 15 mol% MGDG. In this synthetic system, we were able to easily generate vesicles containing 15 mol% MGDG (85:15 mole ratio POPC:MGDG), but higher concentrations yielded unstable vesicles and were not used for this study. When increasing amounts of the Hcf106 APH peptide were incorporated into POPC/MGDG MLVs, the corresponding 31P lineshape broadened compared to that of control POPC/MGDG MLVs (Figure 2B). As with POPC MLVs, the CSA widths decreased as more peptide was added to the POPC/MGDG MLVs. The CSA widths for control POPC/MGDG MLVs and MLVs containing 6 mol % APH peptide were 45 ppm and 41 ppm, respectively. There was a 4 ppm decrease in CSA width when 6 mol % peptide was added when compared to the empty vesicles. However the presence of the control peptide with POPC/MGDG vesicles resulted in a CSA line width of 45±1 ppm, the same as control vesicles (Supplemental Figure 1B). The most obvious difference in 31P spectra lineshape between the POPC MLVs and the POPC/MGDG MLVs was the appearance of an isotropic peak that increased in intensity as the concentration of Hcf106 APH was increased (Figure 2B). The presence of such an isotropic peak suggests that smaller vesicles were induced to form due to the presence of the APH peptides and the contribution from the isotropic phase increased as more Hcf106 APH was incorporated [31].
At least three possible occurrences can explain this change in CSA line width, (1) increased stochastic fluctuations experienced by the lipid head groups due to an increase in the disorder of individual lipids caused by the increasing presence of the APH, but not the control, peptide; (2) increased axial rotational motion as well as lateral diffusion rate of lipid upon peptide incorporation; or (3) increased dynamics of the vesicles, resulting from the interaction between the peptide and head groups of the phospholipids [13, 32–34]. However, an increase in the long axis rotation of the lipid would be unlikely to affect the CSA line width because at the point at which the 31P CSA line width becomes axially symmetric it has reached an average and increasing the rotation at that point will not change those averages. In addition, an increase in the lateral diffusion rate of the lipid is also unlikely to be large enough to affect the CSA line width. More likely the change in CSA line width is due to an increase in the disorder of the individual lipids as the amount of the APH, but not the control, peptide increases. For example, a decrease in the CSA line width may correspond to a change in the orientation of the head groups of the lipid such that they are less upright (i.e., the angle ϑx of the chemical shift tensor increases from the lipid axis of rotation perpendicular to the plane of the phosphorous and oxygens [35]). Regardless, the shift in CSA when the APH peptide was present indicates a perturbation of the liquid crystalline array of the lipids, and a change in averaged orientation of the lipid head groups with respect to the bilayer normal, induced by rotational motion of lipid head group [32, 36]. Regardless, the APH peptide interacted with MLVs containing the thylakoid-specific lipid, MGDG more intensely as compared to MLVs containing only POPC.
3.2 2H NMR studies indicate perturbation of POPC and POPC/MGDG lipids acyl chain upon Hcf106 APH incorporation
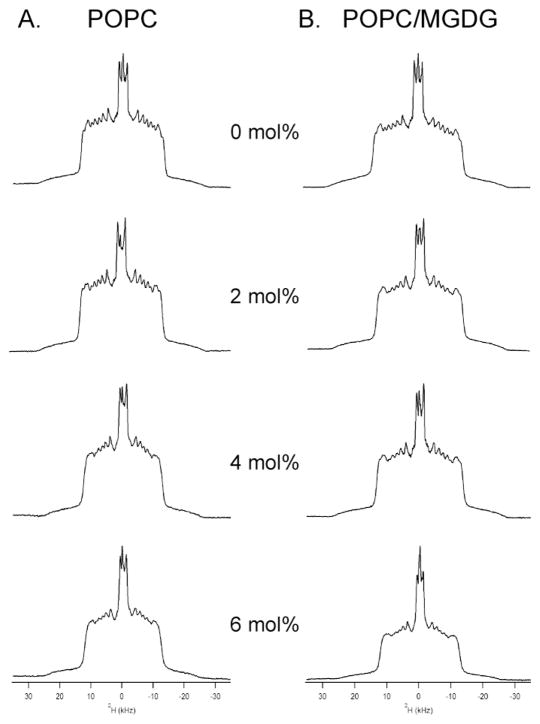
Because static 31P NMR spectra only reflects the perturbation of the peptide to the phospholipid headgroup, 2H solid-state NMR spectroscopy was utilized to investigate the effect of increasing concentration of Hcf106 APH peptide on the order and dynamics of the acyl chain of POPC-d31 phospholipid in POPC and POPC/MGDG MLVs (Figure 3). All spectra display overlapping doublet resonances with the largest splitting resulting from the C-D bond closest to the glycerol backbone and the central splitting arising from the terminal methyl group [13, 31, 34]. Clearly, addition of APH peptide at various concentrations altered the line shape and resolution of 2H NMR spectra of the POPC/POPC-d31 MLVs significantly compared to the control sample lacking peptide (Figure 3A). The spectral widths decreased upon addition of the APH peptide, indicating an increase in the lipid bilayer fluidity caused by perturbation of lipid acyl chains upon peptide incorporation. As the concentration of APH peptide increased there was a loss of resolution of the spectrum as demonstrated by the disappearance of sharp edges of the peaks, also revealing an interaction between the APH peptide and the acyl chains of the lipid bilayer. In addition, this interaction increased the motion of the lipids and/or the dynamics of the vesicles, as evidenced by the increased isotropic peak intensity induced by higher amounts of APH peptide. Similar to the presence of an isotropic peak in the 31P NMR measurements, the increased dynamics of the vesicles could be explained by the formation of small, rapidly tumbling vesicles [31]. The spectral line shape and isotropic component was even more pronounced when Hcf106 APH peptides were added to POPC/POPC-d31/MGDG MLVs (Figure 3B). By contrast the line shape widths of the MLVs (either POPC/POPC-d31 or POPC/POPC-d31/MGDG) were not changed from those of the control vesicles when in the presence of the control peptide (Supplemental Figure 2), although the presence of the control peptide did cause the formation of an isotropic peak. Taken with the static 31P NMR data, these data indicate that the Hcf106 peptide interacts with the POPC/MGDG vesicles, and does so in the same way as it interacts with the POPC vesicles.
Figure 3. Hcf106 APH interacts with the bilayer of POPC or POPC/MGDG MLVs.
2H NMR spectra of MLV samples showing the perturbation of Hcf106 APH on lipids acyl chain of (A) POPC bilayers, and (B) POPC/MGDG bilayers as a function of Hcf106 APH concentration at 25°C. 5mg POPC-d31 phospholipids were used as deuterium probe. MLV samples of different lipids in the absence of Hcf106 APH, and in the presence of 2 mol %, 4 mol %, and 6 mol % Hcf106 APH with respect to lipids were investigated.
However the changes in CSA line widths and in the 2H NMR spectral line shapes due to the presence of the peptide were not very large (barely 10% change) and may be a reflection of how the peptide can engage the membrane. In vesicles containing the thylakoid-specific lipid, the APH peptide may be a membrane breaching peptide at high enough concentrations, similar to antimicrobial peptides, and this is evidenced by the increasing presence of the isotropic peak with increasing peptide amount. The presence of the MGDG lipid may enhance the membrane breaching capabilities of the APH peptide due to its tendency to not form a lamellar bilayer but rather to form a nonlamellar, inverted Hexagonal (Hexagonal II) phase.
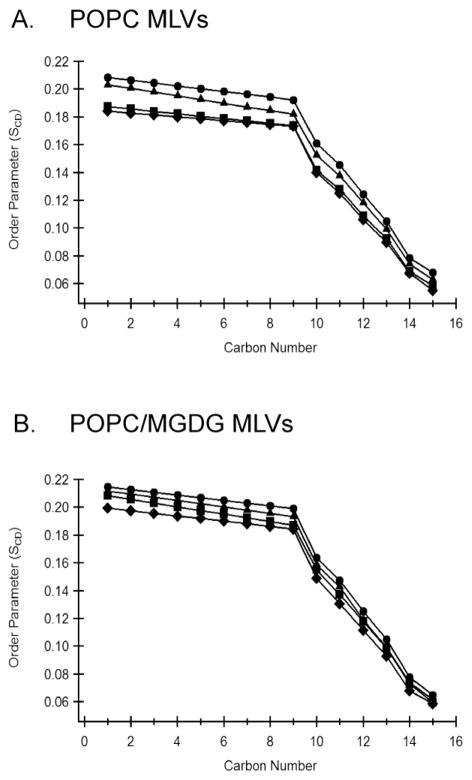
All 2H NMR powder pattern spectra were dePaked to probe the dynamic changes of the acyl chains upon Hcf106 APH peptide incorporation. SCD order parameters are sensitive to intermolecular, intramolecular and collective motions, reflecting the orientation and dynamic change of the C-D bond vector [37, 38]. The smoothed segmental C-D bond order parameters (SCD) were calculated from the dePaked spectra and plotted as a function of acyl chain carbon number for the different APH concentration at 25°C (Figure 4). As distance from the glycerol backbone increased, there was a decrease of SCD for all samples, indicating increased motion and flexibility for the central and terminal acyl chain regions as compared to the proximal headgroup region (Figure 4A, filled circles). In the presence of 2 mol%, 4 mol% and 6 mol% APH peptides, the acyl chains of POPC lipid bilayers were more mobile compared to the control vesicles lacking peptide or vesicles containing the control peptide, as revealed by a decrease in the order of each C-D bond (Figure 4A). In the presence of the APH peptide, there was a larger decrease in the SCD for the first three or four carbons from the ester than at the methyl end of the acyl chains (Figure 4A). Combined with the 31P NMR data, these data suggest that Hcf106 APH peptide mostly sits on the membrane surface or may slightly tilt into the hydrophobic region of the phospholipid bilayers to interact with acyl chains in close proximity to glycerol backbones. It is also possible that Hcf106 APH peptide attached to the membrane surface by its hydrophilic face and induced a relatively compact environment on the membrane surface and more dynamic space in the hydrophobic region of the lipids. Further experiments are needed to verify these explanations. The SCD profile for POPC/MGDG MLVs (Figure 4B) resembles that in POPC MLVs, except that POPC MLVs demonstrate a slightly larger SCD and less perturbation caused by the incorporation of Hcf106 APH peptide.
Figure 4. Hcf106 APH preferentially disturbs the lipid head group/acyl chain interface.
Smoothed acyl chains orientational order SCD profiles calculated by dePaking 2H NMR spectra of Figure 3. (A) POPC MLVs, and (B) POPC/MGDG MLVs with and without Hcf106 APH at various concentrations. SCD profiles calculated in the absence (●) or presence of Hcf106 APH at 2 mol % (▲), 4 mol % (■) and 6 mol % (◆), respectively.
4. Conclusion
This solid-state NMR study clearly shows lipid-peptide interaction. 31P and 2H solid-state NMR static spectra lineshape, CSA widths, and 2H order parameters are utilized to investigate lipid-peptide interaction and the results are complimentary to each other. These data indicate that Hcf106 APH peptide interacts with the head group and acyl chains of phospholipid bilayers. The manipulation of the lipid composition clearly alters the line shape of the spectra, and the peptides interact more intensely with the thylakoid membrane-mimicking environment (POPC/MGDG MLVs). These data show for the first time that Hcf106 APH is by itself a membrane associated peptide, and also imply how the peptide could arrange itself to interact with lipids when incorporated into membrane systems.
Supplementary Material
Highlights.
Twin arginine transport protein Hcf106 contains an amphipathic α-helix (APH)
Interaction of Hcf106 APH peptide and phospholipids was studied by Solid-state NMR
The predicted APH of Hcf106 interacts with vesicle membranes
Acknowledgments
This work was funded by the DOE Office of Science Early Career Research Program (Grant: DE-FG02-10ER16138 to CDS) through the Office of Basic Energy Sciences and National Institutes of Health grant (R01GM080542 and GM080542Z to GAL).
Footnotes
Abbreviations used: cpTat, chloroplast twin arginine transport; TMD, transmembrane domain; APH, amphipathic α-helix; POPC, 1-palmitoyl-2-oleoyl-sn-glycero-phosphocholine; MGDG, monogalactosyl diacylglycerol; MLV, multilamellar vesicles; CSA, chemical shift anisotropy; SCD, segmental C-D bond order parameter.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shi LX, Theg SM. The chloroplast protein import system: From algae to trees. BBA Mol Cell Res. 2013;1833:314–331. doi: 10.1016/j.bbamcr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Celedon JM, Cline K. Intra-plastid protein trafficking: How plant cells adapted prokaryotic mechanisms to the eukaryotic condition. BBA Mol Cell Res. 2013;1833:341–351. doi: 10.1016/j.bbamcr.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cline K, Dabney-Smith C. Plastid protein import and sorting: different paths to the same compartments. Curr Opin Plant Biol. 2008;11:585–592. doi: 10.1016/j.pbi.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fincher V, Dabney-Smith C, Cline K. Functional assembly of thylakoid deltapH-dependent/Tat protein transport pathway components in vitro. Eur J Biochem. 2003;270:4930–4941. doi: 10.1046/j.1432-1033.2003.03894.x. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Zhao E, Li H, Xia B, Jin C. Solution NMR structure of the TatA component of the twin-arginine protein transport system from gram-positive bacterium Bacillus subtilis. J Am Chem Soc. 2010;132:15942–15944. doi: 10.1021/ja1053785. [DOI] [PubMed] [Google Scholar]
- 6.Walther TH, Grage SL, Roth N, Ulrich AS. Membrane alignment of the pore-forming component TatA(d) of the twin-arginine translocase from Bacillus subtilis resolved by solid-state NMR spectroscopy. J Am Chem Soc. 2010;132:15945–15956. doi: 10.1021/ja106963s. [DOI] [PubMed] [Google Scholar]
- 7.Mori H, Summer EJ, Cline K. Chloroplast TatC plays a direct role in thylakoid (Delta)pH-dependent protein transport. FEBS Lett. 2001;501:65–68. doi: 10.1016/s0014-5793(01)02626-6. [DOI] [PubMed] [Google Scholar]
- 8.Rollauer SE, Tarry MJ, Graham JE, Jaaskelainen M, Jager F, Johnson S, Krehenbrink M, Liu SM, Lukey MJ, Marcoux J, McDowell MA, Rodriguez F, Roversi P, Stansfeld PJ, Robinson CV, Sansom MS, Palmer T, Hogbom M, Berks BC, Lea SM. Structure of the TatC core of the twin-arginine protein transport system. Nature. 2012;492:210–214. doi: 10.1038/nature11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin JR, Harwood JH, McCaffery M, Fernandez DE, Cline K. Localization and integration of thylakoid protein translocase subunit cpTatC. Plant J. 2009;58:831–842. doi: 10.1111/j.1365-313X.2009.03816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross TA, Opella SJ. Solid-state NMR structural studies of peptides and proteins in membranes. Curr Opin Struct Biol. 1994;4:574–581. [Google Scholar]
- 11.Nakazawa Y, Asakura T. Structure determination of a peptide model of the repeated helical domain in Samia cynthia ricini silk fibroin before spinning by a combination of advanced solid-state NMR methods. J Am Chem Soc. 2003;125:7230–7237. doi: 10.1021/ja0300721. [DOI] [PubMed] [Google Scholar]
- 12.Watts A. Solid-state NMR approaches for studying the interaction of peptides and proteins with membranes. Biochim Biophys Acta. 1998;1376:297–318. doi: 10.1016/s0304-4157(98)00012-4. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Baker S, Lorigan GA. Phospholamban and its phosphorylated form interact differently with lipid bilayers: a 31P, 2H, and 13C solid-state NMR spectroscopic study. Biochemistry. 2006;45:13312–13322. doi: 10.1021/bi0614028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith ICP, Ekiel IH. Phosphorus-31 NMR of membranes. In: Gorenstein DG, editor. Phosphorus-31 NMR : principles and applications. Academic Press; Orlando, Fla: 1984. pp. 447–475. [Google Scholar]
- 15.Dave PC, Tiburu EK, Damodaran K, Lorigan GA. 31P and 2H Solid-State NMR Spectroscopic Studies of the Transmembrane Domain of the Membrane-Bound Protein Phospholamban. Biophys J. 2004;86:1564–1573. doi: 10.1016/S0006-3495(04)74224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santos JS, Lee DK, Ramamoorthy A. Effects of antidepressants on the conformation of phospholipid headgroups studied by solid-state NMR. Magn Reson Chem. 2004;42:105–114. doi: 10.1002/mrc.1327. [DOI] [PubMed] [Google Scholar]
- 17.Koenig BW, Ferretti JA, Gawrisch K. Site-specific deuterium order parameters and membrane-bound behavior of a peptide fragment from the intracellular domain of HIV-1 gp41. Biochemistry. 1999;38:6327–6334. doi: 10.1021/bi982800g. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi S, Huster D, Waring A, Lehrer RI, Kearney W, Tack BF, Hong M. Orientation and dynamics of an antimicrobial peptide in the lipid bilayer by solid-state NMR spectroscopy. Biophys J. 2001;81:2203–2214. doi: 10.1016/S0006-3495(01)75868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu JX, Damodaran K, Blazyk J, Lorigan GA. Solid-state nuclear magnetic resonance relaxation studies of the interaction mechanism of antimicrobial peptides with phospholipid bilayer membranes. Biochemistry. 2005;44:10208–10217. doi: 10.1021/bi050730p. [DOI] [PubMed] [Google Scholar]
- 20.Arora A, Tamm LK. Biophysical approaches to membrane protein structure determination. Curr Opin Struct Biol. 2001;11:540–547. doi: 10.1016/s0959-440x(00)00246-3. [DOI] [PubMed] [Google Scholar]
- 21.Watts A. Protein-lipid interactions: do the spectroscopists now agree? Nature. 1981;294:512–513. doi: 10.1038/294512a0. [DOI] [PubMed] [Google Scholar]
- 22.Lemmon MA, Engelman DM. Specificity and promiscuity in membrane helix interactions. FEBS Lett. 1994;346:17–20. doi: 10.1016/0014-5793(94)00467-6. [DOI] [PubMed] [Google Scholar]
- 23.Bloom M, Evans E, Mouritsen OG. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Quart Rev Biophys. 1991;24:293–397. doi: 10.1017/s0033583500003735. [DOI] [PubMed] [Google Scholar]
- 24.Jesorka A, Orwar O. Liposomes: technologies and analytical applications. Annu Rev Anal Chem (Palo Alto Calif) 2008;1:801–832. doi: 10.1146/annurev.anchem.1.031207.112747. [DOI] [PubMed] [Google Scholar]
- 25.Stockton GW, Polnaszek CF, Tulloch AP, Hasan F, Smith IC. Molecular motion and order in single-bilayer vesicles and multilamellar dispersions of egg lecithin and lecithin-cholesterol mixtures. A deuterium nuclear magnetic resonance study of specifically labeled lipids. Biochemistry. 1976;15:954–966. doi: 10.1021/bi00650a003. [DOI] [PubMed] [Google Scholar]
- 26.Tiburu EK, Dave PC, Vanlerberghe JF, Cardon TB, Minto RE, Lorigan GA. An improved synthetic and purification procedure for the hydrophobic segment of the transmembrane peptide phospholamban. Anal Biochem. 2003;318:146–151. doi: 10.1016/s0003-2697(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 27.Mccabe MA, Wassall SR. Fast-Fourier-Transform Depaking. J Magn Reson Ser B. 1995;106:80–82. [Google Scholar]
- 28.Lu JX, Caporini MA, Lorigan GA. The Effects of Cholesterol on Magnetically Aligned Phospholipid Bilayers: A Solid-State NMR and EPR Spectroscopy Study. J Mag Res. 2004;168:18–30. doi: 10.1016/j.jmr.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978;515:105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- 30.Douce R, Joyard J. Biochemistry and function of the plastid envelope. Annu Rev Cell Biol. 1990;6:173–216. doi: 10.1146/annurev.cb.06.110190.001133. [DOI] [PubMed] [Google Scholar]
- 31.Dave PC, Tiburu EK, Damodaran K, Lorigan GA. Investigating structural changes in the lipid bilayer upon insertion of the transmembrane domain of the membrane-bound protein phospholamban utilizing P-31 and H-2 solid-state NMR spectroscopy. Biophys J. 2004;86:1564–1573. doi: 10.1016/S0006-3495(04)74224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohler SJ, Klein MP. Orientation and Dynamics of Phospholipid Head Groups in Bilayers and Membranes Determined from P-31 Nuclear Magnetic-Resonance Chemical Shielding Tensors. Biochemistry. 1977;16:519–526. doi: 10.1021/bi00622a028. [DOI] [PubMed] [Google Scholar]
- 33.Abu-Baker S, Qi X, Newstadt J, Lorigan GA. Structural changes in a binary mixed phospholipid bilayer of DOPG and DOPS upon saposin C interaction at acidic pH utilizing 31P and 2H solid-state NMR spectroscopy. Biochim Biophys Acta. 2005;1717:58–66. doi: 10.1016/j.bbamem.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Lu JX, Damodaran K, Lorigan GA. Probing membrane topology by high-resolution 1H-13C heteronuclear dipolar solid-state NMR spectroscopy. J Magn Reson. 2006;178:283–287. doi: 10.1016/j.jmr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Gehman JD, O’Brien CC, Shabanpoor F, Wade JD, Separovic F. Metal effects on the membrane interactions of amyloid-beta peptides. Eur Biophys J. 2008;37:333–344. doi: 10.1007/s00249-007-0251-2. [DOI] [PubMed] [Google Scholar]
- 36.Griffin RG, Powers L, Pershan PS. Head-Group Conformation in Phospholipids - P-31 Nuclear Magnetic-Resonance Study of Oriented Monodomain Dipalmitoylphosphatidylcholine Bilayers. Biochemistry. 1978;17:2718–2722. doi: 10.1021/bi00607a004. [DOI] [PubMed] [Google Scholar]
- 37.Seelig A, Seelig J. The dynamic structure of fatty acyl chains in a phospholipid bilayer measured by deuterium magnetic resonance. Biochemistry. 1974;13:4839–4845. doi: 10.1021/bi00720a024. [DOI] [PubMed] [Google Scholar]
- 38.Seelig J, Seelig A. Deuterium magnetic resonance studies of phospholipid bilayers. Biochem Biophys Res Comm. 1974;57:406–411. doi: 10.1016/0006-291x(74)90945-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.