Abstract
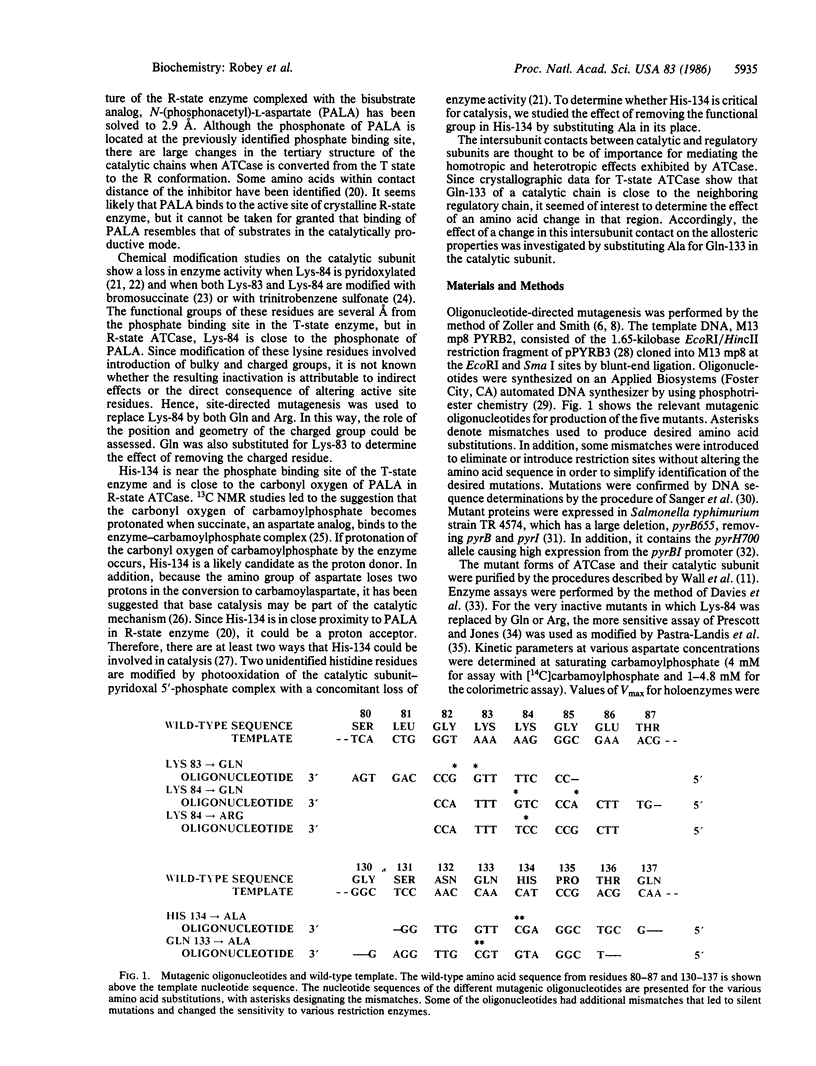
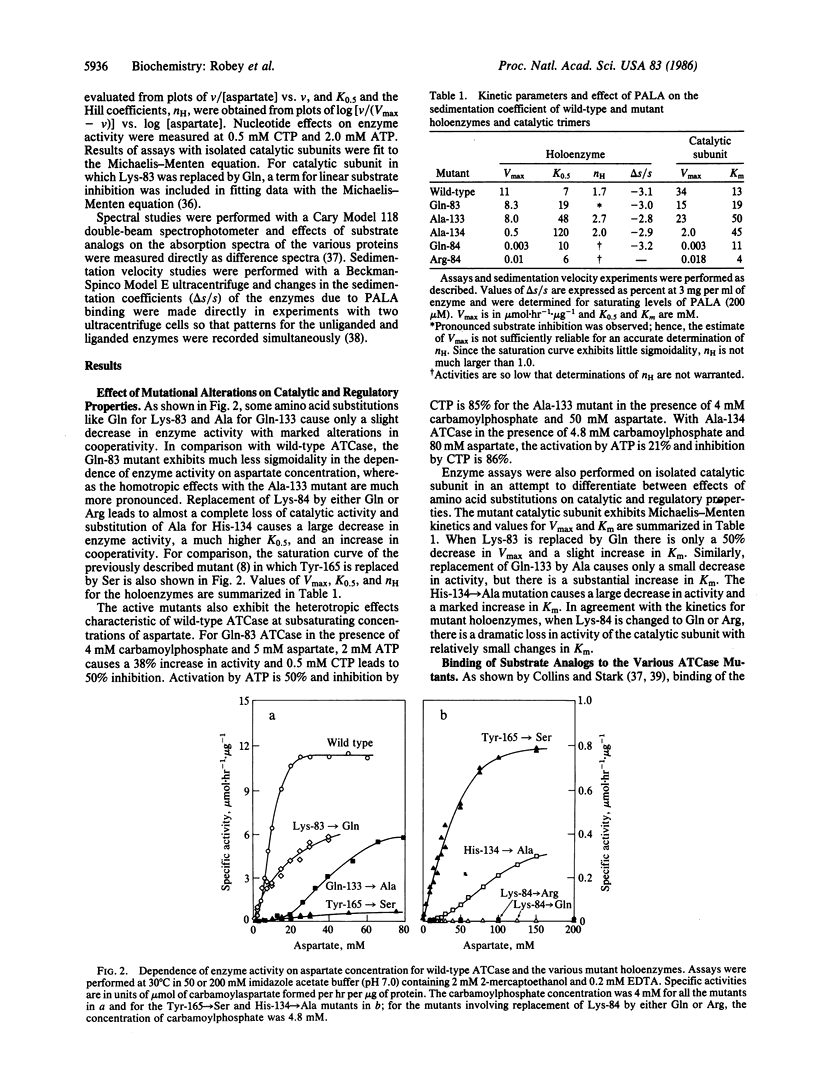
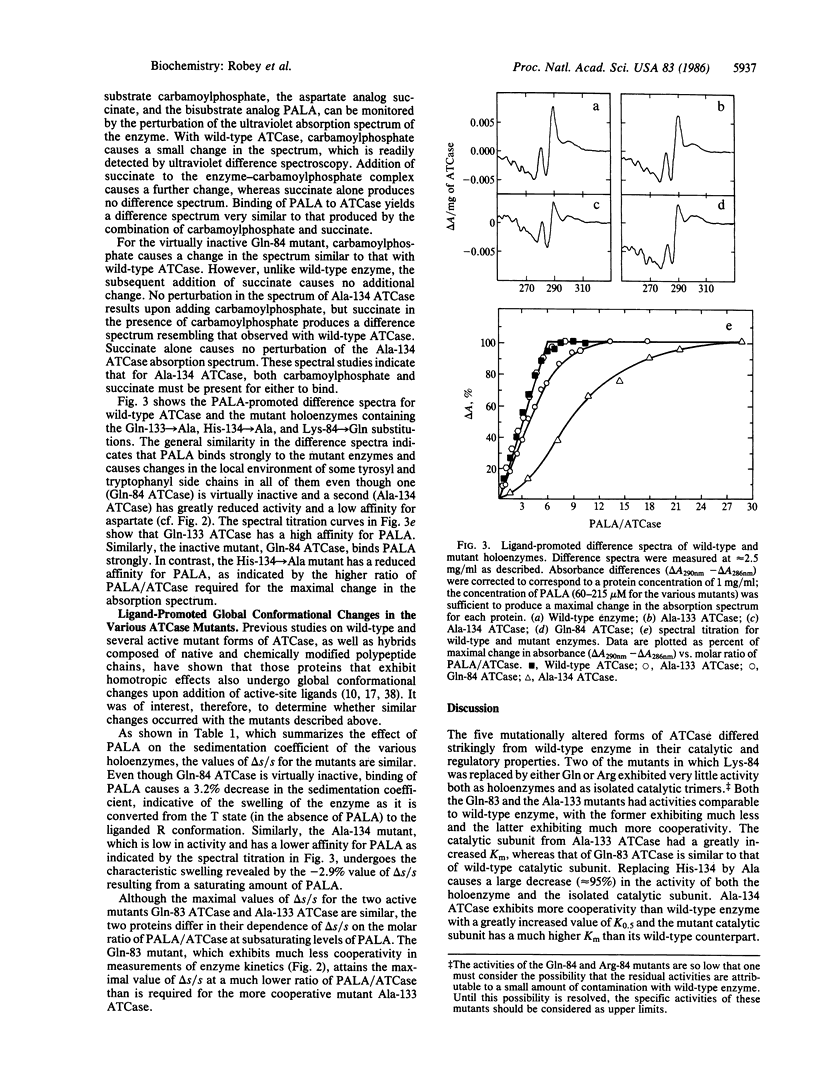
Although intensive investigations have been conducted on the allosteric enzyme, aspartate transcarbamoylase, which catalyzes the first committed reaction in the biosynthesis of pyrimidines in Escherichia coli, little is known about the role of individual amino acid residues in catalysis or regulation. Two inactive enzymes produced by random mutagenesis have been characterized previously but the loss of activity is probably attributable to changes in the folding of the chains stemming from the introduction of charged and bulky residues (Asp for Gly-128 and Phe for Ser-52). Site-directed mutagenesis of pyrB, which encodes the catalytic chains of the enzyme, was used to probe the functional roles of several amino acids by making more conservative substitutions. Replacement of Lys-84 by either Gln or Arg leads to virtually inactive enzymes, confirming chemical studies indicating that Lys-84 is essential for catalysis. In contrast, substitution of Gln for Lys-83 has only a slight effect on enzyme activity, whereas chemical modification causes considerable inactivation. Gln-133, which has been shown by x-ray crystallography to reside near the contact region between the catalytic and regulatory chains, was replaced by Ala. This substitution has little effect on catalytic activity but leads to a marked increase in cooperativity. The Gln-83 mutant, in contrast, exhibits much less cooperativity. Since a histidine residue may be involved in catalysis and His-134 has been shown by x-ray diffraction studies to be in close proximity to the site of binding of a bisubstrate analog, His-134 was replaced by Ala, yielding a mutant with only 5% wild-type activity, considerable cooperativity, and lower affinity for aspartate and carbamoylphosphate. All of the mutants, unlike those in which charged or bulky residues replaced small side chains, bind the bisubstrate analog, which promotes the characteristic "swelling" of the enzymes indicative of the allosteric transition.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K., Smith F. R. Effects of site-specific amino acid modification on protein interactions and biological function. Annu Rev Biochem. 1985;54:597–629. doi: 10.1146/annurev.bi.54.070185.003121. [DOI] [PubMed] [Google Scholar]
- Botstein D., Shortle D. Strategies and applications of in vitro mutagenesis. Science. 1985 Sep 20;229(4719):1193–1201. doi: 10.1126/science.2994214. [DOI] [PubMed] [Google Scholar]
- Collins K. D., Stark G. R. Aspartate transcarbamylase. Interaction with the transition state analogue N-(phosphonacetyl)-L-aspartate. J Biol Chem. 1971 Nov;246(21):6599–6605. [PubMed] [Google Scholar]
- Collins K. D., Stark G. R. Aspartate transcarbamylase. Studies of the catalytic subunit by ultraviolet difference spectroscopy. J Biol Chem. 1969 Apr 10;244(7):1869–1877. [PubMed] [Google Scholar]
- Cunin R., Jacobs A., Charlier D., Crabeel M., Hervé G., Glansdorff N., Piérard A. Structure-function relationship in allosteric aspartate carbamoyltransferase from Escherichia coli. I. Primary structure of a pyrI gene encoding a modified regulatory subunit. J Mol Biol. 1985 Dec 20;186(4):707–713. doi: 10.1016/0022-2836(85)90390-0. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Vanaman T. C., Stark G. R. Aspartate transcarbamylase. Stereospecific restrictions on the binding site for L-aspartate. J Biol Chem. 1970 Mar 10;245(5):1175–1179. [PubMed] [Google Scholar]
- Dennis P. R., Krishna M. V., Di Gregorio M., Chan W. W. Ligand interactions at the active site of aspartate transcarbamoylase from Escherichia coli. Biochemistry. 1986 Apr 8;25(7):1605–1611. doi: 10.1021/bi00355a023. [DOI] [PubMed] [Google Scholar]
- Eaton W. A. The relationship between coding sequences and function in haemoglobin. Nature. 1980 Mar 13;284(5752):183–185. doi: 10.1038/284183a0. [DOI] [PubMed] [Google Scholar]
- Greenwell P., Jewett S. L., Stark G. R. Aspartate transcarbamylase from Escherichia coli. The use of pyridoxal 5'-phosphate as a probe in the active site. J Biol Chem. 1973 Sep 10;248(17):5994–6001. [PubMed] [Google Scholar]
- Howlett G. J., Blackburn M. N., Compton J. G., Schachman H. K. Allosteric regulation of aspartate transcarbamoylase. Analysis of the structural and functional behavior in terms of a two-state model. Biochemistry. 1977 Nov 15;16(23):5091–5100. doi: 10.1021/bi00642a023. [DOI] [PubMed] [Google Scholar]
- Howlett G. J., Schachman H. K. Allosteric regulation of aspartate transcarbamoylase. Changes in the sedimentation coefficient promoted by the bisubstrate analogue N-(phosphonacetyl)-L-aspartate. Biochemistry. 1977 Nov 15;16(23):5077–5083. doi: 10.1021/bi00642a021. [DOI] [PubMed] [Google Scholar]
- Ito H., Ike Y., Ikuta S., Itakura K. Solid phase synthesis of polynucleotides. VI. Further studies on polystyrene copolymers for the solid support. Nucleic Acids Res. 1982 Mar 11;10(5):1755–1769. doi: 10.1093/nar/10.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenness D. D., Schachman H. K. Genetic characterization of the folding domains of the catalytic chains in aspartate transcarbamoylase. J Biol Chem. 1983 Mar 10;258(5):3266–3279. [PubMed] [Google Scholar]
- Justesen J., Neuhard J. pyrR identical to pyrH in Salmonella typhimurium: control of expression of the pyr genes. J Bacteriol. 1975 Sep;123(3):851–854. doi: 10.1128/jb.123.3.851-854.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz E. R., Foote J., Reed H. W., Vensel L. A. Isolation and preliminary characterization of single amino acid substitution mutants of aspartate carbamoyltransferase. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3249–3253. doi: 10.1073/pnas.77.6.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz E. R., Reed H. W., Ferraro R. A., Daigneault J. P. Analysis of mutant Escherichia coli aspartate transcarbamylases isolated from a series of suppressed pyrB nonsense strains. J Mol Biol. 1981 Dec 15;153(3):569–587. doi: 10.1016/0022-2836(81)90408-3. [DOI] [PubMed] [Google Scholar]
- Ke H. M., Honzatko R. B., Lipscomb W. N. Structure of unligated aspartate carbamoyltransferase of Escherichia coli at 2.6-A resolution. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4037–4040. doi: 10.1073/pnas.81.13.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe T. D., Stark G. R. Pyridoxal 5'-phosphate, a fluorescent probe in the active site of aspartate transcarbamylase. J Biol Chem. 1975 Sep 10;250(17):6861–6869. [PubMed] [Google Scholar]
- Krause K. L., Volz K. W., Lipscomb W. N. Structure at 2.9-A resolution of aspartate carbamoyltransferase complexed with the bisubstrate analogue N-(phosphonacetyl)-L-aspartate. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1643–1647. doi: 10.1073/pnas.82.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahue R. S., Schachman H. K. The influence of quaternary structure on the active site of an oligomeric enzyme. Catalytic subunit of aspartate transcarbamoylase. J Biol Chem. 1984 Nov 25;259(22):13906–13913. [PubMed] [Google Scholar]
- Lauritzen A. M., Lipscomb W. N. Modification of three active site lysine residues in the catalytic subunit of aspartate transcarbamylase by D- and L-bromosuccinate. J Biol Chem. 1982 Feb 10;257(3):1312–1319. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Navre M., Schachman H. K. Synthesis of aspartate transcarbamoylase in Escherichia coli: transcriptional regulation of the pyrB-pyrI operon. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1207–1211. doi: 10.1073/pnas.80.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastra-Landis S. C., Foote J., Kantrowitz E. R. An improved colorimetric assay for aspartate and ornithine transcarbamylases. Anal Biochem. 1981 Dec;118(2):358–363. doi: 10.1016/0003-2697(81)90594-7. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Lehmann H. Molecular pathology of human haemoglobin. Nature. 1968 Aug 31;219(5157):902–909. doi: 10.1038/219902a0. [DOI] [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Modified methods for the determination of carbamyl aspartate. Anal Biochem. 1969 Dec;32(3):408–419. doi: 10.1016/s0003-2697(69)80008-4. [DOI] [PubMed] [Google Scholar]
- Roberts M. F., Opella S. J., Schaffer M. H., Phillips H. M., Stark G. R. Evidence from 13C NMR for protonation of carbamyl-P and N-(phosphonacetyl)-L-aspartate in the active site of aspartate transcarbamylase. J Biol Chem. 1976 Oct 10;251(19):5976–5985. [PubMed] [Google Scholar]
- Robey E. A., Schachman H. K. Site-specific mutagenesis of aspartate transcarbamoylase. Replacement of tyrosine 165 in the catalytic chain by serine reduces enzymatic activity. J Biol Chem. 1984 Sep 25;259(18):11180–11183. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., DiMaio D., Nathans D. Directed mutagenesis. Annu Rev Genet. 1981;15:265–294. doi: 10.1146/annurev.ge.15.120181.001405. [DOI] [PubMed] [Google Scholar]
- Silver R. S., Daigneault J. P., Teague P. D., Kantrowitz E. R. Analysis of two purified mutants of Escherichia coli aspartate transcarbamylase with single amino acid substitutions. J Mol Biol. 1983 Aug 25;168(4):729–745. doi: 10.1016/s0022-2836(83)80072-2. [DOI] [PubMed] [Google Scholar]
- Syvanen J. M., Roth J. R. Structural genes for catalytic and regulatory subunits of aspartate transcarbamylase. J Mol Biol. 1973 May 25;76(3):363–378. doi: 10.1016/0022-2836(73)90510-x. [DOI] [PubMed] [Google Scholar]
- Vickers L. P., Compton J. G., Wall K. A., Flatgaard J. E., Schachman H. K. Comparison of active mutants and wild-type aspartate transcarbamoylase of Escherichia coli. J Biol Chem. 1984 Sep 10;259(17):11027–11035. [PubMed] [Google Scholar]
- Wall K. A., Flatgaard J. E., Schachman H. K., Gibbons I. Purification and characterization of a mutant aspartate transcarbamoylase lacking enzyme activity. J Biol Chem. 1979 Dec 10;254(23):11910–11916. [PubMed] [Google Scholar]
- Wall K. A., Schachman H. K. Primary structure and properties of an inactive mutant aspartate transcarbamoylase. J Biol Chem. 1979 Dec 10;254(23):11917–11926. [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]


