Abstract
Study Design
Genetic engineering techniques were used to develop an animal model of juvenile scoliosis during a postnatal skeletal-growth stage.
Objective
To investigate the effect of targeted SHP2 (Src homology-2)-deficiency in chondrocytes on the development of scoliosis during a juvenile growth stage in mice.
Summary of Background Data
Juvenile idiopathic scoliosis can lead to progressive severe spinal deformity. The pathophysiology and molecular mechanisms responsible for this are largely unknown. Here, we investigated the role of SHP2-deficiency in chondrocytes as a potential cause of juvenile scoliosis development.
Methods
Genetically engineered mice with inducible deletion of SHP2 in chondrocytes were generated. The SHP2 function in chondrocytes was inactivated during a juvenile growth stage from the mouse age of 4-weeks. Radiographic, micro-CT, and histological assessments were used to analyze spinal changes.
Results
When SHP2-deficiency was induced during the juvenile stage, a progressive kyphoscoliotic deformity (thoracic lordosis and thoracolumbar kyphoscoliosis) developed within 2 weeks of the initiation of SHP2-deficiency. The 3-dimensional micro-CT analysis confirmed the kyphoscoliotic deformity with a rotational deformity of the spine and osteophyte formation. The histological analysis revealed disorganization of the vertebral growth plate cartilage. Interestingly, when SHP2 was disrupted during the adolescent to adult stages, no spinal deformity developed.
Conclusion
SHP2 plays an important role in normal spine development during skeletal maturation. Chondrocyte-specific deletion of SHP2 at a juvenile stage produced a kyphoscoliotic deformity. This new mouse model will be useful for future investigations of the role of SHP2-deficiency in chondrocytes as a mechanism leading to the development of juvenile scoliosis.
Keywords: a mouse model, scoliosis, kyphosis, lordosis, chondrocyte, cartilage, spine, adolescent, SHP2, loss of function, conditional knockout, age specific gene disruption, RASMAPK signal
Introduction
Scoliosis is a condition affecting children of all ages. It is typically classified as idiopathic (cause unknown or scoliosis without co-existing diagnoses), congenital (vertebral anomalies present at birth), or neuromuscular. Idiopathic scoliosis, which comprises about 80 percent of all cases, is subclassified as infantile (age 0-3), juvenile (age 3-10), adolescent (age 10-18), or adult (age >18), according to when the onset of scoliosis occurs. Progressive early-onset idiopathic scoliosis is a serious, potentially life-threatening condition, and the most clinically challenging form of idiopathic scoliosis. The pathophysiology and molecular mechanisms responsible for the development of idiopathic scoliosis are largely unknown, particularly with regard to the resultant vertebral growth disturbance. One promising strategy to investigate the pathophysiology of scoliosis is to use genetically-engineered animal models to determine which molecular pathways play a role in the pathogenesis of scoliosis. Currently, animal models available to study the molecular mechanisms and the cell types involved with the onset and the subsequent progression of scoliosis are limited.
PTPN11 (Protein-tyrosine phosphatases non-receptor type 11) encodes SHP2 (src homology-2)-containing protein tyrosine phosphate. PTPN11, referred henceforth as SHP2 in this paper, plays a central role in RAS/MAPK signaling downstream of several receptor tyrosine kinases including EGFR (epidermal growth factor receptor) and FGFR (fibroblast growth factor receptor) [1]. In general, an activation of SHP2 has a positive effect on the RAS/MAPK signal transduction. SHP2 is ubiquitously expressed in the body, but the role of SHP2 in the skeleton is largely unknown. In our previous work, we ablated the SHP2 gene in all cells of the body and found skeletal abnormalities including spinal deformity, in which both the trabecular bone mass and the growth plate cartilage in the spine were affected [2]. Given the development of the spinal deformity due to SHP2-deficiency, it is important to determine which tissue (i.e. bone vs. cartilage) and cell type are responsible for the development of scoliosis because such information may provide further new insights into the pathogenesis of scoliosis.
The purpose of this study was to investigate whether the chondrocyte-specific induction of SHP2-deficiency during a juvenile stage can produce scoliosis in mice.
Materials and Methods
Generation of chondrocyte-specific SHP2-deficient mice
The animal protocols for this study were approved by the local IACUC (Institutional Animal Care and Use Committee) at the University of Texas, Southwestern Medical Center. We generated genetically engineered mice with an inducible SHP2 gene deletion in chondrocytes via tamoxifen administration in order to control the cell-type and the time of SHP2-deficiency. In order to conditionally delete the SHP2 gene specifically in chondrocytes, we used a transgenic mouse line expressing Cre recombinase under the control of the Type II collagen promoter (i.e. Col2a1CreERt2 mice, provided by Dr. Chen [3]), which is inducible by tamoxifen administration. We bred the Col2a1-CreERt2 mice with floxed mice for SHP2 (i.e. SHP2fx/fx mice) and obtained mice in the experimental group (i.e. Col2a1CreERt2+:SHP2fx/fx mice) and the control group (i.e. Col2a1CreERt2−:SHP2fx/fx mice) as defined by genotyping. In order to induce a gene disruption in vivo, we injected tamoxifen into mice via intraperitoneal injection at a concentration of 1 mg per mouse per injection following a previous report [3]. Since the Cre recombinase activity that induces the SHP2 gene deletion is controlled under the type II collagen promoter after tamoxifen administration, only the cells expressing type II collagen (i.e. chondrocytes) will develop the SHP2 gene disruption.
Juvenile to adolescent stages: Tamoxifen was administered twice weekly for 5 weeks (i.e. a total of 10 injections) to both the experimental and the control animals from 4 weeks after birth. Mice are normally weaned about 3~4 weeks of age and their reproductivity is matured by 8 weeks of age; thus, we considered 4 weeks of age in mice as a juvenile stage. After the tamoxifen administration, the experimental group was functionally SHP2-deficient, and we referred to the mice in the experimental group as “SHP2-deficient mice” and the mice in the control group as “Control” throughout this study. To confirm the loss of the SHP2 function in the cartilage of the experimental group, we harvested rib cartilage and measured the SHP2 gene expression by quantitative RT-PCR. The SHP2 expression was significantly reduced (i.e. 85% reduction) in the experimental group compared with the control group (data not shown). Note that Cre activity in Col2a1CreERt2 mice is not 100% during postnatal stages [3], resulting in the 85% reduction of SHP2 in the SHP2-deficient mice. We induced gene disruption when the mice were 4 weeks old and analyzed the spinal deformity when they were 12 weeks old. Sixty mice were in each group (i.e. SHP2-deficient mice and Control group).
Adolescent to adult stages: In addition, we also tested another window by initiating gene disruption at 8 weeks (n = 20 per experimental and control group). Tamoxifen was given from 8 weeks for 5 weeks (i.e. a total of 10 injections) to both the experimental and the control animals. We analyzed them at 16 weeks. We did not see any spinal deformity from this window.
Gross morphology, Radiography and micro-CT scan of spine
For the gross morphology, photographs were taken immediately after the mice were euthanized. Radiographic images of the spine were taken using a Faxitron X-ray system (Faxitron). For a micro-CT scan (SkyScan 1172 Micro-CT, Bruker MicroCT, Belgium), each spine was wrapped with gauze soaked in 70% ethanol and scanned with a 50kV and 200μA setting and a 0.5 mm aluminum filter. The resolution was set to 26.6 microns per pixel, and each rotation step was 0.70 degrees over a range of 180 degrees. After reconstruction with NRecon Reconstruction software (version 1.6.8.), all serial section grayscale images were set to a threshold to identify bone that matched the original grayscale x-ray and to remove any soft tissue contribution. The binarized 3D images were visualized with CTVox (version 2.4).
Histological staining (H&E and Safranin O)
Immediately after sacrifice, the skeleton with the spine was harvested and fixed with 10% formalin for 5 days followed by decalcification with 10% EDTA for 5 days. Bones were embedded in paraffin and cut at 3 μm. Hematoxylin and eosin (H&E) staining was performed following a standard protocol as previously described [4, 5]. For the detection of the cartilage matrix (i.e. chondroitin sulfate) in the vertebrae, Safranin O staining with Fast Green counter-staining was performed.
RESULTS
The gross morphology of the spinal deformity in the chondrocyte-specific SHP2-deficient mice
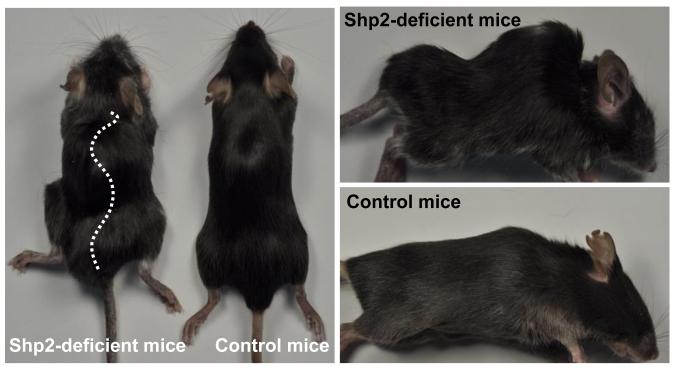
We initiated the target gene disruption at 4 weeks after birth (i.e. juvenile stage). A visible spinal deformity was observed within 2~3 weeks after the initiation of the target gene disruption (SHP2) specifically in chondrocytes (i.e. 6~7 weeks of age). The deformity progressively worsened over time. At 12 weeks old, SHP2-deficient mice had a severe kyphoscoliotic deformity (Figure 1). The spinal deformity together with a gait disturbance was evident in the SHP2-deficient mice. The incidence of spinal deformity in the SHP2-deficient mice was about 40% with varied severity at the endpoint (i.e. 12 weeks).
Figure 1. Gross morphology of spinal deformity in SHP2-deficient mice.
We induced a SHP2 gene disruption in chondrocytes at 4 weeks after birth (i.e. juvenile stage), which produces SHP2 deficiency. Following the induction, a spinal deformity was detected within 2 weeks, which progressed to a severe kyphoscoliotic deformity over time.
In addition, we tested another window to develop scoliosis by changing the timing of the initial gene disruption. We targeted 8-week-old mice which are relevant to puberty in humans because reproductivity is just established. Interestingly, when we kept the mice up to 16 weeks, which is the adult stage of mice, and compared the experimental group (n = 20) with the control group (n = 20), we did not observe development of the spinal deformity (data not shown) although unossified and thick growth plate cartilage is still present at 8 weeks. This finding indicates that the gene disruption during the adolescent to adult stage fails to develop scoliosis and there is a window of susceptibility to the development of spinal deformities in this model (i.e. juvenile to adolescent stages).
Radiographic and micro-CT assessments of the spinal deformity in the chondrocyte-specific SHP2-deficient mice
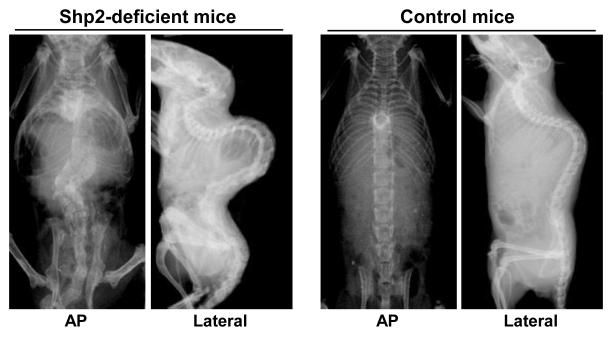
Radiographic assessment revealed severe scoliosis and kyphosis in the SHP2-deficient mice (Figure 2). The scoliosis was in the region of the lower thoracic and lumbar spine. The sagittal plane showed a severe lordosis in the upper thoracic spine and a severe kyphosis in the lower thoracic to lumbar spine. The cobb angle of the lumbar spine in the SHP2-deficient mice was about 90 degrees, while it was zero in the control mice. In the control group, we observed a mild lordosis in the upper thoracic spine and a mild kyphosis in the lower thoracic to lumbar spine, which is anatomically normal for the mouse spine.
Figure 2. Radiographic assessment of spine demonstrating scoliosis and kyphosis in SHP2-deficient mice.
Using mouse genetic-engineering techniques, we induced SHP2-deficiency during a juvenile stage of mouse development. Severe scoliosis and kyphosis were identified in the SHP2-deficient mice at 12 weeks as assessed by x-ray (i.e. AP and lateral views).
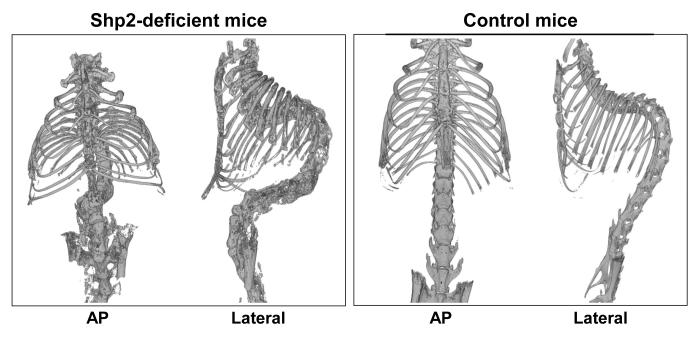
We further investigated the spinal deformity by micro-CT (Figure 3). The 3D-reconstruction images revealed scoliosis, kyphosis, and lordosis in the SHP2-deficient mice in the regions described above, consistent with the radiographic results. In the SHP2-deficient mice, a rotational deformity of the spine was also observed. Micro-CT assessment showed no evidence of spinal or rib fusion. There were no congenital spinal deformities present in the SHP2-deficient spines.
Figure 3. Micro-CT assessment of spinal deformity showing kyphoscoliosis.
The 3D-reconstruction of spine images obtained using micro-CT revealed scoliosis and kyphosis with rotational deformity of the vertebrae in the SHP2-deficient mice at 12 weeks. In addition, minor skeletal abnormalities such as a chestwall deformity and an osteophyte formation on vertebral bodies were identified.
Histological change in the SHP2-deficient mice
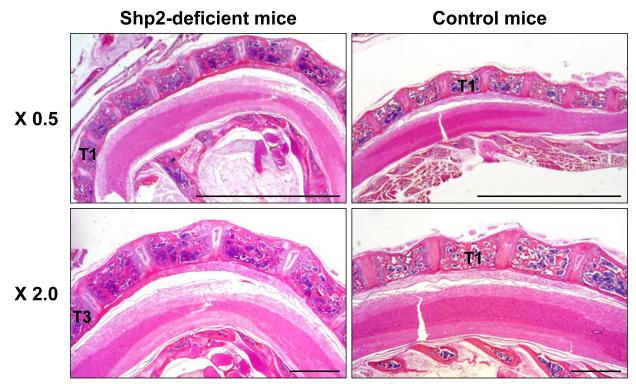
We investigated the histological change in the SHP2-deficient mice at 12 weeks old by H&E staining (Figure 4). The upper thoracic segment was severely lordotic in the SHP2-deficient mice compared to the corresponding region of the control spine, which showed a slight physiological lordosis from the cervical spine to the upper thoracic spine. The segmentation of each individual vertebral body was normal in the SHP2-deficient mice, i.e. no hemivertebrae existed. Using Safranin O staining, examination of the vertebral endplates revealed disorganized growth plate cartilage, variable cartilage thickness, and absent columnar formation of chondrocytes in the SHP2-deficient mice when compared with the controls (Figure 5). Note that the height of the growth plate cartilage appeared to be increased in the SHP2-deficient mice.
Figure 4. A spinal deformity in SHP2-deficient mice assessed by H&E staining.
SHP2-deficient mice demonstrated a severe lordosis in the upper thoracic region compared with only slight lordosis in the control mice at 12 weeks. (T, thoracic vertebrae) Bars; 5 mm (0.5 x), 1 mm (2.0 x)
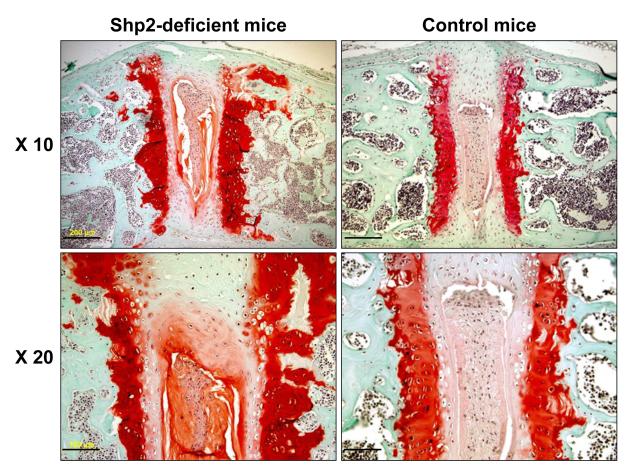
Figure 5. Disorganization of the vertebral growth plate cartilage in SHP2-deficient mice.
Safranin O staining showed a disorganization of growth plate cartilage in SHP2-deficient mice compared with controls at 12 weeks. The thickness of the growth plate cartilage on the vertebral endplate was variable. Bars; 200 μm (10 x), 100 μm (20 x)
Discussion
Scoliosis can be classified by etiology: idiopathic, congenital, or neuromuscular. The spinal deformity identified in the SHP2-deficient mice is relevant for idiopathic scoliosis, but not for congenital or neuromusculare scoliosis. In congenital scoliosis, congenital vertebral abnormalities cause curvature and other deformities of the spine during the embryonic stage leading to asymmetric spinal development. Neuromuscular scoliosis encompasses scoliosis that is secondary to neurological or muscular disorders, including cerebral palsy, spinal cord trauma, muscular dystrophy, spinal muscular atrophy, and spinal bifida. In this study, no congenital vertebral abnormalities such as a failure of formation or segmentation of vertebrae were observed. Furthermore, the SHP2 function is expected to be inactive exclusively in cartilage and the neuromuscular system should not be affected primarily.
The cell types that contribute to the development of idiopathic scoliosis are largely unknown, although several tissue types are involved in the spinal structure including bone, cartilage, blood vessels and the spinal cord. To our knowledge, this is the first study to suggest that chondrocytes are required for the development of normal spinal formation. We found that the single gene ablation (i.e. SHP2) specifically in the cartilage caused a severe spinal deformity. The growth plate cartilage in the spine was disorganized in the SHP2-deficient mice by histological analysis, consistent with a previously report [2]. The abnormality of growth plate cartilage in the SHP2-deficient mice assessed by Safranin O staining, however, did not show a specific pattern of abnormality (Figure 5) and did not appear to correlate with the direction of the spinal deformity (i.e. anterior/posterior, right/left). Further studies are necessary to fully characterize spinal deformities including intervertebral disc, bone, cartilage and related structures and identify specific molecules which could be responsible for the direction of the deformity and the onset/progression of scoliosis.
In this study, we disrupted SHP2 specifically in chondrocytes at 4 weeks after birth and found development of severe scoliosis, kyphosis, and lordosis. Mice are normally weaned about 3 to 4 weeks after birth and their reproductivity is normally established in 8 weeks. Therefore, we considered the mouse age of 4 weeks (i.e. when we induced the target-gene disruption) to be analogous to a juvenile stage of growth in humans. The spinal deformity appeared about 2~3 weeks after the initiation of tamoxifen administration (i.e. about 6~7 weeks old) and the deformity progressed up to 10 to 12 weeks of age, demonstrating that the spinal deformity worsened during the juvenile to adolescent stages. In addition, when we initiated the induction of the SHP2-deficiency at 8 weeks after birth, we did not observe any spinal deformity, indicating that the age has an important bearing on the development of scoliosis in this mouse model.
SHP2 is a positive regulator of RAS-MAPK signaling (Ras-mitogen-activated protein kinase), which is essential for normal skeletal growth. Human mutations involving the RASMAPK signaling pathway have been described with skeletal abnormalities, including scoliosis and kyphosis. It has been reported that excessive RAS-MAPK signaling, as in the patients with neurofibromatosis[6] or Noonan syndrome[7], and reduced RAS-MAPK signaling, as in Leopard syndrome[8], are associated with severe scoliosis[9]. Moreover, Costello syndrome and Cardiofaciocutaneous (CFC) syndrome have been known to develop severe scoliosis due to the dysfunction in RAS-MAPK signaling [10]. In this respect, this mouse model may serve as an important research tool to study how the SHP2-deficiency affects RAS-MAPK signaling and why kyphoscoliosis develops when the RAS-MAPK signaling is altered.
One of the limitations is the incidence of scoliosis (i.e. about 40%). In this study, we generated and analyzed about 60 mice in the experimental group and 25 mice showed scoliosis with a variation of severity at the endpoint of this study (i.e. 12 weeks). In general, the severity and progression is varied in humans. Thus, it would be clinically important to study the detail of development and progression of scoliosis using this mouse model over time as a future direction of this study. As another limitation, we observed a minor phenotype of osteophyte in SHP2-deficient mice; however, it is not typical in juvenile scoliosis in humans. At this time, the significance or the pathophysiology of osteophyte development in this model is unclear. It is possible that SHP-2 deficiency affects peri-articular cartilage remodeling and makes this region more prone to osteophyte development. This will be more closely investigated in our future studies.
There are similarities and differences between the growth plates of mice and humans. One of the differences is that mice are tetra pods and humans are bipeds. The mice also have a more kyphotic spine. In terms of similarities, mice have distinct cervical, thoracic, and lumbar spine segments like humans. Furthermore, studies on the morphology and molecular pathways of mouse growth plates have provided useful insight into our understanding of human growth plate regulation and function [11]. This model does have some differences to human scoliosis and should be used with caution and its clinical relevance remains to be proven. It is, however, a useful model to gain new insights on how genetic disruption can alter the spinal growth plate function and lead to scoliosis.
In conclusion, we developed a murine model of juvenile scoliosis in which SHP2 was deficient specifically in chondrocytes. Because no mouse model of scoliosis and kyphosis was available prior to this, we believe that our genetically-engineered animal model will be useful in future studies of the molecular mechanisms of the spinal growth plate involved with the onset, progression and treatment of juvenile scoliosis.
Key Points.
- We generated a mouse model of scoliosis by inducing SHP2-deficiency specifically in chondrocytes.
- The spinal deformities (scoliosis, kyphosis, and lordosis) were observed when SHP2-deficiency was induced during a juvenile stage in mice.
- The SHP2 function in chondrocytes is required for the normal postnatal development of the spine.
Acknowledgments
This work was supported by the SRS New Investigator Research Grant Award and the Intramural Research Program of TSRH (N. K.). We thank Tracy Wassell and Amanda McLerran for animal care and Reuel Cornelia and Richard Banlaygas for histological preparation.
The Manuscript submitted does not contain information about medical device(s)/drug(s). Scoliosis Research Society New Investigator Research Grant Award and the Intramural Research Program of Texas Scottish Rite Hospital for Children (N. K.) funds were received to support this work. Relevant financial activities outside the submitted work: grants/grants pending, payment for lectures, royalties and stock/stock options.
Abbreviation
- PTPN11
protein-tyrosine phosphatases non-receptor type 11
- SHP2
Src homology-2
- RAS-MAPK
Ras- mitogen-activated protein kinase
Footnotes
Disclosures: none
Level of Evidence: N/A
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tidyman WE, Rauen KA. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev. 2009;19(3):230–6. doi: 10.1016/j.gde.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauler TJ, Kamiya N, Lapinski PE, et al. Development of severe skeletal defects in induced SHP-2-deficient adult mice: a model of skeletal malformation in humans with SHP-2 mutations. Dis Model Mech. 2011;4(2):228–39. doi: 10.1242/dmm.006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen M, Lichtler AC, Sheu TJ, et al. Generation of a transgenic mouse model with chondrocyte-specific and tamoxifen-inducible expression of Cre recombinase. Genesis. 2007;45(1):44–50. doi: 10.1002/dvg.20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamiya N, Ye L, Kobayashi T, et al. Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J Bone Miner Res. 2008;23(12):2007–17. doi: 10.1359/JBMR.080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamiya N, Kobayashi T, Mochida Y, et al. Wnt Inhibitors Dkk1 and Sost are Downstream Targets of BMP Signaling Through the Type IA Receptor (BMPRIA) in Osteoblasts. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bollag G, Clapp DW, Shih S, et al. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nat Genet. 1996;12(2):144–8. doi: 10.1038/ng0296-144. [DOI] [PubMed] [Google Scholar]
- 7.Tartaglia M, Mehler EL, Goldberg R, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29(4):465–8. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 8.Conti E, Dottorini T, Sarkozy A, et al. A novel PTPN11 mutation in LEOPARD syndrome. Hum Mutat. 2003;21(6):654. doi: 10.1002/humu.9149. [DOI] [PubMed] [Google Scholar]
- 9.Lee CK, Chang BS, Hong YM, et al. Spinal deformities in Noonan syndrome: a clinical review of sixty cases. J Bone Joint Surg Am. 2001;83-A(10):1495–502. [PubMed] [Google Scholar]
- 10.Rodriguez-Viciana P, Tetsu O, Tidyman WE, et al. Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science. 2006;311(5765):1287–90. doi: 10.1126/science.1124642. [DOI] [PubMed] [Google Scholar]
- 11.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–6. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]