Abstract
Gamma-amino butyric acid type C (GABAC) receptors inhibit neuronal firing primarily in retina. Maintenance of GABAC receptor protein homeostasis in cells is essential for its function. However, a systematic study of GABAC receptor protein homeostasis (proteostasis) network components is absent. Here, co-immunoprecipitation of human GABAC-ρ1 receptor complexes was performed in HEK293 cells overexpressing ρ1 receptors. To enhance the coverage and reliability of identified proteins, immunoisolated ρ1 receptor complexes were subjected to three tandem mass spectrometry (MS)-based proteomic analyses: namely, gel-based tandem MS (GeLC-MS/MS), solution-based tandem MS (SoLC-MS/MS), and multidimensional protein identification technology (MudPIT). From the 107 identified proteins, we assembled GABAC-ρ1 receptor proteostasis network components, including proteins with protein folding, degradation, and trafficking functions. We studied representative individual ρ1 receptor interacting proteins, including calnexin, a lectin chaperone that facilitates glycoprotein folding, and LMAN1, a glycoprotein trafficking receptor, and global effectors that regulate protein folding in cells based on bioinformatics analysis, including HSF1, a master regulator of the heat shock response, and XBP1, a key transcription factor of the unfolded protein response. Manipulating selected GABAC receptor proteostasis network components is a promising strategy to regulate GABAC receptor folding, trafficking, degradation and thus function to ameliorate related retinal diseases.
Keywords: GABAC receptor, tandem mass spectrometry, interactome, proteostasis, folding, trafficking, degradation, heat shock response, unfolded protein response
INTRODUCTION
Normal organismal physiology depends on the maintenance of protein homeostasis (proteostasis) in each cellular compartment 1, 2, which dictates a delicate balance between protein synthesis, folding, trafficking, and degradation, while minimizing misfolding and aggregation 2–8. For a specific client protein, its interaction with a network of proteins in the crowded cellular environment is critical to maintain its proper folding, trafficking, degradation, and thus function.
GABA is the primary inhibitory neurotransmitter in the mammalian central nervous system and retina. Three types of GABA receptors are reported: GABAA, GABAB, and GABAC receptors. GABAA and GABAC receptors belong to the Cys-loop superfamily of ligand-gated ion channels, whereas GABAB receptors belong to G protein coupled receptor family 9. GABAC receptors, also known as GABA-ρ receptors, are expressed mainly in the retina, although they have been identified in a variety of central nervous systems 10, 11. GABAC receptors are pentameric, sharing common structural characteristics with other Cys-loop receptor members (Figure 1A, left) 12. To date, three isoforms of GABAC-ρ subunits (ρ1, ρ2 and ρ3) have been identified in human. The ρ subunits can form homopentameric GABAC receptors. Most studies focused on homopentameric ρ1 receptors by using HEK293 cells or Xenopus oocytes as the expression system. HEK293 cell lines are extensively used for the expression of ion channels, including GABAC receptors, because of low endogenous ion channel expression level, high transfection efficiency, and good physiological functioning of the expressed ion-channels 10, 13. Each ρ1 subunit has four transmembrane helices (TM1-TM4, with TM2 domain lining the interior of the pore), a large 260-residue extracellular (or the endoplasmic reticulum (ER) luminal) N-terminus after the cleavage of the 21-residue signal peptide, and an extracellular (or the ER luminal) C-terminus (Figure 1A, right). Two GABA-binding sites lie between two adjacent subunits and are located in the N-terminal extracellular domains. GABA binding to GABAC receptors triggers a large conformational change, opens the ion pore to conduct chloride, hyperpolarizes the plasma membrane, and inhibits neuronal firing. Knockout studies in mice demonstrated that elimination of ρ1 subunits led to abnormal visual processing in the mouse retina 14 and resulted in changes in vascular permeability similar to the symptoms in retinal hypoxic conditions 15.
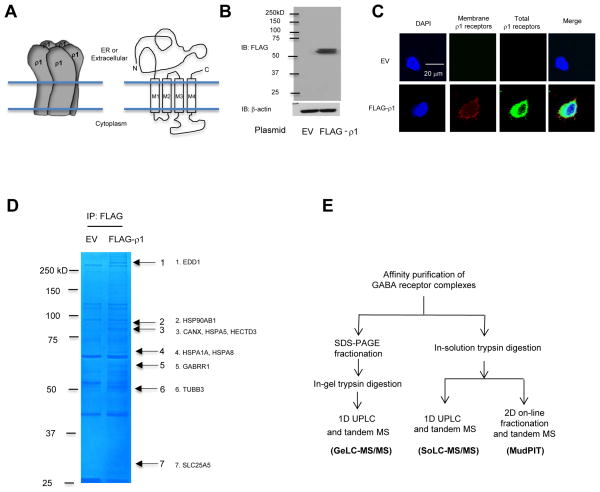
FIGURE 1. Outline of three tandem MS approaches to identify the GABAC-ρ1 receptor interactome in HEK293 cells.
(A) A cartoon of homopentameric ρ1 receptors and the topology of the ρ1 subunit.
(B) Western blot analysis confirms overexpression of FLAG-ρ1 receptors in HEK293 cells. β-actin serves as a loading control. EV: empty vector control plasmid.
(C) Confocal immunofluorescence microscopy confirms the expression of FLAG-ρ1 receptors on the plasma membrane and inside cells.
(D) Visualization of immunoisolated ρ1 receptor complex by SDS-PAGE and Coomassie staining. Representative ρ1 subunit interacting proteins are shown.
(E) Outline of three tandem MS approaches to identify ρ1 receptor interactome. UPLC: ultra-high pressure reverse-phase liquid chromatography.
To function, GABAC receptors need to fold into their native structures and assemble correctly on the ER membrane and traffic efficiently to the plasma membrane. Maintenance of a delicate balance between GABAC receptor folding, trafficking and degradation through specific protein sensing and interactions is critical for its function. However, to date, the identification of proteostasis network components that regulate GABAC receptor folding, trafficking and degradation was not explored. Here, we identified proteins that interact with GABAC receptors using human HEK293 cells overexpressing GABA-ρ1 receptors by immuno-affinity purification tandem mass-spectrometry (MS) proteomics analysis. To enhance the coverage and reliability of the identified proteins, immunoisolated ρ1 receptor complexes were subjected to three tandem MS-based proteomics analyses: namely, gel-based tandem MS (GeLC-MS/MS), solution-based tandem MS (SoLC-MS/MS), and multidimensional protein identification technology (MudPIT). Furthermore, from the identified ρ1 receptor interactome proteins, we focused on assembling proteostasis network components that could potentially regulate GABAC receptor folding, trafficking, and degradation. Manipulation of these protein candidates is a promising strategy to regulate GABAC receptor proteostasis and thus function.
EXPERIMENTAL PROCEDURES
Plasmids
The pCMV6 plasmid containing C-terminal FLAG-tagged human gamma-aminobutyric acid receptor ρ1 subunit (pCMV6-FLAG-ρ1) and pCMV6 Entry Vector plasmid (pCMV6-EV) were obtained from Origene. The human GABAC-ρ1 subunit missense mutations (R89Q or V353D) were constructed using QuickChange II site-directed mutagenesis Kit (Agilent Genomics), and the cDNA sequences were confirmed by DNA sequencing. The pcDNA3.1 plasmid containing N-terminal FLAG-tagged human HSF1 cDNA (pcDNA3.1-FLAG-HSF1) was obtained from Addgene. The pcDNA3.1 plasmid containing a spliced form of XBP1 (pCDNA3.1-XBP1(s)) was a gift from Dr. Richard Sifers (Baylor College of Medicine).
Cell Culture and Transfection
HEK293 cells (ATCC) were maintained in Dulbecco’s Modified Eagle Medium (DMEM) (Hyclone) with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich) and 1% Pen-Strep (Hyclone) at 37°C in 5% CO2. Monolayers were passaged upon reaching confluency with TrypLE Express (Invitrogen). HEK293 cells were grown in 6-well plates or 10-cm dishes and allowed to reach ~70% confluency before transient transfection using Fugene 6 (Roche) or Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Antibodies
The mouse monoclonal anti-FLAG M2 and anti-β-actin antibodies were obtained from Sigma. The rabbit polyclonal anti-matrin 3 antibodies was obtained from Bethyl Laboratories. The rabbit polyclonal anti-calnexin antibody was obtained from Enzo Life Sciences. The rabbit polyclonal anti-Derlin1, anti-DNAJA1 and anti-LMAN1 antibodies, and rabbit monoclonal anti-HSF1 antibody were obtained from Abgent. The rabbit monoclonal anti-BAG-2 and anti-UGGT1 antibodies were obtained from Epitomics. The rabbit polyclonal anti-XBP1 antibody was obtained from Santa Cruz Biotechnology. The rabbit polyclonal anti-C1QBP antibody was obtained from Pierce Antibodies.
Immunoprecipitation and Western Blot
Following 48 hours post-transfection, cells were harvested with TrypLE Express and then lysed with lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, and 1% Triton X-100) supplemented with Roche complete protease inhibitor cocktail. Lysates were cleared by centrifugation (15,000 × g, 10 min, 4°C). Protein concentration was determined by MicroBCA assay (Pierce). To isolate the FLAG-ρ1 subunit complex, the total proteins (1 mg) were pre-cleared with 30 μL of mouse IgG-agarose beads for 1 hr at 4°C to remove nonspecific binding proteins and incubated with 30 μL of anti-FLAG M2 magnetic beads (Sigma-Aldrich) for 1 hr at room temperature. The beads were collected using a magnet separator and washed three times with lysis buffer. The FLAG-ρ1 subunit complex was eluted by either incubation with 30 μL of SDS loading buffer in the presence of DTT or 30 μL of 0.1 M glycine (pH 3.0). The immunopurified eluents or equal amounts of total proteins were separated in 8% SDS-PAGE gel, and Western blot analysis was performed using appropriate antibodies.
Confocal Immunofluorescence
To label cell surface GABAC receptor FLAG-ρ1 subunits, HEK293 cells were incubated on ice in 100 μL of HEPES buffer (HEPES 25 mM, NaCl 140 mM, KCl 5.4 mM, CaCl2 1.8 mM, glucose 15 mM, pH=7.4) containing mouse anti-FLAG M2 antibody (1:100) for 1 hr, and then incubated on ice with 1 mL of buffer containing an Alexa 546-conjugated goat anti-mouse antibody (1:400) for 1 hr. To label total GABAC receptor FLAG-ρ1 subunits, HEK293 cells were then fixed with 4% paraformaldehyde, permeabilized with saponin (0.2%), blocked with goat serum for 0.5 hr at room temperature, labeled with the anti-FLAG antibody (1:100) for 1 hr, and then incubated at room temperature with 1 ml of buffer containing an Alexa 488-conjugated goat anti-mouse antibody (1:400) for 1 hr. For confocal immunofluorescence microscopy, an Olympus IX-81 Fluoview FV1000 confocal laser scanning system was used. A 60× objective was used to collect images using FV10-ASW software.
In-gel Digestion
6 mg of total proteins was immunoprecipitated using anti-FLAG antibody, separated by SDS-PAGE, and Coomassie stained to visualize protein gel bands. The gel was washed in distilled water to remove excess background stain. The whole gel was cut into six pieces containing prominent bands from empty vector (EV) control and GABA-ρ1 receptor overexpression samples, respectively, and destained with 500 μL of 1:1 ACN (acetonitrile) and 100 mM ABC (ammonium bicarbonate) solution for 2–8 hr. Afterwards, 10 mM reductive TCEP (tris(2-carboxyethyl)phosphine) was added, and free cysteines were alkylated with 55 mM IAA (iodoacetamide). ACN and 100 mM ABC were used to dehydrate and rehydrate the gel pieces, respectively, three times. Gel pieces were swelled in 50 mM ABC containing freshly prepared 10 ng/μL trypsin (Promega, sequencing grade) and digested overnight. Peptides were extracted with 60% ACN/5% FA (formic acid) and dried in SpeedVac.
In-solution Digestion
6 mg of total proteins was immunoprecipitated using anti-FLAG antibody, and bound proteins were eluted by 0.1 M glycine (pH 3.0). Proteins from each sample were precipitated using 25% trichloroacetic acid (v/v) and the protein pellet washed using ice-cold acetone. The pellet was air-dried and suspended with 8 M urea in 100 mM Tris-HCl, pH 8.5. Afterwards, 5 mM reductive TCEP was added, and free cysteines were alkylated with 10 mM IAA. The samples were subsequently diluted to 2 M urea with 100 mM Tris-HCl, pH 8.5, and digested by adding trypsin and incubating overnight at 37°C. The digestion reaction was quenched by adding formic acid to 5% (v/v), and peptides were cleaned up using a C18 spin column (Thermo Pierce, Rockford, IL).
1D Ultra-high Pressure Reverse Phase Liquid Chromatography (UPLC) and Tandem Mass Spectrometry Analysis
For both gel-based tandem MS analysis (GeLC-MS/MS) and solution-based tandem MS analysis (SoLC-MS/MS), digested peptides were reconstituted with 0.1% formic acid and analyzed by LC-MS/MS. Separation of peptides via capillary liquid chromatography was performed using Waters nanoAquity system (Waters Corp., Milford, MA). Mobile phase A (aqueous) contained 0.1% formic acid in 5% acetonitrile, and mobile phase B (organic) contained 0.1% formic acid in 85% acetonitrile. Separation was achieved using a C18 column (75 μm × 20 cm, Waters Corp., Ethylene Bridged Hybrid column BEH300) through a 150 min gradient of 6% to 45% mobile phase B at a flow rate of 0.30 μL/min. Mass spectrometry analysis was performed using a hybrid linear ion trap Orbitrap Velos mass spectrometer (LTQ-Orbitrap Velos, Thermo, Waltham, MA). Survey scan was operated at 60,000 resolution, followed by eight standard CID (collision induced dissociation) fragmentations in a data-dependent manner. Dynamic exclusion was enabled as the following: repeat count, 2; repeat duration, 30 sec; exclusion list size, 250; exclusion duration, 120 sec. Both GeLC-MS/MS and SoLC-MS/MS were done as a single experiment at Case Western Reserve University to make a fair comparison to a single-run MudPIT.
Multidimensional Protein Identification Technology (MudPIT)16, 17
Peptide mixture was pressure-loaded onto a 250-μm i.d. fused silica capillary column packed with a 2.5-cm Partisphere strong cation exchanger (Whatman, Clifton, NJ) and a 2.5-cm of 5-μm Aqua C18 material (Phenomenex, Ventura, CA). The column was washed for 30 min with buffer containing 95% water, 5% acetonitrile (ACN), and 0.1% formic acid. After desalting, it was attached to a 100-μm i.d. capillary with a 5-μm pulled tip packed with a 12-cm of 5-μm Aqua C18 material, and the entire column was placed in line with an Agilent 1100 quaternary HPLC (Agilent, Palo Alto, CA). The sample was analyzed using a fully automated 6-step separation procedure. The buffer solutions used for the chromatography were 5% ACN/0.1% FA (buffer A), 80% ACN/0.1% FA (buffer B), and 500 mM ammonium acetate/0.1% FA (buffer C). The first step consisted of a 100 min gradient from 0 to 100% buffer B. Steps 2–5 had the following profile: 3 min of 100% buffer A, 3 min of X% buffer C, a-10 min gradient from 0 to 15% buffer B, and a 97-min gradient from 15 to 55% buffer B. The 3-min buffer C percentages (X) were 20, 40, 60, and 90%. In the final step, the gradient contained: 3 min of 100% buffer A, 10 min of 100% buffer C, a 10-min gradient from 0 to 15% buffer B, and a 107-min gradient from 15 to 100% buffer B. As peptides were eluted from the microcapillary column, they were electrosprayed directly into a linear LTQ ion trap mass spectrometer (ThermoFinnigan, San Jose, CA). A cycle of one full scan mass spectrum (400–1400 m/z) was followed by 5 data-dependent CID MS/MS scans. Dynamic exclusion was enabled as the following: repeat count, 1; repeat duration, 20 sec; exclusion list size, 300; exclusion duration, 90 sec. MudPIT was done as a single experiment at the Scripps Research Institute.
Database Search
Acquired tandem mass spectra were searched against the European Bioinformatics Institute International Protein Index human protein database (version 3.71, released on Mar. 24th, 2010), containing 92,139 protein entries. A decoy database containing the reversed sequences of all the proteins was appended to estimate false discovery rate 18. Protein identification using Sequest 19 or ProLuCID 20 and DTASelect 21, 22 were done through the Integrated Proteomics Pipeline (IP2, Integrated Proteomics Applications, Inc. San Diego, CA). The precursor mass accuracy was limited to 15 ppm for spectra acquired on the Orbitrap instrument and 1 Da on the LTQ instrument; product ions mass accuracy was set at 0.6 Da. Fully tryptic enzyme specificity and up to two missed cleavages were allowed. Static modifications included carbamidomethylation on cysteines (57 Da), and variable modifications included oxidation on methionines (16 Da). Isotopic C13 incorporated ions were automatically included. DTASelect 21, 22 was applied to generate search results of peptide-to-spectra matches (PSMs) with a max false discovery rate (FDR) of 5%, yielding a protein FDR of less than 1% with at least two peptides per protein being assigned. Although accession numbers for protein groups, which share common peptides, are provided, the best annotated from a group, along with its corresponding gene name, is used as an index in this study for clarity. Additionally, listed in Supplemental Table S1 for each protein are protein coverage; unique peptide counts, which merge peptides with the same sequence but multiple precursor charge states; and spectral counts. Furthermore, all assigned peptides are listed in Supplemental Table S2, including precursor charge and mass error, XCorr-, ΔCN- and DTASelect confidence-scores, and spectral counts.
Criteria for the ρ1 receptor interactome and false discovery rate (FDR) analysis
The ρ1 receptor interactome was identified using the spectral counting method 17, 19, 23–27 with arbitrary yet strict criteria to remove potential false positives for ρ1 receptor interacting proteins. We comprehensively considered all three distinct tandem MS methods and used two levels of criteria. For level one criteria, if a protein showed up in two out of three methods, the following was applied: a) if a protein was exclusive in ρ1 receptor overexpression sample, spectra count (SC) ≥ 4 was required; b) if a protein was detected in both EV control and ρ1 receptor overexpression sample, SC ≥ 10 in ρ1 receptor overexpression sample and SC ratio (ρ1/EV) ≥ 2.5 were required. For level two criteria, if a protein showed up only in one in-solution digestion MS method (either SoLC-MS/MS or MudPIT), more strict criteria were applied: c) if a protein was exclusive in ρ1 receptor overexpression sample, SC ≥ 8 and protein sequence coverage ≥ 10% were required; d) if a protein was detected in both EV control and ρ1 receptor overexpression sample, SC ≥ 20 in ρ1 receptor overexpression sample, SC ratio (ρ1/EV) ≥ 2.5, and protein sequence coverage ≥ 10% were required.
To evaluate the FDR of the ρ1 receptor interactome, using the same criteria for the identification of proteins that were enriched in the ρ1 overexpression sample after immunoprecipitation, we applied the following filter to identify proteins that are enriched in EV control samples after immunoprecipitation. We comprehensively considered all three distinct tandem MS methods and used two levels of criteria. For level one criteria, if a protein showed up in two out of three methods, the following was applied: a) if a protein was exclusive in EV control sample, spectra count (SC) ≥ 4 was required; b) if a protein was detected in both EV control and ρ1 receptor overexpression sample, SC ≥ 10 in EV control and SC ratio (EV/ρ1) ≥ 2.5 were required. For level two criteria, if a protein showed up only in one in-solution digestion MS method (either SoLC-MS/MS or MudPIT), more strict criteria were applied: c) if a protein was exclusive in EV control sample, SC ≥ 8 and protein sequence coverage ≥ 10% were required; d) if a protein was detected in both EV control and ρ1 receptor overexpression sample, SC ≥ 20 in EV control sample, SC ratio (EV/ρ1) ≥ 2.5, and protein sequence coverage ≥ 10% were required. We define: FDR (ρ1 receptor interactome) = Number of proteins that are enriched in EV control sample / Number of proteins that are enriched in ρ1 receptor overexpression sample after immunoprecipitation.
Protein abundance analysis of the ρ1 receptor interactome
The human protein abundance information was obtained from PaxDb: Protein Abundance Across Organisms (http://paxdb.org). For our analysis, we used the integrated database, which has a 90% coverage of the human proteome, downloaded on 08/15/2013.
Ingenuity IPA Analysis
Cellular location, molecular and cellular function, and enriched transcription factors of GABA-ρ1 receptor interacting proteins were analyzed using Ingenuity IPA software.
siRNA Transfection
HEK293 cells were seeded at approximately 2.5 × 105 cells per well in 6-well plates for the siRNA treatment. Cells were allowed to reach ~70% confluency before transfection. The small interfering RNA (siRNA) duplexes were obtained from Dharmacon: calnexin (J-003636-07-0005), LMAN1 (J-012122-05-0005), C1QBP (J-011225-13-0005) and Non-Targeting siRNA (D-001810-01-20) as negative control. Cells were transfected with 50 nM siRNA using HiPerfect Transfection Reagent (Qiagen) according to the manufacturer’s transfection protocol prior to protein analyses. Following 48 hours post-transfection, cells were harvested, lysed, and subjected to SDS-PAGE and Western blot analysis.
Statistical analysis
All data are presented as mean ± SEM, and any statistical significance was calculated using two-tailed Student’s t-Test.
RESULTS AND DISCUSSION
Mass Spectrometry identification of affinity-purified GABA-ρ1 receptor interacting proteins
HEK293 cells were transiently transfected with a FLAG-ρ1 plasmid for 48 hr before analysis. A clean double band at ~60 kD represented ρ1 receptors, whereas no band was detected using the negative control empty vector (EV) plasmid for transfection according to Western blot analysis (Figure 1B). The double bands represent different glycosylation states of ρ1 subunits: the top band is a post-ER mature glycoform, whereas the bottom band is an immature ER glycoform of the ρ1 subunits (see below, Figure 4). Cell surface ρ1 receptors were visualized using anti-FLAG antibody without a detergent permeabilization step (Figure 1C, second column, red), and total ρ1 receptors were visualized after a detergent permeabilization step (Figure 1C, third column, green) using confocal immunofluorescence microscopy analysis. No cell surface or total ρ1 receptors were detected for the EV control; both cell surface and total ρ1 receptors were clearly detected using a FLAG-ρ1 plasmid for transfection (Figure 1C), indicating that ρ1 subunits were distributed both on the plasma membrane and inside the cell.
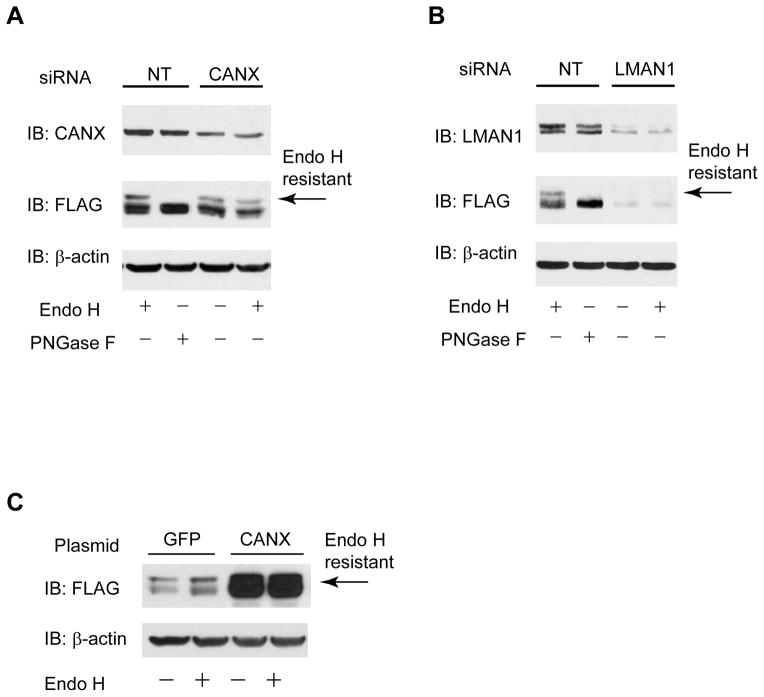
FIGURE 4. Verification of representative GABAC-ρ1 receptor proteostasis network components.
(A and B) Knockdown of calnexin (A), a lectin chaperone in the ER, or LMAN1 (B), a glycoprotein trafficking receptor, decreases the endo H resistant post-ER ρ1 glycoform in HEK293 cells expressing FLAG-ρ1 receptors. PNGase F treatment generates unglycosylated ρ1 subunit (lane 2). β-actin serves as a loading control. NT: non-targeting control siRNA.
(C) Overexpression of calnexin increases the endo H resistant post-ER ρ1 glycoform in HEK293 cells expressing FLAG-ρ1 receptors.
We employed a MS-based proteomics approach to identify ρ1 receptor interacting proteins. GABA-ρ1 receptor complex was enriched by co-immunoprecipitation using anti-FLAG antibody in HEK293 cells overexpressing FLAG-ρ1 receptors. A number of bands were clearly visible in Coomassie-stained gel (Figure 1D). To enhance the coverage and reliability of the identified proteins, immunoisolated ρ1 receptor complexes were subjected to three distinct tandem MS-based proteomic analyses: namely, GeLC-MS/MS, SoLC-MS/MS, and MudPIT (Figure 1E). GeLC-MS/MS employs a SDS-PAGE fractionation step prior to sample loading to a column: the advantages are that ρ1 receptor interacting proteins can be visualized on a Coomassie-stained gel and that proteins within a specific molecular weight range can be identified with high confidence; the disadvantage is that low abundant proteins might be further diluted during sample processing. Both SoLC-MS/MS and MudPIT do not employ a pre-fractionation step before sample loading to a column: the advantage is simpler sample processing and a higher chance to detect low abundant proteins; the disadvantage is that more non-specific proteins might appear in the MS result. Therefore, we combined these three tandem MS methods for our analysis, aiming to achieve the maximum coverage of reliable ρ1 receptor interacting proteins.
GeLC-MS/MS identified 24 proteins as ρ1 receptor interacting proteins (Figure 2A), which are summarized in Table 1 (see also supplemental Tables S1 and S2). Representative proteins are shown in Figure 1D, including molecular chaperones in the ER (CANX, HSPA5) and in the cytosol (HSP90AB1, HSPA1A, HSPA8), E3 ubiquitin ligase (EDD1, HECTD3), and cytoskeleton proteins (TUBB3). To identify more ρ1 receptor interacting proteins, the immunopurified samples were further subjected to SoLC-MS/MS or MudPIT (Figure 1E). The ρ1 receptor interactome was identified using the spectral counting method 17, 19, 23–27. We comprehensively considered all three distinct tandem MS methods with arbitrary yet strict criteria to remove potential false positives for ρ1 receptor interacting proteins (see Experimental Procedures). From SoLC-MS/MS and MudPIT, 50 and 78 proteins were identified, respectively, to interact with ρ1 receptors (Figure 2A and Table 1, see also Supplemental Tables S1, S2 and S4). Along with the 24 interactors identified from GeLC-MS/MS, 107 proteins total were identified as interactors and are listed in Table 1 according to their molecular and cellular function (see also supplemental Table S4). Nine proteins were commonly identified by three tandem MS methods, including two cytoskeleton proteins (TUBB and TUBB3), three proteins that are involved in ubiquitin-related degradation pathway (UBR5, SQSTM1 and UBB), one lectin chaperone (CANX), two metabolic proteins (ATP5A1 and SLC25A6), and overexpressed GABRR1 (Figure 2A). The interacting proteins listed in Table 1 were filtered with carefully defined criteria. Nearly all of them had three or more unique peptides, thus representing strong identification. Nevertheless, an interactor from only one tandem MS method retains higher potential of being a false positive; alternatively, that interaction is transient or unstable.
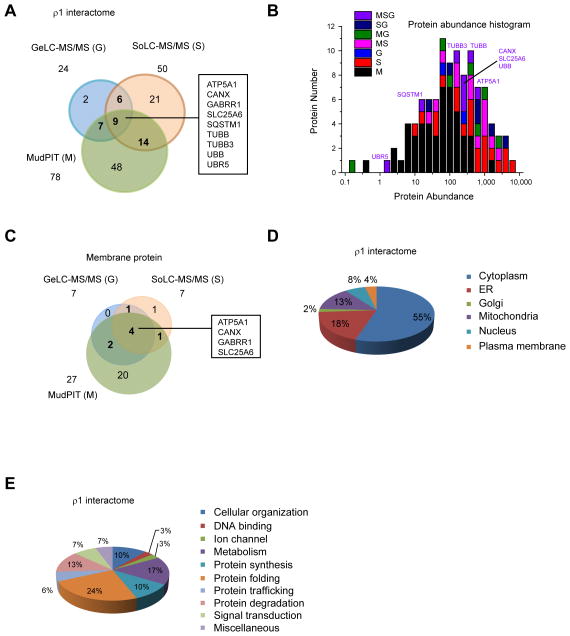
FIGURE 2. Global bioinformatics analysis of GABAC-ρ1 receptor interactome.
(A) A Venn diagram showing the protein number and overlaps of the ρ1 receptor interactome from three tandem MS approaches. Nine proteins, which are commonly identified from three tandem MS methods, are listed.
(B) Protein abundance analysis of the ρ1 receptor interactome. Human protein abundance data is obtained from PaxDb: Protein Abundance Across Organisms (http://pax-db.org). Proteins that are commonly identified using three tandem MS methods are labeled. Tandem MS methods: M, MudPIT; S, SoLC-MS/MS; G, GeLC-MS/MS.
(C) A Venn diagram showing the protein number and overlaps of membrane type ρ1 receptor interactors from three tandem MS approaches.
(D) Cellular location of the ρ1 receptor interactome. Particularly, 18% are in located in the ER, an organelle where membrane protein folding occurs.
(E) Molecular and cellular function of the ρ1 receptor interactome. Particularly, 10% is involved in protein synthesis, 24% in protein folding, 6% in protein trafficking and 13% in protein degradation, composing 53% for the proteostasis network components.
Table 1.
List of GABA ρ1 receptor interactome from three tandem MS approaches
| Function | Gene | Protein | IPI number | Method |
|---|---|---|---|---|
| Protein synthesis | ||||
| Aminoacetyl tRNA synthetase | ||||
| FARSA | Phenylalanyl-tRNA synthetase alpha chain | IPI00031820 | M, G | |
| mRNA binding protein | ||||
| IGF2BP1 | Insulin-like growth factor 2 mRNA-binding protein 1 | IPI00008557 | M | |
| Ribosomal protein | ||||
| RPL14 | Ribosomal protein L14 variant | IPI00555744 | S | |
| RPL7P32 | 60S ribosomal protein L7 | IPI00030179 | M, S | |
| RPS10 | 40S ribosomal protein S10 | IPI00008438 | M, S | |
| RPS24 | 40S ribosomal protein S24 | IPI00029750 | S | |
| RNA processing | ||||
| DDX20 | Probable ATP-dependent RNA helicase DDX20 | IPI00005904 | M | |
| PABPC4 | Polyadenylate-binding protein 4 | IPI00012726 | M | |
| Translation factor | ||||
| EEF1A1 | Elongation factor 1, alpha 1 | IPI00396485 | M, G | |
| EEF2 | Elongation factor 2 | IPI00186290 | M, S | |
| EIF4B | Eukaryotic translation initiation factor 4B | IPI00012079 | G | |
|
| ||||
| Protein folding | ||||
| Folding enzyme | ||||
| P4HA1 | Prolyl 4-hydroxylase, alpha-1 subunit | IPI00009923 | M | |
| P4HB | Prolyl 4-hydroxylase, beta subunit, PDIA1 | IPI00010796 | M | |
| PDIA6 | Protein disulfide-isomerase A6 | IPI00299571 | M | |
| PPIA | Peptidylprolyl isomerase A (cyclophilin A) | IPI00419585 | S | |
| Small HSP family | ||||
| HSPB1 | Heat-shock protein beta-1, Hsp27 | IPI00025512 | M, S | |
| Hsp40 family | ||||
| DNAJA1 | DnaJ homolog subfamily A member 1 | IPI00012535 | M, S | |
| DNAJA2 | DnaJ homolog subfamily A member 2 | IPI00032406 | M | |
| DNAJB11 | DnaJ homolog subfamily B member 11, ERdj3 | IPI00008454 | M | |
| Hsp60 family | ||||
| CCT4 | T-complex protein 1 subunit delta | IPI00302927 | M | |
| HSPD1 | Heat shock 60kDa protein 1 | IPI00784154 | M, S | |
| TCP1 | T-complex protein 1 subunit alpha | IPI00290566 | M | |
| Hsp70 family | ||||
| HSPA1A | Heat shock 70 kDa protein 1A | IPI00304925 | S, G | |
| HSPA5 | Heat shock 70kDa protein 5, BiP | IPI00003362 | S, G | |
| HSPA6 | Heat shock 70 kDa protein 6 | IPI00339269 | S, G | |
| HSPA8 | Heat shock 70kDa protein 8, HSC70 | IPI00003865 | S, G | |
| HYOU1 | Hypoxia up-regulated protein 1, GRP170 | IPI00922127 | M | |
| Hsp90 family | ||||
| HSP90AB1 | Heat shock protein 90kDa beta, cytosolic | IPI00414676 | M, G | |
| HSP90B1 | Endoplasmin, Grp94 | IPI00027230 | M | |
| Hsp105 family | ||||
| HSPH1 | Heat shock 105 kDa protein 1 | IPI00218993 | M | |
| Lectin chaperone | ||||
| CALR | Calreticulin | IPI00020599 | M | |
| CANX | Calnexin | IPI00020984 | M, S, G | |
| N-glycoprotein processing | ||||
| GANAB | Neutral alpha-glucosidase AB precursor | IPI00011454 | M, S | |
| RPN1 | Dolichyl-diphosphooligosaccharide-protein glycosyltransferase subunit 1 | IPI00025874 | M | |
| RPN2 | Dolichyl-diphosphooligosaccharide-protein glycosyltransferase subunit 2 | IPI00028635 | M | |
| DDOST | Dolichyl-diphosphooligosaccharide-protein glycosyltransferase | IPI00297084 | M | |
| UGGT1 | UDP-glucose ceramide glucosyltransferase-like 1 | IPI00024466 | M | |
|
| ||||
| Protein trafficking | ||||
| COPI machinary | ||||
| COPA | Coatomer subunit alpha | IPI00295857 | M | |
| COPE | Coatomer subunit epsilon | IPI00465132 | M | |
| COPII machinary | ||||
| SEC13 | SEC13-related protein | IPI00375370 | M | |
| SEC16A | SEC16 homolog A | IPI00641384 | M | |
| ER Golgi transport | ||||
| LMAN1 | ERGIC-53 protein | IPI00026530 | M | |
| GDP dissociation inhibitor | ||||
| GDI1 | Rab GDP dissociation inhibitor alpha | IPI00010154 | M | |
|
| ||||
| Protein degradation | ||||
| E3 ubiquitin ligase | ||||
| HECTD3 | E3 ubiquitin-protein ligase HECTD3 | IPI00456642 | M, G | |
| HUWE1 | E3 ubiquitin-protein ligase, HUWE1 | IPI00179298 | M | |
| UBR5 | E3 ubiquitin-protein ligase, EDD1 | IPI00026320 | M, S, G | |
| E3 ubiquitin ligase inhibitor | ||||
| BAG2 | BAG family molecular chaperone regulator 2 | IPI00000643 | M, S | |
| ERAD dislocation channel | ||||
| DERL1 | Derlin-1 | IPI00013271 | M | |
| ERAD substrate extraction | ||||
| BAG6 | BCL2-associated athanogene 6 | IPI00465128 | M | |
| VCP | Transitional endoplasmic reticulum ATPase | IPI00022774 | M, S | |
| Protease inhibition | ||||
| SERPINB3 | Serpin B3 | IPI00022204 | S | |
| SERPINB4 | Serpin B4 | IPI00010303 | S | |
| SERPINB5 | Serpin B5 | IPI00783625 | S | |
| Proteasome related | ||||
| PSMD2 | 26S proteasome non-ATPase regulatory subunit 2 | IPI00012268 | M, S | |
| RAD23B | UV excision repair protein RAD23 homolog B | IPI00008223 | M | |
| Ubiquitin-related | ||||
| SQSTM1 | Sequestosome-1 | IPI00179473 | M, S, G | |
| UBB | Ubiquitin B | IPI00179330 | M, S, G | |
|
| ||||
| Ion channel | ||||
| GABRR1 | Gamma-aminobutyric-acid receptor rho-1 subunit | IPI00747264 | M, S, G | |
| KCTD2 | Potassium channel tetramerisation domain containing 2 | IPI00440769 | M | |
| VDAC1 | Voltage-dependent anion-selective channel protein 1 | IPI00216308 | M | |
|
| ||||
| Cellular organization | ||||
| Cytoskeleton | ||||
| ACTA1 | Actin, alpha skeletal muscle | IPI00021428 | S | |
| ACTB | Actin, cytoplasmic 1 | IPI00021439 | S, G | |
| ACTBL2 | Beta-actin-like protein 2 | IPI00003269 | G | |
| TUBA1A | Tubulin alpha-1A chain | IPI00180675 | M, G | |
| TUBB | Tubulin beta, class I | IPI00645452 | M, S, G | |
| TUBB2A | Tubulin beta-2A chain | IPI00013475 | S | |
| TUBB3 | Tubulin beta-3 chain | IPI00013683 | M, S, G | |
| Motor protein | ||||
| MYH10 | Myosin-10 | IPI00397526 | M | |
| MYH9 | Myosin-9 | IPI00019502 | M, S | |
| MYO6 | Myosin VI | IPI00008455 | M | |
| Other | ||||
| SUN2 | Sad1/unc-84-like protein 2 | IPI00295940 | M | |
|
| ||||
| Signal transduction | ||||
| Adapter protein | ||||
| SFN | 14-3-3 protein sigma | IPI00013890 | S | |
| YWHAB | 14-3-3 protein beta/alpha | IPI00216318 | S | |
| YWHAZ | 14-3-3 protein zeta/delta | IPI00021263 | S | |
| Calcium ion binding | ||||
| CALML5 | Calmodulin-like protein 5 | IPI00021536 | S | |
| S100A7 | S100 calcium binding protein A7 | IPI00219806 | M | |
| S100A8 | S100 calcium binding protein A8 | IPI00007047 | S | |
| S100A9 | S100 calcium binding protein A9 | IPI00027462 | M, S | |
| Other | ||||
| PTPLAD1 | Protein-tyrosine phosphatase-like A domain-containing protein 1 | IPI00008998 | M | |
|
| ||||
| DNA binding | ||||
| Histone | ||||
| H2AFV | Histone H2A.V | IPI00018278 | M | |
| HIST1H4A | Histone H4 | IPI00453473 | S | |
| Transcription factor | ||||
| SKP1 | S-phase kinase-associated protein 1 | IPI00172421 | M | |
|
| ||||
| Metabolism | ||||
| Amino acid metabolism | ||||
| DBT | Lipoamide acyltransferase component of branched-chain alpha-keto acid dehydrogenase complex | IPI00003944 | M | |
| Carbohydrate metabolism | ||||
| C1QBP | Complement component 1 Q subcomponent-binding protein | IPI00014230 | M, S | |
| ENO1 | Alpha-enolase 1 | IPI00465248 | S | |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | IPI00219018 | S | |
| PKM2 | Pyruvate kinase isozymes M2 | IPI00220644 | S | |
| Energy production | ||||
| NDUFS1 | NADH-ubiquinone oxidoreductase 75 kDa subunit | IPI00604664 | M | |
| NDUFV1 | NADH dehydrogenase [ubiquinone] flavoprotein 1 | IPI00028520 | M | |
| NDUFV2 | NADH dehydrogenase [ubiquinone] flavoprotein 2 | IPI00291328 | M | |
| Lipid metabolism | ||||
| FABP5 | Fatty acid-binding protein, epidermal | IPI00007797 | S | |
| GSTP1 | Glutathione S-transferase P | IPI00219757 | M, S | |
| Metabolic enzyme | ||||
| ALDOA | Aldolase A, fructose-bisphosphate | IPI00465439 | S | |
| Nucleic acid metabolism | ||||
| ATP5A1 | ATP synthase subunit alpha, mitochondrial | IPI00440493 | M, S, G | |
| ATP5B | ATP synthase subunit beta, mitochondrial | IPI00303476 | S, G | |
| SLC25A3 | Solute carrier family 25, member 3 | IPI00215777 | M, G | |
| SLC25A4 | Solute carrier family 25, member 4 | IPI00022891 | M | |
| SLC25A5 | Solute carrier family 25, member 5 | IPI00007188 | M, G | |
| SLC25A6 | Solute carrier family 25, member 6 | IPI00291467 | M, S, G | |
| Other | ||||
| ATAD3A | ATPase family AAA domain-containing protein 3A | IPI00295992 | M | |
|
| ||||
| Miscellaneous | ||||
| Apoptosis | ||||
| CSE1L | Isoform 1 of exportin-2 | IPI00022744 | M | |
| LGALS7 | Lectin, galactoside-binding, soluble, 7 | IPI00219221 | S | |
| Other | ||||
| AHNAK | AHNAK nucleoprotein isoform 1 | IPI00021812 | M | |
| ANXA2 | Annexin A2 | IPI00418169 | S | |
| IMMT | Isoform 1 of mitochondrial inner membrane protein | IPI00009960 | M | |
| LRRC59 | Leucine-rich repeat-containing protein 59 | IPI00396321 | M | |
| NOMO1 | Nodal modulator 1 precursor | IPI00329352 | M | |
MS/MS methods: M, MudPIT; S, SoLC-MS/MS; G, GeLC-MS/MS
To evaluate the false discovery rate (FDR) of the ρ1 receptor interactome, using the same criteria for the identification of proteins that were enriched in the ρ1 overexpression sample after immunoprecipitation (see Experimental Procedures), we identified 10 proteins that were enriched in the EV control sample after immunoprecipitation (see Supplemental Table S3 for protein list). Most of the 10 proteins have high abundance numbers (Supplemental Table S3), including two ribosomal proteins. We define: FDR (ρ1 receptor interactome) = Number of proteins that are enriched in EV control sample / Number of proteins that are enriched in ρ1 receptor overexpression sample after immunoprecipitation. Therefore, FDR (ρ1 receptor interactome) = 10 / 107 × 100% = 9.3%, which indicates that our selected criteria to identify the ρ1 receptor interactome were reasonably stringent to filter out most of the potential false positives. However, further validation by orthogonal methods is necessary to confirm a bona fide interaction between the ρ1 receptor and all our identified interactors.
The three tandem MS methods identified proteins with different properties. We performed a cellular protein abundance analysis of the ρ1 receptor interactome using the database from PaxDb: Protein Abundance Across Organisms (http://pax-db.org) (Supplemental Table S4). The proteins that were recognized by different tandem MS methods were plotted against their abundance (Figure 2B). Clearly, MudPIT identified more low abundant proteins compared to SoLC-MS/MS and GeLC-MS/MS; SoLC-MS/MS identified more low abundant proteins and more high abundant proteins than GeLC-MS/MS under our experimental conditions. Among the nine proteins that are commonly identified by three tandem MS methods, seven are abundant proteins, including two cytoskeleton proteins (TUBB and TUBB3), CANX, UBB, SLC25A6, ATP5A1 and overexpressed GABRR1 (Figure 2B).
We analyzed the ability of the three tandem MS methods for the identification of membrane proteins. From the 29 membrane proteins identified (Supplemental Table S4), MudPIT identified 27, SoLC-MS/MS identified 7, and GeLC-MS/MS identified 7 (Figure 2C). Among all three tandem MS methods, the 4 common proteins are fairly abundant: ATP5A1 (abundance: 764), CANX (abundance: 296), overexpressed GABRR1 and SLC25A6 (abundance: 226). Furthermore, MudPIT demonstrated much greater capability of identifying low abundant membrane proteins, such as KCTD2 (abundance: 0.4), NOMO1 (abundance: 2.4), SUN2 (abundance: 5.8), SEC16A (abundance: 6.9) and DERL1 (abundance: 7.2).
GABA-ρ1 receptor interacting proteins are distributed in subcellular locations along with protein biogenesis and trafficking pathway
We analyzed the subcellular distribution of GABA-ρ1 receptor interacting proteins (Figure 2D and Supplemental Table S4). The subcellular distribution information was obtained from Gene Ontology Database, the Human Protein Reference Database (http://www.hprd.org) and Pubmed Gene Database (http://www.ncbi.nlm.nih.gov/gene). For a protein with more than one cellular location, only the primary location was chosen for our analysis. 59 GABA-ρ1 receptor interacting proteins are in the cytoplasm, 19 in the ER, 2 in the Golgi, 14 in the mitochondria, 9 in the nucleus, and 4 in the plasma membrane. The GABA-ρ1 receptor interacting proteins are distributed ubiquitously in the cell because the ρ1 subunit is synthesized in the ribosome, co-translationally translocated onto the ER membrane for folding and assembly 28–30, and trafficked to the Golgi and the plasma membrane for function. Biogenesis of GABA-ρ1 receptors requires their interaction with a network of proteins in the cell. It is noteworthy that 18% of GABA-ρ1 receptor interacting proteins are in the ER, an organelle for protein folding, because the ρ1 subunit has a very large 260-residue ER luminal N-terminus after the cleavage of the 21-residue signal peptide.
Enrichment of proteostasis network components from GABA-ρ1 receptor interacting proteins
We analyzed the molecular and cellular function from GABA-ρ1 receptor interacting proteins using Ingenuity IPA analysis and Pubmed Gene Database (http://www.ncbi.nlm.nih.gov/gene) (Figure 2E and Table 1). We identified 11 proteins that are involved in cellular organization, providing structural support; 8 proteins that are involved in signal transduction, including three 14-3-3 family proteins. It is noteworthy that 53% of ρ1 receptor interacting proteins are involved in protein synthesis, folding, trafficking, and degradation functions, suggesting that the proteostasis network components play a critical role in regulating ρ1 receptor protein level. For example, under the protein folding category, major molecular chaperone systems, including Hsp60, Hsp70, and Hsp90 family members and their co-chaperones, were identified to assist the folding of ρ1 receptors in the cell (Table 1). Since the ρ1 subunit is a glycoprotein, lectin chaperones, including calreticulin and calnexin (CANX), and N-glycoprotein processing enzymes, were identified as responsible for assisting the glycosylation and folding of the ρ1 subunit. Vesicle trafficking components, such as COPA, SEC13, and LMAN1, were identified as assisting in transport of the ρ1 subunit between the ER and the Golgi (see below and Figure 7 for more detailed discussion). Misfolded GABAC receptors are recognized by the cellular protein quality-control machinery, leading to ER-associated degradation (ERAD), retrotranslocation into the cytosol, and degradation by the proteasome 5, 31. Therefore, ERAD factors, such as VCP and derlin-1, were identified.
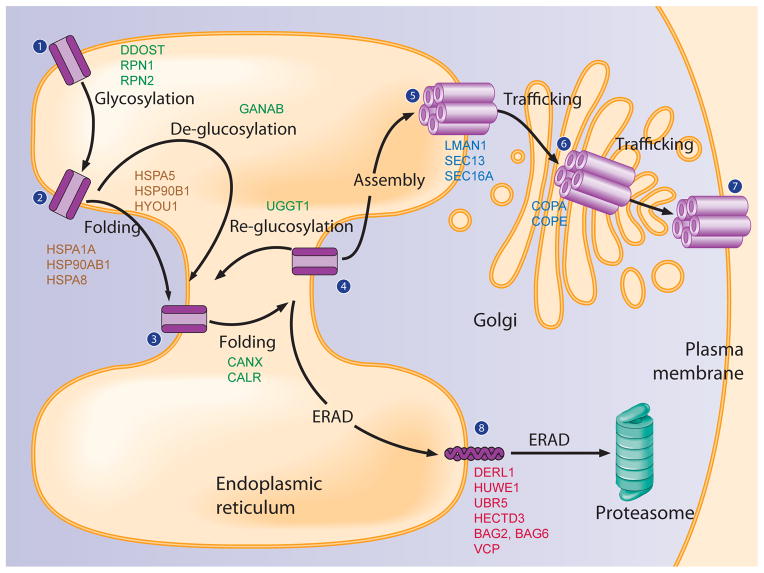
FIGURE 7.
Assembly of the proteostasis network components for GABAC-ρ1 receptors. Proteins that are identified from our MS analysis are included in each step. The ρ1 subunit is co-translationally translocated onto the ER membrane for folding (State 1). Upon entering the ER, it is N-glycosylated (DDOST, RPN1, RPN2) for maturation (State 2). The ρ1 subunit undergoes two independent but collaborative folding pathways from state 2 to stage 4: one is glycan dependent and assisted by lectin chaperones in the ER (CANX, CALR); the other is glycan independent and assisted by heat shock protein family chaperones both in the ER (HSPA5, HYOU1, HSP90B1) and in the cytosol (HSPA1A, HSP90AB1, HSPA8). At stage 4, the ρ1 subunit faces three different pathways: first, if the ρ1 subunits fold properly, they assemble into a homopentamer (State 5) for trafficking to the Golgi (State 6) (LMAN1, SEC13, SEC16A) and plasma membrane (State 7); second, if the ρ1 subunits fail to fold into their native structures, misfolded ρ1 subunits may be re-glucosylated (UGGT1) and re-enter the calnexin / calreticulin folding cycles; third, terminally misfolded ρ1 subunits (State 8) undergo ERAD (DERL1, HUWE1, UBR5, HECTD3, BAG2, BAG6, VCP), being retrotranslocated from the ER into the cytosol and degraded by the proteasome. Proteins that are involved in the N-glycan processing pathways are indicated in green; heat shock protein family members are in brown; trafficking proteins are in blue; and ERAD proteins are in red.
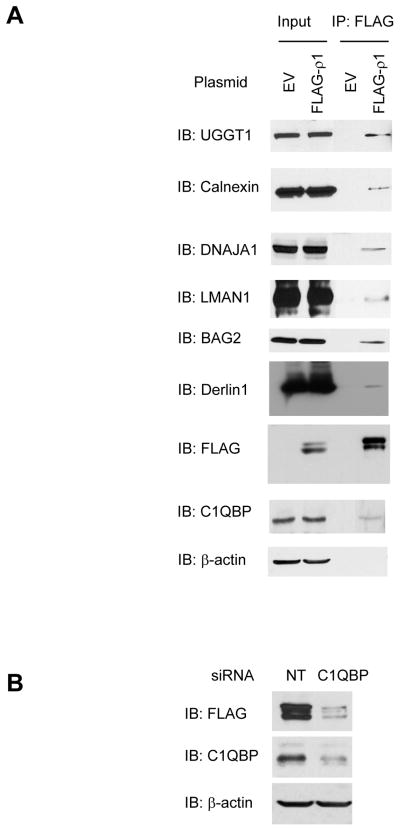
The interaction between representative ρ1 receptor proteostasis network components and ρ1 receptors was verified using immunoprecipitation and Western blot analysis. Folding components (calnexin, UGGT1 and DNAJA1), trafficking components (LMAN1), and degradation components (BAG2 and derlin-1) were shown to interact with ρ1 receptors (Figure 3A).
FIGURE 3. Verification of representative GABAC-ρ1 receptor interactors.
(A) Immunoprecipitation and Western blot analysis verifies the interaction between the ρ1 receptor and selected ρ1 receptor interactors in HEK293 cells expressing FLAG-ρ1 receptors. Proteins that are potentially involved in protein folding (UGGT1, calnexin, and DNAJA1), trafficking (LMAN1), degradation (BAG2 and Derlin1), and metabolism (C1QBP) are shown. β-actin serves as a loading control.
(B) Knockdown of C1QBP, a novel ρ1 receptor interactor, decreases the total FLAG-ρ1 protein level in HEK293 cells expressing FLAG-ρ1 receptors, indicating that C1QBP plays a role in regulating ρ1 receptor proteostasis. NT: non-targeting control siRNA.
We also identified 18 ρ1 receptor interactors that are related to basic metabolism (Table 1 and Supplemental Table S4). Even though similar cases have been reported previously, such as interactors for α7 nicotinic acetylcholine receptors 32 or long form AMPA receptors 33, those proteins could be false positives because most of them are abundant cellular proteins; on the other hand, we were intrigued to investigate whether some of our identified interactors could be novel ρ1 receptor proteostasis network components. We chose to study C1QBP (Complement component 1 Q subcomponent-binding protein), a 34-kDa multifunctional protein, for its potential role in regulating ρ1 receptor proteostasis. The interaction between C1QBP and ρ1 receptors was verified using immunoprecipitation and Western blot analysis (Figure 3A). Furthermore, knockdown of C1QBP using siRNA clearly decreased the total FLAG-ρ1 protein level in HEK293 cells expressing FLAG-ρ1 receptors, indicating that C1QBP enhanced ρ1 receptor proteostasis (Figure 3B). Previously, C1QBP (also called gC1q-R) was identified to interact with β subunits, but not α1 or γ2 subunits of GABAA receptors 34. Although C1QBP was believed to be localized predominantly in the mitochondria, it was reported to appear also in the plasma membrane and in the ER 35. Furthermore, C1QBP was proposed to play a critical role in a chaperone-mediated control of the protein kinase C μ activation 36. Therefore, our data implicated that C1QBP might promote the ρ1 receptor folding and trafficking in the ER or stabilize the ρ1 receptor in the plasma membrane. The detailed mechanism of C1QBP in regulating ρ1 receptor proteostasis merits further investigation.
Verification of critical GABA-ρ1 receptor folding and trafficking components
We selected calnexin (CANX) from ρ1 subunit folding components and LMAN1 from ρ1 subunit trafficking components for more detailed study. We knocked down calnexin or LMAN1 using siRNA and monitored its effect on the maturation of the ρ1 subunit using endoglycosidase H (endo H) enzyme digestion and Western blot analysis. HEK293 cells were co-transfected with FLAG-ρ1 plasmids and siRNA against calnexin, LMAN1, or non-targeting (NT) control for 48 hr before being lysed for endo H digestion and Western blot analysis (Figure 4). The endo H enzyme selectively cleaves after asparaginyl-N-acetyl-D-glucosamine in the N-linked glycans incorporated on the ρ1 subunit in the ER, but it is not possible to remove this oligosaccharide chain after the high-mannose form is enzymatically remodeled in the Golgi. Therefore, endo H resistant ρ1 subunit bands represent properly folded, post-ER ρ1 subunit glycoforms, which traffic at least to the Golgi compartment. The Peptide-N-Glycosidase F (PNGase F) enzyme cleaves between the innermost GlcNAc and asparagine residues from N-linked glycoproteins, serving as a control for unglycosylated ρ1 subunits. PNGase F treatment reduced the double bands for ρ1 subunits, which represents different ρ1 subunit glycosylation states, to one single band (Figure 4A, lane 2). Knockdown of calnexin clearly decreased upper endo H resistant post-ER ρ1 glycoform (Figure 4A, cf. lane 4 to lane 1), indicating that calnexin facilitates the folding of the ρ1 subunit in the ER. Knockdown of LMAN1 clearly decreased upper endo H resistant mature ρ1 glycoform (Figure 4B, cf. lane 4 to lane 1), indicating that LMAN1 plays a critical role in transporting ρ1 receptors from the ER to the Golgi.
We further confirmed calnexin’s role in assisting the folding of the ρ1 subunit in the ER by overexpressing calnexin. HEK293 cells were co-transfected with FLAG-ρ1 plasmids and a calnexin plasmid or a GFP negative control plasmid for 48 hr before being lysed for endo H digestion and Western blot analysis. Clearly, overexpression of calnexin is sufficient to increase the endo H resistant post-ER ρ1 glycoform (Figure 4C, cf. lane 4 to lane 2). Endo H digestion did not decrease the intensity of the upper band for the ρ1 subunit (Figure 4C, cf. lane 2 to lane 1 and lane 4 to lane 3), indicating that the upper band of the ρ1 subunit double bands represents mature post-ER ρ1 subunit glycoform.
The interaction between calnexin and the ρ1 subunit correlates with the maturation level of wild type and mutant ρ1 receptors
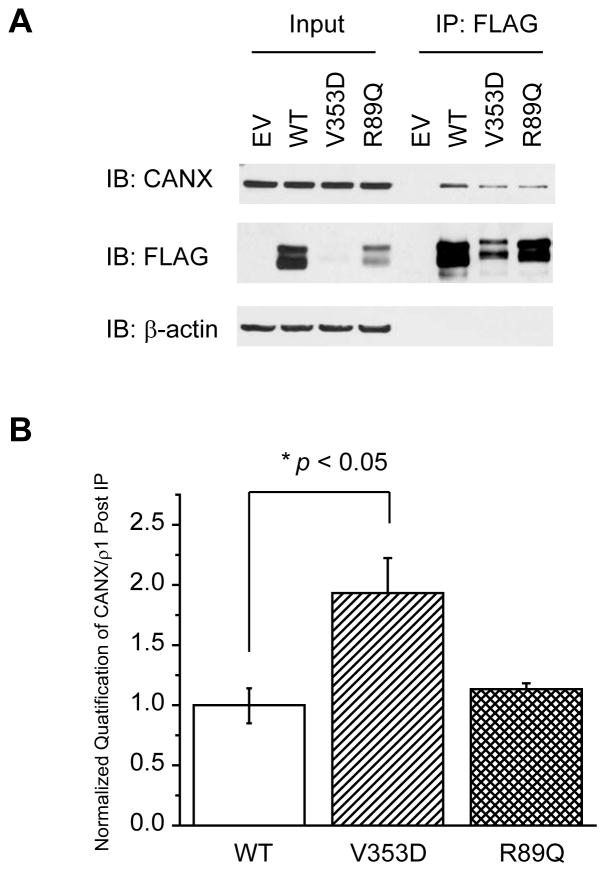
Because calnexin binds to the ρ1 subunit (Figure 3A) and assists its folding (Figures 4A and 4C), we further studied how the interaction between calnexin and the ρ1 subunit is related to the maturation of the ρ1 subunit in the ER. It was reported that the A322D mutation in the GABAA receptor α1 subunit leads to extensive misfolding and ERAD of the α1(A322D) subunit 37, 38 and that the R43Q mutation in the GABAA receptor γ2 subunit leads to inefficient assembly of the γ2(R43Q) subunit with other subunits 39–41. Those two mutations caused trafficking deficiency of corresponding subunits, leading to loss of GABAA receptor function and thus promoting epilepsy. The homologous mutations in the ρ1 subunit are V353D and R89Q. HEK293 cells were transiently transfected with a plasmid containing empty vector (EV) control, FLAG-ρ1, FLAG-ρ1(V353D), or FLAG-ρ1(R89Q) for 48 hr before being lysed. Compared to WT, the V353D or R89Q mutation clearly decreased the total ρ1 subunit protein level as well as the upper mature post-ER ρ1 subunit glycoform (Figure 5A, cf. lanes 3 and 4 to lane 2). The V353D mutation resulted in much less ρ1 subunit proteins than the R89Q mutation, possibly due to more extensive ERAD.
FIGURE 5.
Calnexin binds to WT and mutant GABAC-ρ1 receptors with different interaction capacities. The V353D or R89Q mutation decreases the top mature ρ1 glycoform compared with WT ρ1 receptors. HEK293 cells harboring EV control, WT, V353D, or R89Q ρ1 receptors were immunoprecipitated using anti-FLAG antibody and detected with anti-calnexin antibody. β-actin serves as a loading control. Quantification of (A) is shown in (B) and reported as mean ± SEM. Any statistical significance is calculated using a two-tailed Student’s t-Test. * p < 0.05.
HEK293 cells expressing FLAG-tagged ρ1 subunits were immunoprecipitated using anti-FLAG antibody before being subjected to Western blot analysis (Figure 5A). Clearly, the V353D mutation significantly increased the ratio of immunoprecipitated calnexin/ρ1 by 1.8-fold compared with WT (Figure 5A, cf. lane 7 to lane 6), whereas the R89Q mutation did not significantly change the ratio of immunoprecipitated calnexin/ρ1 compared with WT (Figure 5A, cf. lane 8 to lane 6) (see Figure 5B for quantification). Presumably, the V353D mutation caused extensive misfolding of the ρ1(V353D) subunit; therefore, the interaction between calnexin and the ρ1(V353D) subunit was significantly enhanced to fold this misfolding-prone subunit. On the other hand, the R89Q mutation presumably caused an insufficient subunit assembly after its folding; therefore, the interaction between calnexin and the ρ1(R89Q) subunit was not changed significantly compared with the WT case.
IPA analysis of the ρ1 receptor interactome enriches transcription factors that regulate the heat shock response in the cytosol or the unfolded protein response in the ER
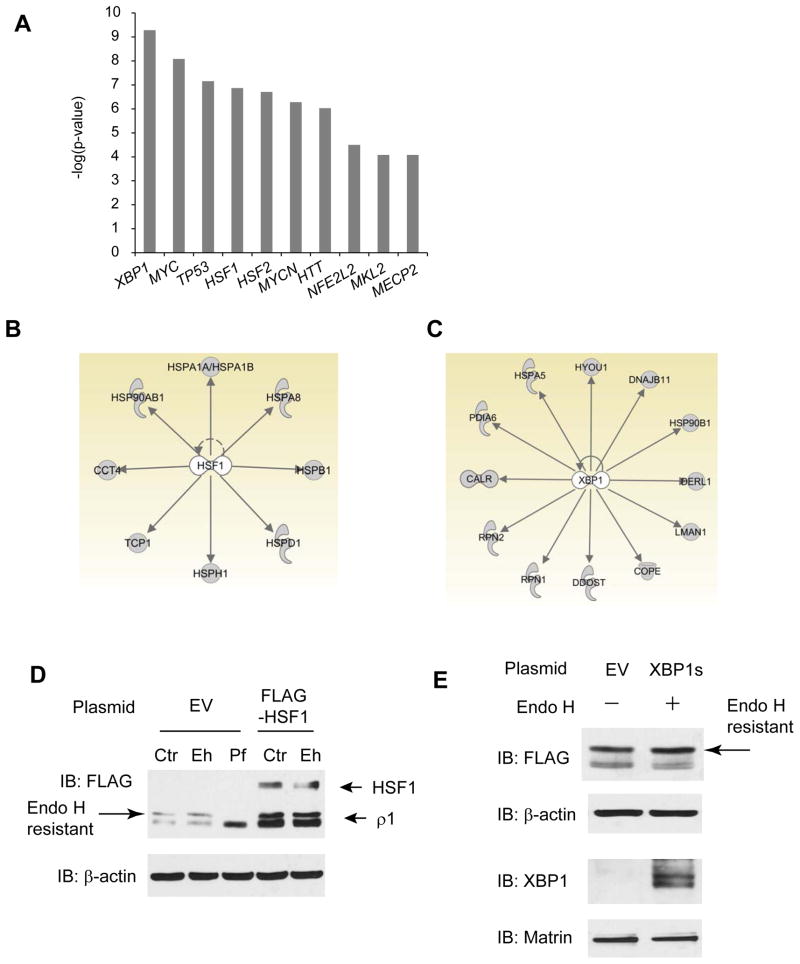
The top ten transcription factors associated by IPA with the ρ1 receptor interactome are: XBP1, MYC, TP53, HSF1, HSF2, MYCN, HTT, NFE2L2, MKL2, and MECP2 (Figure 6A). XBP1 and HSF1 are key transcription factors that enhance cellular folding capacity by up-regulating the expression of molecular chaperones and folding enzymes (see below).
FIGURE 6. Global analysis of transcription factors that regulate the GABAC-ρ1 receptor interactome.
(A) Top 10 transcription factors that are enriched from the GABA-ρ1 receptor interactome by using IPA analysis.
(B and C) HSF1 regulates the expression of 8 cytosolic chaperones within the GABA-ρ1 receptor interactome (B), and XBP1 regulates the expression of 12 proteins belonging to the ER proteostasis network components within the GABA-ρ1 receptor interactome (C).
(D and E) Overexpression of HSF1 (D) or XBP1 (E) increases endo H resistant post-ER ρ1 glycoform in HEK293 cells overexpressing FLAG-ρ1 receptors. EV: empty vector control plasmid; Eh: endo H enzyme; Pf: PNGase F enzyme. β-actin serves as a loading control. Matrin serves as a nuclear protein loading control (E).
Heat shock response transcription factor 1 (HSF1) is a master regulator of the heat shock response (HSR), which is a well-established stress-responsive pathway in the cytosol 23, 42. HSF1 activation upregulates the expression of many heat shock proteins in the cytosol and enhances the protein folding capacity in the cytosol. IPA analysis maps 8 proteins that are regulated by HSF1 activation within our GABA-ρ1 subunit interactome, including CCT4, HSP90AB1, HSPA1A, HSPA8, HSPB1, HSPD1, HSPH1, and TCP1 (Figure 6B).
XBP1 is a key transcription factor during the unfolded protein response (UPR), which is a conserved ER stress-responsive signaling pathway to enhance the ER folding environment 6, 43. The ER responds to the accumulation of unfolded proteins by activating up to three integrated intracellular signaling pathways (IRE1, ATF6 and PERK), collectively referred to as the UPR. UPR activation upregulates the expression of many chaperones and folding enzymes in the ER and promotes protein folding in the ER. IRE1 responds to stress by oligomerization, resulting in trans-autophosphorylation that activates its endonuclease function, which precisely splices the mRNA that encodes the transcription factor XBP1. Spliced XBP1 is translocated to the nucleus, upregulates the expression of many chaperones and folding enzymes in the ER, and promotes protein folding in the ER. IPA analysis indicates 12 proteins that are regulated by XBP1 activation within our GABA-ρ1 subunit interactome, including CALR, PDIA6, HSPA5, HYOU1, DNAJB11, HSP90B1, DERL1, LMAN1, COPE, DDOST, RPN1, and RPN2 (Figure 6C).
IPA pathway analysis predicts that XBP1 and HSF1 play a key role in regulating ρ1 subunit proteostasis. Since ρ1 subunits have both ER components and cytoplasmic components (Figure 1A), the involvement of both the UPR and HSR is expected to be important in the regulation of the ρ1 subunit proteostasis. To test whether the UPR or the HSR indeed influence ρ1 subunit proteostasis, HEK293 cells were transfected with a FLAG-ρ1 plasmid for 48 hr and then with a FLAG-HSF1 plasmid or an EV control plasmid for 18 hr before endo H enzyme digestion and Western blot analysis. HSF1 overexpression clearly increased both total (Figure 6D, cf. lane 4 to lane 1) and endo H resistant (Figure 6D, cf. lane 5 to lane 2) ρ1 subunit protein levels, indicating that HSR activation enhanced the cytosolic folding environment globally to promote ρ1 subunit folding and trafficking. Analogously, HEK293 cells were transfected with a FLAG-ρ1 plasmid for 48 hr and then with a spliced XBP1 (XBP1s) plasmid or an EV control plasmid for 6 hr before endo H enzyme digestion and Western blot analysis. XBP1s overexpression modestly increased the upper post-ER endo H resistant ρ1 subunit protein level (Figure 6E, cf. lane 2 to lane 1), indicating that UPR activation enhanced the ER folding environment globally to promote ρ1 subunit folding and trafficking. Determining the specific downstream proteins that HSF1 (Figure 6B) or XBP1 regulate (Figure 6C) to promote ρ1 subunit folding and trafficking are key future studies in this area.
Assembly of GABA-ρ1 receptor proteostasis network components
The comprehensive identification of GABA-ρ1 receptor interacting proteins from three tandem MS methods enabled us to assemble the proteostasis network components for GABA-ρ1 receptors (Figure 7). We focused on placing ρ1 receptor interacting proteins in the context of assisting protein folding, trafficking, and degradation based on our current knowledge of the general proteostasis network.
The ρ1 subunit (State 1, Figure 7) is co-translationally translocated onto the ER membrane for folding. Asparagine N-linked glycosylation is the most common protein modification in the ER and occurs co-translationally, serving as recognition tags for glycoprotein maturation 44, 45. The ρ1 subunit has three potential N-glycosylation sites (Asn140, Asn234, and Asn274) in the ER lumen. N-linked glycosylation of the ρ1 subunit (State 2, Figure 7) is catalyzed by the oligosaccharyltransferase (OST) complex, which transfers 14-monosaccharide residues Glc3Man9GlcNAc2 (Glc: glucose, Man: mannose, GlcNAc: N-acetylglucosamine) to an Asn residue contained in a Asn-X-Ser/Thr sequence motif 46. The OST complex contains seven subunits; three subunits were successfully identified in our tandem MS analysis, namely, dolichyl-diphosphooligosaccharide-protein glycosyltransferase (DDOST, gene symbol), ribophorin I (RPN1), and ribophorin II (RPN2), indicating that upon entering the ER, the ρ1 subunit uses the OST complex to attach core oligosaccharides. RPN1, RPN2 and DDOST are abundant proteins, all containing large ER lumen domains. Successful identification of RPN1, RPN2 and DDOST, but not the other four OST subunits, might depend on the high cellular abundance, accessible topology by trypsin digestion, and good peptide response to tandem MS analysis of RPN1, RPN2 and DDOST.
From State 2 to State 4, the ρ1 subunit undergoes two independent but collaborative folding pathways: one is glycan dependent and assisted by lectin chaperones in the ER 47; the other is glycan independent and assisted by heat shock protein family chaperones both in the ER and in the cytosol 8, 48.
During the glycan-dependent folding pathway, two outmost glucoses in Glc3Man9GlcNAc2 are removed by ER glucosidase I and II sequentially (State 3 for the ρ1 subunits, Figure 7). The α subunit of ER glucosidase II (GANAB) was identified in our tandem MS analysis, indicating the importance of glucose trimming for the ρ1 subunit folding. The monoglucosylated ρ1 subunit enters the calnexin / calreticulin folding cycles 49. Our tandem MS analysis identified calnexin (CANX), calreticulin (CALR), and several folding enzymes including protein disulfide isomerases (PDIases) (P4HB and PDIA6) in the ER and one peptidylprolyl isomerase (PPIases) (PPIA). Our ρ1 subunit interactome data indicate that calnexin and calreticulin potentially facilitate the folding of the ρ1 subunit with help from other folding enzymes. Calnexin was shown to promote ρ1 subunit folding in the ER (Figures 4A and 4C).
GABA-ρ1 receptors also recruit non-lectin heat shock protein chaperones for their folding. Heat shock proteins bind to hydrophobic patches of unfolded proteins, facilitating their folding while preventing aggregation 23. Because the ρ1 subunit contains both ER and cytosolic components, both heat shock proteins in the ER and in the cytosol contribute to assisting its proper folding. Our tandem MS analysis identified major chaperone systems: BiP (HSPA5) and GRP94 (HSP90B1) in the ER; Hsp70 (HSPA1A), Hsc70 (HSPA8) and Hsp90β (HSP90AB1) in the cytosol. A number of co-chaperones were identified from our tandem MS analysis: DNAJA1 and DNAJA2 (co-chaperones of Hsc70), DNAJB11 and HYOU1 (co-chaperones of BiP). In association with its co-chaperones, our identified heat shock protein chaperones may facilitate ρ1 subunit folding, which will be tested in our future study.
After the collaborative glycan-dependent and glycan-independent folding, the ρ1 subunit enters State 4 (Figure 7). At stage 4, the ρ1 subunit faces three different pathways: first, if the ρ1 subunits fold properly, they assemble into a homopentamer (State 5 for ρ1 receptors, Figure 7) for trafficking; second, if the ρ1 subunits fail to fold into their native structures, misfolded ρ1 subunits may be re-glucosylated by UDP-glucose glycoprotein glucosyltransferase 1 (UGGT1, identified from our tandem MS analysis) and re-enter the calnexin / calreticulin folding cycles; third, terminally misfolded ρ1 subunits (state 8, Figure 7) undergo ER-associated degradation (ERAD), being retrotranslocated from the ER into the cytosol and degraded by the proteasome 50.
Properly folded and assembled ρ1 receptors (State 5 for ρ1 receptors, Figure 7) exit the ER, traffic through the Golgi (State 6 for ρ1 receptors, Figure 7), and reach the plasma membrane (state 7 for ρ1 receptors, Figure 7). Trafficking components that were identified from our tandem MS analysis include SEC13 and SEC16A in the COPII machinery, which is responsible for anterograde (forward) cargo protein vesicle transport from the ER to the Golgi 51, 52; COPA and COPE in the COPI machinery, which is responsible for retrograde (backward) retrieval of cargo proteins 53; and LMAN1 (also called ERGIC-53), which is a cargo receptor for the transport of glycoproteins from the ER to the ER-Golgi intermediate compartment (ERGIC) 54. LMAN1 was shown to facilitate ρ1 subunit trafficking from the ER to the Golgi (Figure 4B).
Terminally misfolded ρ1 subunits (state 8, Figure 7) are subjected to ER-associated degradation (ERAD): the ρ1 subunits are ubiquitinated, retrotranslocated from the ER membrane to the cytosol, and degraded by the proteasome 50. The ρ1 subunit interactome contains three E3 ubiquitin ligases: EDD1 (also called UBR5), HECTD3 and HUWE1. BAG2, a co-factor of an E3 ubiquitin ligase CHIP, was recognized in our tandem MS result. Derlin-1 (DERL1), an integral ER transmembrane protein, is among the ρ1 subunit interactome; it serves as a potential ρ1 subunit retrotranslocation channel. The ρ1 subunit interactome contains VCP (also called p97). VCP is an AAA ATPase protein, serving as a prominent candidate to extract misfolded ρ1 subunits from the ER membrane to the cytosol for degradation, which will be tested in our future study.
Specificity of GABA-ρ1 receptor proteostasis network components
Ion channel proteins utilize a subset of chaperones/co-chaperones, folding enzymes and ERAD factors to regulate their proteostasis, partially depending on their topology. Here, we identified numerous chaperones and ERAD factors both in the ER and in the cytosol that interact with the ρ1 subunit (Table 1 and Figure 7). Although most of our identified ρ1 subunit proteostasis network components have their known general role in protein folding or degradation, their specific role in regulating ρ1 subunit proteostasis might differ from their role for other client proteins and remains to be established.
The classical Hsp70/Hsp90 chaperone system and calnexin / calreticulin lectin chaperone system are the two major chaperone systems in the ER 55. Although cytosolic Hsp90 was reported to interact with and promote the maturation of human ether-a-gogo-related gene (hERG) potassium channels, Grp94 (Hsp90 in the ER) did not interact with hERG channels 56, which might be because hERG channels contain very short ER lumen components. Our identified ρ1 subunit interactome contains both cytosolic Hsp90 (HSPAB1) and ER Grp94 (HSP90B1), implying that both Hsp90 and Grp94 regulate ρ1 subunit proteostasis. Calnexin was reported to interact with both WT and ΔF508 cystic fibrosis transmembrane conductance regulator (CFTR) protein 57; however, overexpression of calnexin did not promote the maturation of WT and ΔF508 CFTR 58. In our case, overexpression of calnexin clearly promoted the maturation of the ρ1 receptors (Figure 4C). This indicates that the role of calnexin in assisting ion channel folding depends on its specific client proteins. Cytosolic Hsp70 (HSPA1A) and Hsc70 (HSPA8) are among the ρ1 receptor interactome (Table 1). Interestingly, although both Hsp70 and Hsc70 associated with hERG channels, it was reported that Hsp70 enhanced the maturation of hERG channels while Hsc70 suppressed their maturation 59. The role of Hsp70 and Hsc70 in regulating the ρ1 receptor proteostasis needs to be explored. The ρ1 receptor interactome contains Hsp40 family members, including DNAJA1 and DNAJA2, and folding enzymes, including PPIA, a PPIase. It was reported that DNAJA1 and DNAJA2 promoted the degradation of hERG channels 60 while FKBP8, a PPIase, promoted the trafficking of hERG channels 61. The role of DNAJA1 and DNAJA2 and PPIA need to be established in the context of regulating ρ1 receptor proteostasis. The GABAC receptors belong to the Cys-loop superfamily of ligand-gated ion channels. To date, the only known specific chaperone in the Cys-loop superfamily is RIC-3 (Resistant to inhibitors of cholinesterase-3) 62. RIC-3 interacts with and regulates the maturation of some subtypes of nicotinic acetylcholine receptors and 5-hydroxytryptamine type 3 receptors, but not GABA receptors. The ρ1 receptor interactome list might contain GABA receptor-specific chaperones: C1QBP might be a promising target (Figure 3B).
Regarding the ERAD factors, we identified three potential E3 ligases for the ρ1 receptors: HECTD3, HUWE1 and UBR5 (Table 1). Interestingly, all three contain a conserved C-terminal HECT (homologous to E6-AP carboxyl terminus) domain, accounting for their E3 ligase function. HECT E3 ligases are one of the two main groups of E3 ligases 63. All three are low abundant proteins: HECTD3 (abundance: 0.17), UBR5 (abundance: 1.33), and HUWE1 (abundance: 8.24). To date, in mammalian cells over 500 potential E3 ligases were identified and E3 ligases are believed to provide substrate specificity during the protein ubiquitination step 64. Ubiquitination of ion channels have been described with limited examples 65, 66. HECTD3 was reported to regulate the ubiquitination of Tara (Trio-associated repeat on actin) 67; UBR5 was reported to regulate the ubiquitination of β-catenin 68; HUWE1 was reported to regulate the ubiquitination of the N-Myc oncoprotein 69. However, the role of HECTD3, UBR5 and HUWE1 in the ubiquitination of ion channels was not well known yet. Therefore, HECTD3, HUWE1 and UBR5 merit further investigation for their specific role in the ubiquitination of the ρ1 receptor.
CONCLUSIONS
Comprehensive consideration of the three tandem MS analyses identified 107 GABAC-ρ1 subunit interacting proteins (Figure 2A). GeLC-MS/MS, SoLC-MS/MS and MudPIT identified proteins with different properties (Figures 2B and 2C). Employment of three tandem MS approaches enabled us to achieve the maximum coverage of reliable ρ1 receptor interacting protein by choosing arbitrary yet comprehensive criteria to include or exclude a protein in the GABAC-ρ1 receptor interactome (see Experimental Procedures). We focused on assembling proteostasis network components from the identified ρ1 subunit interactome (Figure 7). We studied representative individual GABAC proteostasis network components (Figures 4 and 5) and global effectors that regulate protein folding in cells based on bioinformatics analysis of the total ρ1 subunit interactome (Figure 6). The identification of the GABAC receptor proteostasis network components will enable our future efforts to regulate GABAC receptor folding, trafficking, degradation, and thus function, providing a promising strategy to ameliorate related retinal diseases.
Supplementary Material
Acknowledgments
We thank Dr. Richard Sifers (Baylor College of Medicine) for the pcDNA3.1 plasmid containing a spliced form of XBP1. We thank Dr. Mark R. Chance (Case Western Reserve University) for comments on the manuscript. This work was supported by the Research Start-up Fund from Case Western Reserve University School of Medicine (to T.-W. Mu), Epilepsy Foundation of America (225243) (to T.-W. Mu), the Clinical Translational Science Collaborative of Cleveland CTSA (UL1RR024989) from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health (to T.-W. Mu), and the National Institute of Health (P41 GM103533, R01 MH067880, and P01 AG031097 to J.R. Yates 3rd).
ABBREVIATIONS
- GeLC-MS/MS
gel-based tandem MS analysis
- SoLC-MS/MS
solution-based tandem MS analysis
- MudPIT
Multidimensional Protein Identification Technology
Footnotes
Supplemental Information includes four supplemental tables.
References
- 1.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319(5865):916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 2.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475 (7356):324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 3.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125(3):443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Smith MH, Ploegh HL, Weissman JS. Road to Ruin: Targeting Proteins for Degradation in the Endoplasmic Reticulum. Science. 2011;334(6059):1086–1090. doi: 10.1126/science.1209235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9(12):944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walter P, Ron D. The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 7.Christianson JC, Olzmann JA, Shaler TA, Sowa ME, Bennett EJ, Richter CM, Tyler RE, Greenblatt EJ, Harper JW, Kopito RR. Defining human ERAD networks through an integrative mapping strategy. Nat Cell Biol. 2012;14(1):93–105. doi: 10.1038/ncb2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartl FU, Hayer-Hartl M. Protein folding - Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295(5561):1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 9.Lester HA, Dibas MI, Dahan DS, Leite JF, Dougherty DA. Cys-loop receptors: new twists and turns. Trends Neurosci. 2004;27(6):329–336. doi: 10.1016/j.tins.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Enz R, Cutting GR. Molecular composition of GABA(C) receptors. Vision Res. 1998;38(10):1431–1441. doi: 10.1016/s0042-6989(97)00277-0. [DOI] [PubMed] [Google Scholar]
- 11.Johnston GAR, Chebib M, Hanrahan JR, Mewett KN. Neurochemicals for the Investigation of GABA(C) Receptors. Neurochem Res. 2010;35(12):1970–1977. doi: 10.1007/s11064-010-0271-7. [DOI] [PubMed] [Google Scholar]
- 12.Dougherty DA. Cys-loop neuroreceptors: Structure to the rescue? Chem Rev. 2008;108(5):1642–1653. doi: 10.1021/cr078207z. [DOI] [PubMed] [Google Scholar]
- 13.Thomas P, Smart TG. HEK293 cell line: A vehicle for the expression of recombinant proteins. J Pharmacol Toxicol Methods. 2005;51(3):187–200. doi: 10.1016/j.vascn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 14.McCall MA, Lukasiewicz PD, Gregg RG, Peachey NS. Elimination of the rho 1 subunit abolishes GABA(C) receptor expression and alters visual processing in the mouse retina. J Neurosci. 2002;22(10):4163–4174. doi: 10.1523/JNEUROSCI.22-10-04163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng W, Zhao X, Wang J, Lu L. Retinal vascular leakage occurring in GABA Rho-1 subunit deficient mice. Exp Eye Res. 2010;90(5):634–640. doi: 10.1016/j.exer.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Link AJ, Eng J, Schieltz DM, Carmack E, Mize GJ, Morris DR, Garvik BM, Yates JR. Direct analysis of protein complexes using mass spectrometry. Nat Biotechnol. 1999;17(7):676–682. doi: 10.1038/10890. [DOI] [PubMed] [Google Scholar]
- 17.Washburn MP, Wolters D, Yates JR. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19(3):242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 18.Peng JM, Schwartz D, Elias JE, Thoreen CC, Cheng DM, Marsischky G, Roelofs J, Finley D, Gygi SP. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21(8):921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 19.Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass-spectral data of peptides with amino-acid-sequences in a protein database. J Am Soc Mass Spectrom. 1994;5(11):976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 20.Xu T, Venable JD, Park SK, Cociorva D, Lu B, Liao L, Wohlschlegel J, Hewel J, Yates JR. ProLuCID, a fast and sensitive tandem mass spectra-based protein identification program. Mol Cell Proteomics. 2006;5(10):S174–S174. [Google Scholar]
- 21.Cociorva D, Tabb DL, Yates JR. Current Protocols in Bioinformatics. Unit 13.4. Wiley; 2006. Validation of tandem mass spectrometry database search results using DTASelect. [DOI] [PubMed] [Google Scholar]
- 22.Tabb DL, McDonald WH, Yates JR. DTASelect and contrast: Tools for assembling and comparing protein identifications from shotgun proteomics. J Proteome Res. 2002;1(1):21–26. doi: 10.1021/pr015504q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 24.Liu HB, Sadygov RG, Yates JR. A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;76(14):4193–4201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 25.Liao L, Pilotte J, Xu T, Wong CCL, Edelman GM, Vanderklish P, Yates JR. BDNF induces widespread changes in synaptic protein content and up-regulates components of the translation machinery: An analysis using high-throughput proteomics. J Proteome Res. 2007;6(3):1059–1071. doi: 10.1021/pr060358f. [DOI] [PubMed] [Google Scholar]
- 26.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan ZP, Stokes M, Sullivan L, Mitchell J, Wetzel R, MacNeill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131(6):1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 27.Cao R, He QY, Zhou J, He QZ, Liu Z, Wang XC, Chen P, Xie JY, Liang SP. High-throughput analysis of rat liver plasma membrane proteome by a nonelectrophoretic in-gel tryptic digestion coupled with mass spectrometry identification. J Proteome Res. 2008;7(2):535–545. doi: 10.1021/pr070411f. [DOI] [PubMed] [Google Scholar]
- 28.Alder NN, Johnson AE. Cotranslational membrane protein biogenesis at the endoplasmic reticulum. J Biol Chem. 2004;279(22):22787–22790. doi: 10.1074/jbc.R400002200. [DOI] [PubMed] [Google Scholar]
- 29.Green WN, Millar NS. Ion-Channel Assembly. Trends Neurosci. 1995;18(6):280–287. [PubMed] [Google Scholar]
- 30.Skach WR. Cellular mechanisms of membrane protein folding. Nat Struct Mol Biol. 2009;16(6):606–612. doi: 10.1038/nsmb.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romisch K. Endoplasmic reticulum-associated degradation. Annu Rev Cell Dev Biol. 2005;21:435–456. doi: 10.1146/annurev.cellbio.21.012704.133250. [DOI] [PubMed] [Google Scholar]
- 32.Paulo JA, Brucker WJ, Hawrot E. Proteomic Analysis of an alpha 7 Nicotinic Acetylcholine Receptor Interactome. J Proteome Res. 2009;8(4):1849–1858. doi: 10.1021/pr800731z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santos SD, Manadas B, Duarte CB, Carvalho AL. Proteomic Analysis of an Interactome for Long-Form AMPA Receptor Subunits. J Proteome Res. 2010;9(4):1670–1682. doi: 10.1021/pr900766r. [DOI] [PubMed] [Google Scholar]
- 34.Schaerer MT, Kannenberg K, Hunziker P, Baumann SW, Sigel E. Interaction between GABA(A) receptor beta subunits and the multifunctional protein gC1q-R. J Biol Chem. 2001;276(28):26597–26604. doi: 10.1074/jbc.M102534200. [DOI] [PubMed] [Google Scholar]
- 35.Soltys BJ, Kang D, Gupta RS. Localization of P32 protein (gC1q-R) in mitochondria and at specific extramitochondrial locations in normal tissues. Histochem Cell Biol. 2000;114(3):245–255. doi: 10.1007/s004180000191. [DOI] [PubMed] [Google Scholar]
- 36.Storz P, Hausser A, Link G, Dedio J, Ghebrehiwet B, Pfizenmaier K, Johannes FJ. Protein kinase C mu is regulated by the multifunctional chaperon protein p32. J Biol Chem. 2000;275(32):24601–24607. doi: 10.1074/jbc.M002964200. [DOI] [PubMed] [Google Scholar]
- 37.Cossette P, Liu LD, Brisebois K, Dong HH, Lortie A, Vanasse M, Saint-Hilaire JM, Carmant L, Verner A, Lu WY, Wang YT, Rouleau GA. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat Genet. 2002;31(2):184–189. doi: 10.1038/ng885. [DOI] [PubMed] [Google Scholar]
- 38.Gallagher MJ, Shen WZ, Song LY, Macdonald RL. Endoplasmic reticulum retention and associated degradation of a GABA(A) receptor epilepsy mutation that inserts an aspartate in the M3 transmembrane segment of the alpha 1 subunit. J Biol Chem. 2005;280(45):37995–38004. doi: 10.1074/jbc.M508305200. [DOI] [PubMed] [Google Scholar]
- 39.Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE, Berkovic SF. Mutant GABA(A) receptor gamma 2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28(1):49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- 40.Sancar F, Czajkowski C. A GABAA receptor mutation linked to human epilepsy (γ2R43Q) impairs cell surface expression of αβγ receptors. J Biol Chem. 2004;279(45):47034–47039. doi: 10.1074/jbc.M403388200. [DOI] [PubMed] [Google Scholar]
- 41.Hales TG, Tang HY, Bollan KA, Johnson SJ, King DR, McDonald NA, Cheng AX, Connolly CN. The epilepsy mutation, gamma 2(R43Q) disrupts a highly conserved inter-subunit contact site, perturbing the biogenesis of GABA(A) receptors. Mol Cell Neurosci. 2005;29(1):120–127. doi: 10.1016/j.mcn.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Westerheide SD, Anckar J, Stevens SM, Sistonen L, Morimoto RI. Stress-Inducible Regulation of Heat Shock Factor 1 by the Deacetylase SIRT1. Science. 2009;323(5917):1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 44.Hebert DN, Garman SC, Molinari M. The glycan code of the endoplasmic reticulum: asparagine-linked carbohydrates as protein maturation and quality-control tags. Trends Cell Biol. 2005;15(7):364–370. doi: 10.1016/j.tcb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Hebert DN, Molinari M. Flagging and docking: dual roles for N-glycans in protein quality control and cellular proteostasis. Trends Biochem Sci. 2012;37(10):404–410. doi: 10.1016/j.tibs.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohorko E, Glockshuber R, Aebi M. Oligosaccharyltransferase: the central enzyme of N-linked protein glycosylation. J Inherit Metab Dis. 2011;34(4):869–878. doi: 10.1007/s10545-011-9337-1. [DOI] [PubMed] [Google Scholar]
- 47.Helenius A, Trombetta ES, Hebert DN, Simons JF. Calnexin, calreticulin and the folding of glycoproteins. Trends Cell Biol. 1997;7(5):193–200. doi: 10.1016/S0962-8924(97)01032-5. [DOI] [PubMed] [Google Scholar]
- 48.van Anken E, Braakman I. Versatility of the endoplasmic reticulum protein folding factory. Crit Rev Biochem Mol Biol. 2005;40(4):191–228. doi: 10.1080/10409230591008161. [DOI] [PubMed] [Google Scholar]
- 49.Rutkevich LA, Williams DB. Participation of lectin chaperones and thiol oxidoreductases in protein folding within the endoplasmic reticulum. Curr Opin Cell Biol. 2011;23(2):157–166. doi: 10.1016/j.ceb.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Brodsky JL. Cleaning Up: ER-Associated Degradation to the Rescue. Cell. 2012;151(6):1163–1167. doi: 10.1016/j.cell.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Routledge KE, Gupta V, Balch WE. Emergent properties of proteostasis-COPII coupled systems in human health and disease. Mol Membr Biol. 2010;27(8):385–397. doi: 10.3109/09687688.2010.524894. [DOI] [PubMed] [Google Scholar]
- 52.Zanetti G, Pahuja KB, Studer S, Shim S, Schekman R. COPII and the regulation of protein sorting in mammals. Nat Cell Biol. 2012;14(1):20–28. doi: 10.1038/ncb2390. [DOI] [PubMed] [Google Scholar]
- 53.Szul T, Sztul E. COPII and COPI Traffic at the ER-Golgi Interface. Physiology. 2011;26(5):348–364. doi: 10.1152/physiol.00017.2011. [DOI] [PubMed] [Google Scholar]
- 54.Hauri HP, Kappeler F, Andersson H, Appenzeller C. ERGIC-53 and traffic in the secretory pathway. J Cell Sci. 2000;113(4):587–596. doi: 10.1242/jcs.113.4.587. [DOI] [PubMed] [Google Scholar]
- 55.Braakman I, Hebert DN. Protein Folding in the Endoplasmic Reticulum. Cold Spring Harb Perspect Biol. 2013;5:a013201. doi: 10.1101/cshperspect.a013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ficker E, Dennis AT, Wang L, Brown AM. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel hERG. Circ Res. 2003;92(12):E87–E100. doi: 10.1161/01.RES.0000079028.31393.15. [DOI] [PubMed] [Google Scholar]
- 57.Pind S, Riordan JR, Williams DB. Participation of the endoplasmic-reticulum chaperone calnexin (p88, ip90) in the biogenesis of the cystic-fibrosis transmembrane conductance regulator. J Biol Chem. 1994;269(17):12784–12788. [PubMed] [Google Scholar]
- 58.Farinha CA, Amaral MD. Most F508del-CFTR is targeted to degradation at an early folding checkpoint and independently of calnexin. Mol Cell Biol. 2005;25(12):5242–5252. doi: 10.1128/MCB.25.12.5242-5252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li P, Ninomiya H, Kurata Y, Kato M, Miake J, Yamamoto Y, Igawa O, Nakai A, Higaki K, Toyoda F, Wu J, Horie M, Matsuura H, Yoshida A, Shirayoshi Y, Hiraoka M, Hisatome I. Reciprocal Control of hERG Stability by Hsp70 and Hsc70 With Implication for Restoration of LQT2 Mutant Stability. Circ Res. 2011;108(4):458–468. doi: 10.1161/CIRCRESAHA.110.227835. [DOI] [PubMed] [Google Scholar]
- 60.Walker VE, Wong MJH, Atanasiu R, Hantouche C, Young JC, Shrier A. Hsp40 Chaperones Promote Degradation of the hERG Potassium Channel. J Biol Chem. 2010;285(5):3319–3329. doi: 10.1074/jbc.M109.024000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walker VE, Atanasiu R, Lam H, Shrier A. Co-chaperone FKBP38 promotes HERG trafficking. J Biol Chem. 2007;282(32):23509–23516. doi: 10.1074/jbc.M701006200. [DOI] [PubMed] [Google Scholar]
- 62.Millar NS. RIC-3: a nicotinic acetylcholine receptor chaperone. Br J Pharmacol. 2008;153:S177–S183. doi: 10.1038/sj.bjp.0707661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol. 2009;10(6):398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]
- 64.Tsai YC, Weissman AM. Ubiquitylation in ERAD: Reversing to Go Forward? PLoS Biol. 2011;9(3):e1001038. doi: 10.1371/journal.pbio.1001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abriel H, Staub O. Ubiquitylation of ion channels. Physiology. 2005;20:398–407. doi: 10.1152/physiol.00033.2005. [DOI] [PubMed] [Google Scholar]
- 66.Staub O, Rotin D. Role of ubiquitylation in cellular membrane transport. Physiol Rev. 2006;86(2):669–707. doi: 10.1152/physrev.00020.2005. [DOI] [PubMed] [Google Scholar]
- 67.Yu J, Lan J, Zhu Y, Li X, Lai X, Xue Y, Jin C, Huang H. The E3 ubiquitin ligase HECTD3 regulates ubiquitination and degradation of Tara. Biochem Bioph Res Co. 2008;367(4):805–812. doi: 10.1016/j.bbrc.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 68.Hay-Koren A, Caspi M, Zilberberg A, Rosin-Arbesfeld R. The EDD E3 ubiquitin ligase ubiquitinates and up-regulates beta-catenin. Mol Biol Cell. 2011;22(3):399–411. doi: 10.1091/mbc.E10-05-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao X, Heng JIT, Guardavaccaro D, Jiang R, Pagano M, Guillemot F, Iavarone A, Lasorella A. The HECT-domain ubiquitin ligase Huwe1 controls neural differentiation and proliferation by destabilizing the N-Myc oncoprotein. Nat Cell Biol. 2008;10(6):643–653. doi: 10.1038/ncb1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.